Abstract
Objectives
Oxymatrine (OMT), a quinolizidine alkaloid derived from Sophora flavescens Ait., has demonstrated therapeutic potential in type 2 diabetes mellitus (T2DM). This study aimed to investigate its effects on diabetic osteoporosis (DOP) and explore the underlying mechanisms involving gut microbiota and osteogenic regulation.
Methods
In a rat model of T2DM, intragastric Oxymatrine was used to study trabecular bone repair through bone microstructure and histopathology analyses. Changes in gut microbiota, especially Gram-negative bacteria releasing lipopolysaccharides (LPS), were assessed via 16S rRNA sequencing. miRNA sequencing on LPS-induced rat osteoblasts, with and without Oxymatrine, explored osteoblast proliferation, mineralization, and the miR-539-5p/OGN/Runx2 pathway.
Results
The administration of OMT resulted in an enhancement of diabetic osteopathy by reversing trabecular bone loss and modifying the composition of gut microbiota, specifically affecting Gram-negative bacteria that release LPS into the bloodstream. miRNA sequencing revealed that miR-539-5p, which was upregulated in LPS-induced ROBs, was downregulated following OMT treatment. Furthermore, OMT was found to promote osteoblast proliferation and mineralization under conditions of LPS exposure and modulate the miR-539-5p/OGN/Runx2 signaling pathway.
Conclusions
OMT improves diabetic osteoporosis by altering gut microbiota, decreasing LPS release, and enhancing osteoblast growth and differentiation through the miR-539-5p/OGN/Runx2 pathway, suggesting its potential as a treatment.

Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a high incidence of metabolic disease, which is a common disease in the current society. Type 2 diabetes mellitus (T2DM) is a common hormonal metabolic condition in the elderly population, which is increasingly recognized as a public health challenge. Individuals with T2DM experience higher rates of mortality compared to those without the condition. Researches indicate that the risk of all-cause mortality in individuals with T2DM is 1.8 times higher than in those without T2DM, and the medical costs associated with T2DM are reported to be 2.3 times greater than those of individuals without T2DM [1, 2]. The severe outcomes are due to the complications of T2DM, not T2DM on its own. Diabetic osteoporosis (DOP) is one of the potential long-term complications associated with T2DM [3]. The main pathological manifestations of DOP are included decrease bone mass, changed bone microstructure, increased bone fragility and fracture incidence rates [4]. Over the past few years, DOP has emerged as significant danger to both public health and society. It is projected that by 2040, the global prevalence of individuals at high risk of DOP will exceed 300 million, primarily as a result of the ongoing growth in the proportion of elderly individuals within the population [5]. This trend is anticipated to impose a significant economic burden on society. Currently, the drugs used to treat osteoporosis are mainly anti-resorption agents, which have a low treatment rate (19%) following an osteoporotic fracture [6]. In the past, calcium, sex hormones, calcitonin, bisphosphonate were generally used in clinical treatment, but there are adverse drug reactions, intolerance and other problems [7]. Therefore, the identification of efficacious and safe medications for the treatment of osteoporosis that stimulate bone anabolism while minimizing side effects is of paramount importance.
Oxymatrine (OMT), the active ingredient in Sophora flavescens Ait, Sophora tonkinensis Gagnep, Sophora alopecuroides L., and Sophora viciifolia Hance, has been demonstrated to exert anti-inflammatory and anti-tumor effects [8, 9]. According to recent research, it has been shown that OMT can be beneficial in managing diabetes. This is achieved by enhancing the insulin sensitivity of T2DM rats, suggesting that OMT could potentially serve as a novel approach for treating T2DM [10, 11]. Furthermore, OMT exhibited notable anti-inflammatory effects through the modulation of TLR4/NF-κB, NF-κB, MAPKs, and TGF-β signaling pathways [12,13,14,15].
It has been demonstrated that alterations in Toll-like receptor 4 (TLR4) and pathological injury scores are significantly associated with the composition of the gut microbiota. OMT regulates the composition and function of the gut microbiota by inhibiting the activation of the TLR/NF-κB pathway [5]. The degradation and destruction of cartilage ECM leads to increased irregularity and wear of the articular surfaces, which in turn causes destruction and remodeling of the bone microarchitecture. OMT delays cartilage degradation by inhibiting the expression of degradative enzymes and maintaining articular cartilage ECM levels, thereby preserving the bone microarchitecture [16].
The gut microbiome of humans is a collection of microorganisms that live in harmony with the host, consisting of more than 1000 different species [17]. Alterations of the types of gut microbiota can dysregulate metabolic processes, leading to several diseases [18, 19]. Previous researches considered disrupted balance in the intestines as a possible extra hazard to bone health, as well as a fresh target for treatment [20, 21]. Since the gut microbiome and its metabolites are important for the absorption of nutrients, immune function, and energy metabolism, they could potentially impact bone health [22, 23]. In animal models of osteoporosis or diabetes, intestinal infection with pathogenic bacteria can cause bone loss [24, 25]. The relationships between DOP and intestinal microbiota have also been studied [26, 27]. Nevertheless, the complexities and variations of gut microbiota on DOP are highly intricate and require further in-depth investigation.
Numerous metabolites, such as lipopolysaccharide (LPS), bile acids, trimethylamine N-oxide, and short-chain fatty acids have been linked to gut microbiomes and diseases, as supported by evidence [28, 29]. Gram-negative bacteria impact the intestinal epithelia, leading to an increase in both epithelial permeability and serum levels of LPS [30]. The function of the intestinal mucosal barrier is defined by the tight junctions’ integrity and is controlled by the interaction of various signaling pathways [31].
MicroRNAs (miRNAs) are a group of non-coding RNAs that can either break down target mRNAs or hinder their translation. This is achieved through the binding of their seed sequence to the 3’UTR (Untranslated Region) of the target mRNAs, resulting in either degradation or inhibition [32, 33]. A surge in curiosity regarding miRNAs and their target genes in bone growth, osteoblast differentiation, mineralization, and other biological processes has been observed in recent studies [34].
Our research suggests that OMT has the potential to modify the gut microbiota and colonic homeostasis, reducing lipopolysaccharide (LPS) released by the gut microbiota, thereby influencing miRNAs and target genes in osteoblasts, repairing bone trabeculae in patients with type 2 diabetes, and ultimately treating diabetic osteoporosis (DOP). Therefore, the bone structure and gut microbiota variances among the control, model, and OMT treatment groups were examined utilizing DOP animal models. In experiments with rats, it was discovered that Gram-negative bacteria release LPS, which plays a role in the development of DOP. The specificity of candidate miRNAs and target genes in osteoblasts induced by LPS was confirmed. In the end, our study aims to uncover how OMT can engender therapeutic effects for DOP, with a focus on the modulation of gut microbiota diversity, the alteration of gut homeostasis, and the influence on pertinent genes and signaling pathways.
Materials and methods
Animal models and experimental procedure
A total of 50 Sprague-Dawley rats, which were healthy males weighing 200 ± 20 g, were obtained from the Zhejiang Academy of Traditional Chinese Medicine. The protocols used in animal experiments were approved by the Animal Ethics Committee at Zhejiang Academy of Traditional Chinese Medicine, and they adhered to the ARRIVE guidelines. The rats were kept at the animal core of the Zhejiang Academy of Traditional Chinese Medicine’s Institution in Hangzhou, China. They were subjected to a constant room temperature of 23 ± 2 °C, a humidity of 55 ± 5%, and a 12-h light/dark cycle. They were given free access to tap water and were allowed to adapt to the new surroundings for a week prior to the experiment. The rats were randomly assigned to either the control group (n = 10) or the diabetes mellitus group (n = 40). To induce animal models of diabetes mellitus and diabetic osteoporosis, the rats in model group were given a high-sugar and high-fat diet (Diet #MD 31.1%45% fat; 53.3% sugar, total calories 3.95 kcal/g; Mediscience Ltd, China) for a duration of 4 weeks, and then rats were given two consecutive days of intraperitoneal STZ administration (35 mg/kg body weight in 100 mL of sterile citrate buffer, pH 4.5) [35]. Rats that had plasma glucose levels exceeding 16.7 mmol/L were classified as diabetic and selected for additional investigation 72 h after the administration of STZ. Next, the rats in diabetes mellitus group were randomly divided into four groups (n = 10 in each group): the group with diabetic osteoporosis (model group), the group with diabetic osteoporosis and low OMT (Shanghai yuanye Bio-Technology Co., Ltd, Lot: G14N11L130769) (n = 10, OMT-L group, 25 mg/kg/day), the group with diabetic osteoporosis and moderate OMT (n = 10, OMT-M group, 50 mg/kg/day), and the group with diabetic osteoporosis and high OMT (n = 10, OMT-H group, 100 mg/kg/day). The modeling and administration progress was shown in Fig. 1.
Bone microarchitecture and histopathologic analysis
The right femur was examined using micro-CT scan (SCANCO Medical AG, Switzerland). The scanning parameters were configured to 70 kV, 200 A, with a resolution of 15 m and an exposure time of 400 ms. The graphic image resolution was 1024 × 1024 pixels. Three-dimensional (3D) image data were acquired with a voxel size of 20 × 20 μm in all spatial directions. Volumes of interest (VOIs) were selected for data analysis, specifically those located from 1 mm to 2 mm (comprising 100 consecutive slices) below the metaphyseal line. The 3D segmented images were utilized directly to quantify the microarchitecture using the CT analysis software (Evaluation V6.5–3, SCANCO Medical AG, Switzerland). Morphological measurements were conducted, and the following 3D parameters were derived from the analysis of the volumes of interest (VOIs) for the trabecular bone: trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), Bone Mineral Density (BMD), and bone surface density (BS/BV) for each sample [36].
The right femur was collected from each sacrificed rat, and the samples were preserved in 4% (w/v) paraformaldehyde, then embedded in paraffin and subjected to a 1-month decalcification process using a decalcifying solution. Colon tissue was gathered and preserved in a solution containing 4% paraformaldehyde (v/v). The specimens were immersed in paraffin, sliced into 5mm-thick sections, subjected to routine staining with hematoxylin and eosin (H&E). Deparaffinization was achieved using xylene followed by a descending ethanol gradient. The H&E staining procedure was conducted at room temperature, with a total duration of 60–70 min, encompassing the entire process from deparaffinization to completion. Specifically, the application of hematoxylin and eosin required 10 min, with 5 min allocated to each stain. Light microscopy (Olympus Optical Co., Tokyo, Japan) was used for observation.
Immunohistochemistry (IHC) staining was performed on the colon tissue sections to identify the presence of Occludin and Zonula occludens protein 1 (ZO-1) protein expressions. Afterward, the sections were treated with 3% hydrogen peroxide for 10 min to inactivate endogenous peroxidase and subsequently blocked with 5% goat serum for 1 h. Following these preparatory steps, the sections were incubated overnight at 4 °C with Occludin antibody (1:100, Bioss, bs-10011R) and ZO-1 antibody (1:200, Bioss, bs-34023R). On the following day, the sections were incubated with a secondary antibody at a dilution of 1:500 for 1 h at room temperature. Finally, the samples were visualized by the chromogenic substrate diaminobenzidine (DAB) substrate. For the purpose of statistical analysis, three distinct regions were sampled from each section. The mean optical density (calculated as the integral optical density divided by the area) was employed to assess the expression levels of Occludin and ZO-1, while positive cell counting was utilized for the remaining parameters. The analysis was conducted using the ImageJ software (version 2.0.0).
16S rDNA high-throughput sequencing of feces
Following the sacrifice, the excrement was promptly gathered and preserved in liquid nitrogen, then stored at a temperature of −80 °C. To investigate bacterial changes in the intestinal, 16S rDNA high-throughput sequencing was employed to assess the expression of the microbiota flora. PCR was used to amplify the conservative regions by designing general primers and targeting the variation regions through 16S rDNA amplicon sequencing. The hypervariable regions underwent sequencing analysis and species identification. A small fragment library for single-end sequencing was constructed using an IonS5TMXL sequencing platform based on the features of the amplified region.
The reads were cut and filtered, and then operational taxonomic units (OTUs) were clustered. The optimized sequences were subsequently clustered into Operational Taxonomic Units (OTUs) using UPARSE version 7.1, based on a 97% sequence similarity threshold. The most abundant sequence within each OTU was designated as the representative sequence. To mitigate the influence of sequencing depth on alpha and beta diversity metrics, the number of 16S rRNA gene sequences per sample was rarefied to 20,000. This approach maintained an average Good’s coverage of 99.09%. Bioinformatic analysis of the gut microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com). The rarefaction analysis, community richness index, and alpha diversities including Shannon index and Partial Least Squares Discrimination Analysis (PLS-DA) were calculated using QIIME software FLASH (version 1.9.1) and Mothur (version 1.30.2). The taxonomic ranks, in order of size from the largest to smallest, are phylum and genus. The distinctions in genus among the three sets of samples were determined.
LPS concentrations analysis in fecal and plasma
To 2 g of feces, 5 mL of RNAlater Stabilization Solution was introduced, followed by vortexing and two rounds of centrifugation (10 min, 10,000 × g, 4 °C). The liquid above was passed through a filter with a pore size of 0.45 μm and then through another filter with a pore size of 0.22 μm. The total concentrations of plasma and fecal proteins were measured using the Pierce BCA Protein assay kit. Plasma and fecal LPS levels were measured with the LPS RIA Kit (Sinoukbio Beijing, China) in accordance with the manufacturer’s instructions. The subsequent procedures involve the inclusion of specimens, benchmarks, antibodies, and 125I-LPS, followed by vertexing and incubation at 4 °C for duration of 24 h. After combining the serums with the goat anti-rabbit IgG serum and the normal rabbit serum, vigorously mix them and let them sit at room temperature for 15 min. After addition of RIA buffer, the mixture should be vortexed and centrifuged at a speed of 3500 rpm for duration of 20 min. Following this, remove the supernatant and proceed to count the assay tubes.
Chemical components identification in OMT-CS
The control rats were received the same amount of purified water. After the final dose, blood was extracted from the abdominal aorta and then subjected to centrifugation at a speed of 4000 rpm for duration of 15 min. Next, the isolated serum was rendered inactive at a temperature of 56 °C for duration of 30 min. To quantify the main ingredient in OMT containing serum (OMT-CS), a solution of methanol-acetonitrile (900 μL) was added to 300 μL of OMT-CS or blank serum (Con). The mixture was then vortexed for 1 min and centrifuged at 14,000 rpm/min for 15 min at 4 °C to collect the supernatant. To create mother stock solutions, OMT standard substances (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China) were dissolved in methanol. Further, combined spiking stock solutions were prepared by stepwise dilution from the mother stock solutions, and filtered with 0.22 µm membranes. Next, a 2 µL solution was injected into the UPLC-Q/TOF-MS system for analysis.
The ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF-MS) system comprised of an AcquityTM UPLC system (Waters Corporation, Milford, MA, USA) and a Synapt G2 MS (Waters MS-Technologies, Manchester, UK) with an ESI source. The chromatography procedure was conducted using the Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm, Waters Corporation, Milford, MA, United States). The flow rate was set at 0.3 mL/min, and the column temperature was maintained at 40 °C. The mobile phases consisted of A (HCOOH: CH3CN = 0.1: 100, v/v) and B (HCOOH: H2O = 0.1: 100, v/v). The gradient elution was as follows: 0–1 min, 99–90% B; 1–4 min, 99–85% B; 4–8 min, 85–20% B; 8–10 min, 20–1% B.
The ESI source was used for MS analysis in both positive and negative ion modes. The parameters for the ion source gas 1 and gas 2 were set at 55 psi each, while the curtain gas was set at 35 psi. The source temperature was maintained at 600 °C. In positive ion mode, the ion spray voltage was set to float at 5500 V, while in negative ion mode, it was set at −4500 V. IDA MS/MS experiments collected spectra using the high sensitivity mode, employing ±60 psi delustering potential and 35 eV collision energy with ±15 eV collision.
Osteoblasts culture and treatment
Previously published, neonatal (2-day-old) Sprague-Dawley rats were used to isolate Rat calvarial osteoblasts (ROB), as described [37]. Afterwards, the cells were grown in medium consisting of α-MEM (Sigma-Aldrich, Haverhill, UK) with the addition of 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal bovine serum (Sigma-Aldrich) at a temperature of 37 °C with 5% CO2 until they reached confluence. The medium for culture was replaced every week, twice. For all the cellular function tests, passage four osteoblasts were trypsinized, plated at a density of 100,000 cells per well. Prior to treatment, the cells were deprived of nutrients by being cultured in a medium specifically designed for starvation. The medium was prepared with 0.5% fetal bovine serum (Sigma-Aldrich) and incubated for 12–16 h. LPS (Sigma-Aldrich) was dissolved in PBS (Phosphate buffered saline). Next, the cells were exposed to varying amounts of LPS ranging from 10 to 200 μg/mL, along with an equivalent dosage of the control substance (PBS).
The cells were transfected with miR-539-5p mimic, miR-539-5p inhibitor, N.C, and Inhibitor N.C at a concentration of 50 nM using transfection reagent as the manufacturers’ instructions, resulting in the overexpression and inhibition of miR-539-5p. The transfection assays were carried out three times.
MiRNA sequencing of ROBs by treating with OMT
To comprehend the function of miRNAs in different biological processes, it is essential to accurately measure the levels of miRNA by utilizing second-generation sequencing [33]. In order to examine the variation in miRNA expression, ROBs were exposed to LPS (100 μg/mL) and OMT (20 μg/mL) for a duration of 24 h. Total RNAs were extracted from the control, model, and OMT groups using Trizol reagent (Invitrogen, USA). The Bioanalyzer 2100 (Agilent, USA) was used to analyze the amount and quality, ensuring a RIN number greater than 7.0. using the Approximately 1 μg of total RNA was used to prepare a small RNA library TruSeq Small RNA Sample Prep Kits (Illumina, USA). MiRNA-seq was conducted using Illumina Hiseq 2500 (LC-BIO, Hangzhou, China). FastQC was used to verify the quality of the sequence. Normalization and differential expression analyses were conducted utilizing DESeq2 version 4.0. Following the identification of differentially expressed genes (DEGs), the normalized data, including log2 fold changes and p values ≤ 0.05, were employed for subsequent analyses. Genes exhibiting differential expression with a log2 fold change ≥ 2 or ≤−2 and a p value ≤ 0.05 were deemed significant.
Quantitative real-time PCR verification of selected miRNA and target gene
The validation of the miRNA-539-5p sequence data was conducted by performing qRT-PCR on a 7500 real-time PCR system (Applied Biosystems) with the utilization of AceQ qPCR SYBR® Green Master Mix (Vazyme). Table 1 displayed the sequences of the forward and reverse primers. The findings from the experiments were standardized based on the expression of the constant β-actin gene.
Western blot analysis
The ROBs were rinsed two times with PBS that was chilled with ice, and then total cell lysates were prepared by using RIPA lysis buffer. SDS-PAGE was performed on 20 μg of soluble protein, and the resulting proteins were then transferred to a polyvinylidene difluoride membrane (Millipore). Primary antibodies against GAPDH (1:1000 dilution; Boster, A00227-1), Runx2 (1:1000 dilution, Diagbio, db20178), ALP (1:1000 dilution, Bioss, bsm-52252R), and OGN (1:1000 dilution, Abcam, ab73400) were employed to probe the protein bands. Subsequently, the primary antibodies were detected, and the target protein was visualized using EMD Millipore Immobilon™ Western Chemiluminescent HRP Substrate. The ChemiDocTM MP Imaging system (BioRad, USA & Canada) was utilized to obtain images, which were then analyzed using Image J Software.
Dual-luciferase reporter assay
96-well plates were used to seed HEK 293 cells. The OGN 3’-UTR was amplified and inserted into the GPmiRGLO vector provided by GenePharm Pharmaceutical Technology Co., Ltd., located in Shanghai, China. The cells were transfected simultaneously with 50 nM miRNA-539-5p or a mimic with scrambled sequence and 400 ng of a dual luciferase vector that expressed either the wild-type or mutant form of OGN.A dual-luciferase reporter assay kit (Yeasen Biotechnology Co., Ltd., Shanghai, China) was used to conduct a luciferase reporter gene assay after 24 h. To assess the association between miRNA-539-5p and OGN, the renilla luciferase activity to firefly luciferase activity ratios were computed.
ALP and alizarin red staining
The ALP staining was carried out according to the protocols of the ALP staining kit (KGI Biotechnology Co., Ltd. in Jiangsu, China). For a period of 14 days, the osteoblasts were cultured in a medium that promotes calcification. Following induction, the cells were treated with 4% formaldehyde, rinsed with PBS, and then subjected to staining using 0.1% alizarin red. Calcium nodules were identified as either orange or red nodes.
Statistical analysis
The presented data is shown as the average ± the standard error of the average. All data were analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). For the analysis of gut microbiota, Alpha diversity metrics, including Chao1 and Shannon index, were calculated using the mothur software (http://www.mothur.org/wiki/Calculators). The Wilcoxon rank-sum test was employed to analyze differences in Alpha diversity between groups. Principal Coordinate Analysis (PCoA) based on the Bray-Curtis distance algorithm was conducted to assess the similarity of microbial community structures among samples. Additionally, a PERMANOVA non-parametric test was utilized to determine whether significant differences existed in microbial community structures between sample groups. A two-sided Student’s t test was employed to compare differences between two groups, while a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was utilized to analyze differences among multiple groups. Differences were deemed statistically significant at a threshold of p < 0.05 and highly significant at p < 0.01.
Results
OMT ameliorated the microarchitecture of trabecular bone in diabetic rats
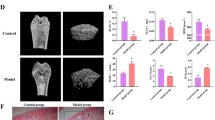
The femur measurements in the DOP group showed a significant decrease compared to the control group, indicating diabetes-induced osteoporosis. However, this decrease was significantly prevented in the OMT-H (100 mg/kg/day) treatment group (p < 0.05). Additionally, it was observed that BMD of femur was reduced in the DOP group. However, this decrease was effectively counteracted by administering OMT (100 mg/kg/day) at the timepoint of 10 weeks (Fig. 2A). From the results of micro-CT and 3D reconstruction, the rats in diabetic group showed lower trabecular bone volume fraction, the trabecular number and trabecular thickness with higher structure model index and trabecular separation than that in the control group. The OMT-H, OMT-M, and OMT-L groups showed higher BMD, BV/TV, and Tb.Th, lower BS/BV values at the distal femur than that in the model groups, and microarchitecture of trabecular bone in the OMT-M groups were superior to OMT-H and OMT-L groups (Fig. 2B). Although the indices of BMD, BV/TV, and Tb.Th were not elevated in the OMT administration treatment groups compared to the control group, the results demonstrated that OMT administration partially preserved these histomorphometric parameters in rats, relative to the DOP group. This indicates that OMT exerts protective effects on DOP animals.
A Three-dimensional reconstruction image of the femur after treating with OMT-H, OMT-M, and OMT-L. B The results of bone microarchitecture results of femur. Data depict the mean ± standard deviation (n = 4 for each group, mean ± SD) and are representative of three independent experiments. Comparison among multiple groups was analyzed by ANOVA (followed by Tukey’s post hoc test). #p < 0.05, compared with the control groups, *p < 0.05, compared with the model groups. All experiments were repeated at least three times.
OMT changed the colon homeostasis in diabetic rats
The hematoxylin-eosin staining of femur (Fig. 3A, D) indicated that the femoral trabeculae in the model group exhibited fractures and a reduction in quantity compared to the control group. However, treatment with OMT successfully prevented the decrease in trabeculae numbers. Statistical analysis of the trabecular percentage indicated a significant difference between the OMT treatment groups and the model group. Furthermore, the OMT-M group demonstrated the most favorable outcome compared to the other OMT treatment groups.
A H&E staining images of femur in each group (×100, scale bar: 200 μm; ×200, scale bar: 100 μm). B H&E staining images of colons in each group (×100, scale bar: 200 μm), red arrows: inflammatory cell infiltration, yellow arrows: thinner muscular layer, Blue hollow arrow: injury of the intestinal epithelial cells and shortened intestinal villi. C ZO-1 and Occludin expression of colons by IHC assay (×100, scale bar: 200 μm). D The percent of trabecular (%) in each group (n = 4 for each group). E The histological score of colons in each group (n = 4 for each group). F, G ZO-1 and Occludin positive expression rates of colons in each group (n = 4 for each group). #p < 0.05, compared with the control groups, *p < 0.05, compared with model groups. Comparison among multiple groups was analyzed by ANOVA (followed by Tukey’s post hoc test). All experiments were repeated at least three times.
It is well-known that the inflammatory reaction and disturbance of colonic equilibrium were associated with bone deterioration. Figure 3B showed that the model group exhibited propria edema and a slight increase in inflammation with noticeable damage or loss of epithelial cells or depletion of cellular mucin, in comparison to the control group. Diabetes caused alterations in the structure of colonic tissue, resulting in the impairment of the intestinal epithelial barrier function. Based on the colitis score, the OMT groups had minimal impact on the healing of epithelial cell damage, whereas OMT-M exhibited a significant therapeutic effect (Fig. 3E, p < 0.05).
Zonula occludens-1 (ZO-1) and Occludin, two proteins that form tight junctions, which play a role in maintaining the integrity and permeability of the intestine, thus contributing to colon homeostasis [38]. The IHC analysis of ZO-1 and Occludin indicated that diabetes inhibited their expression (Fig. 3C, F, G). The findings suggested that the integrity of the intestinal barrier was compromised in the animal models of diabetes, as evidenced by a reduction in the expression of adhesion proteins in the intestines. Additionally, the colons of model groups exhibited a decrease in the expression of ZO-1 and Occludin. Conversely, the expressions of the two molecules were significantly increased in the OMT-M and OMT-L treatments. The findings indicated that OMT had potential to restore the integrity of the intestinal barrier by enhancing the expression of adhesion proteins in the intestines.
OMT was main ingredients in OMT-CS
The UPLC-Q/TOF-MS analysis revealed that OMT was the primary component absorbed into the bloodstream after 10 weeks of administering OMT at a dosage of 100 mg/kg/d. The existing quantitative technique was utilized to ascertain the levels of primary components OMT in OMT-CS. The ingredients quantification results were concluded by matching the accurate mass data of references substances. Figure 4 displayed the Typical total ions current (TIC), MS/MS, and extracted ion chromatogram (XIC) of the OMT. In our subsequent investigation, OMT was regarded as a measure of quality for OMT-CS.
Effects of OMT on gut microbiota diversity
To investigate the impact of OMT on the gut microbiota, we conducted paired-end sequencing of 16S rRNA amplicons. The raw data of the 16S rDNA high-throughput sequencing were uploaded to NCBI (BioProject ID: PRJNA921742). From all samples, a grand total of 101,299,5 high-quality reads were produced after the process of trimming and filtering. At a sequence similarity of 98.87%, a total of 693 OTUs were formed by clustering these reads. The Venn diagram that was generated indicated that there were 317 OTUs that were common to the control, model, and OMT groups. Additionally, there were 151, 20, and 16 OTUs that were exclusive to the control, model, and OMT rats, respectively (Fig. 5A). Moreover, the β-diversity was assessed and the dissimilarity in bacterial composition among each group was visualized using Partial Least Squares Discriminant Analysis (PLS-DA). Figure 5B showed that Comp 1 and Comp 2 accounted for 26.26% and 12.72% of the total variation, respectively. The findings of α-variety and β-variety suggested that OMT therapy enhanced osteoporosis caused by diabetes potentially by regulating the imbalance of gut microorganisms. The analysis of alpha diversity within the sample showed a notable decrease in the richness of the gut microbial community following the administration of OMT in comparison to the model groups, as indicated by the Chao 1 and Sobs indices (Fig. 5C, D).
A Venn diagram of model, control and OMT groups (n = 4 for each group). B β-diversity measured by PLS-DA (Partial Least Squares Discriminant Analysis) (n = 4 for each group). C, D α-diversity analysis based on Chao 1 and Sobs indices (n = 4 for each group), *p < 0.05. E Average composition of the phylum, in each group (n = 4 for each group). F The relative abundance of Firmicutes, Bacteroidota, Actinobacteriota in each group, the different letters represent significant differences between different groups (n = 4 for each group, p < 0.05). G Identified families and genera in the Gram‑negative phylum Bacteroidetes with high relative abundances. H The relative abundance of Bacteroides, Porphyromonas, Prevotella, Paraprevotella and Flavobacterium in each group (n = 4 for each group), **p < 0.01, *p < 0.05. I Effects of OMT on LPS level of Serum and feces (n = 4 for each group), #p < 0.05 compared with the control group (n = 4 for each group), *p < 0.05 compared with the model group. Comparison among multiple groups was analyzed by ANOVA (followed by Tukey’s post hoc test). All experiments were repeated at least three times.
Sequences were identified at both the phylum and genus levels. The data indicated that Firmicutes, Bacteroidota, Actinobacteriota, Patescibacteria, Desulfobacterota, Spirochaetota, Verrucomicrobiota, Campilobacterota, were the most common phyla at the phylum level, accounting for over 90% of the microbial composition in all groups. Additionally, Firmicutes, Bacteroidota, Actinobacteriota exhibited notable variations across the various groups (p < 0.05, Fig. 5E, F). The prevalence of Bacteroidota, a Gram-negative phylum, was considerably greater in the model group compared to the control group, but decreased in the OMT-treated group. The F/B ratio, a widely used parameter, is employed to evaluate the impact of gut microbia on various diseases including obesity, hypertension, and diabetes. In this study, it was observed that the F/B ratio was significantly higher in the model group compared to the control group, whereas it decreased in the OMT-treated group.
In the Bacteroidetes group, there were five families that were recognized: Bacteroidaceae, Porphyromonadaceae, Prevotellaceae, Paraprevotellaceae and Flavobacteriaceae. Additionally, 5 genera (Bacteroides, Porphyromonas, Prevotella, Paraprevotella and Flavobacterium) were found in the human gut, as shown in Fig. 5G. At the genus level, the model group exhibited significantly higher levels (p < 0.05) of the 5 most prevalent intestinal microbiota compared to the control group. Conversely, the OMT group showed significantly lower levels (p < 0.05) of the 5 most prevalent intestinal microbiota compared to the model group (Fig. 5H).
OMT decreased the amount of LPS released by gut microbiotas
Previous studies have shown that Bacteroidetes make up the largest portion of the Gram-negative microbial community in the digestive system [39, 40]. In order to examine the impact of OMT on signaling molecules associated with Gram-negative intestinal microbiota, the concentration of LPS was measured in both serum and fecal samples. According to Fig. 5I, the serum level of LPS was higher in the model group compared to the control group (p < 0.05). However, OMT treatment effectively reduced the release of LPS (p < 0.05). Similar findings were achieved regarding the concentration of LPS in fecal samples. The findings suggested that the decrease in LPS signaling observed in the rats treated with OMT was a result of its impact on the prevalence of Gram-negative microorganisms.
Screening miRNAs in LPS induced osteoblasts treating with OMT
Throughout our investigation, we analyzed the effects of LPS on the expression of genes related to osteoblasts. Different concentrations of LPS were used to induce the ROBs, and the results of the viability assay indicated that the growth viabilities in the cell group treated with 100, 200 μg/mL LPS were 67.47% and 64.28% respectively. However, the 20 μg/mL OMT groups showed an increase in viability to 89.57% at 24 h (p < 0.05, Fig. 6A, B). Hence, the concentration of 100 μg/mL LPS was regarded as the concentration treated with LPS, while the concentration of 20 μg/mL OMT was considered as the concentration treated with OMT. In order to investigate the possible miRNAs in LPS-induced ROBs through the application of OMT, the cells underwent next-generation microRNA analysis. The miRNA-seq findings revealed that 13 miRNAs exhibited differential expression across the three groups (Fig. 6C, p < 0.05). Quantitative real-time PCR was used to validate the expression levels of 13 miRNAs identified from RNA-Seq findings. The analysis revealed that expression of miRNAs, including miR-22-5p_R-1, miR-136-3p, miR-339-5p_R-2, miR-193a-3p, and miR-539-5p, was altered in response to LPS-induced treatment compared to the control groups. Furthermore, the expression levels of miRNAs, such as miR-674-3p_R-2 and miR-539-5p, were differentially modulated in the LPS + OMT treatment group compared to the LPS group. Notably, miR-539-5p was significantly upregulated following LPS-induced treatment, whereas its expression was reduced after OMT treatment, as illustrated in the Fig. 6D. Based on these results, miR-539-5p was identified as the primary target in LPS-induced osteoblasts and OMT treatment. In subsequent studies, miR-539-5p was investigated as a focal biomarker. TargetScan software predicted that miR-539-5p may target OGN, a gene associated with bone development. Figure 6E displayed the indicated binding sites for miR-539-5p and OGN. The luciferase reporter assays confirmed that miR-539-5p inhibited the transcription of OGN in HEK 293 cells. Luciferase activity was inhibited by both OGN wild type and miRNA-539-5p mimics in cells that were co-transfected. However, co-transfecting cells with miRNA-539-5p mimics or OGN mutants did not suppress expression (Fig. 6F, G, p < 0.05).
A ROBs were treated with different concentrations of LPS, the survival rate of osteoblasts was measured by MTT (n = 4 for each group), *p < 0.05, **p < 0.01, compared with the control groups. B MTT results of LPS-induced ROBs were treated with different concentrations of OMT (n = 4 for each group), **p < 0.01, compared with the LPS induced ROBs. C Heatmap of differential expressions of top differential expressed miRNAs among groups (n = 3 for each group). D RT-PCR was conducted to validate the expression levels of predicted miRNAs derived from the sequencing data in each group. (n = 4 for each group), *p < 0.05, compared with the control group, #p < 0.05, compared with the LPS group. E The predicted binding sites for miR miR-539-5p and OGN. F The transfection efficiency of mimics and inhibitor in HEK293 was approximately higher than 80%. G Interaction between miRNA-539-5p and OGN 3’-UTR tested in the luciferase reporter assays (n = 4 for each group). Comparison between the two groups was performed by Student’s t test. *p < 0.05, compared to the N.C groups. H–J RT-PCR results and Western blot assays showed the protein levels of OGN, Runx2, and ALP (n = 4 for each group). **p < 0.001, ***p < 0.0001, compared to the N.C groups, #p < 0.05, ##p < 0.01 compared to the inhibitor N.C groups. K, L ALP staining and ALP activities were used to determine the effect of overexpression of miRNA-539-5p inhibited ALP positive staining (n = 4 for each group). M, N Alizarin red staining and osteocalcin concentrations (ng/mL) were calculated mineralization of osteoblasts in each group (n = 4 for each group), **p < 0.001, compared to the N.C groups, ##p < 0.01 compared to the inhibitor N.C groups. Comparison among multiple groups was analyzed by ANOVA (followed by Tukey’s post hoc test). All experiments were repeated at least three times.
Functional studies were conducted to validate the target gene of miR-539-5p in ROBs through overexpression and knockdown of miR-539-5p. ROBs were transfected with miR-539-5p mimics, miR-539-5p inhibitor, and negative control (NC). RT-PCR and Western blotting were used to evaluate the levels of RNA and protein. Transfecting cells with miR-539-5p mimic led to overexpression and caused a decrease in the mRNA and protein levels of OGN, Runx2, and ALP, compared to cells treated with mimic NC. Conversely, the repression of miR-539-5p increased the expression of OGN, compared to cells treated with inhibitor NC (Fig. 6H–J). Furthermore, the treatment with miR-539-5p mimic demonstrated a significant reduction in ALP activity and formation of calcified nodules. The miR-539-5p inhibitor treatment had oppositive effects (Fig. 6K–N). Our findings indicate that the stimulation of LPS enhanced the expression of miR-539-5p, leading to a simultaneous reduction in OGN levels. Additionally, this stimulation regulated the activity of ALP and the mineralization process in osteogenic cells by targeting the OGN/Runx2 pathway.
OMT protected LPS induced osteoblasts by regulating miR-539-5p/OGN/Runx2 axis
ROBs were transfected with Si-OGN to examine the expression of genes downstream of OGN. As a result, Si-OGN caused a reduction in osteoblast ALP activity and final mineralization in osteoblasts (Fig. 7A, B). In order to assess the impact of OMT on LPS-induced ROBs, we administered varying concentrations of OMT (5 μg/mL, 10 μg/mL, and 20 μg/mL) to the LPS-induced ROBs. The RT-PCR findings indicated that miR-539-5p exhibited upregulation in the LPS-induced ROBs, whereas the OMT-treated groups demonstrated contrasting impacts (Fig. 7C). In contrast, there was a notable reduction in the levels of OGN, Runx2, and ALP expressions in ROBs induced by LPS, while OMT enhanced the expression of these mRNAs (Fig. 7D). Furthermore, the results obtained from western blotting were consistent with the findings from the RT-PCR assay (Fig. 7E, F). Furthermore, LPS treatment led to a decrease in the activity of ALP and the formation of calciferated nodules in ROBs, as well as a reduction in the concentration of osteocalcin. However, these effects were reversed when different concentrations of OMT were administered (Fig. 7G–J). The above findings indicated that OMT has a significant impact on the onset of ROBs after being exposed to LPS.
A, B ALP staining and alizarin red staining after transfection with NC, Si-OGN. ALP activities and osteocalcin concentrations (ng/mL) were calculated after transfection with NC, and Si-OGN (n = 4 for each group). Comparison between the two groups was performed by Student’s t test. *p < 0.05, **p < 0.01, compared with the NC groups. C The expression levels of miR-539-5p in each group analyzed by RT-PCR (n = 4 for each group), *p < 0.05, **p < 0.01, compared with the LPS group, #p < 0.05, compared with the control group. D–F mRNA levels and protein expressions of OGN, Runx2, and ALP in each group (n = 4 for each group). *p < 0.05, **p < 0.01, compared to the LPS groups, #p < 0.05, ##p < 0.01 compared to the control groups. G, H ALP staining and ALP activities were used to determine the effect of OMT on osteoblasts proliferation capability (n = 4 for each group), *p < 0.05, **p < 0.01, compared to the LPS-treated groups. I, J Alizarin red staining and osteocalcin concentrations (ng/mL) were calculated mineralization of osteoblasts (n = 4 for each group), *p < 0.05, **P < 0.01, compared to the LPS-treated groups. Comparison among multiple groups was analyzed by ANOVA (followed by Tukey’s post hoc test). All experiments were repeated at least three times.
Discussion
Type 2 Diabetes (T2D) is a persistent and challenging epidemic that can result in numerous complications, including the emerging public health issue of osteoporosis [41, 42]. The etiology and pathology of osteoporosis in type 2 diabetics is more intricate compared to other types, and this is gaining significance on a global scale. Nevertheless, the detailed mechanisms of diabetic osteoporosis remain unclear.
OMT, an organic alkaloid substance, exhibits a diverse array of biological functions, such as anti-inflammatory, antioxidant, and antineoplastic properties [43, 44]. Recent research has shown that OMT is beneficial in the treatment of diabetes, indicating that OMT could potentially be utilized as a novel therapeutic approach. Our objective in this study was to examine the potential mechanism controlled by OMT in the management of osteoporosis induced by T2DM.
The main treatments currently available for DOP are: bone resorption inhibitors such as bisphosphonates, calcitonin; bone formation stimulators such as parathyroid hormone analogs; and other drugs such as active vitamin D [45]. Bisphosphonates are the most widely used drugs for the prevention and treatment of osteoporosis. When bisphosphonates are used alone, some patients may experience adverse effects such as gastrointestinal upset [46] and skeletal muscle pain. However, OMT can reduce the incidence and severity of these side effects through its anti-inflammatory and analgesic effects, thereby improving patients’ quality of life.
Despite an increasing amount of DOP research, the optimal animal model for studying DOP is still uncertain. To understand the mechanism of diabetes complicated by osteoporosis, it was necessary to have a model that replicated the pathological progression of diabetes. Consequently, we conducted an experiment on rats to simulate T2DM by providing them with a diet high in fat and administering a low dosage of streptozotocin, which replicates the effects of T2DM [47]. This study involved the analysis of bone microstructure using micro-CT, revealing that T2DM effectively leads to decreased bone density in rats, a characteristic associated with DOP. On the other hand, administering OMT orally enhanced the bone microarchitecture parameters BV/TV, Tb.Th, and BMD, while also repaired the fractured femoral trabecular caused by T2DM. These data suggested that OMT is a potential therapeutic drug for DOP treatment.
The role of gut bacteria in bone physiology is important, particularly in relation to the function of the intestinal barrier and the immune response [48]. Recent studies have indicated a strong correlation between the disruption of colon homeostasis, resulting in an inflammatory response and alteration of autoimmune status, and the presence of reduced bone density in the human body [49]. Absorption of dietary nutrients and resistance to pathogens and toxins depends on a complete and dense intestinal barrier [50]. Intestinal epithelial cells are connected by occludin and ZO-1, which are proteins of tight junctions responsible for preserving the integrity and permeability of the intestine [51]. We observed intestinal damage in the colons of DOP rats during our investigation, which resulted in a significant decrease in the expression of Occludin and ZO-1. OMT treatment could repair intestinal damage. We believed that the alteration of DOP animals’ gut microbiota resulted in intestinal damage and subsequent bone deterioration. All these processes mentioned above could be reversed by OMT administration.
In recent years, there has been a rise in studies concerning the gut microbiota, with a specific emphasis on its capacity to stimulate or regulate bone health and disease. This is due to our current comprehension of the role played by the gut microbiota and its metabolites in maintaining the balance of bone structure and function [52]. Our results proved that amounts of OTUs in OMT group were changed, compared with model rats. Also, the microbial composition diversely changed in OMT-treated rats varied considerably from that in model rats. The findings suggest that T2DM leads to imbalances in gut bacteria, which have a direct impact on bone metabolism as a result of alterations in their composition and distribution. The gut microbiota of DOP and OMT-treated individuals contained the Gram-negative phyla, which included Bacteroidetes. Previous studies have suggested that Bacteroidetes are the main microbiota of Gram-negative origin. In our study, five previously unknown genera of Bacteroidetes were identified, including Porphyromonas, Prevotella, Paraprevotella and Flavobacterium. The dominant presence of Bacteroides genus members was reported in the gut microbiota of the OMT treatment. Studies have identified a positive correlation between Bacteroides and bone strength and structure [53]. Additionally, Bacteroides have been shown to facilitate calcium absorption [54]. Notably, research involving ovariectomized mice indicates that the presence of Bacteroides mitigates colonic dysbiosis, thereby aiding in the prevention of lumbar bone deterioration. Additionally, the presence of Firmicutes and Bacteroidetes in the gut plays a crucial role in the differentiation of osteoclasts. The ratio of Firmicutes to Bacteroidetes (F/B ratio) serves as a distinct indicator for osteoporosis [55]. In our research, we noticed a reduction in the proportion of Firmicutes to Bacteroidetes at the phylum level, when compared to the control group.
In recent research, T2DM has been regarded as a persistent low-level inflammation [56]. The progress of DOP involves various inflammatory factors. LPS can be transferred from the gut to the blood when intestinal permeability is lost [57, 58]. Consequently, the degradation of the intestinal lining linked to an imbalance in the gut microbiota may result in increased levels of lipopolysaccharide in individuals with dysmotility of the digestive system. Our study revealed that rats with DOP had a higher prevalence of Gram-negative bacteria in their gut microbiota. DOP may experience chronic inflammation due to an increase in inflammatory biomarkers and elevated levels of LPS caused by Gram negative gut dysbiosis. Systemic inflammation has been shown to be promoted by elevated levels of circulating LPS [59]. The findings we described above suggest that OMT reduced the occurrence of detrimental microorganisms and the generation of LPS.
The repair and remodeling of bones is achieved through the dynamic interaction between osteoblasts and osteoclasts. On the other hand, an imbalance between osteoblasts and osteoclasts can lead to bone diseases. The proliferation and differentiation of osteoblasts could be suppressed by LPS [60]. In vivo, the impact of LPS on bone is typically observed, particularly in cases of diabetic osteoporosis. The clinical cause of osteoporosis is the osteopenic substance glucose, which speeds up the death of osteoblasts and hinders the activity of genes responsible for bone remodeling [55]. MiRNAs play a crucial role in controlling the growth and activity of osteoblasts and osteoclasts, acting as regulatory factors [61, 62]. In a prior study, a subset of specific microRNAs (miRNAs) was identified as promising therapeutic targets for targeted drug therapy, demonstrating potential in addressing osteoporosis by modulating their content and expression levels [61, 63]. In the current study, miRNA sequencing (miRNA-seq) was performed to examine the miRNA profiles in the control group, as well as in the LPS and LPS + OMT-treated osteoblasts (ROBs). We determined that there were 13 miRNAs that exhibited differential expression among these three groups. In the RT-PCR verification experiments, LPS stimulation significantly increased the expression of MiR-539-5p, while OMT treatment decreased it. This suggests that OMT plays a role in enhancing osteogenic differentiation in LPS-induced ROBs. Furthermore, several other miRNAs exhibited significant differential expression, nevertheless, it was miR-539-5p that displayed the most significant differential expression during LPS and OMT treatment. Moreover, our subsequent investigations will involve the validation of the remaining miRNAs. In response to sporadic LPS stimulation, ROBs promoted miR-539-5p while simultaneously reducing the expression of a target gene regulated by these miRNAs. The preceding study established that miR-539 is associated with poor prognosis in osteosarcoma patients and adversely affects osteogenesis by inhibiting the signaling pathway, indicating that this relationship is not coincidental [64, 65].
OGN, a member of the small leucine-rich proteoglycan family, was identified as the predicted target gene for miR-539-5p. This gene has been shown to improve bone formation parameters in osteoblasts [66]. According to previous research, elevated levels of OGN in the blood are linked to a higher risk of reduced bone mineral density (BMD) and vertebral fractures in postmenopausal women with T2DM [54]. Furthermore, OGN has been associated with insulin resistance and cardiovascular disease in clinical studies. OGN could potentially serve as a new indicator of a connection between muscles, pancreas, and bones [67]. Our research findings indicated that OMT effectively restored osteoblast damage caused by LPS. This was achieved by reducing the levels of miR-539-5p and increasing the expression of the target gene OGN, as well as subsequent expressions of Runx2 and ALP in LPS-induced ROBs. Furthermore, our findings indicated that LPS inhibited the growth and mineralization of osteoblasts through the modulation of the miR-539-5p/OGN/Runx2 pathway, while OMT exhibited the contrasting effects. OMT could potentially act as a mediator, enhancing osteoblast activity and maturation via the miR-539-5p/OGN/Runx2 pathway, according to the existing evidence.
However, some limitations exist in this work. Differences in gut microbiota significantly affect how oxymatrine works, impacting metabolism, disease, drug targets, and dosage sensitivity, leading to varied treatment results. The study suggests using high-throughput sequencing to explore oxymatrine’s metabolic interactions with gut microbiota in different intestinal settings for a better understanding of its mechanisms. Research has demonstrated that the gut microbiome and its byproducts, particularly short-chain fatty acids (SCFAs), have an influence on human bone health [68]. Further investigation into oxymatrine’s effect on short-chain fatty acids (SCFAs) is warranted to understand its role in bone metabolism. This research could clarify its impact on bone health and suggest new treatment approaches for osteoporosis and related conditions. Longer studies are needed to evaluate its long-term effects on bone health and gut microbiota. While current research shows it improves gut microbiota in DOP rats by reducing harmful microbes and LPS production, clinical trials are required for validation. Extended follow-up will confirm if oxymatrine provides lasting benefits and is safe over the long term. It has been demonstrated that existing bisphosphonates, which are commonly used in the treatment of osteoporosis, may cause adverse effects such as gastrointestinal discomfort and skeletal muscle pain [46]. Moreover, although hormone replacement therapy is effective in improving bone density, long-term use may increase the risk of breast cancer and cardiovascular disease [69]. In contrast, OMT has a wider scope of application, as it has been shown to reduce the incidence and severity of these side effects through its anti-inflammatory and analgesic effects. There are potential differences in the gut microbiota between individual animals, and our study can only demonstrate that OMT reduces the occurrence of harmful microorganisms in the gut flora and the production of LPS in DOP rats. Despite recent promising research, challenges remain with OMT. Individual gut microbiota variations can affect its efficacy, making personalized dosage and treatment crucial. While studies have identified some molecular mechanisms, further research is needed on other signaling pathways, including those involving inflammatory cytokines and miRNAs. Additionally, OMT’s clinical application is hindered by issues with drug delivery and bioavailability, as its human metabolism is not fully understood. Improving bioavailability through delivery systems like nanoparticles could enhance its effectiveness. Large-scale clinical trials are necessary to confirm OMT’s potential for treating DOP by evaluating its safety, efficacy, long-term effects, and interaction with gut microbiota. Collaboration among clinical researchers, pharmacologists, and microbiome experts is crucial for its advancement.
Conclusions
Collectively, OMT exhibited osteoplastic properties on DOP rats. Furthermore, the gastrointestinal microbiota, particularly the Gram-negative bacteria, significantly contributes to the development of DOP and the functioning of OMT. The results of the activity of miRNA-539-5p and its interaction with the target gene OGN in LPS-induced ROBs suggest a possible role of particular miRNAs in regulating bone remodeling and osteoblast differentiation following damage caused by LPS. Therefore, it is worth considering further research on OMT as a possible treatment for osteoporosis caused by T2DM.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
Gnesin F, Thuesen ACB, Kähler LKA, Madsbad S, Hemmingsen B. Metformin monotherapy for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;6:CD012906. https://doi.org/10.1002/14651858.CD012906.pub2.
Huang Q, Liu Z, Yang Y, Yang Y, Huang T, Hong Y, et al. Selenium nanodots (SENDs) as antioxidants and antioxidant-prodrugs to rescue islet β cells in type 2 diabetes mellitus by restoring mitophagy and alleviating endoplasmic reticulum stress. Adv Sci. 2023;10:e2300880. https://doi.org/10.1002/advs.202300880.
Xiu S, Mu Z, Sun L, Zhao L, Fu J. Hemoglobin level and osteoporosis in Chinese elders with type 2 diabetes mellitus. Nutr Diabetes. 2022;12:19. https://doi.org/10.1038/s41387-022-00198-z.
Yang Y, Lin Y, Wang M, Yuan K, Wang Q, Mu P, et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022;10:26. https://doi.org/10.1038/s41413-022-00198-w.
Liu M, Liu F, Pan Y, Xiong Y, Zeng X, Zheng L, et al. Oxymatrine ameliorated experimental colitis via mechanisms involving inflammatory DCs, gut microbiota and TLR/NF-κB pathway. Int Immunopharmacol. 2023;115:109612. https://doi.org/10.1016/j.intimp.2022.109612.
Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern P, Chung KC. Efectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int. 2017;28:289–300. https://doi.org/10.1007/s00198-017-4175-0.
Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Min Res. 2016;31:1485–7. https://doi.org/10.1002/jbmr.2888.
Jin X, Fu W, Zhou J, Shuai N, Yang Y, Wang B. Oxymatrine attenuates oxidized low‑density lipoprotein‑induced HUVEC injury by inhibiting NLRP3 inflammasome‑mediated pyroptosis via the activation of the SIRT1/Nrf2 signaling pathway. Int J Mol Med. 2021;48:187. https://doi.org/10.3892/ijmm.2021.5020.
Wang W, You R, Qin W, Hai L, Fang M, Huang G, et al. Anti-tumor activities of active ingredients in Compound Kushen Injection. Acta Pharm Sin. 2015;36:676–9. https://doi.org/10.1038/aps.2015.24.
Zhu Y, Hu H, Zuo M, Mao L, Song G, Li T, et al. Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation. Biomed Rep. 2021;15:56. https://doi.org/10.3892/br.2021.1432.
Zuo M, Wang A, Tian Y, Mao L, Song G, Yang Z. Oxymatrine ameliorates insulin resistance in rats with type 2 diabetes by regulating the expression of KSRP, PETN, and AKT in the liver. J Cell Biochem. 2019;120:16185–94. https://doi.org/10.1002/jcb.28898.
Gan P, Ding L, Hang G, Xia Q, Huang Z, Ye X, et al. Oxymatrine attenuates dopaminergic neuronal damage and microglia-mediated neuroinflammation through cathepsin D-dependent HMGB1/TLR4/NF-κB pathway in Parkinson’s disease. Front Pharm. 2020;11:776. https://doi.org/10.3389/fphar.2020.00776.
Xue X, Guo Y, Zhao Q, Li Y, Rao M, Qi W, et al. Weighted gene co-expression network analysis of oxymatrine in psoriasis treatment. J Inflamm Res. 2023;16:845–59. https://doi.org/10.2147/JIR.S402535.
Yang Z, Yin R, Cong Y, Yang Z, Zhou E, Wei Z, et al. Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation. 2014;37:2047–55. https://doi.org/10.1007/s10753-014-9937-7.
Zhao H, Zhang Z, Chai X, Li G, Cui H, Wang H, et al. Oxymatrine attenuates CCl4-induced hepatic fibrosis via modulation of TLR4-dependent inflammatory and TGF-β1 signaling pathways. Int Immunopharmacol. 2016;36:249–55. https://doi.org/10.1016/j.intimp.2016.04.040.
Jiang Y, Sang W, Wang C, Lu H, Zhang T, Wang Z, et al. Oxymatrine exerts protective effects on osteoarthritis via modulating chondrocyte homoeostasis and suppressing osteoclastogenesis. J Cell Mol Med. 2018;8:3941–54. https://doi.org/10.1111/jcmm.13674.
Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 2016;535:85–93. https://doi.org/10.1038/nature18849.
Lv Q, Li Z, Sui A, Yang X, Han Y, Yao R. The role and mechanisms of gut microbiota in diabetic nephropathy, diabetic retinopathy and cardiovascular diseases. Front Microbiol. 2022;13:977187. https://doi.org/10.3389/fmicb.2022.977187.
Shuwen H, Kefeng D. Intestinal phages interact with bacteria and are involved in human diseases. Gut Microbes. 2022;14:2113717. https://doi.org/10.1080/19490976.2022.2113717.
Seely KD, Kotelko CA, Douglas H, Bealer B, Brooks AE. The human gut microbiota: a key mediator of osteoporosis and osteogenesis. Int J Mol Sci. 2021;22:9452. https://doi.org/10.3390/ijms22179452.
de Sire A, de Sire R, Curci C, Castiglione F, Wahli W. Role of dietary supplements and probiotics in modulating microbiota and bone health: the gut-bone axis. Cells. 2022;11:743. https://doi.org/10.3390/cells11040743.
He Y, Chen Y. The potential mechanism of the microbiota-gut-bone axis in osteoporosis: a review. Osteoporos Int. 2022;33:2495–506. https://doi.org/10.1007/s00198-022-06557-x.
Chen Y, Wang X, Zhang C, Liu Z, Li C, Ren Z. Gut microbiota and bone diseases: a growing partnership. Front Microbiol. 2022;13:877776. https://doi.org/10.3389/fmicb.2022.877776.
Yuan Y, Yang J, Zhuge A, Li L, Ni S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022;55:e13194. https://doi.org/10.1111/cpr.13194.
Zhang J, Motyl KJ, Irwin R, MacDougald OA, Britton RA, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–82. https://doi.org/10.1210/EN.2015-1308.
Wang H, Giambini H, Chen J, Wang Q, Hou H, Luo S, et al. Diabetes mellitus accelerates the progression of osteoarthritis in streptozotocin-induced diabetic mice by deteriorating bone microarchitecture, bone mineral composition, and bone strength of subchondral bone. Ann Transl Med. 2021;9:768. https://doi.org/10.21037/atm-20-6797.
Zhang Y, Cao M, Li Y, Zhang R, Wu M, Yu Q, et al. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Min Metab. 2022;40:874–89. https://doi.org/10.1007/s00774-022-01375-x.
Pastori D, Carnevale R, Nocella C, Novo M, Santulli M, Cammisotto V, et al. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to Mediterranean diet. J Am Heart Assoc. 2017;6:e005784. https://doi.org/10.1161/JAHA.117.005784.
Cheng N, Liang Y, Du X, Ye RD. Serum amyloid A promotes LPS clearance and suppresses LPS-induced inflammation and tissue injury. EMBO Rep. 2018;19:e45517. https://doi.org/10.15252/embr.201745517.
Stephens M, von der Weid PY. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes. 2020;11:421–32. https://doi.org/10.1080/19490976.2019.1629235.
Huang L, Sun T, Hu L, Hu S, Sun H, Zhao F, et al. Elevated miR-124-3p in the aging colon disrupts mucus barrier and increases susceptibility to colitis by targeting T-synthase. Aging Cell. 2020;19:e13252. https://doi.org/10.1111/acel.13252.
Dong J, Tai JW, Lu LF. miRNA-microbiota interaction in gut homeostasis and colorectal cancer. Trends Cancer. 2019;5:666–9. https://doi.org/10.1016/j.trecan.2019.08.003.
Long J, Danesh FR. Promises and challenges of miRNA therapeutics. Am J Physiol Ren Physiol. 2022;323:F673–74. https://doi.org/10.1152/ajprenal.00251.2022.
Grillari J, Mäkitie RE, Kocijan R, Haschka J, Vázquez DC, Semmelrock E, et al. Circulating miRNAs in bone health and disease. Bone. 2021;145:115787. https://doi.org/10.1016/j.bone.2020.115787.
Islam MS, Loots du T. Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharm. 2009;31:249–61. https://doi.org/10.1358/mf.2009.31.4.1362513.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Min Res. 2010;25:1468–86. https://doi.org/10.1002/jbmr.141.
Weivoda MM, Hohl RJ. Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation. Endocrinology. 2011;152:3113–22. https://doi.org/10.1210/en.2011-0016.
Wang J, Zhang C, Guo C, Li X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int J Mol Sci. 2019;20:5751. https://doi.org/10.3390/ijms20225751.
d’Hennezel E, Abubucker S, Murphy LO, Cullen TW. Total lipopolysaccharide from the human gut microbiome silences toll-like receptor signaling. mSystems. 2017;2:e00046-17. https://doi.org/10.1128/mSystems.00046-17.
Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. https://doi.org/10.1146/annurev-biochem-060713-035600.
Alalwan TA, Perna S, Mandeel QA, Abdulhadi A, Alsayyad AS, D’Antona G, et al. Effects of daily low-dose date consumption on glycemic Control, lipid profile, and quality of life in adults with pre- and type 2 diabetes: a randomized controlled trial. Nutrients. 2020;12:217. https://doi.org/10.3390/nu12010217.
Bain S, Ramamurthy NS, Impeduglia T, Scolman S, Golub LM, Rubin C. Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone. 1997;21:147–53. https://doi.org/10.1016/s8756-3282(97)00104-x.
Lan X, Zhao J, Zhang Y, Chen Y, Liu Y, Xu F. Oxymatrine exerts organ- and tissue-protective effects by regulating inflammation, oxidative stress, apoptosis, and fibrosis: from bench to bedside. Pharmacol Res. 2020;151:104541. https://doi.org/10.1016/j.phrs.2019.104541.
Xu H, Chen G, Ma Y, Zhang H, Zhou Y, Liu G, et al. Hepatic proteomic changes and Sirt1/AMPK signaling activation by oxymatrine treatment in rats with non-alcoholic steatosis. Front Pharm. 2020;11:216. https://doi.org/10.3389/fphar.2020.00216.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. https://doi.org/10.1016/S2213-8587(17)30188-2.
Schroeder RJ, Staszkiewicz J, O’Quin C, Carroll B, Doan N, Patel S, et al. Oral therapeutics post menopausal osteoporosis. Cureus. 2023;15:e42870. https://doi.org/10.7759/cureus.42870.
Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5:349–58. https://doi.org/10.1111/jdi.12235.
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–32. https://doi.org/10.1038/nri.2017.7.
Yu M, Pal S, Paterson CW, Li JY, Tyagi AM, Adams J, et al. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. 2021;131:e143137. https://doi.org/10.1172/JCI143137.
Luis AS, Jin C, Pereira GV, Glowacki RWP, Gugel SR, Singh S, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature. 2021;598:332–7. https://doi.org/10.1038/s41586-021-03967-5.
Jian Y, Zhang D, Liu M, Wang Y, Xu Z. The impact of gut microbiota on radiation-induced enteritis. Front Cell Infect Microbiol. 2021;11:586392. https://doi.org/10.3389/fcimb.2021.586392.
Tousen Y, Matsumoto Y, Nagahata Y, Kobayashi I, Inoue M, Ishimi Y. Resistant starch attenuates bone loss in ovariectomised mice by regulating the intestinal microbiota and bone-marrow inflammation. Nutrients. 2019;11:297. https://doi.org/10.3390/nu11020297.
Villa CR, Taibi A, Chen J, Ward WE, Comelli EM. Colonic Bacteroides are positively associated with trabecular bone structure and programmed by maternal vitamin D in male but not female offspring in an obesogenic environment. Int J Obes. 2018;42:696–703. https://doi.org/10.1038/ijo.2017.294.
Tanaka KI, Kanazawa I, Kaji H, Sugimoto T. Association of osteoglycin and FAM5C with bone turnover markers, bone mineral density, and vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Bone. 2017;95:5–10. https://doi.org/10.1016/j.bone.2016.11.007.
Rathinavelu S, Guidry-Elizondo C, Banu J. Molecular modulation of osteoblasts and osteoclasts in type 2 diabetes. J Diabetes Res. 2018;2018:6354787. https://doi.org/10.1155/2018/6354787.
Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. https://doi.org/10.1172/JCI92035.
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. https://doi.org/10.3389/fncel.2015.00392.
Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability-a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. https://doi.org/10.1186/s12876-014-0189-7.
Ramachandran G. Gram-positive and gram-negative bacterial toxins in sepsis: a brief review. Virulence. 2014;5:213–8. https://doi.org/10.4161/viru.27024.
Xuan Y, Wang J, Zhang X, Wang J, Li J, Liu Q, et al. Resveratrol attenuates high glucose-induced osteoblast dysfunction via AKT/GSK3β/FYN-mediated NRF2 activation. Front Pharm. 2022;13:862618. https://doi.org/10.3389/fphar.2022.862618.
Bellavia D, Salamanna F, Raimondi L, De Luca A, Carina V, Costa V, et al. Deregulated miRNAs in osteoporosis: effects in bone metastasis. Cell Mol Life Sci. 2019;76:3723–44. https://doi.org/10.1007/s00018-019-03162-w.
Zhang Y, Wang X, Huang X, Shen L, Zhang L, Shou D, et al. Transcriptome sequencing profiling identifies miRNA-331-3p as an osteoblast-specific miRNA in infected bone nonunion. Bone. 2021;143:115619. https://doi.org/10.1016/j.bone.2020.115619.
Yang Y, Wang Y, Wang F, Yuan L, Guo Z, Wei Z, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53:40. https://doi.org/10.1186/s40659-020-00309-z.
Tripathi A, John AA, Kumar D, Kaushal SK, Singh DP, Husain N, et al. MiR-539-3p impairs osteogenesis by suppressing Wnt interaction with LRP-6 co-receptor and subsequent inhibition of Akap-3 signaling pathway. Front Endocrinol. 2022;13:977347. https://doi.org/10.3389/fendo.2022.977347.
Yang W, Qi Y, Si M, Hou Y, Nie L. A comprehensive analysis for associations between multiple microRNAs and prognosis of osteosarcoma patients. PeerJ. 2020;8:e8389 https://doi.org/10.7717/peerj.8389.
Leong I. Osteoglycin—linking bone and energy homeostasis. Nat Rev Endocrinol. 2018;14:379. https://doi.org/10.1038/s41574-018-0036-y.
Starup-Linde J, Viggers R, Handberg A. Osteoglycin and bone—a systematic review. Curr Osteoporos Rep. 2019;17:250–5. https://doi.org/10.1007/s11914-019-00523-z.
Wallimann A, Magrath W, Thompson K, Moriarty T, Richards RG, Akdis CA, et al. Gut microbial-derived short-chain fatty acids and bone: a potential role in fracture healing. Eur Cell Mater. 2021;41:454–70. https://doi.org/10.22203/eCM.v041a29.
Yang X, Liang J, Wang Z, Su Y, Zhan Y, Wu Z, et al. Sesamolin protects mice from ovariectomized bone loss by inhibiting osteoclastogenesis and RANKL-mediated NF-κB and MAPK signaling pathways. Front Pharm. 2021;12:664697. https://doi.org/10.3389/fphar.2021.664697.
Funding
This study was supported by National Natural Science Foundation of China under Grant Nos. 82474240, and 82274547, Co-construction science and technology project of Science and Technology Department of National Administration of Traditional Chinese Medicine and Zhejiang Provincial Administration of Traditional Chinese Medicine under Grant No. GZK-ZJ-KJ-23064, and Research Project of Zhejiang Chinese Medical University under Grant No. 2023JKZKTS34, Zhejiang Provincial Traditional Chinese Medicine Science and Technology Fund under Grant No. 2025ZL621.
Author information
Authors and Affiliations
Contributions
Yang Zhang conceived and designed the experiments; Yiwen Zhu, Mengying Li, and Minjie Zhang performed the experiments, analyzed the data; Yang Zhang wrote the manuscript; Dan Shou and Peijian Tong administered and supervised the experimental work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study was reviewed and approved by Animal Ethics Committee of Zhejiang Academy of Traditional Chinese Medicine, and conformed to the ARRIVE guidelines (Approval No. [2022]004).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Zhu, Y., Li, M. et al. A promising approach to diabetic osteoporosis: oxymatrine’s effects on gut microbiota and osteoblasts. Nutr. Diabetes 15, 19 (2025). https://doi.org/10.1038/s41387-025-00374-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41387-025-00374-x