Abstract
Variation in the gene for apolipoprotein E (APOE) is one of the few variables that is associated with individual differences in age-related cognitive decline in humans. Therefore, it is important to understand the conditions that affect the strength of its effect. Here we examine how the effect size of APOE variation (possession of one or more e4 alleles) on a test of general cognitive ability changes with age from 11–90 years. The data are from the Lothian Birth Cohorts of 1936 and 1921 who took the same cognitive test (the Moray House Test No. 12) at, respectively, ages 11 (N = 954), 70 (N = 1001), 76 (N = 636), 79 (N = 471), and 11 (N = 483), 79 (N = 533), 87 (N = 198), 90 (N = 120). The standardised absolute effect of APOE e4 on general cognitive ability was about zero at ages 11 (beta < 0.05) and 70 (beta ≤ 0.025) and increased linearly to beta = 0.30 (p < 0.001) at age 90. The effect sizes were minimally affected by adjusting for medical conditions (hypertension, diabetes, cardiovascular disease, stroke). However, the results were less robust to removing those participants who developed dementia; effect sizes were reduced by about a third to a half, and were largely non-significant. The results suggest that the negative effect of APOE e4 on cognitive functioning becomes greater with age; this urges more work to understand the mechanisms by which e4 status renders the older person’s brain increasingly vulnerable to cognitive decline and dementia.
Similar content being viewed by others
Introduction
As an outcome variable, cognitive functioning is a moving target. In humans, aspects of cognitive capability show mean declines after young adulthood [1, 2]. Older people score lower on some tests of, for example, processing speed, memory, and reasoning, whereas verbal capability, general knowledge, and some numerical abilities age better [3, 4]. To complicate the picture, people show individual differences in cognitive test scores throughout the life course and, although these differences are moderately stable across several decades [5,6,7,8], there are individual differences in the changes that occur between youth and older age [2, 9, 10]. All of this matters. Even when assessed in childhood or youth, cognitive ability relates to later life outcomes: it predicts how well people perform in educational and occupational settings [11], and higher cognitive ability in youth is related to future better health and longer life [12]. Given this, it is predictable and empirically demonstrated that higher cognitive function in adulthood and older age is related to better management of daily living and a higher likelihood of remaining independent [13, 14]. It follows that it is interesting and practically important to discover the determinants of people’s differences in cognitive functioning. And a moment’s reflection reveals that that is at least two questions. First, what variables are related to cognitive differences at any given age (and we note that a variable related to cognitive functioning at one age might not be associated at some other age)? Second, what variables are related to individual differences in changes from a given age to a later age? Of course, that depends on the stage of the life course that is being investigated; i.e., it is of interest to find out what predicts improvements through childhood to adulthood, what predicts the declines from youth to older age, and what predicts declines within older ages [7].
Focussing on older age, there is much published work that tries to find the variables that are associated with healthy cognitive ageing, i.e., what predicts less decline—or even some improvement—from a given age to a later age [15,16,17]. (Of course, the special hope is to discover modifiable associates, so that people’s cognitive trajectories can be made to proceed less steeply downward.) The list of candidate predictors is long, taking in genetics, demographics, physical and mental health, fitness, personality, lifestyle, diet, physical and social environment, and so forth. Popular media, books, and sundry sources of advice beyond the academic journals abound with ideas to keep one’s thinking sharp [18,19,20,21]. Though this looks encouraging at a distance, the reality is more stark. There are few replicated determinants of people’s differences in age-related cognitive changes [15, 16, 22]. Most effect sizes are small. Some so-called associates of cognitive ageing are either outcomes rather than causes of cognitive capability, or cognition and the putative predictor are both related to some prior confounder [15, 23]. In a stringent test, when many putative predictors of cognitive ageing are entered together in a multivariate setting, there are few significant results [16].
The equation that tries to solve the problem of what contributes to cognitive ageing differences is simple; i.e., beyond the level of cognitive ability that each person had on a previous occasion, what predicts their cognitive score on subsequent occasions? To be clear, it is important that the predictor is set as an exposure to the outcome of cognitive change, not just a one-time cognitive test score. In older age—the 70 s, for example—cognitive test scores from adolescence account for about half of the variance in cognitive ability. Beyond prior cognitive ability [6, 7], one variable that stands out as a frequently-reported additional predictor of cognitive ability test scores in older age is possession of the e4 allele of the gene for apolipoprotein E (APOE) [16, 24]. The approximately quarter of the population who have at least one copy of the APOE e4 allele are more likely to develop dementia and also to have more age-related cognitive decline that is short of dementia than those who don’t possess it [24,25,26]. There are studies demonstrating that variation in APOE genotype is associated with cognitive function in older age, cognitive change from childhood to older age [27], and cognitive change within older age [16, 28]. Few studies in the field are directly comparable, owing to differences including the age-range studied, the cognitive tests used, and the categories of APOE genotype that are used. The Whitehall II study’s examination of APOE’s association with global cognitive function from age 45–85 across five waves of testing is comparable in its concerns to the present study [29]. They found that APOE e4 heterozygotes had poorer cognitive function and steeper cognitive decline from age 75 onwards, compared to non-APOE carriers, with no differences between 60 and 70 years. Similarly, in the Chicago Health and Ageing Project, those who were aged about 84 years and possessed e3e4 APOE genotypes declined twice as much (about 1 versus 0.5 standard deviations) in global cognitive function over ten years than those with e3e3 [30]. No such differences in decline were found in those aged about 66 years.
The need for more research was the conclusion of a review that assembled results from 65 cross-sectional and 46 longitudinal studies of the association between APOE variants and cognitive test scores in people without cognitive impairment or dementia [24]. The reviewed studies were published between 1994 and 2017. The results were presented across a number of cognitive phenotypes and constructs. Neither the cross-sectional results nor the longitudinal results were consistent. Sometimes people with the e4 allele of APOE had lower cross-sectional/baseline test scores and sometimes not; ditto with accelerated cognitive change. There was an indication that episodic memory might be particularly affected by the e4 allele. The authors suggested that any detrimental effects of APOE e4 on cognitive function might be less in the oldest old. They recognised that a meta-analytic review had found overall detrimental effects of e4 allele possession on global cognition, episodic memory, executive function, and perceptual speed [31]. They also discussed methodological challenges at length, aiming to encourage studies that might be more informative. Recommendations included having a large (e.g., >1000) sample size, starting with a sample that is older than their 60 s, conducting longitudinal research with a sufficient follow-up period, trying to rule out prodromal dementia among apparently healthy people, using sensitive rather than brief cognitive tests, and using one or a small number of cognitive phenotypes that are affected by ageing and dementia to minimise multiple comparisons.
The present study accords with the above recommendations. We declare that, among the APOE-cognition studies mentioned heretofore, there are several that employed data from the Lothian Birth Cohorts whose data are used in the present study [16, 27, 32,33,34,35,36,37]. Only one study of APOE variants in the Lothian Birth Cohorts reported on the cognitive phenotype that is used in the present study (the Moray House Test) and that included only two time points—age 11 and age 79—in the Lothian Birth Cohort 1921 [27]. Moreover, the present study is the first to include all testing waves to date of both cohorts in the same study. Notably, the younger (LBC1936) and older (LBC1921) cohorts’ data collection has proceeded substantially since that publication; the two cohorts overlap almost perfectly in sample size at age 79, allowing a two-cohort, uninterrupted follow-up from age 70 to age 90.
The present study addresses a specific question that has not been addressed to date, i.e., what is the effect size of the e4 allele of APOE on general cognitive ability on the same people taking the same cognitive test from age 11 to age 90?
Materials and methods
Participants
The Lothian Birth Cohorts of 1921 and 1936 (LBC1921 and LBC1936) are two longitudinal studies based in Scotland, investigating cognitive ageing and health trajectories across the lifespan [10, 16, 38, 39]. Most of the participants in these cohorts took part in the Scottish Mental Surveys of 1932 [40] or 1947 [41], respectively, which tested the intelligence of nearly all Scottish school children born in 1921 and 1936, using a validated IQ-type test—The Moray House Test No. 12 (MHT). These early-life assessments provide rarely-available baseline data for studying cognitive ageing.
The LBC1921 recruited 550 participants at baseline in older age (age ~79), with follow-ups until age 90. The LBC1936 recruited 1091 participants at baseline in older age (age ~70) and continues ongoing follow-ups at the time of writing (2025). Both cohorts provide rich longitudinal data, including cognitive tests, health measures, genetic profiles, and psycho-social and sociodemographic variables [39].
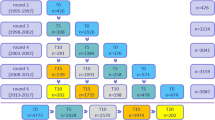
For the current study, ‘All comers’ included participants with APOE e4 and Moray House Test data from at least one time point (LBC1921: n = 457; LBC1936: n = 1010). ‘Completers’ were those who completed the MHT at all three later-life waves (LBC1921: ages 79, 87, 90, n = 119; LBC1936: ages 70, 76, 79, n = 457). Participant flowcharts are presented in Fig. 1.
APOE e4 status
APOE e4 carrier status (no = 0, yes = 1) was determined through genotyping of DNA extracted from blood or saliva samples. Genotyping focused on two key single nucleotide polymorphisms (SNPs), rs429358 and rs7412, which define the e2, e3, and e4 alleles of the APOE gene. APOE was genotyped in LBC1921 by polymerase chain amplification restriction fragment length polymorphism (PCR-RFLP) analysis as described in [33]. Genotyping of LBC1936 was conducted by the Genetics Core at the Wellcome Trust Clinical Research Facility at the Western General, Edinburgh using TaqMan technology [34].
The Moray House Test (MHT)
The MHT (No. 12, revised) is a group-administered cognitive test originally developed to measure general intelligence in Scottish school children. It was administered to almost all 1921-born and 1936-born children in Scotland as part of the Scottish Mental Surveys of 1932 [40] and 1947 [41], respectively, under standardised conditions. Most of the Lothian Birth Cohorts’ participants—those born in 1921 and 1936—completed the MHT at age about 11 years, providing a childhood measure of cognitive ability that serves as a baseline in the lifelong study of cognitive ageing. These data, archived by the Scottish Council for Research in Education were made available for the LBC studies. The MHT includes a variety of items assessing verbal reasoning (which predominates), spatial awareness, arithmetic, and other cognitive skills. Across the life course, MHT scores are highly correlated with concurrently-administered measures of general intelligence (g factor), making it a validated measure of childhood and older-age cognitive ability [40, 42]. The same MHT test was re-administered to Lothian Birth Cohorts participants in older age, at study baseline and two further later-life waves (see above), with identical wording, instructions, and time limits. For the present study, raw MHT score (maximum = 76) served as the dependent variable.
Covariates
In the LBC1921 and LBC1936, several demographic and health-related variables were assessed at each wave of testing. Age (calculated in days), sex (male = 1, female = 2), and health variables (self-reported history of cardiovascular disease, stroke, hypertension, and diabetes; all coded as no = 0, yes = 1) were included as covariates.
Participants were free from dementia at recruitment and subsequent presence of dementia was ascertained using medical consensus based on health records and some in-person visits [37, 43,44,45]. A similar multi-source approach was used in both cohorts: electronic medical records were examined in detail for all consenting participants by a team of clinicians; dementia recorded as a cause of death on death certificates (in any position) was noted; and a subset of participants were visited at home for an assessment by a research doctor if they expressed concerns about their memory at any wave of follow up or if the research team judged that there were reasons to be concerned about cognitive decline. Separate multi-specialty consensus meetings were held for each cohort where each individual’s dementia status (probable, possible, or no dementia) was agreed, along with subtype where it was possible to identify this (again, probable or possible, depending on the amount of information available). In LBC1921 and LBC1936, respectively, 117/527 (in December 2016) and 125/865 (in August 2022) individuals were identified as having probable (110 and 118, respectively) or possible dementia. The n = 865 denominator is the number who consented (because they were asked at Wave 2) to our accessing their medical records. Dementia ascertainment was used in sensitivity analyses (see below).
Statistical analysis
Two sets of statistical analyses tested two related, but slightly different questions.
First, we used linear regression simply to ask whether there was a widening of any cognitive gap between APOE e4 carriers vs non-carriers as people grew older. Thus, we conducted separate regression analyses for each testing instance (wave). Specifically, linear regression was used to examine cross-sectional associations between APOE e4 status and MHT score in childhood (age 11) and in later life (LBC1936: ages 70, 76, 79; LBC1921: ages 79, 87, 90). Model 1 adjusted for age and sex, whereas model 2 included additional adjustments for health variables (CVD, stroke, hypertension, and diabetes) in the later-life data collection waves. Analyses were stratified by cohort and by subgroup (‘all comers’ and ‘completers’). A meta-analytic estimate for MHT scores at age 79 combined results across both the LBC1921 and LBC1936 because both cohorts had been tested at that age. Sensitivity analyses excluded participants with dementia diagnosed post-baseline (both cohorts were dementia-free at baseline), up to the end of the dementia ascertainment follow-up period (which was completed after the data analysed in the present study were collected).
Second, we asked, using the same data but this time applying a longitudinal analytical approach, whether possession of APOE e4 was associated with steeper declines in MHT score in older age. In a structural equation modelling setting, latent growth curve models estimated individuals’ trajectories of MHT scores over time, focussing on the association between APOE e4 carrier status and cognitive performance in older age. These models provide estimates for the intercept (baseline cognitive ability) and the slope (rate of cognitive change over time), with adjustments for covariates, to test whether APOE e4 carriers differ in their initial cognitive performance and/or experience a steeper decline compared to non-carriers. The structural equation modelling-based approach was consistent with previous work on the Lothian Birth Cohorts [16, 46, 47]. Path weights corresponded to average time lags between waves: LBC1936 (0, 6.75, 9.81 years) and LBC1921 (0, 7.53, 11.04 years).
Linear regression and growth curve models were conducted in R (v4.3.3, “Tidyverse Teachings”, R Core Team, Vienna, Austria), using the ‘lavaan’ package [48], with standardised estimates, p-values, and confidence intervals reported. Model fit for growth curve modelling was tested using absolute fit indices: Root Mean Square Error of Approximation (RMSEA; values <0.06 considered acceptable), Comparative Fit Index (CFI; values > 0.95 considered acceptable), and Standardized Root Mean Square Residual (SRMR; values <0.08 considered acceptable).
Principal component analysis was used in SPSS version 29 to examine the loadings of the Moray House Test in the setting of the other 13 varied cognitive tests used in the first testing wave (age 70) of the Lothian Birth Cohort 1936 [49]. Owing to their high correlation, the National Adult Reading Test and the Wechsler Test of Adult Reading (which share the same maximum score) were averaged to form a single indicator of verbal ability. Component number was determined using the eigenvalues >1 criterion and scree slope inspection. This analysis was carried out to offer more information about the Moray House Test as an indicator of general cognitive functioning.
In response to a referee’s request to compare the Moray House Test scores of participants who had zero, one, or two APOE e4 alleles, we conducted one-way ANOVA tests. Where ANOVA results were significant, the Tukey HSD (Honestly Significant Difference) test was used to test for pairwise differences in MHT scores across the three groups. Analyses were run for all comers and for the subset of completers only.
Results
Considering the all comers of both cohorts, 30% of the LBC1936 had the e4 allele of APOE as did 27% of the LBC1921 (Table 1). A sizeable minority of both cohorts reported hypertension and/or cardiovascular disease with much smaller minorities reporting diabetes or stroke (Table 1).
Across both the LBC1921 and LBC1936 analytic samples, N = 216 developed dementia. Of the N = 1010 in the LBC1936 analytic sample, N = 107 developed dementia (medically confirmed) by the end of the follow-up period. The LBC1936 sample with dementia exclusions was N = 903. Of the N = 539 in the LBC1921 analytic sample, N = 109 developed dementia (medically confirmed) by the end of the follow-up period. The LBC1921 sample with dementia exclusions was N = 430.
The moray house test scores from age 11 to age 90
Mean Moray House Test scores for both LBC1921 and LBC1936 were always higher in older ages than at age 11 years (Table 2). Within older age, the MHT scores declined for both cohorts whether one considers the all comers or the completers. The LBC1936 had a higher mean score on MHT at age 11 than the LBC1921. The difference is 2.7 points, roughly reflecting the whole-population difference found between the Mental Surveys of 1932 and 1947 [41]. Both cohorts were tested at the same older age—79 years—at which time the MHT score difference was similar to that at age 11, i.e., 3.2 points; we note that this was the third exposure to this test within older age for the LBC1936 but only the first for the LBC1921. Considering completers only, the mean decline in LBC1936 from age 70 to age 79 was 0.44 SD (0.048 SD per year), and for LBC1921 from age 79 to age 90 was 0.98 SD (0.089 SD per year).
The stability coefficients for the Moray House Test are shown in Fig. 2 (and Supplementary Table 1). Some of these have been published by us previously [6]. The age 11 versus age 79 association—the only comparison that is at the same ages for the two Lothian Birth Cohorts—is 0.64 for both LBC1921 and LBC1936. Correlations within older age are high, i.e., almost at or well above 0.7.
Colour and size of circle indicate the magnitude of correlation between the MHT scores. All correlations are significant at the P < 0.01 level. For standard errors see Supplementary Table S1.
Linear regression: the APOE e4 effect sizes on Moray House Test scores at each testing occasion from age 11 to age 90
For descriptive purposes we show the mean Moray House Test scores for the LBC1921 and LBC1936 split by whether or not people possess the e4 allele of APOE (Table 2, Fig. 3). This is done for all comers and completers. There is little difference in the scores of e4− and e4+ groups at age 11 and age 70. Thereafter, the e4+ group appears to decline more steeply than the e4− group; mostly, the differences between e4− and e4+ scores are greater at older ages. The completers’ panel of Fig. 3 shows that the LBC1921 and LBC1936 have similar values at age 79 with the e4+ group scoring lower in both cohorts. Overall—including both cohorts—the mean decline in Moray House Test score from age 70 to age 90 for all comers is 20.3 points (21.8 for completers) for those with an APOE e4 allele, and 11.0 points (12.9 for completers) for those without one.
Dotted lines denote the change in MHT score from age 11 to the first MHT score in older age. A continuous line denotes the change in MHT score across older age. The ticks representing individual years between childhood (age 11) and age 70 have been compressed for visual clarity. Error bars represent standard errors.
Data were first analysed from all comers using regression models to test cross-sectional effects. There were significant effects of the e4 allele of APOE on Moray House Test scores at ages 76 and 79 in the LBC1936 and ages 79, 87, and 90 in the LBC1921 (all p values at or lower than 0.009) (Table 3). There was no significant effect at age 11 in either cohort nor at age 70 in the LBC1936. For all comers and adjusting for age, sex and health covariates (model 2), the absolute effect size (standardised beta [standard error]) of e4 on MHT scores is as follows: 0.03 (0.03) at 70 (this is the only non-significant result); 0.13 (0.04) at 76; 0.13 (0.03) at 79 (meta-analysis of LBC1921 and LBC1936); 0.23 (0.07) at age 87; and 0.33 (0.09) at age 90 (Table 3, Fig. 4). The pattern for completers is similar: 0.08 at 70 (non-significant); 0.13 at 76; 0.16 at 79 (meta-analysis of LBC1921 and LBC1936); 0.20 at age 87; and 0.31 at age 90 (Table 4, Fig. 4). After excluding those who subsequently developed dementia, the pattern is similar in the all-comers analysis (Supplementary Table 2, Fig. 4). However, for the completers most results become non-significant and the pattern is less clear; we note that the N is small at older ages in the LBC1921 (Supplementary Table 3, Fig. 4).
Solid lines (plots A and C) denote results for the full cohort (corresponding to Model 2 in Tables 3 and 4) and dashed lines (model B and D) denote results for the subsamples without dementia up to the end of the follow-up period (corresponding to Model 2 in Supplementary Tables S2 and S3). Note that both cohorts were dementia free at baseline. Effect sizes are presented as positive values for the purposes of showing the magnitude of associations between APOE e4 status and MHT sores at each age. Error bars represent standard errors.
Growth curve modelling: the APOE e4 effect sizes on intercepts (age 70 and 79 for LBC1936 and LBC1921, respectively) and slopes (age 70 to age 79 for LBC1936, and age 79 to age 90 for LBC1921)
We applied growth curve modelling to the Moray House Test data from LBC1936 and LBC1921. This affords an estimate of the effect size of the e4 APOE allele on the intercepts (age 70 and age 79, respectively, for the two cohorts) and the cognitive-change slopes (age 70–79 for LBC1936 and age 79–90 for the LBC1921). Age, sex, and health variables (cardiovascular disease, diabetes, stroke hypertension) were included as covariates. There was no significant effect of APOE e4 possession on the LBC1936 intercept (absolute standardised estimate = 0.03, p = 0.33), which accords with the cross-sectional analyses (Table 5, Fig. 5). There was a significant association between APOE e4 possession and steeper cognitive ageing in LBC1936 slope (age 70 to age 79); the absolute effect size was 0.22 (p < 0.001). In LBC1921, those who were carriers of the APOE e4 allele had lower MHT baseline scores at age 79 (intercept) and steeper declines (slope) in MHT scores from age 79–90. The APOE e4 effects for LBC1921 were: intercept effect size = 0.11 (p = 0.010); slope effect size = 0.31 (p < 0.001). Although the LBC1921 slope effect size is numerically larger than that of the LBC1936, their 95% confidence intervals overlap (Table 5). After excluding those who subsequently developed dementia, of the four results reported above only the intercept of the LBC1921 analysis had a p value less than 0.05 (i.e., p = 0.045, absolute effect size = 0.10; (Supplementary Table 4).
Values in white are P-values. Standardised effect sizes are derived from longitudinal growth curve models which have been adjusted for age, sex, CVD, stroke, diabetes, and hypertension (see Table 5).
Component loadings of the Moray House Test
An analysis was carried out to show the reader the cognitive credentials of the Moray House Test as an indicator of general cognitive functioning (g) in older age. Principal components analysis was conducted on the Moray House Test and 13 other diverse cognitive tests administered to the Lothian Birth Cohort 1936 at mean age of about 70 (their Wave 1 in older age). This allows us to contextualise the MHT (a measure of general cognitive function designed over a century ago) within an extensive multi-domain cognitive battery of currently-used cognitive tests. The N was 930. The scree slope and Eigenvalues greater than 1 criterion indicated the presence of two components, though the first unrotated component was much larger than the second (Supplementary Information and Supplementary Fig. 1). The first unrotated component accounted for 39% of the total variance in the tests. The Moray House Test had the highest loading, at 0.83; all tests loaded at 0.4 or greater (Supplementary Fig. 2). The next four highest-loaded tests after Moray House Test were Wechsler Symbol Search (0.73; assessing the cognitive domain of processing speed), the average of National Adult Reading Test and Wechsler Test of Adult Reading (0.73; verbal knowledge/crystallised ability), Wechsler Matrix Reasoning (0.70; non-verbal reasoning), and Wechsler Block Design (0.69; spatial ability). Therefore, the general component, here, has a several high loadings from tests assessing diverse cognitive domains, and the Moray House Test has a very high loading on general cognitive ability.
Additional analyses on APOE status
At the request of referees, we provide some additional information and analyses: first, on the proportions of the specific APOE genotypes; second on the cognitive differences between participants who had zero, one, or two APOE alleles; and, third, on associations between APOE e4 status and dropout, death, and dementia.
The numbers of LBC1921 and LBC1936 participants with specific APOE genotypes is:
LBC1936 (N = 1010), e2e2 = 5 (0.5%), e2e3 = 118 (11.7%), e2e4 = 23 (2.3%), e3e3 = 587 (58.1%), e3e4 = 257 (25.4%), e4e4 = 20 (2.0%);
LBC1921 (N = 539), e2e2 = 2 (0.4%), e2e3 = 80 (14.8%), e2e4 = 19 (3.5%), e3e3 = 314 (58.3%), e3e4 = 119 (22.1%), e4e4 = 5 (0.9%).
The numbers of LBC1921 and LBC1936 participants with no, one, or two APOE e4 alleles and their mean (SD) Moray House Test scores at each wave of testing is shown in Supplementary Table 5. With only 4 people in LBC1921 having two e4 alleles, comparisons were carried out on only the LBC1936, where 20 people at age 70 had two e4 alleles. Comparisons of mean Moray House Test scores (one way ANOVA) for LBC1936 are shown in Supplementary Table 6a, for all-comers and completers. In all-comers there was a significant overall difference between means at age 76 (p = 0.002; eta squared = 0.019) and age 79 (p = 0.002; eta squared = 0.026). In all-comers, post-hoc Tukey HSD comparisons revealed that participants with no APOE e4 alleles scored significantly higher on the MHT than those with one e4 allele at both Wave 3 (mean difference = 2.779, p = 0.003) and Wave 4 (mean difference = 3.389, p = 0.002) (Supplementary Table 6b). No other pairwise differences between genotype groups were statistically significant.
The associations between APOE e4 status (carrier or not carrier) and dropout, death, and dementia are shown in Supplementary Table 7. Those with one or two e4 alleles were more likely to develop dementia (p < 0.001); in LBC1921, 16.7% of those without an e4 allele and 30.0% of those with one or two; in LBC1936, 8.0% of those without an e4 allele and 25.7% of those with one or two. Possession of the APOE e4 allele was positively and significantly associated with death in the LBC1921 sample but not LBC1936. In LBC1921, 59.3% of those with no e4 allele died during the follow-up period whereas 70.1% of those with one or two e4 alleles died. There was no significant association between APOE e4 status and dropout.
Discussion
The effect size of APOE e4+ status on general cognitive ability rises from about zero at age 70 (and age 11) to about a standardised effect of 0.3 at age 90. If one tends to think of genetic variation offering a static effect, APOE variation’s effect on cognitive function rebuts that (as do heritability results across childhood to adolescence [50]). A main novelty in the present study was the use of the same test in the same people from age 11 to age 90, albeit that that was achieved by bolting together two (very similar) cohorts at age 79. We found no differences between APOE e4 carriers and Moray House Test scores at ages 11 or 70; of course, that is a large age gap and we may not state that differences did not appear and then disappear during that epoch [29]. The results agree with the Whitehall II study’s results on global cognitive function, i.e., that there is no difference between APOE e4 heterozygotes at about age 70 but the APOE e4 carriers score lower by age 75 and decline faster [29]. It also accords with studies that indicate inconclusive effects of APOE e4 at younger ages on cognitive and brain-imaging markers [51, 52], although there is evidence for an effect of e4 (especially for e4e4 homozygotes) on poorer brain white matter health in middle to older aged people without dementia [53]. The present study extends previous research with a validated test of general cognitive ability administered in youth and on six occasions from age 70–90. Growth curve modelling found that possession of at least one e4 allele of APOE was associated with cognitive slope (decline) in the LBC1921 (age 79–90) and 1936 (age 70–79). This effect was also present in the LBC1936 when tested at test-specific, domain, and general levels of cognitive functioning using 13 cognitive tests (age 70–83) [16]. The effect size was numerically larger in the LBC1921, although the difference between the two betas was not significant; a larger N would be required to establish whether the slope is greater in the ninth versus eighth decades. We showed that the Moray House Test is strongly loaded on general cognitive ability which was our intended outcome construct.
The study meets recommendations [24] for this field of research by, e.g., having a large starting sample with a narrow age range, having a long follow-up period (including 20 years in older age), focussing on one cognitive phenotype that is affected by ageing and dementia, and trying to rule out dementia subsequent to the first wave of testing in older age [24]. The latter was done by clinical assessment based on medical records and by some in-person visits. This was done to try to discover if all of the effect of APOE variation (here, possession of one or two e4 alleles) on individual differences in apparently healthy cognitive ageing was due to preclinical dementia; this has been referred to as the “prodromal effect” [24]. The possibility that people with the e4 allele of APOE might have an “altered neural endophenotype” and, as a result of that, undergo steeper age-related cognitive decline, has been referred to as the “phenotype effect” [24]. In the present study, for the phenotype that we studied, there is some evidence for both effects operating, though larger numbers of participants at the older ages are required more clearly to decide between these possibilities or to demonstrate that they are both operating. That is, if the present study’s effect sizes for the APOE e4 association with cognitive function after excluding those with dementia are approximately correct, we shall have made a type 2 statistical error by not being able robustly to demonstrate a ‘phenotype effect’ here. This will have to be corrected by a larger sample size or a meta-analysis with another sample(s).
The present study suggests that the effects of the APOE e4 on cognitive functioning might increase in size through the eighth and ninth decades of human life (though it is not definitive on whether the effect is on dementia risk alone or on both that and on non-dementia cognitive decline). Evidence that genetic influences—including that of APOE e4—on cognitive functioning might increase with age was gathered and then interpreted via the, “resource modulation hypothesis” [54]. That overview article—which included some non-APOE genetic effects that have not been robustly replicated—called for more longitudinal studies such as the present report provides. The authors summarised the hypothesis as follows: “losses of anatomical and neurochemical brain resources in normal aging modulate the effects of common genetic variations on cognitive functioning… This notion is based on the assumption that the function relating brain resources to cognition is non-linear, and that genetic differences therefore exert increasingly larger effects on performance as resources recede from high to medium levels”. This possibility suggests that, as the structure and/or functioning of the brain deteriorates with age, the cognitive and brain differences between those with different genotypes expands. Imagine how this might work. Suppose that a gene expressed a protein that was involved in neural repair after some neural damage. Also, suppose that the different genotypes varied in how well the repair was implemented. The consequence would be that a person with one genotype would have, through the life course, a series of good repairs and a person with the other genotype would suffer an accumulation of less-good repairs. Therefore, the effect size of the genetic differences would expand with age. The explanation of the increasing effect size between genotype and cognitive functioning would be their different abilities to repair neural damage, originating either from the external or internal environment. Might there be alternative account? Suppose that the genotypes in question contributed to formation of neural architecture through development to young adulthood and that those with one genotype built a more robust brain than did the other genotype (there is equivocal support for there being subtle structural and functional connectivity differences in the brains of young adults who differ in APOE e4 status [51]). Suppose, too, that both types of architecture could survive the external and internal environments’ insults without a lowering of cognitive functioning until an ageing-related threshold was reached. Thereafter, as the slings and arrows continue, we might see that the less robust structure would show increasing deterioration of functioning compared with the better-built one. To summarise, the first account hypothesises that genetic effects on cognitive functioning might increase because of differences in the response to neural challenges (here, the gene produces a repairing resource), and the second account hypothesises that the cause is differences in the quality of the original assembly (here, the gene produces a constructing resource). Both might apply, even to the same genetic variation (constructions are sometimes repaired by agents with the same skills that made them) and different genetic variations might contribute relatively more to one process than the other. This is relevant to the concept of the ‘last in first out’ hypothesis (LIFO); it suggests that brain networks which develop relatively late in adolescence are also those that are vulnerable to ageing [55, 56]. That is, it, “posits that the process of age-related brain decline mirrors developmental maturation” [55].
The mechanistic trail from the current results should be interesting and practically valuable. It is important to discover what it is about possessing one or more APOE e4 alleles that results in steeper cognitive decline. There is much that is known about the biological actions of apolipoprotein E from work on humans and other research models, and there are hints at therapeutic approaches [57]. Further human-based research could usefully search for brain-imaging-based variables that mediate the association between APOE variation and general cognitive functioning [51, 58], though these variables would need to be available nearer to the ages at which APOE variation has a significant effect on cognitive functioning. There is already a suggestion that brain white matter health might show increasing negative effects of APOE variation with older age and that this might occur at ages prior to the APOE-cognition associations [53]. In addition to the increasing range of brain-imaging-based variables that are available, various ‘omics panels will offer clues to the ways by which APOE variation affects cognitive functioning [59], including the ‘protein signatures’ of dementia risk [60].
This study has imitations. Here, we concentrated on providing a clean result for the effect of APOE e4 variation on general cognitive functioning in older age. A substantial review emphasised that research on APOE’s association with cognitive function should adopt a design and choice of cognitive phenotype that is appropriate to the neurobiological mechanism being investigated [24]. We chose to investigate general/global cognitive functioning as it is the factor that accounts for much of the variance in age-associated cognitive decline across diverse cognitive domains and is affected in dementia [61,62,63]. By using a single, well-validated cognitive test in two very similar and geographically co-located, narrow-age samples the results are relatively free from possible moderators of the effect. Of course, this means that there are other investigations that would be valuable to broaden the results. It will be useful to examine further: different cognitive domains such as episodic memory, processing speed, and fluid reasoning/executive functioning (although much of the effect of ageing on cognitive capability is on general cognitive ability) [64]; sex differences and geographically varied samples [65,66,67]; and the possible moderating effect of medical conditions. It will also be useful to have longer follow-ups so that even older people may be included and later incident cases of dementia can be used for exclusion. Although, we should introduce some caution in being too conscientious here: that is, if the goal is to exclude those who were already on a dementia trajectory at study entry, then, say, 20 years of subsequent observation might be too long a window—one might be excluding cognitively healthy individuals who, much later, aged into dementia and who were not in a prodromal state when the APOE e4-cognition association was examined. A balance might be to exclude only those who developed dementia within a ‘preclinical window’ (e.g., within 5–10 years of baseline), as these cases are more likely to have had undetected pathology at age 70 or 79. We had insufficient power to include tests of other types of APOE variation, such as being homozygous for e4 and possession of one or more e2 alleles, though we provide information about these in the paper and its Supplement. Thus, compared with the Whitehall II study, owing to our later starting age of 70 in older age and a smaller sample size, we were not able to test their findings of specific effects of APOE e4 homozygotes and possibly better cognitive scores of APOE e4 heterozygotes between ages 45 and 55 years [29]. Larger sample sizes—especially at the older ages covered herein—are needed formally to test for differences in the effect size of APOE e4 status on cognitive test scores between different ages. We note that our additional analyses showed the expected association between APOE e4 possession and subsequent dementia, but that there was no association between e4 possession and dropout. In the LBC1921 there was a slightly greater proportion of deaths among the subsample with an e4 allele. Beyond APOE variation it will be important to explore whether there are age-heterogeneous (probably increasing) genetic effects on cognitive functioning. This could be done with plausible candidate genes or with polygenic risk scores for related phenotypes, including those for dementias and other neurodegenerative disorders. We note that our principal components analysis of the Moray House Test with 13 other tests had a strong first unrotated principal component (general cognitive ability). The presence of a small second component was probably a result of the battery of tests containing four assessments of processing speed.
Concluding remarks
APOE variation is a relative rarity in cognitive ageing research in being a robust predictor of people’s differences in age-related cognitive decline. Here, we find that its effects are around null in childhood and at about age 70 and increase to a large effect [68] through the ninth decade. As is known from phenylketonuria, genetic variation is not destiny; therefore, research that explores the pathways through which APOE variation works could provide useful information toward alleviating some of the health inequalities in cognitive ageing.
Data availability
The datasets analysed during the current study are not publicly available owing to the nature of the ethical approvals given by the participants but are available from the Lothian Birth Cohorts Director (Professor S. Cox; Simon.Cox@ed.ac.uk) or via a data request form that is available on the Lothian Birth Cohorts website.
References
Salthouse TA Major issues in cognitive aging. Oxford, New York: Oxford University Press; 2010. ix, p. 246.
Tucker-Drob EM. Cognitive aging and dementia: a life span perspective. Annu. Rev. Dev. Psychol. 2019;1:177–96. https://doi.org/10.1146/annurev-devpsych-121318-085204
Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5:87–96. https://doi.org/10.1038/nrn1323
Salthouse TA. Trajectories of normal cognitive aging. Psychol. Aging. 2019;34:17–24. https://doi.org/10.1037/pag0000288
Schwartzman AE, Gold D, Andres D, Arbuckle TY, Chaikelson J. Stability of intelligence: a 40-year follow-up. Can. J. Psychol. 1987;41:244–56. https://doi.org/10.1037/h0084155
Deary IJ. The stability of intelligence from childhood to old age. Curr. Dir. Psychol. Sci. 2014;23:239–45. https://doi.org/10.1177/0963721414536905
Breit M, Scherrer V, Tucker-Drob EM, Preckel F. The stability of cognitive abilities: a meta-analytic review of longitudinal studies. Psychol. Bull. 2024;150:399–439. https://doi.org/10.1037/bul0000425
Plassman BL, Welsh KA, Helms M, Brandt J, Page WF, Breitner JC. Intelligence and education as predictors of cognitive state in late life: a 50-year follow-up. Neurology. 1995;45:1446–50. https://doi.org/10.1212/wnl.45.8.1446
Underwood E. Starting young. Science. 2014;346:568–71. https://doi.org/10.1126/science.346.6209.568
Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish Mental Surveys of 1932 and 1947. J. Pers. Soc. Psychol. 2004;86:130–47. https://doi.org/10.1037/0022-3514.86.1.130
Strenze T. Intelligence and socioeconomic success: a meta-analytic review of longitudinal research. Intelligence. 2007;35:401–26. https://doi.org/10.1016/j.intell.2006.09.004
Deary IJ, Hill WD, Gale CR. Intelligence, health and death. Nat. Hum. Behav. 2021;5:416–30. https://doi.org/10.1038/s41562-021-01078-9
Jacob L, Smith L, Thoumie P, Haro JM, Stickley A, Koyanagi A. Association between intelligence quotient and disability: the role of socioeconomic status. Ann. Phys. Rehabil. Med. 2020;63:296–301. https://doi.org/10.1016/j.rehab.2019.07.010
Tucker-Drob EM. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25:368–77. https://doi.org/10.1037/a0022348
Walhovd KB, Lovden M, Fjell AM. Timing of lifespan influences on brain and cognition. Trends Cogn. Sci. 2023;27:901–15. https://doi.org/10.1016/j.tics.2023.07.001
Corley J, Conte F, Harris SE, Taylor AM, Redmond P, Russ TC, et al. Predictors of longitudinal cognitive ageing from age 70–82 including APOE e4 status, early-life and lifestyle factors: the Lothian Birth Cohort 1936. Mol. Psychiatry. 2023;28:1256–71. https://doi.org/10.1038/s41380-022-01900-4
Salthouse TA. Selective review of cognitive aging. J. Int. Neuropsychol. Soc. 2010;16:754–60. https://doi.org/10.1017/S1355617710000706
Prince JB, Davis HL, Tan J, Muller-Townsend K, Markovic S, Lewis DMG, et al. Cognitive and neuroscientific perspectives of healthy ageing. Neurosci. Biobehav. Rev. 2024;161:105649 https://doi.org/10.1016/j.neubiorev.2024.105649
American Geriatrics Society, Parker TE, Agin B, Perkins S, Arden JB, Bodian S, et al. Staying sharp for dummies. Hoboken, NJ: John Wiley & Sons, Inc.; 2016. xx, p.596.
Castel AD Better with age : the psychology of successful aging. New York, NY: Oxford University Press; 2019. xiii, p. 236.
Emmons H, Alter D Staying sharp : 9 keys for a youthful brain through modern science and ancient wisdom. New York: Touchstone, a imprint of Simon & Schuster; 2015. p. 288.
Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann. Intern. Med. 2010;153:182–93. https://doi.org/10.7326/0003-4819-153-3-201008030-00258
Corley J, Cox SR, Deary IJ. Healthy cognitive ageing in the Lothian Birth Cohort studies: marginal gains not magic bullet. Psychol. Med. 2018;48:187–207. https://doi.org/10.1017/S0033291717001489
O’Donoghue MC, Murphy SE, Zamboni G, Nobre AC, Mackay CE. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: a review. Cortex. 2018;104:103–23. https://doi.org/10.1016/j.cortex.2018.03.025
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. https://doi.org/10.1126/science.8346443
Palmer JM, Huentelman M, Ryan L. More than just risk for Alzheimer’s disease: APOE epsilon4’s impact on the aging brain. Trends Neurosci. 2023;46:750–63. https://doi.org/10.1016/j.tins.2023.06.003
Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, et al. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932 https://doi.org/10.1038/418932a
Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M. Apolipoprotein-E (Apoe) epsilon4 and cognitive decline over the adult life course. Transl. Psychiatry. 2018;8:18 https://doi.org/10.1038/s41398-017-0064-8
Gharbi-Meliani A, Dugravot A, Sabia S, Regy M, Fayosse A, Schnitzler A, et al. The association of APOE epsilon4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res. Ther. 2021;13:5 https://doi.org/10.1186/s13195-020-00740-0
Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Apolipoprotein E genotypes, age, race, and cognitive decline in a population sample. J. Am. Geriatr. Soc. 2019;67:734–40. https://doi.org/10.1111/jgs.15727
Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiol. Aging. 2011;32:63–74. https://doi.org/10.1016/j.neurobiolaging.2009.02.003
Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewald DC, et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive ageing. Mol. Psychiatry. 2014;19:76–87. https://doi.org/10.1038/mp.2012.159
Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, et al. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol. Aging. 2004;19:367–71. https://doi.org/10.1037/0882-7974.19.2.367
Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, et al. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: the Lothian Birth Cohort 1936 study. Psychol. Aging. 2009;24:129–38. https://doi.org/10.1037/a0014780
Luciano M, Gow AJ, Taylor MD, Hayward C, Harris SE, Campbell H, et al. Apolipoprotein E is not related to memory abilities at 70 years of age. Behav. Genet. 2009;39:6–14. https://doi.org/10.1007/s10519-008-9236-x
Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry. 2012;17:315–24. https://doi.org/10.1038/mp.2010.137
Sibbett RA, Russ TC, Pattie A, Starr JM, Deary IJ. Does incipient dementia explain normal cognitive decline determinants? Lothian birth cohort 1921. Psychol. Aging. 2018;33:674–84. https://doi.org/10.1037/pag0000241
Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. Int. J. Epidemiol. 2012;41:1576–84. https://doi.org/10.1093/ije/dyr197
Taylor AM, Pattie A, Deary IJ. Cohort profile update: the Lothian Birth Cohorts of 1921 and 1936. Int. J. Epidemiol. 2018;47:1042–r. https://doi.org/10.1093/ije/dyy022
Scottish Council for Research in Education. The intelligence of Scottish Children. London, UK: University of London Press; 1933.
Scottish Council for Research in Education. The trend of Scottish intelligence: a comparison of the 1947 and 1932 surveys of the intelligence of eleven-year-old pupils. London, UK: University of London Press; 1949.
Deary IJ, Johnson W, Starr JM. Are processing speed tasks biomarkers of cognitive aging? Psychol. Aging. 2010;25:219–28. https://doi.org/10.1037/a0017750
Mullin DS, Stirland LE, Buchanan E, Convery CA, Cox SR, Deary IJ, et al. Identifying dementia using medical data linkage in a longitudinal cohort study: Lothian Birth Cohort 1936. BMC Psychiatry. 2023;23:303 https://doi.org/10.1186/s12888-023-04797-7
Sibbett RA, Russ TC, Deary IJ, Starr JM. Dementia ascertainment using existing data in UK longitudinal and cohort studies: a systematic review of methodology. BMC Psychiatry. 2017;17:239 https://doi.org/10.1186/s12888-017-1401-4
Sibbett RA, Russ TC, Deary IJ, Starr JM. Risk factors for dementia in the ninth decade of life and beyond: a study of the Lothian birth cohort 1921. BMC Psychiatry. 2017;17:205 https://doi.org/10.1186/s12888-017-1366-3
Cox SR, Harris MA, Ritchie SJ, Buchanan CR, Valdes Hernandez MC, Corley J, et al. Three major dimensions of human brain cortical ageing in relation to cognitive decline across the eighth decade of life. Mol. Psychiatry. 2021;26:2651–62. https://doi.org/10.1038/s41380-020-00975-1
Ritchie SJ, Tucker-Drob EM, Cox SR, Corley J, Dykiert D, Redmond P, et al. Predictors of ageing-related decline across multiple cognitive functions. Intelligence. 2016;59:115–26. https://doi.org/10.1016/j.intell.2016.08.007
Rosseel Y. lavaan: an R package for structural equation modeling. J. Stat. Softw. 2012;48:1–36. https://doi.org/10.18637/jss.v048.i02
Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, et al. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28 https://doi.org/10.1186/1471-2318-7-28
Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol. Psychiatry. 2010;15:1112–20. https://doi.org/10.1038/mp.2009.55
Kucikova L, Xiong X, Reinecke P, Madden J, Jackson E, Tappin O, et al. The effects of APOEe4 allele on cerebral structure, function, and related interactions with cognition in young adults. Ageing Res. Rev. 2024;101:102510 https://doi.org/10.1016/j.arr.2024.102510
Lyall DM, Ward J, Ritchie SJ, Davies G, Cullen B, Celis C, et al. Alzheimer disease genetic risk factor APOE e4 and cognitive abilities in 111,739 UK Biobank participants. Age Ageing. 2016;45:511–7. https://doi.org/10.1093/ageing/afw068
Heise V, Offer A, Whiteley W, Mackay CE, Armitage JM, Parish S. A comprehensive analysis of APOE genotype effects on human brain structure in the UK Biobank. Transl. Psychiatry. 2024;14:143 https://doi.org/10.1038/s41398-024-02848-5
Papenberg G, Lindenberger U, Backman L. Aging-related magnification of genetic effects on cognitive and brain integrity. Trends Cogn. Sci. 2015;19:506–14. https://doi.org/10.1016/j.tics.2015.06.008
Manuello J, Min J, McCarthy P, Alfaro-Almagro F, Lee S, Smith S, et al. The effects of genetic and modifiable risk factors on brain regions vulnerable to ageing and disease. Nat. Commun. 2024;15:2576 https://doi.org/10.1038/s41467-024-46344-2
Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging. 2004;25:5–18. https://doi.org/10.1016/j.neurobiolaging.2003.03.001. author reply 49-62
Jackson RJ, Hyman BT, Serrano-Pozo A. Multifaceted roles of APOE in Alzheimer disease. Nat. Rev. Neurol. 2024;20:457–74. https://doi.org/10.1038/s41582-024-00988-2
Lyall DM, Cox SR, Lyall LM, Celis-Morales C, Cullen B, Mackay DF, et al. Association between APOE e4 and white matter hyperintensity volume, but not total brain volume or white matter integrity. Brain Imaging Behav. 2020;14:1468–76. https://doi.org/10.1007/s11682-019-00069-9
Perez-Gonzalez AP, Garcia-Kroepfly AL, Perez-Fuentes KA, Garcia-Reyes RI, Solis-Roldan FF, Alba-Gonzalez JA, et al. The ROSMAP project: aging and neurodegenerative diseases through omic sciences. Front. Neuroinform. 2024;18:1443865 https://doi.org/10.3389/fninf.2024.1443865
Walker KA, An Y, Moghekar A, Moaddel R, Duggan MR, Peng Z, et al. Proteomic analysis of APOEepsilon4 carriers implicates lipid metabolism, complement and lymphocyte signaling in cognitive resilience. Mol. Neurodegener. 2024;19:81 https://doi.org/10.1186/s13024-024-00772-2
Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Dev. Psychol. 2011;47:331–43. https://doi.org/10.1037/a0021361
Tucker-Drob EM, Briley DA, Starr JM, Deary IJ. Structure and correlates of cognitive aging in a narrow age cohort. Psychol. Aging. 2014;29:236–49. https://doi.org/10.1037/a0036187
Ghisletta P, Rabbitt P, Lunn M, Lindenberger U. Two thirds of the age-based changes in fluid and crystallized intelligence, perceptual speed, and memory in adulthood are shared. Intelligence. 2012;40:260–8. https://doi.org/10.1016/j.intell.2012.02.008
Daly J, De Luca F, Berens SC, Field AP, Rusted JM, Bird CM. The effect of apolipoprotein E genotype on spatial processing in humans: a meta-analysis and systematic review. Cortex. 2024;177:268–84. https://doi.org/10.1016/j.cortex.2024.05.006
Beydoun MA, Weiss J, Beydoun HA, Hossain S, Maldonado AI, Shen B, et al. Race, APOE genotypes, and cognitive decline among middle-aged urban adults. Alzheimers Res. Ther. 2021;13:120 https://doi.org/10.1186/s13195-021-00855-y
Granot-Hershkovitz E, Tarraf W, Kurniansyah N, Daviglus M, Isasi CR, Kaplan R, et al. APOE alleles’ association with cognitive function differs across Hispanic/Latino groups and genetic ancestry in the study of Latinos-investigation of neurocognitive aging (HCHS/SOL). Alzheimers Dement. 2021;17:466–74. https://doi.org/10.1002/alz.12205
Walters S, Contreras AG, Eissman JM, Mukherjee S, Lee ML, Choi SE, et al. Associations of sex, race, and apolipoprotein E alleles with multiple domains of cognition among older adults. JAMA Neurol. 2023;80:929–39. https://doi.org/10.1001/jamaneurol.2023.2169
Funder DC, Ozer DJ. Evaluating effect size in psychological research: sense and nonsense. Adv. Meth Pract. Psych. 2019;2:156–68.
Acknowledgements
We thank the participants of the Lothian Birth Cohorts of 1921 and 1936 (LBC1921, 1936). We thank the LBC1921 and LBC1936 research team members, past and present, who collected, entered, checked, and provided the data. We thank members of the Alzheimer Scotland Dementia Research Centre who helped with the dementia ascertainment. We are grateful for the following sources of support: National Institutes of Health (NIH) research (R01AG054628, U01AG083829); BBSRC and ESRC (BB/W008793/1); Wellcome and the Royal Society (221890/Z/20/Z); MRC (MR/X003434/1); Milton Damerel Trust; Alzheimer Scotland; and the University of Edinburgh.
Author information
Authors and Affiliations
Contributions
Conceptualisation, IJD; Analysis plan, IJD, JC; Formal analysis, JC; Writing—original draft, IJD, JC; Writing—review and editing, all authors; Creation of Tables and Figures, JC; Supervision, SRC, IJD, TR; project administration, IJD, SRC, TCR; Funding acquisition, IJD, SRC, TCR, JC, SEH.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All participants provided written informed consent. Ethical approval for the LBC1921 study was provided by the Lothian Research Ethics Committee for test waves 1–3 at ages 79, 83 and 87 (LREC/1998/4/183, LREC/2003/7/23, 1702/98/4/183) and the Scotland A Research Ethics Committee for test wave 4 at age 90 (10/MRE00/87, 10/MRE00/87). Ethical approval for the LBC1936 study was obtained from the Multicentre Research Ethics Committee for Scotland (baseline, MREC/01/0/56), the Lothian Research Ethics Committee (age 70, LREC/2003/2/29), and the Scotland A Research Ethics Committee (ages 73, 76, 79, 82, 07/MRE00/58). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41380_2025_3274_MOESM1_ESM.docx
Supplementary information for: Effect sizes of APOE e4 on the same general cognitive ability test taken by the same people from age 11 to age 90: The Lothian Birth Cohorts 1921 and 1936
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deary, I.J., Harris, S.E., Russ, T.C. et al. Effect sizes of APOE e4 on the same general cognitive ability test taken by the same people from age 11 to age 90: The Lothian Birth Cohorts 1921 and 1936. Mol Psychiatry 31, 27–38 (2026). https://doi.org/10.1038/s41380-025-03274-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03274-9