Abstract
Intestinal inflammatory lesions are inherently hypoxic, due to increased metabolic demands created by cellular infiltration and proliferation, and reduced oxygen supply due to vascular damage. Hypoxia stabilizes the transcription factor hypoxia-inducible factor-1α (HIF) leading to a coordinated induction of endogenously protective pathways. We identified IL12B as a HIF-regulated gene and aimed to define how the HIF-IL-12p40 axis influenced intestinal inflammation. Intestinal lamina propria lymphocytes (LPL) were characterized in wild-type and IL-12p40−/− murine colitis treated with vehicle or HIF-stabilizing prolyl-hydroxylase inhibitors (PHDi). IL12B promoter analysis was performed to examine hypoxia-responsive elements. Immunoblot analysis of murine and human LPL supernatants was performed to characterize the HIF/IL-12p40 signaling axis. We observed selective induction of IL-12p40 following PHDi-treatment, concurrent with suppression of Th1 and Th17 responses in murine colitis models. In the absence of IL-12p40, PHDi-treatment was ineffective. Analysis of the IL12B promoter identified canonical HIF-binding sites. HIF stabilization in LPLs resulted in production of IL-12p40 homodimer which was protective against colitis. The selective induction of IL-12p40 by HIF-1α leads to a suppression of mucosal Th1 and Th17 responses. This HIF-IL12p40 axis may represent an endogenously protective mechanism to limit the progression of chronic inflammation, shifting from pro-inflammatory IL-12p70 to an antagonistic IL-12p40 homodimer.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) is characterized by chronic immune-mediated mucosal inflammation. During inflammatory episodes, increased metabolic demands, reduced oxygenated blood supply due to vascular damage and oxygen depletion by infiltrating immune cells combine to create a localized tissue-oxygen deficit (hypoxia). To survive repeated hypoxic insults, eukaryotic cells have evolved a number of hypoxia-responsive protective pathways, many of which are mediated by the oxygen-sensing heterodimeric transcription factor hypoxia-inducible factor-1 (HIF-1). The HIF-1α subunit is subjected to oxygen-dependent proteasomal degradation by prolyl-hydroxylase-domain enzymes (PHD) under normal oxygen tensions. The oxygen deficit in inflamed tissues facilitates stabilization of HIF-1α allowing translocation to the nucleus, where it forms a functional complex with the HIF-1β subunit and activates hypoxia-response elements (HRE) of target gene promoters.1 The HIF response has been widely associated with both mucosal protection and healing, and PHD inhibitors (PHDi) that pharmacologically stabilize HIF are of interest as a potential therapy for IBD, infectious disease, chronic kidney disease and wound healing.2, 3, 4, 5, 6 Despite this growing interest in HIF as a therapeutic target for inflammatory diseases, much of our knowledge of HIF biology is limited to the epithelium and innate responses, while the role of HIF in shaping T helper cell responses within inflammatory tissue is comparatively poorly defined.
Aberrant CD4+ T-cell responses have been ascribed a central role in colitis-associated pathology,7, 8 with differentiation of naive T cells into T helper (Th) subsets, such as Th1, Th2 and Th17 largely dictated by the production of polarizing cytokines.9, 10 In IBD patients, two important IL-12 family cytokines, IL-12p70 and IL-23, are key to the development of pathogenic Th cell responses. IL-12 and IL-23 are heterodimers with a shared subunit, p40 which pairs with IL-12p35 or IL-23p19, respectively. Both IL-12 and IL-23 are secreted by innate immune cells, such as monocytes and antigen presenting cells, and bind to corresponding receptors on naive T cells.11 While IL-12 plays a key role in the differentiation of naive T cells to Th1 cells, IL-23 promotes the expansion of Th17 cells. A high proportion of genetic risk loci for IBD are involved in IL-12 family signaling and recent approaches to IBD therapy have employed monoclonal antibodies targeting p40 in an attempt to inhibit both the IL-12 and IL-23 signaling pathways, with these approaches showing efficacy in randomised human trials.8, 12 Given that aberrant Th1 and Th17 activities have the potential to trigger chronic inflammatory and autoimmune diseases,13 understanding how Th1/Th17 differentiation and expansion are controlled is likely to provide an explanation of how inflammation is both initiated and sustained in chronic inflammatory diseases, such as IBD.
Although much research has focused on the epithelial contribution to HIF-mediated mucosal protection, studies outlining HIF-mediated adaptive immune responses have been relatively overlooked. On the basis of precursor studies identifying differential IL-12 family gene expression, we hypothesized that mucosal HIF-stabilization during murine colitis would regulate T helper cell populations via IL-12 cytokine family signaling. Employing murine models of colitis, we demonstrated suppression pro-inflammatory T helper cell subsets concurrent with selective induction of IL-12p40, but not IL-12p35 or IL-23p19. In vitro analysis identified conserved hypoxia-responsive elements in the IL-12p40 gene promoter facilitating IL-12p40 homodimer secretion from murine and human monocytes treated with PHDi. IL-12p40 homodimer was sufficient to ameliorate colitis in mice, while PHDi efficacy was lost in IL-12p40-deficient mice. Taken together our results demonstrate a novel regulatory pathway in IL-12 signaling and suggest that pharmacological HIF stabilization indirectly suppresses pathogenic mucosal lymphocyte responses during inflammation.
Results
PHDi-treatment ameliorates DSS colitis-associated pathology
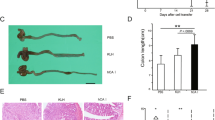
Following our previous work examining the mucosal protection afforded by PHDi compounds in the 2,4,6-trinitrobenzenesulfonic acid (TNBS) and TNFΔARE models of colitis,2, 3, 4 we examined the therapeutic efficacy of PHDi-treatment in the dextran sulfate sodium (DSS) model of colitis. Administration of PHDi ameliorated disease, as measured by body weight and mucosal thickening (Figure 1a), with significant differences between the PHDi- and vehicle-treated disease groups. Reduced disease was also evident by histopathology inflammation and epithelial injury score on day 10 (Figure 1b,c). PHDi-treated animals had significantly lower endoscopic scores by day 15 (Figure 1d) and this reduced mucosal inflammation in DSS-treated animals receiving PHDi was reflected in percentage weight change from baseline (Figure 1e) and histopathology scoring (Figure 1f,g) on day 15. Because histological disease preceded endoscopic mucosal healing, we examined mucosal cytokine levels, in order to identify whether PHDi-treatment led to an overall suppression of immune responses. Tissue cytokine analysis confirmed a reduction in IFN-γ, IL-12p70, and IL-23 levels with PHDi-treatment (Figure 2a). Surprisingly, levels of IL-12p40, the common subunit for IL-12p70 and IL-23 were significantly elevated with PHDi-treatment compared with vehicle-treated DSS mice, suggesting an independent role for PHDi in IL-12p40 expression. To ascertain whether PHDi regulated IL-12p40 at a transcriptional level, we examined the expression of genes for IL-12 family subunits (Figure 2b). PHDi-treatment of DSS-treated animals resulted in a 75±17.8% reduction in il12a transcript compared with vehicle-treated animals. In contrast, and unexpectedly, il12b expression was significantly increased (3.1±0.3-fold increase) in tissue from mice that had received PHDi compared with vehicle-treated mice. Expression of il23a, the gene for IL-23p19, was not significantly increased in DSS or altered by PHDi-treatment. To examine whether these responses were a facet of the DSS colitis model, we examined IL-12 family gene expression in the TNBS model of colitis (Figure 2c) and found, similarly to DSS, that il12b was elevated by PHDi-treatment while il12a and il23a were reduced. Taken together these data suggest that HIF-stabilization differentially regulates IL-12 subunit genes in murine models of colitis and that IL-12p40 could be a HIF target gene.
The influence of pharmacological HIF stabilization on the development of DSS colitis associated pathology. Groups were treated at 48 h intervals with PHDi (5 mg kg−1 AKB-4924) or vehicle (veh) (cyclodextrin) from day 0. (a) Weight change (%) from initial weight and pathological shortening and thickening of the colon expressed as weight (mg):length (cm) (Vehicle n=6, PHDi n=6, DSS+Veh n=11, DSS+PHDi n=11). (b) Representative H&E-stained colon sections at 10 × magnification from healthy treated with vehicle or DSS-treated animals treated with vehicle or PHDi. (c) Sections were scored to provide inflammation and epithelial injury scores. (d) Endoscopic scores from day 4 to 15 post DSS for animals treated with vehicle or PHDi. (e) % weight change from initial weight, (f) representative H&E-stained colon sections (10x) and (g) inflammation and epithelial injury scores on day 15 post DSS for animals treated with vehicle or PHDi. Data expressed as mean ±s.e.m., n=6-11, (a, e) two-way ANOVA, (c, d, g) one-way ANOVA, *P<0.05 **P<0.01. Scale bar, 100 μm. ANOVA, analysis of variance; DSS, dextran sulfate sodium; H&E, hematoxylin and eosin; HIF, hypoxia-inducible factor-1α.
Analysis of PHDi-driven Th1 and Th17 responses in the colonic mucosa and MLNs of mice with chemically-induced colitis. (a) Colon tissue-cytokine analysis of IFN-γ, IL-12p70, IL-23 and IL-12p40 protein levels from healthy or DSS-treated animals treated with PHDi (5 mg kg−1 AKB-4924) or vehicle (veh) (cyclodextrin). (b) RT-PCR analysis of il12a, il12b and il23a expression in whole colon tissue of DSS mice receiving vehicle or PHDi, compared with healthy animal receiving vehicle (n=6–11). Flow cytometric analysis of intracellular cytokine production from LPL and MLNs in PHDi treated (AKB-4924 5mg/kg) or vehicle (veh) mice on day 10 of DSS colitis (n=18). (c) Relative expression of il12a, il23a and il12b mRNA levels in the colon of control and TNBS animals treated with vehicle of PHDi (d) Representative dot plots of IFN-γ and IL-17 producing CD3+CD4+ T cells from LPL. (e) LPL+T cells producing IFN-γ or IL-17, expressed as total number and % live CD3+CD4 population. (f) Representative dot plots of MLN CD3+CD4+ T cells producing IFN-γ or IL-17. (g) MLN CD3+CD4+ T cells producing IFN-γ or IL-17 expressed as % live CD3+CD4+ cells. (n=6, 5mg/kg AKB-4924 every 48 hours). Values are presented as mean ±s.e.m. (a, b, g) one-way ANOVA, (d, f) student’s t-test, ns=not significant, *P<0.05, **P<0.01. ANOVA, analysis of variance; DSS, dextran sulfate sodium; LPL, lamina propria lymphocyte; TNBS, 2,4,6-trinitrobenzenesulfonic acid; MLN, mesenteric lymph node; RT-PCR, real-time PCR.
PHDi-treatment suppresses Th1 differentiation in the intestinal mucosa and mesenteric lymph nodes of DSS mice
Given that IL-12 family gene signaling was altered in PHDi-treated DSS colitis, we investigated whether this altered gene expression resulted in changes to Th1 and Th17 cell populations in the intestinal mucosa and mesenteric lymph nodes (MLN) of DSS-treated animals. Flow cytometry analysis of IFN-γ and IL-17 expressing CD3+CD4+ lamina propria lymphocytes (LPLs; Figure 2d) indicated a significant reduction in CD3+CD4+IFN-γ+ lymphocytes in PHDi-treated DSS-treated animals in terms of both total number (35.9±5.9%, P=0.0055) and percentage of total population (39.0±9.8%, P=0.0145) (Figure 2e). By contrast, there was no significant reduction in CD3+CD4+IL-17+ cells by either metric (Figure 2e). Analysis of MLN cell populations (Figure 2f) revealed a similar reduction in the percentage of in CD3+CD4+IFN-γ+ (37.0±8.4%, P=0.0277) but no reduction in CD3+CD4+IL-17+ cells (Figure 2g). These data suggest that PHDi-driven changes in the IL-12p40 gene results in altered Th1 responses in DSS colitis models.
PHDi significantly enhances LPS-induced il12b expression and alters IL-12 protein secretion in vitro
To investigate the mechanism behind the suppression of Th1 responses by PHDi, we utilized an in vitro model incorporating RAW 246.7 murine macrophages to directly analyze the influence of PHDi on IL-12 signaling. Exposure of cultured RAW 246.7 to PHDi resulted in a significant but transient increase of il12b expression at 8 h (Figure 3a). PHDi treatment also led to a suppression of il12a expression by RAW 246.7 macrophages after 10 h, while PHDi had no influence on il23a expression. To investigate whether this increased il12b induction was influenced by activating stimuli, we co-incubated RAW 246.7 cells with lipopolysaccharide (LPS) and PHDi over 10 h. Cells treated with PHDi had a significantly enhanced il12b induction in response to LPS stimulation as early as 4 h after treatment (Figure 3b), while again, PHDi treatment also led to a suppression of il12a expression after 10 hours and il23a expression remained unaffected. To assess whether these changes in gene expression influenced IL-12 protein secretion, we analyzed the supernatants of LPS-stimulated RAW 246.7 cells that had or had not been treated with PHDi, by western blot. Although no changes could be detected under reducing and denaturing conditions, when examined under native gel conditions, PHDi-treated supernatants showed three distinct bands at 50, 75 and 92 kDa, (Figure 3c), corresponding to molecular weights for IL-23, IL-12 and IL-12p40 homodimer, respectively, with the 92 kDa protein corresponding to a recombinant homodimer-loading control. Densitometry analysis revealed a significant increase in IL-12p40 homodimer in the supernatants of PHDi-treated cells, when compared with vehicle treated groups (Figure 3c). To ascertain whether these findings may have translational relevance, we examined whether LPS-stimulated human peripheral blood monocyte cells (PBMCs) (Figure 4a) and lamina propria mononuclear cells (LPMCs; Figure 4b) secreted IL-12p40 homodimer after PHDi treatment. Supernatants were analyzed by western blot and 92 kDa IL-12p40 homodimer protein was only detectable under non-denaturing conditions and transfer conditions without the addition of methanol. Increased IL-12p40 homodimer levels in PHDi-treated and hypoxic cultures were statistically significant upon densitometry analysis (Figure 4c), confirming that this response is conserved across species. Together these data demonstrate that PHDi-mediated induction of both murine and human IL-12p40 at the gene level, results in increased IL-12p40 homodimer production.
Regulation of IL-12 and IL-23 subunit expression by PHDi in vitro. RAW 246.7 macrophages were cultured in vitro with either vehicle or PHDi (AKB-4924 5 μM) for 10 hours, either in (a) medium alone or (b) with LPS (100 ng ml−1). RT-PCR was used to determine the expression of il12b, il12a, or il23a in these cultures. Supernatants were collected from LPS-stimulated RAW 246.7 macrophages and (c) examined by immunoblot and (d) densitometry for IL-12p40-containing proteins under denaturing and non-denaturing conditions. Densitometry analysis was performed with ImageJ to examine IL-12p40-containing. Data expressed as mean±s.e.m. (a,b) or fold change over vehicle±s.e.m. (c) (n=6). ***P<0.005. (a,b) two-way ANOVA, (c) students t-test, ns, not significant, *P<0.05. ANOVA, analysis of variance; LPS, lipopolysaccharide; RT-PCR, real-time PCR.
Analysis of hypoxia-responsive elements in the human and murine il12b promoter. Immunoblot analysis of IL-12p40 homodimer secretion from human (a) peripheral blood monocytes or (b) lamina propria monocyte cells stimulated with LPS in the presence of absence of cyclodextran vehicle (Veh), PHDi (AKB-4924, 5 μM) or hypoxia (Hx), with (c) densitometry analysis representing the ratio of IL-12p40 homodimer to IL-12p70. (d) Schematic representation of hypoxia-responsive elements (HRE) in the il12b promoter in mouse and human and (e) subsequent analysis of the influence of hypoxia or PHDi-treatment on iL12b promoter activity in U937 cells transfected with an il12b-luciferase promoter reporter vector. (f) In silico analysis of HBS and HAS in the murine and human il12b promoter with predicted similarity to HRE function (Matrix similarity, max=1). (g) Site-directed mutagenesis of HIF-binding sites in the human (U937-transfected cells) and murine (RAW 264.7 transfected cells) il12b promoter and subsequent response to PHDi-treatment or hypoxia, in comparison to normal oxygen tensions (normoxia). For reporter transfection studies, renilla was employed as a co-transfection control and data expressed as mean RLU ratio il12b:renilla±s.e.m. (n=4). For densitometry analysis, data expressed as mean±s.e.m. (c, g) one-way ANOVA, (e) student’s t-test, †P<0.05, *P<0.05. ANOVA, analysis of variance; HAS, HIF ancillary sites; HIF ancillary sites, HIB; HIF, hypoxia-inducible factor-1α; LPS, lipopolysaccharide; RLU, relative luminescence units.
Il12B and IL-12p40 secretion is regulated by hypoxia-inducible factor in murine and human monocytes
PHDi inhibition can influence a number of transcriptional pathways, including HIF and NFκB.14, 15 Therefore, we investigated whether il12b induction was directly related to HIF transcriptional activity. Initially we performed in silico analysis of both the murine and human il12b promoter in order to identify potential hypoxia-responsive elements, canonical binding sites for HIF. Bioinformatic analysis with MatInspector, identified two potential HRE sites on the murine il12b promoter and four potential binding sites on the human IL12B promoter (Figure 4d). On the basis of these analyses, we cloned genomic fragments extending from positions extending 1000 bp from the IL12B TSS into pGL3 luciferase reporter vector. IL12B-transfected U937 cells revealed a 4.5±0.04-fold increase (P=0.0059) in luciferase activity in response to hypoxia. In addition, we observed a concentration-dependent increase in luciferase activity with PHDi treatment, with a maximal increase of 2.6±0.10-fold at 50 μM PHDi (Figure 4e). To further identify the contribution of HIF to induction of IL12B with PHDi treatment or hypoxia, we performed site-directed mutagenesis of the IL12B putative HIF-binding sites designated in Figure 4f and examined transcriptional activity following transient transfection into RAW 267.4 (murine) or U937 (human) cells. Because the mil12bΔHRE2 site did not have a corresponding HIF ancillary site, we excluded it from investigation. Each HIF-binding region of the murine il12b and human IL12B promoter was mutated to a non-HIF-binding sequence and examined for transcriptional activity compared with the wild-type promoter following transient transfection into RAW267.4 or U937 cells. The hIL12BΔHRE1 and hIL12BΔHRE2 transfected cells showed a 62±8% and 39±5% decrease in activity elicited by PHDi respectively and 30±8% and 21±6% decrease in activity elicited by hypoxic conditions (all P<0.05 compared with wild-type plasmid) (Figure 4g). There was no reduction in activity for hIL12BΔHRE3 or hIL12BΔHRE4 constructs. Mutagenesis of the murine HRE promoters showed a similar reduction in mil12bΔHRE1 activity of 42±2% for PHDi and 32±3% for hypoxia (Figure 4g). These data indicate that the induction of IL-12p40 due to PHDi treatment is mediated by the HIF-dependent regulation of the IL-12p40 gene.
PHDi-treatment is not protective against DSS colitis in the absence of IL-12p40
Given the conserved nature of HIF-mediated IL-12p40 induction, we investigated the importance of this response in DSS colitis and whether IL-12p40 was necessary for the protective action of PHDi treatment. In order to test this, we treated IL-12p40−/− mice with PHDi or vehicle during DSS colitis. In contrast to WT mice, the absence of IL-12p40 negated the therapeutic benefit of PHDi treatment and IL-12p40−/− mice receiving DSS colitis showed no recovery from colitis with PHDi treatment, as measured by weight loss (Figure 5a) or colonic thickening (Figure 5b). Histological analysis of colon sections from IL-12p40−/− animals (Figure 5c) showed no significant change in histological score in DSS IL-12p40−/− mice compared with WT animals receiving DSS, but in contrast to WT animals, treatment with PHDi did not reduce inflammation and epithelial injury, as assessed by histopathology scoring in IL-12p40−/− animals (Figure 5d). Analysis of LPL populations (Figure 5e) revealed a lack of CD3+CD4+IFN-γ+ lymphocytes compared with WT in both vehicle and PHDi-treated DSS IL-12p40−/− animals at total cell and % live CD4 population (Figure 5f). These animals showed a population of CD3+CD4+IL-17+ cells that were not significantly affected by PHDi treatment (Figure 5f). There are a number of IL-23 independent pathways for Th17 cell differentiation, but IL-23 is critical for maintaining these populations. In line with these reports, we identified increased IL-1β and IL-6 transcript and protein (Figure 5g) levels in IL-12p40−/− DSS-treated animals compared with controls and both cytokines are capable of driving Th17 differentiation. Thus these data suggest that, PHDi affords protection against pathology in an IL-12p40-dependent manner.
Influence of PHDi treatment on colitis-associated pathology Th responses in the absence of IL-12p40. Groups were treated at 48 hour intervals with PHDi (AKB-4924, 5 mg kg−1) or vehicle (veh, cyclodextrin). (a) Disease progress was assessed by weight change (%) from initial weight and (b) mucosal thickening of the colon expressed as weight (mg)/length (cm). Upon sacrifice, pathology was assessed by (c) blinded histological inflammation and colitis score. Panel (d) shows representative H&E-stained histological images of colons from naive IL-12p40−/− (Naive KO) animals and DSS IL-12p40−/− (DSS KO) animals with and without PHDi treatment. Flow cytometric analysis was performed to examine intracellular cytokine production from LPL restimulated in vitro in DSS WT or IL-12p40−/− mice receiving PHDi (AKB-4924 5 mg kg−1) or vehicle treatment (Veh). (e) Representative dot plots of LPL CD3+CD4+ T cells producing IFN-γ, or IL-17 in DSS wildtype or IL-12p40−/− mice. (f) LPL CD3+CD4+ T cells producing IFN-γ or IL-17, expressed as total number and % live (g) Analysis of IL-1β and IL-6 mRNA transcript and protein levels in the colonic mucosa of control and DSS willdtype or IL-12p40−/− mice. Data expressed as mean ±s.e.m., (n=6-9 per group). (a) Two-way ANOVA, (b, g) student’s t-test, (d, f) one-way ANOVA, *P<0.05, **P<0.01. Scale bar,100 μm. ANOVA, analysis of variance; DSS, dextran sulfate sodium; H&E, hematoxylin and eosin; KO, knockout; WT, wild type.
PHDi-treatment suppresses Th17 responses in the Citrobacter rodentium model of infectious colitis
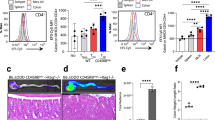
Because our DSS model of colitis did not exhibit a clear Th17 response, we examined next whether PHDi-mediated induction of IL-12p40 was capable of suppressing disease in a well-defined Th17 driven model of colitis.16 To this end, we investigated Th17-expressing LPLs in the C. rodentium model of infectious colitis with and without PHDi treatment (Figure 6a). Citrobacter rodentium infection led to an increase in CD3+CD4+ cells expressing IFN-γ, IL-17 and IL-22 (Figure 6b), as well as IL-17+ cells co-expressing IFN-γ or IL-22 (Figure 6c). PHDi treatment reduced IFN-γ+, IL-17+ cell populations while increasing IL-22+ cells (Figure 6b) and co-expressing cell populations were reduced with PHDi treatment (Figure 6c). Changes in IL-17 and IL-22 expression were verified by qPCR (Figure 6d). These changes in cell population coincided with reduced disease, as measured by colon thickening (Figure 6e) spleen weights (Figure 6f) and histological colitis score (Figure 6g,h), suggesting that PHDi treatment can supress IL-23-mediated disease.
The influence of PHDi on Th17 responses in the Citrobacter rodentium model of infectious colitis. Flow cytometric analysis of intracellular cytokine production from LPL re-stimulated in vitro. (a) Representative dot plots of LPL CD3+CD4+ T cells producing IL-17 and IFN-γ or IL-22 from naive (N) uninfected or C. rodentium-infected mice receiving vehicle (Veh) or PHDi (AKB-4924 5mg kg−1) 14 days post infection. (b) % live CD3+CD4+ T cells expressing IFN-γ, IL-17 and IL-22 or (c) IL-17/IFN-γ and IL-17/IL-22 double-expression. (d) Real-time PCR analysis of il17a and il22 expression in isolated LPL populations. (e) Changes in mucosal thickening (f) spleen weight and (g) histological colitis score in C. rodentium-infected animals with vehicle or PHDi treatment, compared with naive animals. (h) Representative H&E-stained histological images of colons from uninfected naive animals, or C. rodentium infected animals treated with vehicle or PHDi. Data expressed as mean ±s.e.m., (n=6 per group). **P<0.01; ***P<0.005. One-way ANOVA, *P<0.05. ANOVA, analysis of variance.
IL-12p40 homodimer requires IL-12p40 driven disease to exert therapeutic benefit.
Based on the necessity of IL-12/IL-23-mediated disease for the benefit of PHDi treatment, and the secretion of IL-12p40 homodimer in vitro, we examined whether recombinant IL-12p40 homodimer (rIL12p40h) requires IL-12/IL-23 driven disease for therapeutic efficacy. In agreement with previous reports, recombinant p40 homodimer suppressed disease activity in DSS-treated animals (Figure 7a). Treatment with rIL12p40h was sufficient to protect against DSS colitis at a level comparable to PHDi treatment in WT but not IL-12p40−/− DSS-treated animals, as measured by weight (Figure 7a), mucosal thickening, and colon shortening (Figure 7b). Examination of LPLs revealed a significant reduction in CD3+CD4+IFN-γ+ cells in WT DSS-treated animals treated with rIL12p40h that was not evident in IL-12p40−/− DSS-treated animals (Figure 7c) while il12b expression was not affected by treatment (Figure 7d). In order to assess whether the protection afforded by PHDi or rIL12p40h was IL-23-dependent, we examined the efficacy of these treatments in animals subjected to IL-23 depletion over the course of DSS (starting at day 0). Depletion of IL-23 with monoclonal antibody attenuated the severity of colitis as measured by histopathology scoring (Figure 7e) and weight change (Figure 7f) when compared with Rat IgG2a control, treatment, but at a level significantly lower than with the addition of PHDi or rIL-12p40h (Figure 7e,f). IL-23 depletion status made no difference to the protection against colitis afforded by rIL-12p40h (Figure 7e,f). Treatment with rIL12p40h showed similar protection in the TNBS model of colitis, attenuating weight loss (Figure 7f), mucosal thickening (Figure 7g) and this protection was mirrored in disease activity scores (data not shown). These data indicate that IL-12p40 homodimer selectively supresses Th1 mucosal inflammation in the DSS model of colitis.
The influence of recombinant IL-12p40 homodimer treatment on the development of DSS colitis associated pathology. Groups received daily i.p. treatments with 10 ng of recombinant IL-12p40 homodimer (rIL-12p40h), PHDi or vehicle (Veh) as indicated. (a) Weight change (%) from initial weight in the wildtype (WT) or IL-12p40−/− mice receiving DSS and treated with vehicle, PHDi or rIL-12p40h (* denotes DSS WT+PHDi and † denotes DSS WT+rIL-12p40h compared against DSS WT+Veh). (b) Pathological mucosal thickening of the colon after DSS treatment, expressed as weight (mg)/length (cm) and DSS-induced colon-shortening. (c) Percentage of LPL CD3+CD4+ T cells producing IFN-γ in the colon of DSS WT or IL-12p40−/− mice treated with vehicle or rIL-12p40h. (d) il12b mRNA expression in tissue isolated from the colonic mucosa of DSS wild-type mice treated with vehicle or rIL-12p40 homodimer. (e) Representative H&E colon sections and inflammation and epithelial score for animals receiving anti-IL-23p19 (20 mg kg−1, i.p) or control (Rat IgG2a, 20 mg/Kg, i.p) daily from day 0 and treated with vehicle, PHDi or rIL-12p40h from day 5 as indicated. (f) Weight change (%) from initial weight in DSS-treated animals receiving anti-IL-23p19 (daily 20 mg/kg−1, i.p) or control (Rat IgG2a, 20 mg kg−1, i.p) and treated with vehicle, PHDi or rIL-12p40h as indicated. (g) Weight change (%) from initial weight in TNBS models of colitis treated with vehicle, PHDi or rIL-12p40h. (h) Pathological mucosal thickening of the colon expressed as weight (mg)/length (cm) in the TNBS model of colitis. Data expressed as mean±s.e.m., (a, e and f) two-way ANOVA (n=6 per group), (b–d) one-way ANOVA (n=6 per group) (g, h) student’s t-test, (n=6 per group) * and †P<0.05 ** and †† P<0.01, ***P<0.005. Scale bar, 100 μm. ANOVA, analysis of variance; DSS, dextran sulfate sodium; H&E, hematoxylin and eosin; TNBS, 2,4,6-trinitrobenzenesulfonic acid; WT, wild-type.
Discussion
This study aimed to examine mechanisms of HIF-mediated suppression of T helper cell responses in animal models of colitis. We have previously shown the importance of epithelial HIF to the protective action of the HIF prolyl-4-hydroxylase inhibitors (PHDi); AKB-4924 in models of colitis.2 However, little is known about how HIF influences mucosal myeloid populations despite previous observations of a reduction in mucosal pro-inflammatory cytokines.2, 4, 17 Guided by the initial observation that PHD inhibition differentially regulates genes of the IL-12 family, we identified IL12B as a HIF-responsive gene in human and murine monocyte populations resulting in IL-12p40 homodimer secretion with PHDi-treatment. We demonstrate that PHDi-treatment is sufficient to suppress Th1 and Th17 inflammation in an IL-12p40-dependent manner and describe a novel level of HIF-1α-mediated regulation of T-cell responses via IL-12 family cytokines. HIF-1α-mediated responses have emerged as a powerful signaling pathway involved in a wide range of biological processes.14, 15 For instance, recent studies have demonstrated that the influx of neutrophils during experimental colitis induces mucosal hypoxia and HIF-1α stabilization and that this process is critical for the resolution of colitis.18 Although much of our knowledge of inflammatory HIF-responses relates to the epithelium, HIF has also been shown to regulate neutrophil survival and phagocytic capacity.19, 20 HIF-responses in leukocytes is an expanding and important area of research,21 which is unsurprising, given that cells recruited to inflamed tissue will migrate from a relatively oxygen rich environment to the anoxic environment of inflamed tissue.
In considering how HIF stabilization may influence leukocyte responses in inflamed tissue, we observed that PHDi enhanced LPS-mediated induction of IL12B. This may suggest a synergistic relationship between hypoxia and TLR4 signaling, and indeed HIF-mediated enhancement of LPS responses have been previously identified in pancreatic ductal adenocarcinomas.22 In support of this, we identified HREs in both human and murine IL12B promoters. In the human promoter, transcriptional regulation of IL12B was restricted to the two HREs most distal to the transcriptional start site. Of note, the proximal HRE also coincided with an AHR binding site and may represent xenobiotic response elements rather than canonical HREs.23 Interestingly, AHR has been identified as crucial to IL-17/22 innate lymphoid cell (ILC) responses24 and in this way hypoxia, and HIF, may have a role in regulating ILC homeostasis via the HIF/IL-12 axis.
Targeting the IL-12 family pathway has shown promise for the treatment of IBD.12, 25 IL-12 and IL-23 are heterodimers with a shared subunit, p40, and unique subunits IL-12p35 and IL-23p19, respectively. Given the therapeutic approach of targeting the IL-12p40 subunit, it is surprising that IL-12p40−/− DSS mice suffered severe disease in models of colitis comparable to wild-type and this observation is consistent with other animal models of IBD.26, 27 Although IL-12 and IL-23 are key regulators of Th1/Th17 responses, respectively, their importance in initiating and sustaining mucosal inflammation appear to be distinct. In acute or infectious models of colitis, deletion or inhibition of IL-12p35 (refs 26,27) or IL-23p19 (refs 28–31) leads to reduced pathology, suggesting that both of these cytokines are important in initiating innate inflammatory cascades. In contrast, IL-23 appears to have a regulatory role in the adaptive response as specific depletion of IL-23 from T lymphocytes or dendritic cells increases pathology in colitis models,28, 32 with a loss of balance between IL-23 and IL-12 contributing to disease.32, 33 In addition, IL-12 appears to have greater influence on systemic rather than mucosal immune responses as inhibition of IL-12, but not IL-23, signaling led to reduced wasting and serum cytokines in CD40-stimulated colitis.34 These findings are supported by work in the C. rodentium model showing that loss of either IL-12 or IL-23 impairs resistance to infection.35, 36 In reviewing the sometimes-conflicting reports on IL-12 and IL-23 signaling in mucosal inflammation, it is worth considering that much of our knowledge on the interplay of these cytokines in innate mucosal responses precedes our current understanding of innate lymphocytes such as ILCs, for which IL-12 and IL-23 are also critical.37 Contrasting roles for innate and adaptive lymphoid responses would explain why IL-23 depletion is protective in acute colitis, but when crossed with RAG2−/− mice, IL-23 deficient animals exhibit more severe disease,28 as ILCs would be unaffected in these animals. In support of a role for innate cell responses is the regulation of IL-17 and IL-22 observed in C. rodentium mice treated with PHDi. Recently identified innate Th17 (iTH17) cells are a source of pathogenic IL-17A and IL-22 in murine infection models38 and the loss of IL-17 in favor of IL-22 may reflect a suppression of these cells. In addition, the novel finding that IL-22 is a transcriptional target for HIF may indicate that hypoxia and hypoxia-responses are globally important in regulating mucosal cytokine responses39 Given that IL-22 has a dual nature in promoting both inflammation and resolution, contextually coupling both IL-12p40 and IL-22 to a hypoxia response would support the hypothesis that HIF signaling acts to limit the extent of the immune response. Future studies identifying contrasting regulation of HIF/IL-12/23 responses in innate vs. adaptive cells, are likely to be important. For instance, the recent discovery of a GM-CSF/IL-23 axis that regulates eosinophils in IL-23/Th17 models of colitis40 may indicate that an imbalance of IL-12/23 could also promote a T helper Type-2 rather than Th17 response, which in the GI tract, may be important in the context of homeostasis, and indeed, eosinophils are important in both IBD41 and functional GI disorders (FGIDs).42 In addition, both Th1 and Th17 populations can arise independently of IL-12/IL-23. For instance, inflammasome activation of IL-18 signaling is sufficient to drive IFN-γ secretion and Th1 responses43 while Th17 differentiation can occur independent of IL-23 via TGF-β and IL-6 signaling44 or HMGB-1 and IL-1β,45 suggesting that there are redundancies for IL-12 family-mediated T helper cell responses. Nevertheless, the fact that some studies report that IL-12p40-deficient mice actually exhibit more severe disease26 suggests that IL-12p40 may be involved in inflammatory suppression.
The IL-12p40 monomer can dimerize to form a homodimer46 that acts antagonistically against IL-12 and IL-23 (47ref. 47) and has been shown to suppress inflammatory pathways in a number of disease models.48, 49 For instance, recombinant IL-12p40 homodimer prevents IL-12-mediated shock in murine models48 while an IL-12p40-IgG fusion protein has been shown to be protective in the TNBS murine model of colitis49 and this protection occurs due to excess levels of homodimer that inhibit Th1/Th17 pathways.50 The antagonistic properties of IL-12p40 homodimers may therefore act as an inflammatory suppressor, promoting inflammatory resolution, and this hypothesis has been suggested to explain the observation of increased IL-12p40 in the serum of IBD patients in remission.51, 52 The biological role of IL-12p40 homodimer has not been well-defined and remains controversial.53 Indeed, many studies erroneously suggest that IL-12p40 homodimer is not secreted by human immune cells; however, IL-12p40 homodimer has been identified in human airway epithelia54 and microglia.53 In agreement with these studies, we identified homodimer secretion from both mouse and human monocyte populations in response to PHDi or hypoxia. A mechanism for the concurrent reduction in IL-12p35 was not identified in this study and no obvious HRE was identified in the IL12A promoter. It is likely that the IL-12p35 subunit is indirectly regulated by hypoxia and there is evidence to suggest that hypoxia-associated microRNAs55 can suppress IL-12p35 expression.56 Whether PHDis promote this response is yet to be determined. Important in this context, previous work by Kim et al.57 has demonstrated in vitro suppression of IL-12 and IL-23 by IL-12p40 homodimer, while delivery of adenovirus expressing IL-IL-12p40 suppressed IL-17 while enhancing IFN-γ responses in DSS colitis. Crucially, the study by Kim et al. had a number of methodological differences to the current study and of the IL-12p40 produced by adenovirus delivery only 16.9% was IL-12p40 homodimer. In our study, both recombinant IL-12p40 homodimer and combined homodimer and IL-23 blockade was more effective than blockade of IL-23 alone, suggesting that the influence of IL-12p40 homodimer is not based solely on IL-23 inhibition. In the context of mucosal inflammation, the observation that IL-12p35 was suppressed by PHDi may indicate that in the setting of mucosal inflammation, a concurrent suppression of IL-12p35 is important for the secretion of biologically active IL-12p40 homodimer.
IL-12p40 is particularly interesting in the context of IBD, as a large proportion of single-nucleotide polymorphisms (SNPs) identified as risk loci for IBD are involved in IL-12 family signaling.51, 58 The functional nature of IL-12 family SNPs have not been identified, but polymorphisms that influence the HIF-IL12B axis could promote chronic inflammation. In addition, at least two SNPs for HIF-1 have been identified as risk factors for colorectal cancer.59 Recent studies have suggested that HIF-1 does not have a role in tumorigenesis;60 however, it is possible that SNPs that drive over-activation of the HIF-IL12B axis after tumorigenesis could promote immune-evasion by colorectal cancers. One functionally defined polymorphism related to IL-12p40 results in a stronger binding affinity of IL-12p40 to the p35 and p19 dimers,61 and in this regard may allow acute inflammation to proceed to chronic disease due to an absence or reduction of antagonistic homodimer formation. Conversely, evidence of a pathogenic role for IL-12p40 homodimer lies in the observation that the airway epithelia of asthma patients have increased levels of IL-12p40, manifesting as homodimer, that skews inflammation towards Th2 signaling54 by suppressing Th1 responses, particularly during viral infections. Interestingly, epithelial HIF stabilization is also a feature of asthma62 and myeloid HIF-1 expression is associated with Th2-skewing63 suggesting that the HIF-IL12B axis this may represent a maladaptive pathway in certain conditions. In the context of cancer, in vitro studies suggest that excessive IL-12p40 secretion from breast cancer cells may promote tumor development via inhibition of NK cells.64 Thus homodimer secretion, in a maladaptive setting, may be a further link between hypoxia and cancer. Our observation that HIF-mediated IL-12p40 induction led to reduction of both Th1 and Th17 cell populations in the lamina propria of colitis models in a manner similar to treatment with homodimer, offers a possible mechanism linking both HIF and IL-12/23 SNPs with IBD.
Our work demonstrates that IL-12p40 is selectively regulated by HIF and this transcriptional pathway may represent an endogenous “off switch” for inflammation, activated when immune infiltration reaches the tissue’s hypoxic threshold and signaling outward to limit the extent of immune cell influx into the inflamed tissue, therefore acting as an “inflammatory brake” (Figure 8). This observation may explain the increases of serum IL-12p40 in remitting IBD patients52 and may therefore represent a mechanism which dictates whether inflammation resolves or progresses to chronicity. While antagonism of IL-12/IL-23 may offer similar translational potential as monoclonal antibodies targeting IL-12p40, these monoclonal therapies will also target p40 homodimer, thus the potential of supplementing antibody therapies with recombinant homodimer may improve the efficacy of this approach. Importantly, our findings offer two new therapeutic strategies for mucosal inflammatory disease; prolyl-hydroxylase inhibition and IL-12/IL-23 antagonism to target inflammatory conditions.
Potential role for IL-12p40 homodimer as an “inflammatory brake” during hypoxic inflammation. Activation of HIF via hypoxia or therapeutic PHDi treatment leads to a selective induction of IL-12p40 and secretion of IL-12p40 homodimer by myeloids cells, which suppress the differentiation of naive T helper cells into Th1cells or the stabilization of Th17 cells, limiting the progression of Th1/th17 inflammation. PHDi: prolyl-hydroxylase inhibitor, Hx: hypoxia Th0: naive T helper cell. HIF, hypoxia-inducible factor-1α.
Methods
Bacterial and cell culture
The RAW264.7 murine macrophage cell line (ATCC T1B-71, Manassas, VA) was cultured in DMEM and the human U937 monocyte cell line (ATCC CRL-1593.2) was cultured in RPMI 1640. Both media were supplemented with 10% heat-inactivated fetal bovine serum (FBS, Bovogen Biologicals, Kellor East, Victoria, Australia) and penicillin (100 units ml−1), streptomycin (0.1 mg ml−1), HEPES (25 mM), nonessential amino acids, L-glutamine (4 mM). Cells were seeded at a density of 5 × 104 cells cm−2 and maintained in 5% C02, 37 °C. U937 cells were differentiated to macrophages by the addition of phorbol 12-myristate 13-acetate (Sigma-Aldrich, St. Louis, MO) at a final concentration of 160 nM, 48 h before assay. The PHDi AKB-4924 was a generous gift from Aerpio Therapeutics, Cincinnati, OH, USA. Cells were treated with cyclodextrin vehicle or 5 uM AKB-4924 in medium alone or in the presence of lipopolysaccharide (Escherichia coli LPS, 100 ng ml−1, Sigma-Aldrich). C. rodentium was obtained from the ATCC. C. rodentium were cultured overnight and resuspended in sterile PBS for murine infection, as previously described.65
Animal models
Wildtype (WT) female BALB/c, C57Bl/6 and C57Bl/6-IL-12p40−/− (KO) mice (Australian BioResources, Moss Vale, New South Wales, Australia) (6–8 week old) were housed for one week to allow microflora equilibration. For the DSS model, mice received 2.5% w/v dextran sodium sulfate (DSS; M.Wt. 36,000–50,000; MP Biomedicals, Illkirch, France) in drinking water ab libitum for 5 days, followed by 5 days of drinking water. TNBS colitis was initiated as previously described.17 For the C. rodentium model, mice received an oral gavage with 0.1 ml of 2.5 × 108 CFU of C. rodentium. The PHDi AKB-4924 (Aerpio Therapeutics (5 mg kg−1)was administered at 48 h intervals via 100 μl intraperitoneal injection. Cyclodextrin (100 μl) was administered as a vehicle. Body weight was monitored daily over the course of the experiment from day-1 relative to disease induction. All experiments were performed in accordance with institutional approvals. Recombinant IL-12p40 homodimer (#499-ML-025) and Mouse Anti-IL-23 p19 Antibody (#AF1619) were obtained from R&D Systems (Minneapolis, MN, USA).
Clinical samples
Mucosal biopsies and blood samples were obtained from human volunteers (N=10, 22–45 years of age, six male and four female) undergoing routine endoscopic screening for non-inflammatory disorders. All clinical studies were performed in accordance with institutional ethics approvals.
IL12B promoter analysis
In silico analysis of the IL12B promoter sequence was carried out using MatInspector (https://www.genomatix.de) and revealed the existence of two potential binding sites for HIF-1 (core sequence 5-RACGTG-3) in the murine IL12B promoter and 4 potential HIF-1-binding sites in the human IL12B promoter. The human IL12B promoter construct, cloned into a pGL-3 basic luciferase expression vector was originally purchased from Switchgear Genomics (Carlsbad, CA). The murine IL12B promoter was generated from RAW264.7 cell genomic DNA using standard PCR with Accuprime DNA polymerase (Invitrogen, Carlsbad, CA) and cloned into pGL-3 basic luciferase expression vector (Promega, Madison, WI, USA) as previously described. Homology to published sequence was confirmed by sequencing. Site-directed mutagenesis was performed as previously described.3 Cells were transfected with plasmid constructs using Fugene-6 transfection reagent (Promega). All activity was normalized with respect to co-transfected Renilla plasmid and Dual-Luciferase Reporter assay (Promega) was used to measure the ratio of firefly Renilla/luciferase.
RT-PCR
RNA was extracted from colon segments or purified cells with TRIzol Reagent (Invitrogen) and complementary DNA (cDNA) generated (Bio-Rad iScript). mRNA analysis was carried out by real-time-PCR assays using previously validated primers (Realtime Primers, Elkins Park, PA) and normalized to the housekeeping gene ACTB for generation of ΔCt values. Relative expression was quantified with the 2−ΔCt method.
Isolation of colonic lamina propria monocytes (LPMC) and peripheral blood monocyte cells (PBMC)
Murine colonic tissue or colonic biopsies were washed with PBS. For removal of the epithelium, colons were incubated with Hank’s balanced salt solution (HBSS) without Mg2+ or Ca2+, supplemented with 10% heat-inactivated FBS, 10 mM HEPES and 5 mM EDTA. Tissue was then digested using collagenase D (25 μg ml−1, Roche) in HBSS (Life Technologies, Carlsbad, CA) supplemented with 10 μg ml−1 DNase I (Roche, Basel, Switzerland) for 45 min in a shaking water bath (37 °C). Cell suspensions were strained and the digestion repeated. Cells were re-suspended in 40% Percoll (GE Healthcare, Parramatta, New South Wales, Australia) and layered on 80% Percoll before centrifugation at 900g at r.t. to purify lymphocyte populations. PBMCs were similarly isolated by density centrifugation as previously described.66
In vitro and ex vivo cell culture assays
RAW 264.7 cells, murine LPMCs or human PBMCs were plated at a density of 5 × 105 in RPMI media, supplemented with 10% FBS in U-bottom 96-well plates. Cells were stimulated with 10 ng LPS in the presence or absence of PHDi (AKB-4924, 5 μM) and incubated in either hypoxic conditions (37 °C, 1%O2, 5%CO2, PLAS-Labs hypoxia chamber) or normal culture conditions. At 24 and 48 h time-points supernatants were collected for assay.
Cytokine protein analysis
Mucosal cytokine levels were assayed by cytometric bead array (CBA) assay (BD Bioscience, San Jose, CA) as per the manufacturer’s instructions.
Flow cytometry
Combinations of the following anti-mouse antibodies were used for flow cytometry; CD3-efluor780, CD4-FITC, CD8-PerCP,IL-17-efluor710, anti-IL22-PE and IFN-γ-PeCy7. Live/Dead staining was performed with Live/Dead efluor506 (EBioscience, San Diego, CA). For intracellular cytokine staining, 1 × 106 cells isolated from the lamina propria or MLN were stimulated with Phorbol myristate acetate (PMA, 50 ng ml−1)+Ionomycin (1 μg ml−1) for 6hr in the presence of Brefeldin A (5 μg ml−1). Samples were fixed and permeabilized, and acquired using BD CANTO II or FACS ARIA II (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed with FlowJo software (Treestar, Ashland, OR).
Western blotting
IL-12p40 homodimer was identified by native polyacrylamide gel electrophoresis and western blot. Cell supernatants were resolved on 7% SDS-polyacrylamide gels. Recombinant IL-12p40 homodimer (rIL-12p40h) was used as a standard. Proteins were transferred to membranes by semi-dry blotting in methanol-free transfer buffer. IL-12p40 homodimers were detected by incubation with a primary anti-IL-12p40 mAb (clone EPR5739; ab133752) (Abcam, Cambridge, UK) followed by Goat-anti-rabbit IgG secondary (Abcam #HAF008). The membranes were then visualized by chemi-luminescence (SuperSignal WestPico Chemi-luminescence, Thermo Fisher Scientific, Waltham, MA).
Histological scoring
Histological grading of severity of inflammation and epithelial injury was scored blinded and then unblinded by a gastrointestinal pathologist as previously published.17 The degree of inflammation and epithelial injury was graded semi-quantitatively by a system modified from Neurath et al.67 For the C. rodentium model colitis score was assessed as previously described by Nordlander et al.68 and Simmons et al.36
Endoscopic scoring
Animals were examined using a small animal gastrointestinal endoscope (Karl Storz Endoscopy Australia Pty, Lane Cove West, New South Wales, Australia) as previously described.17 Images and videos were recorded to characterize the extent of disease throughout the length of the colon. Disease was assessed by the presence and/or extent of disease pathologies based on a specific criteria previously published.17 Each pathological feature was assigned a numerical value between 0 and 3 representative of the presence and/or extent of a specific disease feature in the animal. Each animal’s recording was carefully examined and scored under blind conditions to avoid bias. Animals were given a total score out of 15 as a representation of disease severity, with 0 representing negligible disease present to 15 representing the presence of severe disease.
Statistical analysis
Due to the time-dependent nature of immune cell isolation protocols, all colitis experimental data is representative of at least two experiments, where groups of N=3 were pooled. For cell culture experiments each N represents a passage of cells with three technical replicates per passage. Data was analyzed by Student’s t-test, Mann–Whitney U-test or analysis of variance as appropriate using GraphPad Prism (La Jolla, CA). Mean data are expressed with error bars denoting s.e.m. *P<0.05, **P<0.01, ***P<0.001.
References
Balligand, J.L., Feron, O. & Dessy, C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 89, 481–534 (2009).
Keely, S. et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 7, 114–123 (2014).
Keely, S. et al. Selective induction of integrin beta1 by hypoxia-inducible factor: implications for wound healing. FASEB J. 23, 1338–1346 (2009).
Robinson, A., Keely, S., Karhausen, J., Gerich, M.E., Furuta, G.T. & Colgan, S.P. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Okumura, C.Y. et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J. Mol. Med. (Berl.) 90, 1079–1089 (2012).
Tanaka, T. & Nangaku, M. Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylase inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J. Pharmacol. Sci. 109, 24–31 (2009).
Funderburg, N.T. et al. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology 140, 87–97 (2013).
Fuss, I.J. et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 157, 1261–1270 (1996).
Mosmann, T.R., Cherwinski, H., Bond, M.W., Giedlin, M.A. & Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J. Immunol. 175, 5–14 (2005).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
McKenzie, B.S., Kastelein, R.A. & Cua, D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 27, 17–23 (2006).
Sandborn, W.J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 367, 1519–1528 (2012).
Zenewicz, L.A., Antov, A. & Flavell, R.A. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol. Med. 15, 199–207 (2009).
Goggins, B.J., Chaney, C., Radford-Smith, G.L., Horvat, J.C. & Keely, S. Hypoxia and integrin-mediated epithelial restitution during mucosal inflammation. Front. Immunol. 4, 272 (2013).
Taylor, C.T. & Colgan, S.P. Hypoxia and gastrointestinal disease. J. Mol. Med. 85, 1295–1300 (2007).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Marks, E. et al. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm. Bowel Dis. 21, 267–275 (2015).
Campbell, E.L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014).
McGovern, N., Willcocks, L., Walmsley, S., Condliffe, A., Chilvers, E. & Chilvers, E.R. The effects of hypoxia on neutrophil phagocytosis, respiratory burst activity and bactericidal capacity. Am. J. Resp. Crit. Care 179 (2009).
Walmsley, S.R. et al. Hypoxia-induced neutrophil survival is mediated by HIF-1 alpha-dependent NF-kappa B activity. J Exp Med 201, 105–115 (2005).
Jantsch, J. & Schodel, J. Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology 220, 305–314 (2015).
Zhang, J.J., Wu, H.S., Wang, L., Tian, Y., Zhang, J.H. & Wu, H.L. Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J. Gastroenterol. 16, 2881–2888 (2010).
Matsushita, N., Sogawa, K., Ema, M., Yoshida, A. & Fujii-Kuriyama, Y. A factor binding to the xenobiotic responsive element (XRE) of P-4501A1 gene consists of at least two helix-loop-helix proteins, Ah receptor and Arnt. J. Biol. Chem. 268, 21002–21006 (1993).
Lee, J.S., Cella, M. & Colonna, M. AHR and the transcriptional regulation of type-17/22 ILC. Front. Immunol. 3, 10 (2012).
Sandborn, W.J. et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastro 135, 1130–1141 (2008).
Camoglio, L. et al. Contrasting roles of IL-12p40 and IL-12p35 in the development of hapten-induced colitis. Eur. J. Immunol. 32, 261–269 (2002).
Takagi, H. et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand. J. Gastroenterol. 38, 837–844 (2003).
Cox, J.H. et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 5, 99–109 (2012).
Hue, S. et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006).
Kullberg, M.C. et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 (2006).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Becker, C. et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J. Immunol. 177, 2760–2764 (2006).
Khader, S.A. et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175, 788–795 (2005).
Uhlig, H.H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Mangan, P.R. et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441, 231–234 (2006).
Simmons, C.P. et al. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168, 1804–1812 (2002).
Goldberg, R., Prescott, N., Lord, G.M., MacDonald, T.T. & Powell, N. The unusual suspects—innate lymphoid cells as novel therapeutic targets in IBD. Nat. Rev. Gastroenterol. Hepatol. 12, 271–283 (2015).
Geddes, K. et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17, 837–844 (2011).
Budda, S.A., Girton, A., Henderson, J.G. & Zenewicz, L.A. Transcription factor HIF-1alpha controls expression of the cytokine IL-22 in CD4 T cells. J. Immunol. 197, 2646–2652 (2016).
Griseri, T. et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43, 187–199 (2015).
Keely, S. & Foster, P.S. Stop press: eosinophils drafted to join the Th17 team. Immunity 43, 7–9 (2015).
Keely, S., Walker, M.M., Marks, E. & Talley, N.J. Immune dysregulation in the functional gastrointestinal disorders. Eur. J. Clin. Invest. 45, 1350–1359 (2015).
Smeltz, R.B., Chen, J. & Shevach, E.M. Transforming growth factor-beta1 enhances the interferon-gamma-dependent, interleukin-12-independent pathway of T helper 1 cell differentiation. Immunology 114, 484–492 (2005).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487 (2007).
Hasegawa, E. et al. IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J. Immunol. 190, 1778–1787 (2013).
Dasgupta, S., Bandopadhyay, M. & Pahan, K. Generation of functional blocking monoclonal antibodies against mouse interleukin-12 p40 homodimer and monomer. Hybridoma (Larchmt) 27, 141–151 (2008).
Wang, X. et al. Characterization of mouse interleukin-12 p40 homodimer binding to the interleukin-12 receptor subunits. Eur. J. Immunol. 29, 2007–2013 (1999).
Mattner, F. et al. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect. Immun. 65, 4734–4737 (1997).
Stallmach, A. et al. An interleukin 12 p40-IgG2b fusion protein abrogates T cell mediated inflammation: anti-inflammatory activity in Crohn's disease and experimental colitis in vivo. Gut 53, 339–345 (2004).
Ha, S.J. et al. Engineering N-glycosylation mutations in IL-12 enhances sustained cytotoxic T lymphocyte responses for DNA immunization. Nat. Biotechnol. 20, 381–386 (2002).
Abraham, C. & Cho, J.H. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 60, 97–110 (2009).
Kader, H.A. et al. Protein microarray analysis of disease activity in pediatric inflammatory bowel disease demonstrates elevated serum PLGF, IL-7, TGF-beta1, and IL-12p40 levels in Crohn's disease and ulcerative colitis patients in remission versus active disease. Am. J. Gastroenterol. 100, 414–423 (2005).
Jana, M. & Pahan, K. Induction of lymphotoxin-alpha by interleukin-12 p40 homodimer, the so-called biologically inactive molecule, but not IL-12 p70. Immunology 127, 312–325 (2009).
Walter, M.J., Kajiwara, N., Karanja, P., Castro, M. & Holtzman, M.J. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J. Exp. Med. 193, 339–351 (2001).
Mace, T.A., Collins, A.L., Wojcik, S.E., Croce, C.M., Lesinski, G.B. & Bloomston, M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. J. Surg. Res. 184, 855–860 (2013).
Lu, T.X., Munitz, A. & Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 182, 4994–5002 (2009).
Kim, D.J. et al. Delivery of IL-12p40 ameliorates DSS-induced colitis by suppressing IL-17A expression and inflammation in the intestinal mucosa. Clin. Immunol. 144, 190–199 (2012).
McGovern, D. & Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 56, 1333–1336 (2007).
Knechtel, G. et al. Single nucleotide polymorphisms in the hypoxia-inducible factor-1 gene and colorectal cancer risk. Mol. Carcinog. 49, 805–809 (2010).
Xue, X., Ramakrishnan, S.K. & Shah, Y.M. Activation of HIF-1alpha does not increase intestinal tumorigenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G187–G195 (2014).
Zwiers, A. et al. A polymorphism in the coding region of Il12b promotes IL-12p70 and IL-23 heterodimer formation. J. Immunol. 186, 3572–3580 (2011).
Ahmad, T. et al. Hypoxia response in asthma: differential modulation on inflammation and epithelial injury. Am. J. Respir. Cell Mol. Biol. 47, 1–10 (2012).
Crotty Alexander, L.E. et al. Myeloid cell HIF-1alpha regulates asthma airway resistance and eosinophil function. J. Mol. Med. (Berl.) 91, 637–644 (2013).
Heckel, M.C. et al. Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol. 266, 143–153 (2011).
Linden, S.K., Florin, T.H. & McGuckin, M.A. Mucin dynamics in intestinal bacterial infection. PLoS One 3, e3952 (2008).
Ulmer, A.J., Scholz, W., Ernst, M., Brandt, E. & Flad, H.D. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology 166, 238–250 (1984).
Neurath, M.F. et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 195, 1129–1143 (2002).
Nordlander, S., Pott, J. & Maloy, K.J. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal Immunol. 7, 775–785 (2014).
Acknowledgements
We acknowledge funding from the National Health and Medical Research Council (NHMRC) Project Grants APP1021582 and APP1128487 and the Hunter Medical Research Institute, Bowel of the Ball charity award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Marks, E., Naudin, C., Nolan, G. et al. Regulation of IL-12p40 by HIF controls Th1/Th17 responses to prevent mucosal inflammation. Mucosal Immunol 10, 1224–1236 (2017). https://doi.org/10.1038/mi.2016.135
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/mi.2016.135
This article is cited by
-
CD4+ T cells mediate CAR-T cell-associated immune-related adverse events after BCMA CAR-T cell therapy
Nature Medicine (2026)
-
High glucose/ChREBP-induced Hif-1α transcriptional activation in CD4+ T cells reduces the risk of diabetic kidney disease by inhibiting the Th1 response
Diabetologia (2025)
-
Over supplementation with vitamin B12 alters microbe-host interactions in the gut leading to accelerated Citrobacter rodentium colonization and pathogenesis in mice
Microbiome (2023)
-
Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome
Mucosal Immunology (2019)