Abstract
Objectives
Ketogenic conditions have gained attention due to their favorable metabolic prognoses. We aimed to investigate the association between blood levels of beta-hydroxybutyrate (βHB), the most abundant form of ketone body, and type 2 diabetes (T2D) incidence in patients with impaired fasting glucose (IFG).
Methods
We randomly selected 500 patients with IFG from the prospective Korean Cancer Prevention Study II biobank. Blood levels of βHB were measured from the stored samples, and the diagnostic data from the Korean National Health Insurance Service were used to determine the probability of T2D-free survival. A multivariable Cox regression analysis was performed to assess the association between blood βHB levels and the incidence of new-onset T2D.
Results
A total of 453 patients with IFG were included, and 105 (23%) developed T2D during a mean follow-up period of 10.9 years. Higher blood βHB levels in patients with IFG were associated with improved T2D-free survival, although it was not statistically significant (log-rank test, p = 0.058). In multivariable Cox regression models, βHB levels showed a tendency toward a lower risk of T2D, but it was not statistically significant (HR 0.70; 95% CI 0.47–1.04; p = 0.07).
Conclusions
In patients with IFG, the blood βHB level showed a tendency to be associated with the risk of new-onset T2D; however, this tendency was not statistically significant.
Similar content being viewed by others
Introduction
Ketone bodies, synthesized in the liver from fatty acids and transported to extrahepatic tissues, play a crucial role in energy metabolism [1]. Conventionally, they are regarded as thrifty fuel for peripheral tissues. Circulating ketone bodies levels vary depending on the underlying disease and may also vary according to age and fasting duration in the healthy population [2]. Beta-hydroxybutyrate (βHB) is the most abundant form of ketone body, followed by acetoacetate and acetone.
Recently, ketone bodies have gained attention, as studies have been published showing their association with favorable metabolic conditions. A ketogenic, low-carbohydrate diet was reported to be effective for weight loss [3]. The presence of ketonuria after fasting was associated with metabolic superiority in patients without type 2 diabetes mellitus (T2D) [4]. Fasting ketonuria was also reported to be associated with a lower risk of non-alcoholic fatty liver disease [5, 6]. Furthermore, in a longitudinal prospective study, fasting ketonuria was reported to be statistically associated with a lower incidence of T2D in a population without diabetes in Korea [7]. These findings are supported by candidate mechanisms such as induction of the peroxisome proliferator-activated receptor alpha (PPARα)–fibroblast growth factor 21 (FGF21) axis, suppression of the tricarboxylic acid (TCA) cycle and gluconeogenesis in the ketogenic process [8,9,10], and the anti-inflammatory effects of the ketone bodies [11, 12].
However, many previous studies have limitations in using categorical tests for ketonuria as a marker for ketogenesis, although one of these studies showed a meaningful relationship between urinary ketone and blood βHB level via ancillary analysis [7]. Moreover, some studies reported that blood βHB levels were reportedly more effective than urine ketones in detecting ketosis [13]. In 2023, the first longitudinal study conducted by Dutch and Swedish researchers on blood ketone bodies and the risk of developing T2D among general populations in the Netherlands showed a positive correlation between these two variables [14], contradicting the studies mentioned above. As such, previous research on the association between blood βHB and the incidence of T2D is scarce and controversial. In particular, research on patients with prediabetes, a high-risk group for developing T2D, is lacking. Considering the global prevalence of over 520 million people with diabetes, an increase in prevalence of approximately 90% over the last 30 years, and the burden of diabetes reaching 79.2 million disability-adjusted life-years[15], research investigating whether candidate markers such as blood βHB predict the occurrence of T2D is clinically meaningful. Therefore, we designed a longitudinal observational study to assess the relationship between blood βHB levels and the incidence of new-onset T2D in patients with impaired fasting glucose (IFG).
Subjects and methods
Study design and participants
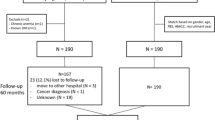
Study participants were recruited from the prospective Korean Cancer Prevention Study II (KCPS-II) biobank. Detailed descriptions of the KCPS-II with respect to questionnaires, anthropometric measurements, and blood collection are provided in a previous publication [16]. The KCPS-II comprises 156,701 participants (94,840 men and 61,861 women) who underwent routine health assessments between 2004 and 2013, provided blood samples, and gave informed consent for long-term follow-up [16, 17]. From the KCPS-II cohort database, we randomly selected 500 patients (250 men and 250 women) with baseline impaired fasting glucose (IFG) levels for a pilot study. Random sampling is a statistical technique where each member of a population has an equal chance of being included in the sample, ensuring unbiased and representative selection. We used random sampling (The PROC SURVEYSELECT statement of SAS software was used, and the METHOD is simple random sampling.) to minimize selection bias and enhance the generalizability of our findings. We included patients with IFG who fulfilled all of the following criteria: (1) fasting plasma glucose (FPG) levels between 100 and 125 mg/dL, (2) no documentation of the use of glucose-lowering agents or insulin, and (3) glycated hemoglobin (HbA1c) levels <6.5% [18]. Patients without baseline laboratory data were excluded from the study. Additionally, patients who had service claims with a diagnosis of diabetes (outpatient or inpatient care) within 1 year prior to enrollment were also excluded. Finally, we assigned a total of 453 subjects (215 men and 238 women) (Fig. 1). The study protocol was approved by the Institutional Review Board of the Yonsei University College of Medicine (No. 4-2019-1228). This study adhered to the tenets of the Declaration of Helsinki.
Ketone body measurements
The biochemical assessment process employed in this study has been described previously [19]. According to the KCPS-II study protocol, patients were fasted overnight, and blood was sampled between 7 a.m. and 11 a.m. An Autokit Total Ketone Bodies assay (FUJIFILM Wako Pure Chemical Corporation, Japan) [20] was used to measure the levels of total ketone bodies and the kinetic enzymatic method (Ranbut, Randox Laboratories Ltd., United Kingdom) [21] was used to measure the levels of total ketone bodies βHB in stored blood samples.
Based on the βHB values, participants were divided into two groups. Since βHB values below 0.05 mmol/L were undetectable with the βHB measurement method used, we divided the baseline and high βHB groups based on 0.05 mmol/L instead of the median. As a result, individuals with βHB values below 0.050 mmol/L were assigned to the reference group (n = 240, 53%), while the other group consisted of individuals with βHB levels above 0.05 mmol/L (n = 213, 47%).
Outcomes
During a mean follow-up period of 10.9 ± 3.0 years, the endpoint of the study was the diagnosis of new-onset T2D. To identify new cases of T2D, we merged the study data with records maintained by the Korean National Health Insurance Service (NHIS), which were based on the International Classification of Diseases, 10th Revision (ICD-10) codes. This study employed diabetes diagnosis code from the National Health Information Database (NHID) of the Korean National Health Insurance Service (NHIS) between 2002 and 2020 [22]. The Korean NHID contains nationwide claims data [23]. As the single insurer, the NHIS covers health care costs based on the billing records of health care providers [22]. As of 2020, this service covers almost the entire population of South Korea, over 50 million individuals [24]. T2D was defined as having at least one service claim with a diagnosis of diabetes (in outpatient or inpatient care). The principal and additional diagnoses were determined using the ICD-10 code for diabetes (E11).
Statistical analysis
Baseline characteristics are presented as means (standard deviation [SD]) for continuous variables or numbers (percentages) for categorical variables. The Kaplan-Meier estimator, a non-parametric survival function estimation method, was used to plot the T2D-free survival curve, and the log-rank test was used to compare the distribution of T2D-free survival between the low and high ßHB groups. Between-group differences in outcome were assessed for statistical significance with the use of a Cox proportional-hazards model, with prespecified covariates of age, sex, high-density lipoprotein cholesterol (HDL-C) levels, triglyceride (TG) levels, family history of diabetes mellitus, history of hypertension, and behavioral risk factors (smoking status, alcohol consumption, and physical activity) at baseline. The results of the Cox regression analysis are expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). We also examined the association between βHB levels and the risk of T2D according to age ( < 65 or ≥65), sex (male or female), BMI ( < 25 or ≥25), and FBS ( < 110 or ≥110).
All data analyses were performed using SAS version 9.4 for Windows (SAS Institute Inc.). A two-sided p-value < 0.05 was considered statistically significant.
Results
Characteristics of study participants
In this study, we finally included 453 subjects with IFG at baseline, with the exclusion of 31 patients due to insufficient blood samples for measuring the levels of total ketone bodies and βHB. Additionally, we excluded 16 patients who had service claims with a diagnosis of diabetes (in outpatient or inpatient care) within 1 year prior to enrollment (Fig. 1). The median age of the patients was 53.8 years, and the median body mass index (BMI) was 24.9 kg/m2 (Table 1); 47.9% of patients had obesity ( ≥ 25 kg/m2). 47.5% of patients were male, and 21.9% had a family history of T2D. The median HbA1c and FPG levels were 5.8% and 105.6 mg/dL, respectively.
Participants with higher βHB levels had significantly lower TG levels (176.3 vs. 139.1 mg/dL, p = 0.004; Table 1). However, no significant differences were observed between the two groups on other variables. This trend was also observed in the classification according to serum total ketone body levels. Baseline characteristics according to the levels of total ketone bodies are summarized in Table A.1.
Association of new-onset type 2 diabetes mellitus with βHB levels
During a mean follow-up of 10.9 years, T2D developed in 105 participants. The higher βHB group showed a trend toward a lower incidence of T2D, although it was not statistically significant (log-rank test, p = 0.058) (Fig. 2). In the Cox proportional hazards model, the result was similar to that of the log-rank test; the high βHB group seemed to be weakly associated with a lower risk of T2D, but it was not statistically significant (crude HR, 0.69; 95% CI, 0.46–1.02; p = 0.06) (Table 2). In the multivariable adjusted Cox proportional hazards models, βHB levels did not show any significant association with a decreased risk of T2D (adjusted HR, 0.70; 95% CI, 0.47-1.04; p = 0.07) (Table 2). The FPG level and family history of diabetes were significantly associated with the development of T2D in both crude and adjusted models. No significant interactions were found between subgroups (age, BMI, FPG level) and βHB level, except for sex (Fig. 3). The association between total ketone body level and incidence of T2D was similar to the association between βHB level and incidence of T2D, which was also not statistically significant. (Table A.2.) The association between βHB levels and the risk of T2D was more prominent in women than men (adjusted HR, 0.47; 95% CI, 0.27–0.81 in females vs. adjusted HR, 1.15; 95% CI, 0.62–2.14 in males; p for interaction = 0.02).
Discussion
In this longitudinal study, blood βHB levels showed a tendency toward a lower risk of T2D in IFG patients during a mean follow-up duration of 10.9 years, but this was not statistically significant. In the multivariable regression analysis, patients with higher βHB levels tended to have a lower risk of new-onset T2D, but it was not statistically significant, and this trend was maintained in the subgroup analyses; this was not completely in line with previous reports. Only FPG levels and family history of T2D were significantly correlated with an increased risk of T2D in this study.
Blood TG levels showed a tendency to decrease significantly in the group with higher βHB levels. Since blood TG levels were measured at a similar level regardless of the fasting time if the subjects fasted for more than 8 h [25, 26], it is unlikely that these differences in blood TG levels were due to inhomogeneous fasting periods in our study, in which participants fasted for more than 8 h. Referring to other previous studies [9, 27], we speculate that this trend reflects the ability of the liver to dispose of delivered fatty acids in ketogenic subjects.
Recent studies have examined the metabolic consequences of ketone bodies, including βHB, acetoacetate, and acetone, in ketogenic diets. A ketogenic diet, characterized by a very low intake of carbohydrates with unrestricted fat intake, is widely known to be effective for weight loss [28,29,30]. In addition, accumulating evidence indicates that a ketogenic diet improves lipid profiles [30], insulin resistance [31], and non-alcoholic fatty liver disease [32,33,34]. Furthermore, in the process of studying the mechanisms of the pleiotropic effects of sodium-glucose cotransporter-2 (SGLT-2) inhibitors, the hypothesis that ketogenic conditions can have a favorable metabolic effect has gained strength [35,36,37].
Some researchers have studied the prognosis of individuals with ketogenic tendencies not caused by specific diets or drugs. In 2010, Korean researchers conducted a cross-sectional analysis comparing the clinical characteristics of subjects with and without ketonuria using health check-up data [4]. In that study, the ketonuria group showed favorable metabolic features, such as lower body weight, waist circumference, blood pressure, and blood glucose, and had a lower prevalence of obesity, central obesity, and metabolic syndrome. Logistic regression analysis showed that the presence of ketonuria after fasting was significantly associated with a lower prevalence of obesity, central obesity, and metabolic syndrome. Several years later, another research team used large-scale data and compared the difference in the incidence of new-onset T2D during a long-term follow-up period between groups classified by ketonuria [7]. They recruited 8,703 individuals who were free of diabetes at baseline and defined individuals with ketonuria as those with morning ketonuria, classified as trace, 1+ (150 mg/L), or 2+ (400 mg/L). Then, using 1:4 propensity score matching, the corresponding non-ketonuria group was recruited. Over 12 years, compared to those without ketonuria, individuals with spontaneous ketonuria showed a significantly low incidence of T2D, with an HR of 0.66 (95% CI 0.45–0.96). The researchers also showed a meaningful relationship between urinary ketones and blood βHB levels in an ancillary dataset consisting of patients with T2D using SGLT-2 inhibitors. Except for the ancillary study, the majority of previous studies used ketonuria as a variable since the qualitative or categorical ketonuria test was included in the routine urinalysis set in various clinics or health check-up centers.
In 2023, researchers in the Netherlands and Sweden published the results of a long-term follow-up study on fasting plasma ketone bodies and the development of T2D for the first time [14]. They recruited 3,307 participants from the general population, free of diabetes or IFG at baseline. During a mean follow-up of 7.3 years, fasting plasma ketone bodies showed a significant association with the incidence of T2D, with an HR of 1.62 (95% CI 1.19–2.19) in the final multivariate regression model. This result contradicts previous studies and goes against the direction of our research. We attribute this discrepancy to the metabolic characteristics and racial differences of the study participants. While all our study participants were prediabetics, the study in 2023 excluded subjects with diabetes or IFG. Consequently, the median fasting glucose level was approximately 4.7 mmol/L (84.6 mg/dL), much lower than ours, 105.6 mg/dL. In addition, Asians, including Koreans, tend to exhibit a relatively more severe decline in their insulin secretion function as they progress from normoglycemia to prediabetes and then to diabetes [38]. Therefore, in the Korean population with IFG, ketogenesis may occur in different circumstances, and its clinical meaning could be interpreted differently compared to normoglycemic Dutch people who have intact insulin secretory function. We assume that the ketogenic pathway may act as a kind of protective mechanism to prevent progression to T2D in Koreans with IFG, whose insulin secretory function is relatively weaker than their insulin resistance.
A clear mechanism of association of ketone bodies with the risk of developing T2D has not yet been identified. However, several putative mechanisms have been proposed. First, PPARα and its downstream target, FGF21, were reported to be induced during the ketogenesis [8, 10]. These are considered important regulators of energy metabolism, and their activation is believed to confer protective effects against T2D [39,40,41]. Second, ketogenesis is an efficient pathway for the disposal of free fatty acids in the liver, including the production of acetyl-CoA by β-oxidation, serial conversion to acetoacetyl-CoA, 3-hydroxy-3-methylglutaryl-CoA, and ketone bodies [2, 42]. If the ketogenic pathway is impaired, acetyl-CoA enters the TCA cycle instead, and the anaplerotic pathway, including pyruvate carboxylase flux, increases, which leads to gluconeogenesis [9, 43]. Therefore, impaired ketogenesis is associated with hyperglycemia. Third, the ketone bodies themselves may have beneficial effects on protection against T2D. In particular, previous research suggest that βHB acts as a signaling substance. It reduces inflammatory responses [11, 12], regulates the expression of genes related to gluconeogenesis [44, 45], and modulates several reactions, which improve insulin resistance [46, 47].
In this study, the occurrence of new-onset T2D tended to be lower in the higher βHB group, but it was not statistically significant. The reason for the statistical insignificance might be the small scale of the study. According to the Kaplan–Meier survival curve presented in Fig. 2, the difference in the probability of T2D-free survival between the groups gradually widened over time. We presumed that the results could not reach statistical significance due to the lack of long-term follow-up subjects in our study. In addition, considering that meaningful ketonuria was present in a very low percentage of the population (approximately 2%) in the previous study [7], we assumed that the number of patients with significant ketonuria or hyperketonemia in this study was low. This may have resulted in the lack of statistically significant trends in this study. Our assumption also supported by the finding that the median βHB level in groups II was 0.074 mmol/L, which was within the range of the non-ketonuric group in the previous study [7].
This study had several limitations. First, as mentioned above, our sample size might have been too small to show statistically significant differences between the two groups. Large-scale studies including more subjects with long-term data may be required to show the differences in T2D incidence between groups classified by blood βHB levels. Second, our study did not include a dataset of urinary ketone levels. Although blood βHB or total ketone body levels were distinctive variables in our study, comparison with the findings of previous studies would be more reliable if data on ketonuria had been available. Third, due to the limitations of our equipment, we were unable to measure the exact value of blood βHB in cases of blood βHB < 0.05 mmol/L. Therefore, analyzing the differences in T2D incidence according to all blood βHB levels had limitations. Fortunately, when dividing by blood βHB levels of 0.05 mmol/L, we were able to divide the patients into two groups relatively evenly. Fourth, this study did not include data on plasma insulin levels. Therefore, the indices for insulin resistance, such as the homeostasis model assessment [48] or quantitative insulin sensitivity check index [49], could not be calculated and compared in our analysis. Fifth, we could not assess the dietary information of the participants or the exact duration of fasting, as the latter was recorded as “at least 8 h.” These factors could have affected ketogenesis. In addition, the lack of dietary information may cause a lack of information about the nutritional status of participants. Diet changes during the study were also unavailable. Although we tried to match the general conditions of the participants, including their age, BMI, and physical activity, more accurate analyses would have been possible if data on nutritional status were available.
Despite these limitations, our study is clinically meaningful as, to our knowledge, it is the first study to examine the relationship between blood ketone levels and the risk of new-onset T2D in patients with IFG. This study also found that higher βHB levels were more significantly associated with an increased risk of T2D in women (p = 0.02 for sex interaction). The mechanisms explaining the sex differences in this association require further study.
In conclusion, in patients with IFG, blood βHB levels tended to be associated with the incidence of new-onset T2D but did not show statistical significance. Large-scale, long-term observational studies with precise nutritional data, accurate information on fasting duration, and more accurate repeated measurements of ketone bodies are needed in the future.
Data availability
The data that support the findings of this study are from the KCPS-II cohort data, which is linked to the Korean National Health Insurance Service (NHIS) database, and access to these data is restricted. As a result, these data cannot be publicly available. However, data may be provided by the corresponding author upon reasonable request and with permission from the NHIS.
References
McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420.
Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15:412–26.
Astrup A, Larsen Meinert, Harper T. A. Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet. 2004;364:897–9.
Joo NS, Lee DJ, Kim KM, Kim BT, Kim CW, Kim KN, et al. Ketonuria after fasting may be related to the metabolic superiority. J Korean Med Sci. 2010;25:1771–6.
Kim Y, Chang Y, Kwon MJ, Hong YS, Kim MK, Sohn W, et al. Fasting Ketonuria and the Risk of Incident Nonalcoholic Fatty Liver Disease With and Without Liver Fibrosis in Nondiabetic Adults. Am J Gastroenterol. 2021;116:2270–8.
Lim K, Kang M, Park J. Association between Fasting Ketonuria and Advanced Liver Fibrosis in Non-Alcoholic Fatty Liver Disease Patients without Prediabetes and Diabetes Mellitus. Nutrients 2021;13:3400.
Kim G, Lee SG, Lee BW, Kang ES, Cha BS, Ferrannini E, et al. Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study. Diabetologia. 2019;62:779–88.
Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37.
Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, Browning JD. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 2019;5:e127737.
Kim JH, Lee M, Kim SH, Kim SR, Lee BW, Kang ES, et al. Sodium-glucose cotransporter 2 inhibitors regulate ketone body metabolism via inter-organ crosstalk. Diabetes Obes Metab. 2019;21:801–11.
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7:66444–54.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9.
Klocker AA, Phelan H, Twigg SM, Craig ME. Blood β-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in Type 1 diabetes: a systematic review. Diabet Med. 2013;30:818–24.
Szili-Torok T, de Borst MH, Garcia E, Gansevoort RT, Dullaart RPF, Connelly MA, et al. Fasting Ketone Bodies and Incident Type 2 Diabetes in the General Population. Diabetes. 2023;72:1187–92.
Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023;402:203–34.
Jee YH, Emberson J, Jung KJ, Lee SJ, Lee S, Back JH, et al. Cohort Profile: The Korean Cancer Prevention Study-II (KCPS-II) Biobank. Int J Epidemiol. 2018;47:385–386f.
Jee SH, Kim M, Kim M, Yoo HJ, Kim H, Jung KJ, et al. Metabolomics Profiles of Hepatocellular Carcinoma in a Korean Prospective Cohort: The Korean Cancer Prevention Study-II. Cancer Prev Res (Phila). 2018;11:303–12.
Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical Practice Guidelines for Type 2 Diabetes Mellitus in Korea. Diabetes Metab J. 2019;43:398–406.
Yoo HJ, Jung KJ, Kim M, Kim M, Kang M, Jee SH, et al. Liver Cirrhosis Patients Who Had Normal Liver Function Before Liver Cirrhosis Development Have the Altered Metabolic Profiles Before the Disease Occurrence Compared to Healthy Controls. Front Physiol. 2019;10:1421.
Lee N, Ha C, Yun H, Woo K. Performance Evaluation of the Autokit Total Ketone Bodies. Journal Laboratory Medicine Quality Assurance. 2017;39:178–80.
Kraus FB, Kocijancic M, Kluttig A, Ludwig-Kraus B. Test validation, method comparison and reference range for the measurement of β-hydroxybutyrate in peripheral blood samples. Biochem Med (Zagreb). 2020;30:010707.
Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15.
Kim MK, Han K, Lee SH. Current Trends of Big Data Research Using the Korean National Health Information Database. Diabetes Metab J. 2022;46:552–63.
Sun-Min Kim Y-IK. 2020 National Health Insurance Statistical Yearbook. Health Insurance Review & Assessment Service, National Health Insurance Service: Health Insurance Review & Assessment Service, National Health Insurance Service, 2020.
Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172:1707–10.
Pongsuthana S, Tivatunsakul N. Optimal Fasting Time before Measurement of Serum Triglyceride Levels in Healthy Volunteers. J Med Assoc Thai. 2016;99:S42–46.
Balasse EO. Kinetics of ketone body metabolism in fasting humans. Metabolism. 1979;28:41–50.
Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178–87.
Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5–16.
Yancy WS Jr., Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77.
Colica C, Merra G, Gasbarrini A, De Lorenzo A, Cioccoloni G, Gualtieri P, et al. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. Eur Rev Med Pharmacol Sci. 2017;21:2274–89.
Tendler D, Lin S, Yancy WS Jr., Mavropoulos J, Sylvestre P, Rockey DC, et al. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:589–93.
Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024.
Pérez-Guisado J, Muñoz-Serrano A. The effect of the Spanish Ketogenic Mediterranean Diet on nonalcoholic fatty liver disease: a pilot study. J Med Food. 2011;14:677–80.
Prattichizzo F, De Nigris V, Micheloni S, La Sala L, Ceriello A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component? Diabetes Obes Metab. 2018;20:2515–22.
Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPA-REG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care. 2016;39:1108–14.
Ferrannini E. Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell Metab. 2017;26:27–38.
Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul). 2015;30:263–9.
Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69.
Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654–67.
Velingkar A, Vuree S, Prabhakar PK, Kalashikam RR, Banerjee A, Kondeti S. Fibroblast growth factor 21 as a potential master regulator in metabolic disorders. Am J Physiol Endocrinol Metab. 2023;324:E409–e424.
Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18:193–216.
Cotter DG, Ercal B, Huang X, Leid JM, d’Avignon DA, Graham MJ, et al. Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest. 2014;124:5175–90.
Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. Embo j. 2008;27:1017–28.
Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, Alvarez JG, et al. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–21.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17.
Veech RL. Ketone esters increase brown fat in mice and overcome insulin resistance in other tissues in the rat. Ann N. Y Acad Sci. 2013;1302:42–8.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Contributions
JHB, YEK, SHJ, KJJ, and BWL contributed to the conception and design of the manuscript. JHB, YEK, KJJ, and BWL contributed to the interpretation of the data. JHB and YEK contributed to the drafting of the manuscript. KJJ and BWL contributed to the critical revision of the article, supervised the study, and are the guarantors of this work. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the Yonsei University College of Medicine (No. 4-2019-1228). This study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants prior to their inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bae, J., Kim, Ye., Jung, K.J. et al. Association between serum beta-hydroxybutyrate levels and risk of type 2 diabetes mellitus in patients with impaired fasting glucose. Nutr. Diabetes 15, 16 (2025). https://doi.org/10.1038/s41387-025-00364-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41387-025-00364-z