Abstract
T cell progenitors are known to arise from the foetal liver in embryos and the bone marrow in adults; however different studies have shown that a pool of T cell progenitors may also exist in the periphery. Here, we identified a lymphoid population resembling peripheral T cell progenitors which transiently seed the epidermis during late embryogenesis in both wild-type and T cell-deficient mice. We named these cells ELCs (Epidermal Lymphoid Cells). ELCs expressed Thy1 and CD2, but lacked CD3 and TCRαβ/γδ at their surface, reminiscent of the phenotype of extra- or intra- thymic T cell progenitors. Similarly to Dendritic Epidermal T Cells (DETCs), ELCs were radioresistant and capable of self-renewal. However, despite their progenitor-like phenotype and expression of T cell lineage markers within the population, ELCs did not differentiate into conventional T cells or DETCs in in vitro, ex vivo or in vivo differentiation assays. Finally, we show that ELC expressed NK markers and secreted IFN-γ upon stimulation. Therefore we report the discovery of a unique population of lymphoid cells within the murine epidermis that appears related to NK cells with as-yet-unidentified functions.
Similar content being viewed by others
Introduction
The process of T cell differentiation from hematopoietic precursors has been studied for many years and is relatively well defined. Precursors leave the adult bone marrow (BM), or foetal liver in the case of embryonic T cell development and arrive in the thymus as lymphoid progenitor cells. These progenitors proliferate and populate the thymus with immature thymocytes, which lack expression of the mature T cell markers CD3, CD4 and CD8 1,2. The dual absence of CD4 and CD8 during this phase characterizes the cells as “double-negative” (DN) T cell precursors and their DN status is maintained through four further stages of differentiation named DN 1–4 1,2.
Movement through the DN stages is accompanied by progressive rearrangement of three out of the four T cell receptor (TCR) loci: γ, δ and β. If the TCRβ rearrangement is productive, it permits expression of the TCRβ chain, complexed with the germline-encoded invariant pre-TCRα (pTα)3,4,5,6. Upon expression of the TCRβ/pTα complex, called the pre-TCR, immature thymocytes are licensed to proliferate and rapidly progress to a CD4+CD8+ double-positive (DP) stage7. At this time, rearrangement of the TCRα locus takes place, resulting in expression of mature αβTCR complexes on DP thymocytes. Then DP thymocytes are subjected to positive and negative selection, which will result in death of 95% of thymocytes7. During this selection process, surviving thymocytes begin to down-regulate either CD4 or CD8 expression to become single-positive (SP) CD4+ or CD8+ thymocytes ready for export from the thymus to the periphery as naïve T cells8.
While this is clearly the route followed by the vast majority of T cells, it has recently become apparent that other mechanisms of generating specific T cell populations do exist, both prior to the development of the thymus in the foetus and independent of the thymus after birth. Rodewald et al. first identified a pro-thymocyte in murine foetal blood at a pre-thymic stage of development9 and later work described a phenotypically similar population in the adult mice, defining them as Committed T-cell Progenitors (CTPs)10. The CTPs, which are thought to be the precursors of the more-recently characterised Committed Intermediate Progenitors (CIPs)11, were first detected as an early T cell progenitor in adult murine BM that had the potential to give rise to mature T cells by an extra-thymic pathway12. Consistently, Nude (Foxn1-deficient) mice lack a functional thymus, yet retain some capacity to generate T cell populations in the mucosae13. Even within the populations of T cells that are generated within the thymus during embryonic development, some sub-populations appear capable of thymus-independent self-maintenance in the adult: the skin is a particularly interesting site in this regard. Murine Dendritic Epidermal T Cells (DETCs) are CD3+ T cells characterized by expression of Thy1 (Thymocyte antigen 1) and a unique γδ TCR comprising Vγ3/Jγ1-Cγ1 and Vδ1/Dδ2/Jδ2-Cδ chains14,15,16,17. DETCs are the first TCR-bearing cells to be generated in the foetal thymus, as early as embryonic day 14 (E14.0) of the 20 days of murine gestation18,19. These cells, expressing CD3 and the Vγ3/Vδ1 TCR, then expand, mature and migrate to the skin as early as E16.019,20,21. Within the epidermis, the unique TCR from DETCs is thought to recognize a putative self-antigen on damaged, stressed, or transformed keratinocytes22,23 and this population of T cells has now been shown to have roles in tissue and wound healing24,25,26. Early work on the properties of DETCs uncovered their ability, unusual amongst T cells, to maintain themselves locally, independently from blood circulating precursors19,20. Upon irradiation, the DETC population restores itself without input from any circulating or transplanted BM progenitor27. However, the exact mechanisms underlying the renewal of the DETC population are unknown and the question of whether a DETC progenitor exists in the adult murine epidermis remains without answer.
Here, we used a combination of immune -sufficient and -deficient mouse models to reveal a further layer of complexity within the epidermal lymphoid compartment. We identified a novel population of lymphoid cells, named Epidermal Lymphoid Cells (ELCs), that appears in the epidermis during late embryogenesis and declines after birth in wild-type (WT) animals, but accumulates in mice with either extrinsic or intrinsic defects in T cell generation. Intriguingly, while these cells exhibit a phenotype suggestive of a T cell progenitor, in vivo, ex vivo and in vitro assays suggest they lack the capacity to proceed further in the T cell differentiation pathway and thus may represent a distinct sub-lineage. Expression of specific NK markers and cytokine production profile points towards ELCs to be related to the NK lineage, however the exact nature of this cell population and their potential immune functions remains to be revealed.
Results
Murine epidermis contains a population of Thy1+ cells that are distinct from DETCs
Mice deficient in mature T and B cells through genetic ablation of either Recombination-Activating Gene (Rag) -1 or -2, completely lack DETCs in their epidermis28,29 (Fig. 1a). Here, the absence of DETCs in 8 week old (wo) Rag1- and Rag2-deficient mice revealed the existence of a minor population of Thy1+ cells in the epidermis that, unlike DETCs, did not express either CD3 or TCRβ (Fig. 1a). Detailed examination of epidermal suspensions from WT mice confirmed the presence of a minor population of these cells, representing approximately 1% of the CD45+Thy1+ population, which similarly lacked CD3 or TCRβ expression (Fig. 1a).
Thy1+ cells are present in the Rag1−/− epidermis.
(a) Flow cytometry of adult mouse epidermal cell suspension. Gating strategy to identify a Thy1+CD3− population in both WT and Rag-deficient models is shown. (b). Histograms show relative expression intensity of CX3CR1 and CD103 among Thy1.2+CD3− cells. Representative data from n > 5 mice is shown for a–b. (c–d) Two-photon imaging of the epidermis of WT and Rag1−/−Cx3cr1gfp/+ mice. In green are the CX3CR1-positive cells, scale 200 μm (c) and 20 μm (d). (e) Cell morphology analysis of CX3CR1-positive cells from WT (black) and Rag1−/− (grey) mice. Representative data of n = 3.
Phenotypic characterisation of the minor Thy1+ epidermal population from WT, Rag1−/− and Rag2−/− mice revealed uniformly high expression of the integrin CD103 (Fig. 1b), a marker normally associated with intraepithelial T cells30,31,32. Using the Cx3cr1gfp/+ reporter model33, our data revealed that the fractalkine receptor was expressed by 100% of the epidermal Thy1+ population from both Rag-deficient models, while the Thy1+CD3− population from WT epidermis expressed varying levels of CX3CR1 and was restricted to only 25% of the total cells (Fig. 1b).
We also investigated the physical distribution of the epidermal Thy1+ cells in Cx3cr1gfp/+ mice. Rag-sufficient mice exhibited a uniform network of GFP+ cells since DETCs express CX3CR1. However, despite the fact that Rag1−/− mice lack DETCs, Rag1−/−Cx3cr1gfp/+ epidermis clearly contained a population of GFP+ cells (Fig. 1c). In addition to their patchy distribution, Thy1+ cells in the Rag1−/− mice were more markedly spherical, compared to the highly dendritic morphology characteristic of DETCs in WT mice (Fig. 1d,e).
Differential CD2 expression sub-divides the minor epidermal Thy1+ population
Having identified a minor Thy1+ population that is distinct from DETCs, we next explored the possibility that these cells were related to Innate Lymphoid Cells (ILCs). ILCs are a diverse group of cells that exists as 3 main sub-populations, all of which resemble lymphoid cells in terms of morphology but lack the expression of a mature TCR34,35. Within the murine dermis, a population of ILC2 has been identified, which exhibits a characteristic phenotype, expressing Thy1 and ICOS (Inducible T-cell COStimulator), while lacking expression of CD3 and the inter-cellular adhesion/T cell activation molecule CD236.
Using the same gating strategy as Roediger et al.36 (Fig. S1), we identified both CD2+ (blue) and CD2− (red) cell populations within the Thy1+CD3− epidermal compartment of Rag1−/− and WT mice (Fig. 2a,b). The CD2− sub-population also expressed ICOS and therefore was likely ILC2, while the CD2+ sub-population of the minor epidermal Thy1+ cells was ICOS− (Fig. 2a) and expressed high levels of CX3CR1 in both WT and Rag1−/−. The ILC compartment had a heterogeneous profile of expression for the same fractalkine receptor (Fig. 2b). Thus, the majority of the murine epidermal CD3−Thy1+ population, which expresses CD2 and lacks ICOS, is distinct from both DETCs and ILCs. They represent a novel and discrete population that we named Epidermal Lymphoid Cells (ELCs).
The epidermal Thy1+ population is heterogeneous.
(a) Flow cytometry of mouse epidermal cell suspension. Gating strategy and histograms to identify ICOS expression across the two populations: Thy1+, ILCs (CD3−CD2−, red) and ELCs (CD3−CD2+, blue) in both WT and Rag-deficient models is shown. (b) Histograms show relative expression intensity of CX3CR1 among ELCs and ILCs from WT and Rag1−/− mice. Representative data from n > 3 mice is shown a–b. (c) Identification of ELCs and ILCs in different mouse models. c. Absolute numbers of ELCs and ILCs in different mouse models. Representative data from n > 3 is shown, except for NSG where n = 2.
The genetic ablation of the Rag genes causes an intrinsic defect in T and B cell maturation in the context of otherwise normal immune anatomy28,29. In contrast, the Nude mouse model is rendered effectively athymic by a mutation in the Foxn1 gene that results in defective development of the thymic epithelium and the almost complete absence of T cells37,38. Interestingly, we also detected a population of Thy1+CD3− cells in the epidermis of Nude mice, which, similar to the Rag1−/− and WT, contained both ELCs and ILCs (Fig. 2c,d). Therefore, we conclude that ELCs arise independently of classical thymus-dependent pathways of differentiation.
Given the thymic-independence of ELC generation, we asked which other components of the immune differentiation pathways were these cells reliant upon. The common gamma chain (γc), also known as IL-2R gamma (IL-2Rγ), is a cytokine receptor subunit that is part of the receptor complexes for at least six different interleukin receptors: IL-2, IL-4 IL-7, IL-9, IL-15 and IL-2139,40,41,42,43,44,45. As a consequence, the γc-deficient mouse model, such as NOD scid gamma (NOD/SCID/γc null or NSG), lack all mature T cells46, including DETCs47. Hence, we tested if ELC were also γc-dependent. Examining the epidermis of NSG mice revealed the complete absence of Thy1+ cells, including ELCs. Hence while ELCs are not dependent on the thymus for their derivation, they do require γc signaling for either their differentiation, survival or maintenance.
ELCs are radioresistant and possess self-renewal capacity
Unlike most immune cell populations, which are derived from the BM, within the epidermis, both DETCs and Langerhans cells (LCs), are resistant to depletion by irradiation and renew themselves locally27,48,49. Using BM chimeras, we asked whether the same was true for the ELC population. We lethally-irradiated CD45.2 Rag1−/− host mice before infusing flushed BM cells from CD45.1 WT donors. After 2 months, 99.8 ± 0.1% T cells and 77.8 ± 4.1% of ILCs were of donor origin (Fig 3a,b), in agreement with published data36, while most ELCs were still of host origin. By 6 months post-reconstitution, ILCs were almost exclusively of donor origin (Fig. 3b), while 86 ± 4.6% of ELCs remained of host origin. Thus, like DETCs and LCs in the epidermis, ELCs are radioresistant and do not undergo replenishment from the BM cell pool following irradiation.
ELCs cells are radioresistant and renew themselves locally.
(a–b) Flow cytometry analysis showing percentage of CD45.2 and CD45.1 T cells, ILCs and ELCs in mouse epidermal cell suspension from BM chimeric mice (CD45.1 WT: CD45.2 Rag1−/−) (2 months n = 6; 6 months n = 7). (c–d) WT CD45.1 mice were joined surgically with CD45.2 Rag1−/− mice to create parabiont pairs. 4 months later flow cytometric analysis was performed on the Rag1−/− epidermal cell suspensions. n = 4. (e–f) CD45.1 Rag1−/− mice were joined surgically with CD45.2 Rag1−/− mice to create parabiont pairs. 4 months later flow cytometric analysis was performed on epidermal cell suspension from both mice (n = 4).
While BM chimeras are often informative, the process of irradiation and reconstitution results in an inflammatory environment that may introduce experimental artefacts50. Therefore we also assessed the homeostatic turnover of epidermal cell populations using parabiotic mice. In this model, two adult congenic mice differing in expression of CD45 alleles are surgically attached in order to link their circulatory systems. This technique enables measurement of the extent of the contribution made by blood-borne cells from each parabiont to the immune cell populations in the other, over prolonged periods and without the need for irradiation. We joined congenic WT CD45.1 and Rag1−/− CD45.2 mice and assessed the cellular origin of the CD45+ hematopoietic cell compartment in the epidermis of the Rag1−/− mice 4 months later. As expected from the inability of Rag1−/− mice to produce their own T cells, all CD3+ cells in the Rag1−/− epidermis were of donor origin, while ILCs exhibited marked heterogeneity in source (Fig. 3c,d). In contrast, 99.1 ± 0.3% of ELCs remained of host origin (Fig. 3c,d). To exclude the possibility that WT mice, which bear fewer ELCs than their Rag1−/− counterparts (Fig. 1a), were simply unable to provide the appropriate blood-circulating precursors for ELCs, we confirmed our findings in Rag1−/− CD45.1/Rag1−/− CD45.2 parabionts. Similarly, after 4 months of parabiosis, ELCs in the epidermis of both mice remained of host origin (Fig. 3e,f). In summary, ELCs, similar to DETCs, are epidermal-resident cells that do not undergo replenishment from precursors arising from BM or borne in the blood, either following irradiation or during prolonged periods of parabiosis.
ELCs express heterogeneous levels of T lineage markers
Since ELCs share several characteristics with DETCs and also express lymphoid markers, we wondered whether they might represent local DETC precursors. Several studies have shown that T cell progenitors in the adult or embryonic thymus express CD3 intracellularly51,52 and that cytoplasmic CD3 might be considered a hallmark of the T cell lineage53. Therefore, we investigated whether ELCs expressed CD3 in their cytoplasm. Intracellular expression of CD3 in the ELC population varied from 25–40% in WT, Rag1−/− and Nude mouse models, while ILCs were devoid of cytoplasmic CD3 (Fig. 4a,b). Having suggestive properties of a T cell identity, as intracellular CD3 expression was heterogeneous within the ELC population, we next extended our phenotypic analysis to other markers of the T cell lineage.
ELCs have a T cell progenitor-like phenotype.
(a) Flow cytometry of mouse epidermal cell suspensions with intracellular labelling for CD3. Histograms show the expression profile of intracellular CD3 for DETCs, ELCs and ILCs in several mouse models. (b) Plot shows the percentage of ELCs from WT (red), Rag1−/− (blue) and Nude (green) mice that express CD3 intracellularly. n = 5 for WT and Rag1−/− mice, n = 6 for Nude mice. (c) Gating scheme to identify pTα expressing cells from the mouse epidermis. The histograms and dot plots show the frequency of current or past pTα expression within the αβT-cell (red), DETC (blue), ELC (green) and ILC (orange) populations. Each data point represents an individual mouse. Data were obtained from n = 4 of Rosa26tdRFP/tdRFP control mice, Ptrcaicre/+ x Rosa26tdRFP/+ n = 10 on a WT background and n = 8 on a Rag1−/− background.
The pre-TCRα (pTα) chain is an essential and invariant subunit of the pre-TCR, whose known physiological function is to associate with nascent TCRβ chains in T lineage committed progenitors54. Accordingly pTα is almost-exclusively expressed by immature T cell precursors from both thymic and and extra-thymic T cell development pathways55. Therefore, we employed a fate-mapping mouse model expressing CRE recombinase under the control of the endogenous pTα-encoding gene locus (Ptcra) enabling all cells that have expressed pTα to be heritably marked56. To trace the progeny of pTα expressing cells, we crossed PtcraiCre knockin mice with Rosa26tdRFP reporter line to produce a model in which all cells expressing pTα or deriving from pTα-expressing cells express RFP. As negative control, Rosa26tdRFP/tdRFP mice lacking iCre expression were used (Fig. 4c). In 8 week-old mice, all αβ T cells in the skin have history of pTα expression, as expected56, as did the vast majority of DETCs (97 ± 0.8%)(Fig. 4c). In contrast, few cells with an ILC-like phenotype expressed pTα, 4.8 ± 1.2% (Fig. 4c) and the ELC population exhibited a heterogeneous profile of expression (12 ± 2.7%) (Fig. 4c). These data suggest that ELCs are unlikely to represent a homogeneous population of DETC progenitors; and that the heterogeneity of T-lineage molecule expression within the ELC population may reflect the presence of distinct maturation stages of ELC, with some belonging to a stage before pTα expression. In agreement with the latter, ELCs from PtcraiCre knockin mice with a Rag1−/− background crossed with the Rosa26tdRFP reporter line were not RFP-labelled in this model, leading us to speculate that the absence of Rag expression limits the ability of ELCs to develop normally. Taken together, the observed degrees of expression of intracellular CD3 and pTα across ELCs in the WT background mice led us to hypothesise that the ELC population might comprise a heterogeneous pool of T-lineage-committed cells.
Adult ELCs do not differentiate into T cells
The fate mapping data showed that some of the ELCs from WT mice had differentiated sufficiently far down the T cell pathway to express pTα. However, this was not the case in Rag1−/− mice, perhaps because the lack of Rag expression created an intrinsic block at a stage preceding pTα expression. To attempt to rescue ELC development in Rag1−/− mice, we developed a Rag inducible model (Fig. S2 and Methods). DNA fragment containing 2A peptide sequences between Rag1 and Rag2 cDNA after the loxP flanked Stop cassette was inserted into the Rosa26 locus of WT Embryonic Stem (ES) cells. The resulting mouse model was crossed to Rag1−/− Rosa26Cre-ERT2/+ (Ref. 57), resulting in Rag1+/− mice harboring an inducible Cre-ERT2 transgene or the stop-Rag1A2 fragment in their Rosa26 locus, respectively. Mice were then further intercrossed to obtain the desired genotype, the Rag-inducible model on Rag1−/− background. Upon tamoxifen administration that activates the Cre recombinase, these mice will produce both Rag1 and Rag2 protein from Rosa26Rag1A2 allele as well as express GFP as a reporter of recombination (Fig. S2 and Methods), allowing identification of Rag-expressing cells by flow cytometry (Fig. 5). We hypothesized that by inducing expression of the Rag genes that the cells were missing, the developmental potential of ELC would be restored, with the possibility of proceeding from their double negative (DN) CD4−CD8− status to the DP4, or subsequent single positive CD4 (CD4+CD8−, SP CD4+) or CD8 (CD4−CD8+, SP CD8+) stages8.
ELCs do not develop into mature T cells upon Rag re-expression.
WT, Rag1−/− and Rag inducible model were injected i.p. with tamoxifen daily for 5 days and tissues were analysed 30 days later by flow cytometry. GFP signal indicates Rag recombination and expression. Flow cytometry of mouse thymus (a) and epidermis (b) cell suspensions from WT and Rag1−/− controls and the Rag inducible mouse model. Data are representative of n = 3.
Rag gene expression in our inducible model was induced by intraperitoneal administration of Hydroxytamoxifen (4’OHT) over 5 consecutive days. One month later, both thymus and epidermis from WT, Rag1−/− and Rag-inducible mice were collected and their cell populations analysed by flow cytometry. Approximately one third of thymocytes in the Rag-inducible model expressed GFP and 12% of these thymocytes had proceeded to the DP stage, while all thymocytes in the Rag1−/− were arrested at the DN stage (Fig. 5a). Furthermore, in the Rag-inducible mice, 0.5–1% of thymocytes achieved SP expression of CD4 or CD8 with TCRβ expression (Fig. 5a), showing that our rescue model was able to partially overcome the blockade to thymic T cell development seen in Rag1−/− mice. However, in the epidermis of the same mice, ELCs exhibited comparable phenotypes regardless of the induction of Rag expression (Fig. 5b). Even upon reinstatement of the Rag genes, no change in expression of markers linked to T cell maturation, such as the TCR or CD3, was observed. Later time points and topical delivery of 4’OHT revealed similar results (data not shown). Hence, ELCs either are not T cell progenitors or cannot differentiate in the adult skin environment.
ELCs are present in the murine epidermis prior to birth
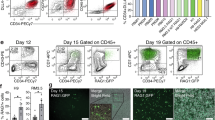
DETCs arise from embryonic precursors and self-maintain into adulthood18,19,27. Given the homeostatic similarities we uncovered between ELCs and these cells, we asked whether ELCs might also seed the skin prior to birth. In the WT E17.5 epidermis (the earlier time point to separate dermis from epidermis), we detected a population of CD3+ cells that were confirmed as DETCs according to expression of the Vγ3 TCR and in agreement with early reports19. However, alongside DETCs, a population of Thy1+CD3−Vγ3− cells was present in both WT and Rag1−/− embryos (Fig. 6a). Furthermore, the relative frequency of this Thy1+CD3−Vγ3− population increased in the Rag1−/− epidermis similarly to what we observe in WT mice with the DETC compartment (Fig. 6b). To further characterize this Thy1+CD3−Vγ3− population in terms of their relation to ILCs, we measured expression of CD2 and ICOS. Before birth, the majority (70%) of the Thy1+CD3−Vγ3− cells in the WT and Rag1−/− epidermis were CD2+, likely corresponding to the ELCs observed in the adult (Fig. 6c). At the newborn (NB) stage, the number and frequency of ELCs increased in both groups of mice, but while this increase was maintained into adulthood in the Rag1−/−, in the WT animals the ELC population decreased dramatically (Fig. 6d). In addition, Wright-Giemsa staining showed that neonatal ELCs were small in size and had a round shape, dark nucleus and scanty cytoplasm (Fig. 6e) - all characteristics of lymphoid cells. Of note, we did not find ELC-like populations in tissues (lung, liver, gut, kidney) other than neonatal epidermis (data not shown).
ELCs are present before birth in both WT and Rag1−/− mice.
(a) Flow cytometry of mouse epidermal cell suspensions. Gating strategies for DETC and Thy1+CD3−Vγ3− populations are shown throughout mouse development (among CD45+Thy1+ cells). (b) Absolute numbers of both populations before birth, in newborns and in adult WT (•) and Rag1−/− (■) mice. Representative data from n > 3. (c) Dot plots show the gating scheme revealing the heterogeneity of the Thy1+CD3−Vγ3− population, which is mainly composed of ELCs, in the mouse epidermis. (d) Absolute numbers of ELCs and ILCs, before birth, in newborns and in adult WT (•) and Rag1−/− (■) mice. Representative data from n > 3 (e) Purified neonatal ELCs were spun onto cytospin slides for Wright-Giemsa staining (Scale bar of 10 μm). Data are representative of 3 independent experiments. (f–g) Plots identify cells with a history of pTα-expression in E18.5 epidermis. The histograms and dot plot identify pTα-expressing cells within the DETC (blue) as well as ELC (green) populations. Each data point represents an individual embryo. Data were obtained from n = 36 embryos from 6 independent experiments of Ptrcaicre/+ x Rosa26tdRFP/+ mice.
Having established the presence of ELCs in the embryos of both WT and Rag1−/− mice, we again asked whether these cells were bound to the T cell lineage prior to birth (Fig. 6f, g). In the E18.5 embryos from the pTα fate mapping model used previously, we observed that 87.2 ± 0.7% of DETCs exhibited a history of pTα-expression, while ELCs were again heterogeneous for prior or current pTα expression (average 34.5 ± 5%). These findings further support the notion that the ELC population might be a heterogeneous pool of cells, some of which are committed to the T cell lineage, while some remain T cell-uncommitted.
Neonatal ELCs do not differentiate into T cells
We hypothesized that the skin might not provide the adequate environment for these cells to further develop along the T cell pathway. Hence we tested if the ELCs had T cell differentiation potential in vitro. We purified ELCs from WT NB epidermis and co-cultured them with TSt-4 stromal cells expressing Delta-like 1 (TSt4/DL1), a foetal thymic fibroblastoid cell line commonly used in T cell differentiation assays58. In addition, a cytokine cocktail including IL-2, IL-7 and IL-15 was also added to the culture to reveal any inherent potential of the ELCs to develop towards a more mature phenotype58. However, after 9–12 days co-culture in the cytokine-enriched stromal environment, ELCs maintained their CD3− TCR− phenotype and did not differentiate into T cells or more mature T cell progenitors (Fig. 7a).
Neonatal ELCs do not develop into mature T cells.
(a) Progeny of ELC co-culture with TSt-4 stromal cells after 9–12 days were analysed for expression of maturation markers of the T cell lineage, including CD4, CD8, CD3 and TCR. n = 6. (b–c) Progeny of the thymic DN cells (b) and ELCs (c) after culture in foetal thymic organ culture for 12–15 days, analysed for CD4 and CD8 expression by flow cytometry. Data are representative of n = 3.
We also tested the T cell potential of neonatal ELCs using foetal thymic organ cultures (FTOCs), an ex-vivo system that supports the complete T-cell development program, including positive and negative selection steps59. We cultured E15.5 thymic lobes from CD45.1 WT mice with 2’-deoxyguanosine (dGuo) for 7 days selectively depleting their hematopoietic compartment. At this time, ELCs and thymic DN cells (positive control) were isolated from WT NB mice (Fig. S3). Purified cell populations were added to the hematopoietic cell-depleted lobes and cultured for 12 to 15 days. After this time, the thymic DN cells had developed as in vivo: both DP and SP stages were evident, with abundant expression of TCRβ and CD3 (Fig. 7b). Some γδ T cells were also detected. In contrast, ELCs in FTOC maintained their Thyhi profile, down-regulated CD2 expression and did not express TCR, CD3 or CD8 (Fig. 7c). While approximately 5% of cultured ELCs did express CD4, as their in vivo counterpart (data not shown), they did not exhibit any other aspects of late DN, DP or SP thymocyte stages (Fig. 7c). Therefore, we conclude that neonatal ELCs are not T cell progenitors.
Neonatal ELCs have a NK-like phenotype
Both CD2 and Thy1 markers are not restricted to the T cell lineage since expressed by other cell types, including NK cells60. Therefore, we hypothesized that the ELC population could be related to NK cells. Accordingly, both WT and Rag1−/− ELC populations expressed low levels of CD49b and had a partial expression of NK1.1 (Fig. 8a,b), specific markers found on the surface of murine NK cells. In addition, low levels of IL-2Rβ were also found in neonatal ELCs of both mouse models (Fig. 8a,b). Interleukin 2 (IL-2) is a major growth factor for mature NK cells61,62. Freshly isolated NK cells preferentially express IL-2Rβ, through which IL-2 plays a pivotal role in proliferation and induction of cytolytic activity63. In addition, the CD2/SLAM family includes several members such as CD2, 2B4 (CD244) and CD48. Murine CD244 is expressed on different subsets of cells, such as T cells and all NK cells64,65,66. In addition, CD48 is expressed widely on hematopoietic cells including T cells and NK cells and has been identified as a ligand for CD244 and CD266. Interestingly, neonatal ELCs expressed both CD244 and CD48 in the WT and Rag1−/− models (Fig. 8a,b).
Neonatal ELCs express NK cell marker and secrete IFN-γ and IL-2.
(a) Representative histograms of the level of expression of NK markers by ELCs present in epidermal cell suspensions of NB from both WT and Rag1−/− models. (b) Mean Fluorescence Intensity (MFI) with standard deviation (SD) bars of NK marker expression by ELC population from both WT and Rag1−/− mice. Data representative n = 3. (c–d) Representative dot plots and bar graphs of the production of IFN-γ and IL-2 from ELCs from WT and Rag1−/− NB after 4 h stimulation with PMA and ionomycin. Data representative of n = 3.
IFN-γ secretion after simulation is a hallmark of NK cells67. Hence, we stimulated NB epidermal cell suspensions in vitro and tested their capacity to secrete IFN-γ as well as other relevant cytokines. After stimulation with PMA plus ionomycin, around 20% of ELCs from both WT and Rag1−/− expressed IFN-γ (Fig. 8c,d). Similar percentage of ELCs expressed IL-2 as well, a cytokine crucial for NK differentiation and function61,62. Production of IL-4, IL-5, IL-9, IL-10, IL-13, IL-17, IL-22, TNF-α, granzyme and perforin were also analysed, revealing that ELCs did not produce any of them with or without stimuli (data not shown).
Discussion
Cutaneous lymphoid populations have been extensively studied under both steady state and inflammatory conditions. DETCs are the major lymphoid cell type in the murine epidermis in the steady state14, while following inflammation epidermal CD8+ T cells become part of the immune pool found in this tissue68. In contrast the dermis is populated by different types of T cells including αβ and γδ T cells, NK cells and the recently-described ILC236,69,70. Here we identified a new lymphoid population that is present at low frequencies in the adult WT epidermis, but accumulates in the epidermis of adult Rag-deficient models and athymic mice - the Epidermal Lymphoid Cells (ELCs).
ELCs share several homeostatic features with DETCs, including radioresistance and independence from circulating progenitors for their maintenance. Considering these similarities and the expression of lymphoid markers by ELCs, we first hypothesized that they could represent DETC local progenitors, belonging to the T cell or T cell progenitor family. It has been reported that cells belonging to T cell lineage express CD3 intracellularly51,52,53 and variable proportions of the ELC population were positive for cytoplasmic CD3. Moreover, prior or current expression of the T lineage-restricted marker pTα was detected in some ELCs as well as all DETCs. Taken together, the expression of CD3 and pTα by some cells within the ELC population is indicative of a T cell lineage identity. ELCs also expressed CD2, leading us to hypothesize that these cells might be related to the CIPs described by Dejbakhsh-Jones and collaborators10. If true, these intermediate progenitors should be able to develop through an extrathymic pathway into mature T cells. However, using a Rag-inducible mouse model that enabled reinstatement of normal thymocyte development, ELCs retained their existing phenotype. One possible explanation is that the adult skin simply does not provide the correct environment for these cells to further differentiate: maybe, similarly to DETCs20, ELCs are restricted to a specific time line during development. In order to understand their true developmental potential we isolated neonatal ELCs and attempted to reveal their differentiation capacity via in vitro and ex vivo approaches. In co-culture with TSt4/DL1 cells or FTOC cultures, ELC did not express T cell maturation markers such as CD3 or TCR, but maintained the same phenotype throughout the culture period. Hence, despite exhibiting T lineage characteristics in both adult and foetal skin, ELC might not belong to the T cell lineage or represent peripheral T cell progenitors.
The true nature of ELCs remains unknown. As lymphoid-like cells, they could be related to ILC. However, their phenotype is different from bona fide ILC, lacking expression of surface markers characteristic of the different ILC groups such as ICOS, NKp46, ST2 and c-Kit (data not shown). More importantly, ELC are radioresistant, unlike the ILC2 described by Roediger36 and other ILC71,72. Accordingly, all the immune population resident of the epidermis, such as DETCs and Langerhans cells (LCs), are resistant to depletion by irradiation and renew themselves locally27,48,49. Alternatively, ELCs could be NK-like cells, which also express Thy1 and CD2 on their surface. However, neonatal ELCs only partially express NK classical markers such as CD49b and NK1.1. Another hallmark of NK cells is their rapid secretion of IFN-γ and IL-2 upon stimulation, exhibiting a Th1-like profile of cytokine73. Importantly, neonatal ELCs from both WT and Rag1−/− are able to produce IFN-γ and IL-2, supporting their NK-like nature. Further studies regarding ELC function, transcription factor’ profile and dependency might provide further insights into their true nature.
ELCs were also present in E17.5 epidermis in WT and represent a significant population. Up to the NB stage, the frequency of ELCs was the same in both WT and Rag1−/− and similar to the frequency of DETCs. However, in adult mice, ELCs were increasingly abundant in the Rag1−/−, while they became rare in the WT epidermis. Combined with the knowledge that ELCs are able to self-maintain locally independent of circulating progenitors, it is likely that embryonic ELCs that seed the skin prenatally give rise to the ELCs found in adulthood, in particular the population found in the Rag1−/−. Thus, we hypothesize that the disappearance of ELCs in WT postnatal epidermis might be due to direct competition with DETCs for niche occupation or cytokine requirements. IL-15 produced by keratinocytes has been reported to induce growth of the DETC population47. Thus, IL-15 arises as a possible candidate for the cytokine competition hypothesis drawn between DETCs and ELCs. Nevertheless, localization studies would have to be addressed in order to further understand the exact proximity of these two groups of cells that might lead to cell competition.
Finally, the specific time frame in which these cells are present in the WT epidermis, plus the expression of ligand/receptor CD48/CD244/CD2 (as CD244 and CD48 expression was been reported to be expressed by DETCs74 and LCs75 respectively) indicates a possible cooperative role for ELCs alongside LCs and DETCs in supporting the establishment of a mature epidermal immune system at birth. Of note that skin-resident DCs have recently been shown to have an active role in remodelling the skin microbiome76. Therefore, we hypothesize that these three immune compartments might cooperate on a tolerogenic role towards skin microbiota, but additional investigations would be required in order to further understand the mechanisms behind such collaboration. Additionally, other possible hypotheses would be that ELCs could interact with keratinocytes and be involved in the maturation mechanisms of LCs or could mediate the LC/DETC activation upon microbiome establishment. Thus the role and lineage relations of the ELC population remain an enigma at this stage and should prove a productive avenue for future research into immunity in the skin.
Methods
Mice
C57BL/6 (CD45.2) mice were purchased from the Biological Resource Center (BRC), Agency for Science, Technology and Research (A*STAR), Singapore. C57BL/6 (CD45.1), B6.Cg-Foxn1nu/J (Nude), B6.129S7-Rag1tm1Mom/J (Rag1−/−), B6.129P-Cx3cr1tm1Litt/J mice (Cx3cr1gfp/+), were purchased from the Jackson Laboratory (Jackson Laboratory, Bar Harbor, USA). B6.129S6-Rag2tm1Fwa N12 (Rag2−/−) were purchased from Taconic (Taconic Farms, USA). Rag1−/− mice were crossed with C57BL/6 CD45.1 to give rise to congenic Rag1−/− CD45.1 mice, or with Cx3cr1gfp/+ mice to generate Rag1−/− Cx3cr1gfp/+ mice. All PtrcaiCre/+ × Rosa26tdRFP/+ mice were kindly provided by Dr. Hans Jörg Fehling (Institute of Immunology, University Clinics, Ulm, Germany). NOD scid gamma (NSG) mice were kindly provided by the National University of Singapore. All mice were bred and maintained in our animal facility and adult mice were analysed between 8-12 weeks of age. All experiments and procedures were approved by the Institutional Animal Care and Use Committee of A*STAR (Biopolis, Singapore) in accordance with the guidelines of the Agri-Food and Veterinary Authority and the National Advisory Committee for Laboratory Animal Research of Singapore.
Flow cytometry, cell sorting and intracellular cytokine staining
Flow cytometry was performed on a LSR II with 5 lasers, or an Aria II with 4 or 5 lasers (Becton Dickinson, San Jose, USA) and analysed with FlowJo software (Tree Star, Ashland, USA). Fluorochrome-conjugated monoclonal antibodies (mAbs) specific to mouse CD45 (30F11), CD45.1 (A20), CD45.2 (104), CD2 (RM2-5), CD3 (145-2C11), CD4 (GK1.5), CD8 (53-6.7), CD48 (HM48-1), CD49b (DX5), CD90.2 (53-2.1), TCRβ (H57-597), TCRγ/δ (GL3), Vγ3 (536), CD103 (2E7), CD244 (244F4), CD278 (C398.4A), NK1.1 (PK136), IL-2Rβ (TMb1), IL-2 (JES6-5H4), IFN-γ (XMG1.2) were purchased either from BD Biosciences (San Jose, USA) or Ebiosciences (San Diego, USA). Intracellular labelling for CD3, IL-2 and IFN-γ was performed on cells previously labelled for surface markers, following fixation and permeabilisation with Fix/Perm solutions (BD Biosciences, Mountain View, CA) according to the manufacturer’s instructions. Intracellular cytokine staining was performed on epidermal cell suspensions after stimulation for 4 hr with phorbol myristate acetate (PMA) (500 ng/mL) and ionomycin (500 ng/mL) (Sigma-Aldrich).
Mouse skin cell preparation
Mouse skin cells were isolated as described previously77. Briefly, embryonic/neonatal skin was detached from the body or mouse ears were split into dorsal and ventral halves and floated on RPMI-1640 medium (Sigma) containing 1 mg/ml dispase (Invitrogen) for 60 min to allow separation of epidermal and dermal sheets. Epidermal and dermal sheets were then cut into small pieces and incubated in RPMI containing 10% feotal calf serum (FCS), 0.8 mg/ml collagenase type IV (Sigma) and 50 μg/ml DNAse I (Roche) for 90 min. Cells were then passed through 19 G syringe and filtered through a 70 μM cell strainer (BD Falcon) to obtain a homogenous single cell suspension. For purpose of cell number normalisation, analyses were performed on both ears of the adult mice and the corresponding area, 3 cm2, of the body skin from the embryos and newborn.
Intravital multiphoton imaging of mouse ear skin
Mice were anaesthetized with a cocktail of 150 mg/kg ketamine and 10 mg/kg xylazine, before ear hair was removed with the depilatory lotion Veet. Anaesthetized mice were immobilized on a custom-made stage78, with a heating pad attached to maintain the animal at 37 °C. To label blood vessels in vivo, mice were retro-orbitally injected with 40 μl of a 10 mg/ml solution of Evans Blue dye (Sigma-Aldrich). Images were acquired using a multiphoton microscope system (LaVision Biotec) with a tunable Chameleon Ultra II Ti:Sapphire laser (Coherent) at 950 nm and the following long pass mirrors and bandpass filters: 495 LPXR (Chroma), 560 LPXR (Chroma); 475/42 (Semrock), 525/50 (Chroma), 655/40 (Semrock). Data sets generated were analysed by IMARIS imaging software (Bitplane).
Generation of bone marrow chimeras
Recipient 7 to 8 week old CD45.2 Rag1−/− mice were lethally irradiated (2 × 600 rad, 3 hr apart using a Caesium source) and reconstituted by intravenous injection with CD45.1 WT BM. Engraftment was assessed by measuring the relative numbers of donor cells among blood CD45+ cells 4 weeks after transplantation. Epidermal cell suspensions from mice were analysed 2 or 6 months post-transplant and the proportion of DETCs, ELCs and ILCs derived from WT (CD45.1) cells was determined.
Generation of parabiotic mice
CD45.2 Rag1−/− mice were sutured to either CD45.1 WT or CD45.1 Rag1−/− mice and remained together for 4 months. All mice were 5 to 6 weeks old at the time of surgery. Mice received ketamine/xylazine as an anaesthetic during surgery, plus buprenophine and baytril for analgesia for the few days following the procedure.
Wright-Giemsa staining
For cytospin, purified cells were spun onto glass slides, fixed and stained using the Hema 3 System (Thermo Fisher Scientific) and rinsed in distilled water. Images were analyzed using an Olympus BX43F conjugated with an Olympus DP21 digital camera. Snapshots were taken at a 10 × 100-fold magnification.
Generation of Rosa26-Rag mice for inducible Rag1/2 activity
In order to generate a mouse line in which Rag1 and Rag2 expression could be induced from the Rosa26 locus by removal of the STOP cassette upon Cre expression, we used 2A peptides79 for bicistronic expression. We amplified the mouse Rag2 coding region from genomic DNA by PCR reaction with primers (Rag2-5′ and Rag2-2A-3′) which allowed us to add 2A peptide sequences after the stop codon for Rag2 translation. We also amplified Rag1 cDNA by PCR. These PCR products were cloned into the pCR-TOPOII vector (Invitrogen) and their sequences verified. We then inserted the Rag2-2A fragment in front of the Rag1 cDNA fragment in pCR-TOPOII, followed by preparation of an entire Rag2-2A-Rag1 fragment by NotI digestion and a ligation into the NotI site of the pCTV vector (Addgene) (Supp Fig. 2a). The linearized targeting vector was transfected into M1 embryonic stem (ES) cells by electroporation. After G418 selection, ES clones that underwent homologous recombination were screened by PCR as previously described80. Primers: Rag2-5′ (5′- CGGCGCGCC AGCATAATTACCAATATGAAAAGATATTC-3′), Rag2-2A-3′ (5′- CGGATCCCCTGGGCCAGGATTCTCCTCGACGTCACCGCATGTTAGCAGACTTCCTCTGCCCTCTCCACTGCCATCAAACAGTCTTCTAAGGAAGGATTTC-3′), Rag1-5′ (5′-GGATCCTATGGCTGCCTCCTTGCCGTCTACCCTGAGC-3′) and Rag1-3′ (5′- CGGCGCGCCATGTGGAGATCCTATTTAAAACTCCATTGA -3′).
In vitro recombination assay
To measure Rag1/2 activity from the Rosa26-Rag1A2 locus that was induced upon Cre expression, we used a recombination template vector, pJH20081. Embryonic feeder cells generated from E13.5 embryos, which harbour WT or heterozygous Rosa26-Rag alleles, were transfected with pJH200 with or without the Cre expression vector, pMC-Cre, using FuGENE reagent (Promega)(Supp Fig. 2b). Three days after transfection, cell lysates were prepared and were analysed for Rag-dependent recombination events by PCR, as previously described81.
Stromal culture
Stromal cell culture methods were modified from58. In brief, TSt4/DL1 cells (kindly gifted by Dr. Ikawa) were co-cultured with sorted ELCs and DETCs from newborn WT epidermis, in the presence of 10 ng/ml IL-2, 10 ng/ml IL-7 and 10 ng/ml IL-15 (R&D systems), in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 2 mg/ml sodium bicarbonate, 0,1 mM non-essential amino acids, 50 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 mg/ml streptomycin.
Foetal thymic organ cultures (FTOCs)
The experiment was performed as described82. 2-deoxyguanosine-treated E15.5 foetal thymic lobes from CD45.1 WT mice were cultured for 7 days before reconstitution with purified ELCs from CD45.2 WT newborns. Around 10 to 15 × 103 ELCs were added to the thymic culture by the Hanging-Drop technique over 24 h and then cultured for 12–15 days. RPMI medium (Sigma) supplemented with 10% Hyclone FCS (GE Healthcare Life Sciences) 5 μM 2-mercaptoethanol, 10 mM HEPES, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/streptomycin was used throughout the culture. Medium was additionally supplemented with 1.35 mM of 2’-deoxyguanosine (dGuo) (Sigma) to treat the E15.5 thymic lobes. Treatment with dGuo selectively eliminates thymocytes, interdigitating cells and dendritic cells from the thymic cultured tissue, allowing the introduction of new hematopoietic progenitors to the foetal thymic stromal environment.
Statistics
Statistical analysis was performed on GraphPad Prism6. Mann-Whitney tests were performed using unpaired experimental designs with non-parametric tests. Significance was defined at p < 0.05 (ns p > 0.05, *p ≤ 0.5, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001). Error bars in graphs represent Standard Error of the Mean (SEM).
Additional Information
How to cite this article: Almeida, F. F. et al. Identification of a novel lymphoid population in the murine epidermis. Sci. Rep. 5, 12554; doi: 10.1038/srep12554 (2015).
References
Shortman, K. & Wu, L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 14, 29–47 (1996).
Adkins, B. et al. Early events in T-cell maturation. Annu. Rev. Immunol. 5, 325–365 (1987).
Petrie, H. T. & Zúñiga-Pflücker, J. C. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 25, 649–679 (2007).
Rothenberg, E. V., Moore, J. E. & Yui, M. a. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21 (2008).
Yang, Q., Jeremiah Bell, J. & Bhandoola, A. T-cell lineage determination. Immunol. Rev. 238, 12–22 (2010).
Koch, U. & Radtke, F. Mechanisms of T cell development and transformation. Annu. Rev. Cell Dev. Biol. 27, 539–562 (2011).
Ciofani, M. & Zúñiga-Pflücker, J. C. Determining γδ versus αß T cell development. Nat. Rev. Immunol. 10, 657–663 (2010).
Germain, R. N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 (2002).
Rodewald, H. R., Kretzschmar, K., Takeda, S., Hohl, C. & Dessing, M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. EMBO J. 13, 4229–4240 (1994).
Dejbakhsh-Jones, S., Garcia-Ojeda, M. E., Chatterjea-Matthes, D., Zeng, D. & Strober, S. Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway. Proc. Natl. Acad. Sci. USA 98, 7455–7460 (2001).
García-Ojeda, M. E. et al. Stepwise development of committed progenitors in the bone marrow that generate functional T cells in the absence of the thymus. J. Immunol. 175, 4363–4373 (2005).
Dejbakhsh-Jones, S. & Strober, S. Identification of an early T cell progenitor for a pathway of T cell maturation in the bone marrow. Proc. Natl. Acad. Sci. USA 96, 14493–14498 (1999).
Nonaka, S. et al. Intestinal γ δ T cells develop in mice lacking thymus, all lymph nodes, peyer’s patches and isolated lymphoid follicles. J. Immunol. 174, 1906–1912 (2005).
Tschachler, E. et al. Expression of Thy-1 antigen by murine epidermal cells. J. Invest. Dermatol. 81, 282–285 (1983).
Stingl, G. et al. Thy-1+ dendritic epidermal cells express T3 antigen and the T-cell receptor gamma chain. Proc. Natl. Acad. Sci. USA 84, 4586–4590 (1987).
Koning, F. et al. Identification of a T3-associated gamma delta T cell receptor on Thy-1+ dendritic epidermal Cell lines. Science 236, 834–837 (1987).
Kuziel, W. a. et al. Regulation of T-cell receptor gamma-chain RNA expression in murine Thy-1+ dendritic epidermal cells. Nature 328, 263–266 (1987).
Leclercq, G., Plum, J., Nandi, D., De Smedt, M. & Allison, J. P. Intrathymic differentiation of Vgamma3 T cells. J. Exp. Med. 178, 309–315 (1993).
Havran, W. L. & Allison, J. P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature 335, 443–445 (1988).
Havran, W. L. & Allison, J. P. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 344, 68–70 (1990).
Payer, E., Elbe, A. & Stingl, G. Circulating CD3+/T cell receptor V gamma 3+ fetal murine thymocytes home to the skin and give rise to proliferating dendritic epidermal T cells. J. Immunol. 146, 2536–2543 (1991).
Payer, E., Elbe, a & Stingl, G. Epidermal T lymphocytes–ontogeny, features and function. Springer Semin. Immunopathol. 13, 315–331 (1992).
Jameson, J. M., Cauvi, G., Sharp, L. L., Witherden, D. a. & Havran, W. L. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201, 1269–1279 (2005).
Jameson, J. et al. A role for skin gamma delta T cells in wound repair. Science 296, 747–749 (2002).
Sharp, L. L., Jameson, J. M., Cauvi, G. & Havran, W. L. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat. Immunol. 6, 73–79 (2005).
Jameson, J. & Havran, W. L. Skin gamma delta T-cell functions in homeostasis and wound healing. Immunol. Rev. 215, 114–122 (2007).
Honjo, M. et al. Thymus-independent generation of Thy-1+ epidermal cells from a pool of Thy-1- bone marrow precursors. J. Invest. Dermatol. 95, 562–567 (1990).
Mombaerts, P. et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877 (1992).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Russell, G. J. et al. Distinct structural and functional epitopes of the alpha E beta 7 integrin. Eur. J. Immunol. 24, 2832–2841 (1994).
Cerf-Bensussan, N. et al. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur. J. Immunol. 17, 1279–1285 (1987).
Kilshaw, P. J. & Baker, K. C. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol. Lett. 18, 149–154 (1988).
Jung, S. et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Diefenbach, A., Colonna, M. & Koyasu, S. Development, differentiation and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Nehls, M., Pfeifer, D., Schorpp, M., Hedrich, H. & Boehm, T. New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372, 103–107 (1994).
Pantelouris, E. M. Absence of thymus in a mouse mutant. Nature 217, 370–371 (1968).
Kondo, M. et al. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science 262, 1874–1877 (1993).
Miyazaki, T. et al. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science 266, 1045–1047 (1994).
Noguchi, M. et al. Interleukin-2 receptor gamma chain: A functional component of the interleukin-7 receptor. Science 262, 1877–1880 (1993).
Giri, J. G. et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13, 2822–2830 (1994).
Kimura, Y. et al. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int. Immunol. 7, 115–120 (1995).
Russell, S. M. et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science 262, 1880–1883 (1993).
Asao, H. et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001).
Kovanen, P. E. & Leonard, W. J. Cytokines and immunodeficiency diseases: Critical roles of the gc-dependent cytokines interleukins 2, 4, 7, 9, 15 and 21 and their signaling pathways. Immunol. Rev. 202, 67–83 (2004).
Edelbaum, D., Mohamadzadeh, M., Bergstresser, P. R., Sugamura, K. & Takashima, a. Interleukin (IL)-15 promotes the growth of murine epidermal gamma delta T cells by a mechanism involving the beta- and gamma c-chains of the IL-2 receptor. J. Invest. Dermatol. 105, 837–843 (1995).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Merad, M. et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3, 1135–1141 (2002).
Ferrara, J. L. M. & Reddy, P. Pathophysiology of graft-versus-host disease. Semin. Hematol. 43, 3–10 (2006).
Wilson, A., Capone, M. & MacDonald, H. R. Unexpectedly late expression of intracellular CD3epsilon and TCR gammadelta proteins during adult thymus development. Int. Immunol. 11, 1641–1650 (1999).
Levelt, C. N., Carsetti, R. & Eichmann, K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor beta CD3 epsilon and maturation to the CD4+8+ stage are highly correlated in individual thymocytes. J. Exp. Med. 178, 1867–1875 (1993).
Smith, E. et al. T-lineage cells require the thymus but not VDJ recombination to produce IL-17A and regulate granulopoiesis in vivo. J. Immunol. 183, 5685–5693 (2009).
Von Boehmer, H. Unique features of the pre-T-cell receptor alpha-chain: not just a surrogate. Nat. Rev. Immunol. 5, 571–577 (2005).
Bruno, L., Rocha, B., von Boehmer, H. & Rodewald, H.-R. Intra- and extra-thymic expression of the pre-T cell receptor alpha gene. Eur. J. Immunol. 25, 1877–1882 (1995).
Luche, H. et al. In vivo fate mapping identifies pre-TCRα expression as an intra- and extrathymic, but not prethymic, marker of T lymphopoiesis. J. Exp. Med. 210, 699–714 (2013).
Seibler, J. et al. Rapid generation of inducible mouse mutants. Nucleic Acids Res. 31, e12 (2003).
Ikawa, T. et al. An Essential Developmental Checkpoint for Production of the T Cell Lineage. Science 329, 93–96 (2010).
Anderson, G. & Jenkinson, E. J. Thymus organ cultures and T-cell receptor repertoire development. Immunology 100, 405–410 (2000).
K, K. & M, C. Specificity, Function and Development of NK cells. Immunology 28, (1998).
Biron, C. a., Young, H. a. & Kasaian, M. T. Interleukin 2-induced proliferation of murine natural killer cells in vivo. J. Exp. Med. 171, 173–188 (1990).
Ishida, B. Y. Y., Nishi, M., Taguchi, O., Kawaichi, M. & Honjo, T. Expansion of Natural Killer Cells but not T cells in Human Interleukin2/Interleukin 2 Receptor (Tac) Transgenic Mice. J. Exp. Med. 170, 1103–1115 (1989).
Philips, J. H., Takeshita, T., Sugamuraj, K. & Lanier, L. L. Activation of Natural Killer Cells via the p75 Interleukin 2 Receptor. J. Exp. Med. 170, (1989).
Garni-Wagner, B. A., Purohit, A., Mathew, P. A., Bennett, M. & Kumar, V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J. Immunol. 151, 60–70 (1993).
Mathew, P. a. et al. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J. Immunol. 151, 5328–5337 (1993).
Brown, M. H. et al. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J. Exp. Med. 188, 2083–2090 (1998).
Kubota, a., Lian, R. H., Lohwasser, S., Salcedo, M. & Takei, F. IFN-gamma production and cytotoxicity of IL-2-activated murine NK cells are differentially regulated by MHC class I molecules. J. Immunol. 163, 6488–6493 (1999).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).
Nestle, F. O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Sumaria, N. et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 208, 505–518 (2011).
Serafini, N. et al. Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J. Exp. Med. 211, 199–208 (2014).
Geiger, T. L. et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J. Exp. Med. 211, 1723–1731 (2014).
Schoenborn, J. R. & Wilson, C. B. Regulation of Interferon-gamma During Innate and Adaptive Immune Responses. Adv. Immunol. 96, 41–101 (2007).
Schuhmachers, G. et al. 2B4, a new member of the immunoglobulin gene superfamily, is expressed on murine dendritic epidermal T cells and plays a functional role in their killing of skin tumors. J. Invest. Dermatol. 105, 592–596 (1995).
Ozawa, H., Aiba, S., Nakagawa, S. & Tagami, H. Murine epidermal Langerhans cells express CD48, which is a counter-receptor for mouse CD2. Arch. Dermatol. Res. 287, 524–528 (1995).
Naik, S. et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 (2015).
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007).
Li, J. L. et al. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 (2012).
Trichas, G., Begbie, J. & Srinivas, S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 6, 40 (2008).
Muroi, S. et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat. Immunol. 9, 1113–1121 (2008).
Cortes, P. et al. In vitro V(D)J recombination: signal joint formation. Proc. Natl. Acad. Sci. USA 93, 14008–14013 (1996).
Nitta, T., Ohigashi, I. & Takahama, Y. The Development of T Lymphocytes in Fetal Thymus Organ Culture. 946, 85–102 (2013).
Acknowledgements
This work was supported by core grants of the Singapore Immunology Network to F.G., startup funds from NUS to NRJG and Deutsche Forschungsgemeinschaft (FE 578/3-1) from G.A. & H.J.F. We wish to thank Dr. Manfred Kopf and Dr. Jan Kisielow for scientific discussions. We also wish to thank Dr. Lucy Robinson of Insight Editing London for her assistance in preparing the manuscript.
Author information
Authors and Affiliations
Contributions
F.G. and I.T. conceived the study; F.F.A., M.T., J.B., J.L.Y.L., G.H. and P.S. performed experiments; F.G., F.A.F, M.T., J.L.Y.L. and I.T. analysed data; G.A., H.J.F. and I.T. provided reagents; L.G.N., H.J.F. and N.R.J.G. provided intellectual guidance; F.G. and F.F.A. wrote the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Almeida, F., Tenno, M., Brzostek, J. et al. Identification of a novel lymphoid population in the murine epidermis. Sci Rep 5, 12554 (2015). https://doi.org/10.1038/srep12554
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep12554
This article is cited by
-
Establishment and function of tissue-resident innate lymphoid cells in the skin
Protein & Cell (2017)