Abstract
Tumor-associated Macrophages (TAMs) are highly plastic immune cells that shape the tumor microenvironment (TME) and influence cancer progression. However, the molecular determinants governing their functional heterogeneity remain incompletely understood. In this study, we identify Rab37 as a key regulator that remodels the states of macrophages within the lung TME. Single-cell RNA sequencing revealed that Rab37 wild-type (WT) tumors were enriched in immunosuppressive Spp1+ TAMs, whereas Rab37 knockout (KO) tumors contained a higher proportion of Thbs1+ TAMs, suggesting Rab37-dependent shifts in macrophage programming. Mechanistically, Rab37 promoted osteopontin (OPN) secretion, which activated STAT3 signaling to establish an autocrine feedback loop that sustained Spp1 expression and induced M2-like polarization. Paracrine OPN signaling further enhanced lung cancer cell proliferation, migration, and invasion. In clinical lung cancer specimens, CD163+/Rab37+/OPN+ TAMs correlated with recurrence and poor survival, and multivariate analysis confirmed their independent prognostic value. Together, these findings demonstrate that Rab37 governs macrophage phenotype and function by orchestrating OPN/STAT3 signaling, thereby reinforcing an immunosuppressive TME and promoting lung cancer progression. Targeting the Rab37–OPN axis may thus represent a promising therapeutic strategy.
Similar content being viewed by others
Introduction
Lung cancer remains a leading cause of cancer-related mortality worldwide, characterized by high heterogeneity and limited responsiveness to conventional therapies [1, 2]. Lung cancer progression is intricately associated with dynamic interactions within the tumor microenvironment (TME). The TME comprises diverse cell types, including tumor cells, immune cells, fibroblasts, and endothelial cells, whose interactions influence tumor growth, metastasis, and treatment responses [3, 4].
Rab37, a member of the Rab GTPase family, is a key regulator of vesicle trafficking, mediating the transport and secretion of cargo [5]. Our previous observations in orthotopic mouse models suggest that Rab37 deficiency limits tumor growth and enhances infiltration of anti-tumor immune cells [6]. In macrophages and T cells, Rab37-mediated exocytosis of secreted proteins and surface receptor presentation promotes immunosuppressive phenotypes and reduces therapeutic efficacy [7, 8]. However, the regulated cargo proteins, cell type-specific functions, and context-dependent effects of Rab37 in the TME remain poorly defined. Understanding these mechanisms is crucial for defining the role of Rab37 in regulating interactions within the TME and its impact on lung cancer progression.
Among the immune populations in the TME, tumor-associated macrophages (TAMs) are particularly important drivers of tumor progression [9]. TAMs often adopt an M2-like phenotype that triggers immune suppression, angiogenesis, extracellular matrix remodeling, and metastasis [10, 11]. A key effector of these pro-tumor activities is secreted phosphoprotein 1 (SPP1), also known as osteopontin (OPN). In the TME, OPN-expressing TAMs are recognized as a defining feature of immunosuppressive macrophage populations [12]. OPN mediates its effects through integrins (e.g., αvβ3, α4β1) and CD44, activating JAK2-STAT3, PI3K-AKT, MAPK, and NF-κB pathways. These signals strengthen immunosuppressive cytokine networks, enhance tumor cell survival, and metastasis [13,14,15]. Additionally, TAM-derived OPN stimulates VEGF-driven angiogenesis, induces matrix metalloproteinases (e.g., MMP9) to facilitate invasion, and attracts or sustains other suppressive immune cells [16]. High OPN expression in macrophages has been associated with aggressive disease features and poor clinical outcomes in lung cancer [17], while hypoxic conditions (e.g., HIF-1α activation) can further elevate SPP1 expression in myeloid cells [18]. Notably, OPN can directly promote M2 macrophage polarization through autocrine or paracrine activation of the JAK2-STAT3 pathway, thereby amplifying immunosuppressive functions and sustaining pro-tumor TME [19, 20]. Collectively, these findings highlight OPN as a pivotal mediator connecting macrophage polarization to the progression of malignant disease.
In this study, we used single-cell RNA sequencing (scRNA-seq) to demonstrate that Spp1+ macrophages are enriched in the Rab37 wild-type (WT) lung TME. We further demonstrated that Rab37 mediates OPN release in macrophages, thereby enhancing the formation of an immunosuppressive TME and accelerating tumor progression. Importantly, we observed that elevated infiltration of CD163+/Rab37+/OPN+ macrophages is closely linked to poor prognosis in lung cancer patients, suggesting their potential as a valuable prognostic biomarker and underscoring the importance of elucidating Rab37-mediated pathways in TAMs in advance therapeutic development.
Materials and methods
Mouse, cell lines, and culture conditions
C57BL/6 wild-type (WT) and Rab37 knockout (KO) mice were maintained under specific pathogen-free conditions with free access to food and water under a 12-h light/dark cycle in the animal facility of National Cheng Kung University. All animal experiments were performed in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee (IACUC; #112062).
Human THP-1 monocytes, lung cancer cell lines (H460, H460, H1299, and LLC), and murine RAW264.7 macrophages were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI-1640 or DMEM (Gibco, Waltham, MA, US) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere with 5% CO2. THP-1 cells were differentiated into macrophages by stimulation with 40 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma Aldrich, USA) for 24 h prior to experiments.
Single-cell RNA sequencing (scRNA-seq) analysis
CD45+ immune cells were isolated from Rab37 WT and KO C57BL/6 mice injected with Matrigel (Matrigel-injected) or tumor cells (LLC-injected) 14 days after tumor establishment. Single-cell suspensions were subjected to scRNA-seq using the 10x Genomics Chromium platform according to the manufacturer’s instructions. Libraries were prepared using the Chromium Single Cell 3’ Reagent Kit v2 and sequenced on an Illumina NovaSeq 6000 system.
Downstream analysis of scRNA-seq data was conducted in R version 4.1.3 using RStudio version 2025.05.1 + 513 and the Seurat package version 4.3.0. Genes detected in fewer than three cells were excluded, as were cells with fewer than 200 or more than 4,000 detected genes, fewer than 500 or more than 50,000 transcripts, or more than 10% mitochondrial content. Gene expression was normalized using the NormalizeData function with the “LogNormalize” method and a scale factor of 10,000. Highly variable genes were identified with FindVariableFeatures. For datasets generated on different run dates, batch effects were corrected using the IntegrateData workflow in Seurat [21]. All genes were then scaled and centered with ScaleData. Dimensionality reduction was performed using principal component analysis (RunPCA), and clusters were defined using FindNeighbors and FindClusters, selecting dimensions that accounted for approximately 90% of the variance. Uniform manifold approximation and projection (UMAP) was applied for visualization using RunUMAP.
Ligand–receptor interactions were inferred using the R package CellChat (version 2.1.2) following the standard workflow [22]. Differential gene expression was assessed with the Wilcoxon rank-sum test and Bonferroni correction, implemented through the identifyOverExpressedGenes function, with the following thresholds: thresh.pc = 0.1 (gene expressed in at least 10% of cells within a cluster), thresh.fc = 0.1 (minimum log fold-change), and thresh.p = 0.05 (P value cutoff). Ligands with a fold-change below 0.25% were excluded from downstream visualization. Interaction networks between ligand-expressing clusters and receptor-expressing populations were displayed using netVisual_chord_gene with slot.name = “netP”, and manually annotated with corresponding gene names from slot.name = “net.”
Vesicle isolation and immunoprecipitation (IP)
The vesicle isolation protocol was modified from Hendrix’s report [23]. RAW264.7 cells were transfected with either an empty vector (EV) or GFP-tagged Rab37 wild-type (GFP-Rab37WT) plasmid and subsequently lysed by sonication. Cell debris was removed by centrifugation at 3000 × g for 10 min at 4 °C, and the resulting supernatants were subjected to ultracentrifugation at 30,000 × g for 60 min at 4 °C using a 40Ti rotor (Beckman Coulter, Brea, CA, USA) to enrich vesicle fractions. Vesicle-containing suspensions were then incubated with an anti-GFP antibody to selectively isolate Rab37-associated vesicles. Plasmids and antibodies used in the study are listed in Supplementary Tables 1 and 3.
Patient samples and clinical information
A total of 36 lung cancer patients from National Cheng Kung University Hospital were recruited with institutional review board permission approval (#A-ER-111-517) and patient informed consent. Overall survival was calculated as the time from the day of surgery to the date of death or the last follow-up. Disease-free survival was calculated as the time from the day of surgery to either the date of disease recurrence or the last follow-up. Tumor type and disease staging were performed according to the World Health Organization classification and the TNM classification system, respectively.
Multiplex fluorescence immunohistochemistry (IF-IHC)
Multiplex IF-IHC was performed to evaluate the co-localization of CD163, Rab37, and OPN in tumor specimens from 36 lung cancer patients. Staining was performed using the Opal Multiplex IHC kit (#NEL810001KT, Akoya Biosciences, Marlborough, MA, USA) according to the manufacturer’s instructions. Quantification was defined as the average percentage of positive cells across the entire image. A cut-off value of 0.98%, determined from the mean of patient data, was applied for correlation analysis. Images were acquired using an Olympus FV3000 confocal microscope and analyzed with FV31S-SW software (Olympus). The primary antibodies used are listed in Supplementary Table 3.
Statistical analysis
Cell studies were done in triplicate, except as noted. Statistical analysis included two-tailed Student’s t tests for mean ± SD data, Pearson χ2 for protein expression correlation in patients, Kaplan-Meier for survival curves with log-rank tests, and Cox regression for patient outcome risk analysis. The level of statistical significance was taken as p value, *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Results
Rab37 shapes immune cell composition and drives anti-inflammatory macrophage polarization in lung TME
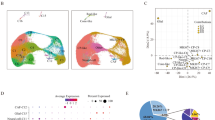
To investigate the role of Rab37 in shaping the lung immune microenvironment, we performed scRNA-seq using the 10× Genomics platform on CD45+ immune cells isolated from Rab37 WT and KO C57BL/6 mice injected with Matrigel or tumor cells (LLC-injected) (Fig. 1A). After quality control, 12,426 high-quality single-cell transcriptomes were retained (Fig. 1A; Supplementary Fig. 1A). Harmony-corrected principal components were then used to construct a unified Uniform manifold approximation and projection (UMAP) embedding, followed by graph-based clustering to define immune subsets. Based on canonical cell markers and SingleR annotation with reference to the ImmGen database [24] and PanglaoDB [25], seven major immune cell types were identified (Fig. 1B, C, and Supplementary Fig. 1B). These included T cells (Cd3d, Trbc2, Cd8a, Cd4), natural killer cells (Nkg7, Gzma, Klra4), B cells (Cd79a, Igkc, Ighm, Ly6d), monocytes (Cd68, Ifitm3, Ms4a6c, Lst1), macrophages (Marco, Chil3, Spp1, Mrc1), neutrophils (S100a9, S100a8, Retnlg), and dendritic cells (H2-Eb1, Cst3, Ckb) (Supplementary Fig. 1C). Although T-cell proportions decreased in the Rab37 KO TME compared with WT, CD8+ T cells in Rab37 KO TME displayed higher cytotoxicity signatures, suggesting enhanced effector activity despite reduced abundance (Supplementary Fig. 1D).
A Schematic workflow of the experimental design. CD45+ immune cells were isolated from lung tissues of Rab37 WT and KO C57BL/6 mice injected with Matrigel (control) or tumor cells (LLC tumor-bearing), followed by scRNA-seq using the 10× Genomics platform. B UMAP plot showing clustering of immune cells into major populations. C Stacked bar plots illustrating the distribution of immune cell subsets across experimental groups. D Dot plot showing the expression of representative anti-inflammatory and pro-inflammatory markers in macrophages. CellChat chord diagrams depicting macrophage outgoing signaling interactions with other immune subsets in Rab37 WT (E) and KO (F) lung TME.
Myeloid cells are the dominant population in the TME and critically regulate tumor progression [26]. In particular, TAMs establish an immunosuppressive milieu by suppressing cytotoxic T cell activity [27]. Consistent with this, our data suggest that Rab37-dependent macrophage activity contributes to reduced T cell cytotoxicity in WT TME. Differential expression analysis showed that Rab37 WT macrophages exhibited elevated anti-inflammatory genes, including Trem2 and Spp1, along with Ctsb, Gpr183, Pparg, Gpnmb, and Trem2—a conserved SPP1+ TAM signature reported by Palma et al. [28] (Fig. 1D, Supplementary Fig. 1E). In contrast, Rab37 KO macrophages displayed decreased anti-inflammatory and increased pro-inflammatory markers, indicating a tumor-specific shift toward a pro-inflammatory state (Supplementary Fig. 1E, F).
Since macrophages play a central role in immune regulation, we performed CellChat [22] analysis to investigate Rab37-dependent macrophage communication with other immune subsets in the lung TME. Rab37 WT macrophages in the lung TME exhibited strong outgoing and incoming interactions with multiple immune populations, predominantly mediated by the Spp1, ApoE, and Fn1 pathways (Supplementary Fig. 1G). In contrast, Rab37 KO macrophages displayed reduced interactions, with signaling mainly restricted to the Thbs, App, and Cxcl pathways (Supplementary Fig. 1H). Analysis of outgoing communication further revealed that Rab37 WT macrophages actively interacted with macrophages, T cells, NK cells, B cells, dendritic cells, and neutrophils, with Spp1 emerging as the dominant signaling axis (Fig. 1E, Supplementary Fig. 1I). By comparison, Rab37 KO macrophages exhibited a marked reduction in outgoing Spp1 signals (Fig. 1F, Supplementary Fig. 1J). Predicted receptors of Spp1, including Cd44 and multiple integrins (Itga4, Itgb1, and Itgb5), were broadly expressed across macrophages in both Rab37 WT and KO TMEs (Supplementary Fig. 1K). Notably, Spp1 expression was substantially higher in Rab37 WT macrophages, whereas receptor expression levels were comparable between genotypes (Supplementary Fig. 1K), suggesting that Rab37 primarily enhances Spp1-mediated signaling through increased ligand availability rather than alterations in receptor abundance.
Spp1 + macrophages are enriched by Rab37 and associated with poor prognosis in lung cancer
To further characterize the heterogeneity of macrophages within the lung TME, we performed subclustering analysis and identified three distinct macrophage subpopulations based on gene marker expression (Fig. 2A). One subset exhibited strong expression of Spp1, which encodes the glyco-phosphoprotein osteopontin (OPN), representing an immunosuppressive macrophage population [17, 29]. A second subset was defined by high expression of Thbs1, while the third subset expressed canonical alveolar macrophage markers (Ear1, Ear2), consistent with a homeostatic alveolar macrophage phenotype. Notably, the macrophage subpopulation composition showed a striking shift, with tumors from Rab37 WT mice predominantly enriched with Spp1+ TAM, whereas tumors from KO mice contained more Thbs1+ TAM (Fig. 2B; Supplementary Fig. 2A). Multiplex immunofluorescence (IF) staining of orthotopic lung tumor tissues from Rab37 WT and KO mice further validated these findings, showing abundant infiltration of CD163+/OPN+ TAMs in WT TME, whereas CD163+/THBS1+ TAMs were enriched in KO TME (Fig. 2C, D; Supplementary Fig. 2B, C). In addition, TNFα staining revealed that CD163+/THBS1+ macrophages in the Rab37 KO TME exhibited higher TNFα expression, indicative of a pro-inflammatory phenotype (Supplementary Fig. 2B, C). These results support the notion that Rab37 regulates macrophage phenotype by promoting the accumulation of immunosuppressive Spp1+ TAM.
A UMAP visualization of macrophage subclusters, showing three distinct populations defined by gene marker expression. B Distribution of macrophage subclusters across Rab37 WT and KO normal and tumor lung tissues. Multiplex IF-IHC staining (C) and quantitative analysis (D) of orthotopic lung tumor tissues from Rab37 WT and KO mice, showing CD163 (blue) co-localization with OPN (red). Nuclei were counterstained with DAPI (white). Yellow arrows indicate CD163+/ OPN+ cells. Scale bar, 20 μm. E, F Representative images of multiplex IF-IHC staining of lung cancer patient samples showing triple labeling of CD163 (blue), Rab37 (green), and OPN (red) in tumor sections (Scale bar, 20 μm), with enlarged views highlighting co-localization. Yellow arrows indicate CD163+/Rab37+/OPN+ cells, while white arrows denote CD163+/OPN+ cells (E). Quantification of CD163+Rab37+OPN+ tumor-associated macrophages in patients without recurrence and with recurrence (F). Kaplan–Meier survival curves comparing overall (G) and recurrence-free (H) survival of patients stratified by the percentage of OPN+/Rab37+/CD163+ cells. I Pathway enrichment analysis based on differentially expressed genes in Spp1+ macrophages. Data are presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s ttest.
The clinical relevance of these findings was first assessed using the TCGA-LUAD dataset. SPP1 expression was positively correlated with M2 macrophages (Supplementary Fig. 2D), and patients with high SPP1 expression exhibited significantly poorer overall survival compared with those with low expression (p = 0.044, log-rank test; Supplementary Fig. 2E). In contrast, although THBS1 expression was also correlated with M2 macrophages (Supplementary Fig. 2F), it was not associated with overall survival in lung cancer patients (Supplementary Fig. 2G), suggesting that SPP1, but not THBS1, holds prognostic value.
To further validate these observations, we analyzed our lung cancer patient cohort using immunofluorescent-immunohistochemistry (IF-IHC). Quantitative assessment revealed that increased infiltration of CD163+/Rab37+/OPN+ cells was significantly associated with cancer recurrence (Fig. 2E, F). High levels of CD163+/Rab37+/OPN+ cells ( ≥ 0.98%) were detected in 69.2% of recurrent patients (p = 0.001; Supplementary Table 1), supporting a significant association between triple-positive macrophage infiltration and tumor recurrence. Consistently, Kaplan–Meier survival analysis demonstrated that patients with high infiltration of CD163+/Rab37+/OPN+ cells had significantly poorer overall survival (p = 0.014; Fig. 2G) and recurrence-free survival (p = 0.024; Fig. 2H) compared to those with low infiltration. Notably, stratification based on CD163+/OPN+ macrophage infiltration showed only a trend toward worse overall survival and recurrence-free survival in the high infiltration group, but the differences did not reach statistical significance (Supplementary Fig. 2H, I). Finally, to evaluate whether infiltration of triple-positive macrophages served as an independent prognostic factor, we conducted Cox regression analysis (Table 1). Univariate analysis showed that patients with high infiltration of CD163+/Rab37+/OPN+ cells ( ≥ 0.98%) had a significantly increased risk of poor outcome compared with those with low infiltration ( < 0.98%) (Hazard Ratio [HR] = 2.691, 95% CI: 1.182–6.126, p = 0.015). This prognostic significance remained robust in multivariate analysis after adjusting for clinicopathological parameters (HR = 2.691, 95% CI: 1.182–6.126, p = 0.018), indicating that CD163+/Rab3+/OPN+ cell infiltration represents an independent prognostic biomarker in lung cancer. Among 36 patients analyzed, 12 were positive for both CD163+/Rab37+/OPN+ and CD163+/OPN+ groups, showing partial overlap but no significant correlation (Rho = 0.1842, p = 0.43; Supplementary Fig. 2J). Consistently, CD163+/OPN+ infiltration alone did not correlate with survival (HR = 1.110, 95% CI: 0.506–2.433, p = 0.794) (Table 1), underscoring the critical role of Rab37 in defining the prognostic relevance of OPN⁺ TAMs.
Pathway enrichment analysis of differentially expressed genes between Spp1+ TAMs and Thbs1+ TAMs further demonstrated that Spp1+ TAMs were strongly associated with myeloid and lymphocyte differentiation, regulation of the inflammatory response, and the JAK–STAT signaling pathway (Fig. 2I). In contrast, Thbs1+ TAMs exhibited enrichment in pathways related to viral response, type I interferon signaling, and cytokine-mediated immune activation, reflecting a more pro-inflammatory transcriptional program (Supplementary Fig. 2K). Notably, vesicle transport–related pathways were also significantly enriched in Spp1+ TAMs, suggesting a potential mechanistic link between Rab37, SPP1, and macrophage vesicular trafficking programs. Together, these results identify Spp1+ TAM as a Rab37-dependent immunosuppressive subset with both prognostic significance and mechanistic relevance in lung cancer progression.
Rab37 mediates OPN secretion in a GTP-dependent manner to drive M2 macrophage polarization
Rab37 GTPase is a key regulator of vesicle trafficking [5]. To examine its role in OPN secretion, Rab37 KO THP-1 macrophages were generated using CRISPR/Cas9. Both Rab37 WT and KO macrophages were stimulated with lung cancer cell–derived conditioned medium (LCM), then the secreted proteins were analyzed by Western blot. OPN secretion was progressively increased in Rab37 WT cells with prolonged LCM stimulation, whereas KO cells showed minimal secretion (Fig. 3A). To determine whether this release occurred through the exocytotic pathway, THP-1 macrophages were treated with Brefeldin A (BFA), an ER-to-Golgi transport inhibitor. LCM-induced OPN secretion was reduced by BFA, indicating that OPN release occurs via exocytosis (Supplementary Fig. 3A). We next examined whether OPN is directly trafficked within Rab37-associated vesicles. GFP-tagged Rab37WT or empty vector (EV) was overexpressed in LCM-educated RAW264.7 macrophages, and vesicles were isolated by immunoprecipitation with GFP magnetic beads (Fig. 3B). Western blot analysis demonstrated that OPN was enriched in Rab37-specific vesicle compared with the EV control (Fig. 3C). Confocal microscopy confirmed the co-localization of OPN (green) and Rab37 (red), with increased yellow puncta in LCM-stimulated THP-1 cells compared with controls (Supplementary Fig. 3B), supporting the role of Rab37 in mediating OPN vesicular trafficking. Rab37 is a small GTPase, and its activity depends on GTP binding and hydrolysis [30]. Therefore, we further examined the mechanism of Rab37-mediated OPN secretion by overexpressing EV, Rab37WT, constitutively active Rab37Q89L, or dominant-negative Rab37T43N in LCM-educated RAW264.7 macrophages. Confocal microscopy showed prominent co-localization of OPN and Rab37 in cells expressing Rab37WT or Rab37Q89L, as indicated by yellow puncta in the merged images, whereas EV and Rab37T43N-expressing cells displayed minimal co-localization, which was supported by quantitative analysis (Fig. 3D, E). CM-Western blot and ELISA analyses revealed elevated OPN secretion in Rab37WT cells, further enhanced in Rab37Q89L cells, and reduced in Rab37T43N cells (Supplementary Fig. 3C, D).
A Western blot analysis of OPN secretion in CM from THP-1 Rab37 WT and KO macrophages stimulated with LCM for the indicated durations. Rab37-specific vesicle isolation of GFP-tagged Rab37WT or EV in LCM-educated RAW264.7 macrophages (B), followed by western blot analysis of associated vesicles (C). Confocal microscopy images (D) and quantitative analysis (E) showing the subcellular localization of Rab37 (red) and OPN (green) in LCM-educated macrophages. Nuclei are stained with DAPI (blue). Scale bar, 10 μm. F Schematic representation of the CM transfer assay. Rab37 WT and KO macrophages were stimulated with LCM, CM was collected, and then applied to recipient macrophages. G Western blot analysis of p-STAT3 in THP-1 macrophages stimulated with CM derived from Rab37 WT or KO THP-1 macrophages, with or without OPN neutralization (nOPN, 5 µg/ml) or recombinant OPN (rOPN, 100 ng/ml) supplementation. RT-qPCR analysis of SPP1 mRNA expression (H) and FIZZ1 mRNA expression (I) in recipient THP-1 macrophages treated with CM under the indicated conditions. Data are presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s ttest.
Rab37 regulates OPN/STAT3 signaling that drives SPP1 upregulation and M2-like macrophage polarization
Since our scRNA-seq analysis revealed predominant Spp1 expression in Rab37 WT macrophages, we next examined whether Rab37 regulates SPP1 transcription. RT-qPCR analysis showed that Rab37 WT macrophages exhibited higher basal SPP1 levels than KO cells, even without LCM stimulation. Upon LCM treatment, SPP1 expression was further upregulated in Rab37 WT but not in KO macrophages (Supplementary Fig. 3E), indicating that Rab37 is essential for enhancing SPP1 expression in response to tumor-derived signals.
Our previous study demonstrated that Rab37 regulates IL-6 secretion in macrophages, promoting M2 polarization [6]. Additionally, tumor-derived IL-6 has been reported to drive monocyte differentiation toward SPP1+ TAMs [31]. To further investigate the relationship between IL-6 and OPN, we performed time-course experiments in macrophages stimulated with recombinant IL-6. IL-6 treatment transiently increased THBS1 expression at 6 h (Supplementary Fig. 3F), followed by a progressive rise in SPP1 levels at 24–48 h (Supplementary Fig. 3G), accompanied by induction of the M2 marker FIZZ1 (Supplementary Fig. 3H). These results indicate that IL-6 promotes a temporal transition from a THBS1+ to a SPP1+ macrophage state.
CellChat analysis suggested that Spp1 signals from Rab37 WT macrophages may act in an autocrine or paracrine manner to influence macrophage function (Fig. 1E). Based on reports that OPN activates the JAK2–STAT3 pathway to enhance SPP1 transcription [15], we hypothesized that Rab37-mediated OPN secretion drives STAT3–SPP1 upregulation in an autocrine or paracrine manner, whereas Rab37 deficiency abolishes this effect. To test whether OPN contributes to sustaining SPP1 expression after IL-6 induction, macrophages were first treated with IL-6 for 24 h and then cultured for another 24 h with or without nOPN. Removal of IL-6 followed by nOPN treatment reduced SPP1 levels (Supplementary Fig. 3I). These findings indicate that IL-6 functions as an early initiator that promotes the transition of THBS1+ macrophages toward an SPP1+ phenotype, while Rab37 may further regulate OPN secretion to maintain and amplify SPP1 expression through a positive feedback mechanism.
To further validate whether Rab37 regulates a positive feedback loop in macrophages, in which OPN secretion enhances SPP1 expression, we designed a CM transfer assay. Rab37 WT and KO THP-1 macrophages were stimulated with LCM for 24 h, after which the media was replaced with fresh complete media and cultured for an additional 24 h to collect CM. CM collected from Rab37 WT or KO cells was subsequently used to stimulate THP-1 macrophages (Fig. 3F). Western blot analysis was performed to assess STAT3 activation under these conditions. CM derived from Rab37 WT THP-1 macrophages induced a marked increase in STAT3 phosphorylation (p-STAT3) in recipient THP-1 cells compared with CM from KO cells. Furthermore, OPN neutralization (nOPN) in Rab37 WT CM reduced p-STAT3, whereas supplementation of recombinant OPN (rOPN) to KO CM restored STAT3 activation (Fig. 3G). RT-qPCR analysis further confirmed that CM derived from Rab37 WT THP-1 macrophages significantly increased SPP1 expression in THP-1 macrophages compared with CM from KO cells. Moreover, neutralization of OPN in Rab37 WT CM suppressed SPP1 induction, whereas supplementation of rOPN to KO CM restored SPP1 expression (Fig. 3H).
OPN has been shown to drive M2 macrophage polarization [32], prompting us to assess whether Rab37-dependent OPN secretion influences M2 polarization in macrophages. To evaluate M2 polarization, we measured FIZZ1 mRNA expression and CD206 surface levels, which are canonical M2 macrophage markers [33]. Macrophages treated with Rab37 WT CM exhibited higher FIZZ1 and CD206 expression compared with those treated with KO CM. nOPN in Rab37 WT CM reduced this induction, whereas rOPN supplementation to KO CM restored both FIZZ1 and CD206 expression (Fig. 3I; Supplementary Fig. 3J, K). Collectively, these results demonstrate that Rab37-mediated OPN secretion activates STAT3 signaling and enhances SPP1 expression to promote M2-like macrophage polarization in a positive feedback loop.
Rab37-mediated OPN secretion from macrophages promotes lung cancer progression
OPN promotes tumor progression by enhancing cancer cell proliferation, migration, and invasion through extracellular matrix remodeling and oncogenic signaling [13, 34, 35]. To assess the functional impact of Rab37-mediated OPN secretion in macrophages on tumor progression, CM transfer experiments were performed. CM from Rab37 WT or KO macrophages stimulated with LCM was applied to lung cancer cells with or without nOPN or rOPN supplementation. Western blot and IF analyses showed that Rab37 WT CM strongly activated STAT3 phosphorylation and nuclear localization, effects that were reduced by nOPN and restored by rOPN, whereas KO CM failed to induce STAT3 activation (Supplementary Fig. 4A, B).
To evaluate the role of Rab37-mediated OPN secretion in lung cancer progression, CM transfer assays were conducted using H460 and H1299 cells treated with CM from Rab37 WT or KO macrophages with or without nOPN or rOPN supplementation (Fig. 4A). CM from Rab37 WT macrophages significantly promoted tumor cell proliferation, migration, and invasion compared with KO CM. These effects were suppressed by nOPN and restored by rOPN (Fig. 4B–H). Thus, Rab37-dependent OPN secretion by macrophages enhances lung cancer aggressiveness through paracrine signaling.
A Schematic workflow of the CM transfer assay. Rab37 WT or KO THP-1 macrophages were stimulated with LCM for 24 h, followed by collection of CM with or without nOPN (5 µg/ml) or rOPN (100 ng/ml) supplementation, and subsequently applied to lung cancer cells. Cell proliferation assays of H460 (B) and H1299 (C) cells treated with the indicated CM, measured at different time points. D Transwell migration assay schematic illustrating lung cancer cells seeded in the upper chamber, with Rab37 WT or KO THP-1 macrophages placed in the lower chamber, in the presence or absence of nOPN (5 µg/ml) or rOPN (100 ng/ml) supplementation. Representative images (E) and quantification (F) of migration assays of H460 cells. Representative images (G) and quantification (H) of invasion assays of H1299 cells. Data are presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t–test.
Discussion
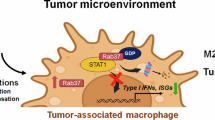
Our study reveals a novel role for Rab37 in promoting an immunosuppressive lung TME through regulation of OPN secretion from macrophages. By integrating scRNA-seq, molecular and functional analyses, we show that Rab37-mediated OPN release establishes an autocrine feedback loop that sustains Spp1 expression and exerts paracrine effects resulting in M2-like polarization and enhance tumor growth and metastasis. These findings uncover a mechanism by which Rab37 modulates macrophage-driven immunosuppression and highlight the Rab37–OPN–STAT3 axis as a potential therapeutic target in lung cancer (Fig. 5).
Diagram showing Rab37-dependent vesicle trafficking of OPN in macrophages, followed by its secretion into the extracellular space. Secreted OPN activates the JAK2–STAT3 pathway in macrophages to enhance Spp1 transcription, establishing an autocrine positive feedback loop. In parallel, OPN acts in a paracrine manner to promote M2 macrophage polarization and to drive lung cancer cell proliferation and migration.
scRNA-seq analysis revealed distinct macrophage compositions between groups, with Spp1+ TAMs enriched in Rab37 WT TME and Thbs1+ TAMs more abundant in KO TME (Fig. 2B), defining two divergent macrophage programs. Spp1+ TAMs secrete OPN, a glyco-phosphoprotein linked to immunosuppressive signaling, matrix remodeling, angiogenesis, and tumor progression [13,14,15, 34]. In contrast, Thbs1+ TAM express thrombospondin-1, a matricellular protein that has been reported to inhibit angiogenesis by counteracting VEGF activity and to modulate immune responses through pathways such as CD47 [36, 37]. Among the transcriptional changes following Rab37 deletion, Ctss downregulation in TAMs was a prominent feature with functional implications. Ctss encodes cathepsin S, a cysteine protease involved in antigen presentation and tumor invasion, and its elevated expression has been associated with pro-tumor, alternatively activated macrophages [38, 39]. In our scRNA-seq analysis, Ctss was reduced in Rab37 KO TAMs compared with WT, indicating attenuation of the pro-tumor phenotype (Fig. 1D). Notably, Ctss and Ctsb are canonical markers of SPP1+ TAM, along with Gpr183, Pparg, Gpnmb, and Trem2 [28]. These findings further support that Rab37 promotes the formation of immunosuppressive Spp1+ TAM in the TME.
Previous studies using single-cell transcriptomics in colorectal cancer identified SPP1+ TAMs as a central immunosuppressive population that engages in crosstalk with FAP+ fibroblasts, with RNA velocity analyses in that context suggesting a potential origin from a THBS1+ precursor state [40]. Consistent with this model, our time-course experiments demonstrated that IL-6 stimulation transiently increased THBS1 expression (Supplementary Fig. 3F), followed by sustained SPP1 induction (Supplementary Fig. 3G), suggesting a sequential transition from THBS1+ to SPP1+ TAMs during polarization. The presence of more Spp1+ TAM in Rab37 WT TME and more Thbs1+ TAM in KO TME supports the notion that Rab37 governs macrophage fate decisions within the lung TME. Therefore, we propose that Rab37 participates in a two-step regulatory mechanism: Rab37-mediated IL-6 release serves as an early cue that drives THBS1+ macrophages toward an SPP1+ state, while Rab37-dependent OPN secretion subsequently reinforces SPP1 expression through a STAT3-mediated positive feedback loop. This coordinated regulation positions Rab37 as a key modulator linking cytokine-driven initiation and OPN-mediated stabilization of the SPP1+ TAM phenotype, thereby promoting immunosuppressive macrophage programming within the tumor microenvironment.
In addition to mechanistic regulation, our findings highlight the pivotal role of Rab37 in defining the prognostic significance of OPN+ TAM. OPN has been reported to enhance M2-like macrophage polarization [32, 41], and suppress cytotoxic CD8 T-cell activation [42], thereby reinforcing a tolerogenic niche. In our study, we observed that only the subset of TAMs co-expressing Rab37 (CD163+/Rab37+/OPN+) correlated with recurrence and poor survival outcomes (Fig. 2G, H), and acted as an independent prognostic biomarker (Table 1). Interestingly, CD163+/OPN+ TAMs alone did not fully capture the prognostic impact (Supplementary Fig. 2I, J), as OPN expression without Rab37-mediated exocytosis may be insufficient to sustain the pathological feedback loop driving tumor progression. Together, these results highlight Rab37 as a molecular determinant that integrates vesicle trafficking machinery with immunosuppressive signaling, positioning Rab37+/OPN+ TAMs as a mechanistically relevant subset contributing to lung cancer recurrence and progression.
Several strategies have been explored to therapeutically target OPN in cancer. Neutralizing monoclonal antibodies, including AOM1 [43], 100D3 [44], and hu1A12 [45], demonstrated favorable efficacy in preclinical models by blocking OPN–integrin interactions, inhibiting proteolytic cleavage, or suppressing tumor cell adhesion, migration, and metastasis. Although the neutralizing antibody ASK8007, which targets the SVVYGLR sequence of OPN, failed to show clinical benefit in patients with rheumatoid arthritis, it demonstrated an acceptable safety profile [46]. This indicates that OPN-targeting antibodies could be highly promising candidates for cancer therapy, where the pathogenic contribution of OPN is more profound. Importantly, OPN also regulates immune evasion by modulating PD-L1, thereby providing a rationale for OPN blockade as an immunotherapy sensitizer [47]. Moreover, inhibition of WDR-5 reduced OPN expression and enhanced anti-PD-1 efficacy in pancreatic cancer models, while in breast cancer, OPN induction by low-dose anti-VEGFR2 synergized with PD-1 blockade, highlighting the context-dependent and multifaceted roles of OPN in immune regulation [48].
In light of these findings, our study identifies that Rab37-mediated OPN secretion drives STAT3–SPP1 upregulation in an autocrine or paracrine manner to promote M2-like macrophage polarization and cancer progression. Notably, the CD163⁺/Rab37⁺/OPN⁺ TAM subset is a clinically relevant biomarker linked to poor prognosis, suggesting that patients with high enrichment of this population may particularly benefit from therapeutic strategies targeting OPN, either alone or in combination with immune checkpoint inhibitors.
Data availability
Raw and processed scRNA-sequencing data have been deposited in the NCBI Gene Expression Omnibus (GEO) under accession number GSE311076. Additional datasets and materials that support the conclusions of this study are available from the corresponding author upon reasonable request.
Code availability
The code used will be made available upon request.
References
Laguna JC, Garcia-Pardo M, Alessi J, Barrios C, Singh N, Al-Shamsi HO, et al. Geographic differences in lung cancer: focus on carcinogens, genetic predisposition, and molecular epidemiology. Ther Adv Med Oncol. 2024;16:17588359241231260.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Desharnais L, Sorin M, Rezanejad M, Liu B, Karimi E, Atallah A, et al. Spatially mapping the tumour immune microenvironments of non-small cell lung cancer. Nat Commun. 2025;16:1345.
Sorin M, Rezanejad M, Karimi E, Fiset B, Desharnais L, Perus LJM, et al. Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature. 2023;614:548–54.
Wang YS, Tzeng HT, Tsai CH, Cheng HC, Lai WW, Liu HS, et al. VAMP8, a vesicle-SNARE required for RAB37-mediated exocytosis, possesses a tumor metastasis suppressor function. Cancer Lett. 2018;437:79–88.
Kuo IY, Yang YE, Yang PS, Tsai YJ, Tzeng HT, Cheng HC, et al. Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics. 2021;11:7029–44.
Yang YE, Hu MH, Zeng YC, Tseng YL, Chen YY, Su WC, et al. IL-33/NF-kappaB/ST2L/Rab37 positive-feedback loop promotes M2 macrophage to limit chemotherapeutic efficacy in lung cancer. Cell Death Dis. 2024;15:356.
Yang PS, Yu MH, Hou YC, Chang CP, Lin SC, Kuo IY, et al. Targeting protumor factor chitinase-3-like-1 secreted by Rab37 vesicles for cancer immunotherapy. Theranostics. 2022;12:340–61.
Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207.
Puttock EH, Tyler EJ, Manni M, Maniati E, Butterworth C, Burger Ramos M, et al. Extracellular matrix educates an immunoregulatory tumor macrophage phenotype found in ovarian cancer metastasis. Nat Commun. 2023;14:2514.
Zhang C, Li K, Zhu H, Cheng M, Chen S, Ling R, et al. ITGB6 modulates resistance to anti-CD276 therapy in head and neck cancer by promoting PF4(+) macrophage infiltration. Nat Commun. 2024;15:7077.
Vanmeerbeek I, Naulaerts S, Sprooten J, Laureano RS, Govaerts J, Trotta R, et al. Targeting conserved TIM3(+)VISTA(+) tumor-associated macrophages overcomes resistance to cancer immunotherapy. Sci Adv. 2024;10:eadm8660.
Wu Q, Li L, Miao C, Hasnat M, Sun L, Jiang Z, et al. Osteopontin promotes hepatocellular carcinoma progression through inducing JAK2/STAT3/NOX1-mediated ROS production. Cell Death Dis. 2022;13:341.
Hao C, Cui Y, Chang S, Huang J, Birkin E, Hu M, et al. OPN promotes the aggressiveness of non-small-cell lung cancer cells through the activation of the RON tyrosine kinase. Sci Rep. 2019;9:18101.
Yu W, Gui S, Peng L, Luo H, Xie J, Xiao J, et al. STAT3-controlled CHI3L1/SPP1 positive feedback loop demonstrates the spatial heterogeneity and immune characteristics of glioblastoma. Dev Cell. 2025;60:1751–67.e1759.
Su Z, He Y, You L, Chen J, Zhang G, Liu Z. SPP1+ macrophages and FAP+ fibroblasts promote the progression of pMMR gastric cancer. Sci Rep. 2024;14:26221.
Trehan R, Huang P, Zhu XB, Wang X, Soliman M, Strepay D, et al. SPP1 + macrophages cause exhaustion of tumor-specific T cells in liver metastases. Nat Commun. 2025;16:4242.
Matusiak M, Hickey JW, van IDGP, Lu G, Kidzinski L, Zhu S, et al. Spatially segregated macrophage populations predict distinct outcomes in colon cancer. Cancer Discov. 2024;14:1418–39.
Huang Z, Li Y, Liu Q, Chen X, Lin W, Wu W, et al. SPP1-mediated M2 macrophage polarization shapes the tumor microenvironment and enhances prognosis and immunotherapy guidance in nasopharyngeal carcinoma. Int Immunopharmacol. 2025;147:113944.
Hou J, Ji J, Chen X, Cao H, Tan Y, Cui Y, et al. Alveolar epithelial cell-derived Sonic hedgehog promotes pulmonary fibrosis through OPN-dependent alternative macrophage activation. FEBS J. 2021;288:3530–46.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–87.e3529.
Jin S, Plikus MV, Nie Q. CellChat for systematic analysis of cell-cell communication from single-cell transcriptomics. Nat Protoc. 2025;20:180–219.
Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010;102:866–80.
Huang Q, Liu Y, Du Y, Garmire LX. Evaluation of cell type annotation R packages on single-cell RNA-seq Data. Genomics Proteom Bioinforma. 2021;19:267–81.
Franzen O, Gan LM, Bjorkegren JLM PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford). 2019; 2019:baz046.
van Vlerken-Ysla L, Tyurina YY, Kagan VE, Gabrilovich DI. Functional states of myeloid cells in cancer. Cancer Cell. 2023;41:490–504.
Waibl Polania J, Hoyt-Miggelbrink A, Tomaszewski WH, Wachsmuth LP, Lorrey SJ, Wilkinson DS, et al. Antigen presentation by tumor-associated macrophages drives T cells from a progenitor exhaustion state to terminal exhaustion. Immunity. 2025;58:232–46.e236.
Palma A. The landscape of SPP1 (+) macrophages across tissues and diseases: A comprehensive review. Immunology. 2025;176:179–96.
Liu C, Wu K, Li C, Zhang Z, Zhai P, Guo H, et al. SPP1+ macrophages promote head and neck squamous cell carcinoma progression by secreting TNF-alpha and IL-1beta. J Exp Clin Cancer Res. 2024;43:332.
Homma Y, Hiragi S, Fukuda M. Rab family of small GTPases: an updated view on their regulation and functions. FEBS J. 2021;288:36–55.
van Baarle L, De Simone V, Schneider L, Santhosh S, Abdurahiman S, Biscu F, et al. IL-1R signaling drives enteric glia-macrophage interactions in colorectal cancer. Nat Commun. 2024;15:6079.
Xing C, Hu W, Zhao L. Osteopontin derived from hypoxia-induced M2 macrophages promotes osteosarcoma progression through modulation of EGR3/ISG15 signaling and RIG-I expression. J Transl Med. 2025;23:950.
Yu T, Gan S, Zhu Q, Dai D, Li N, Wang H, et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat Commun. 2019;10:4353.
Jia R, Liang Y, Chen R, Liu G, Wang H, Tang M, et al. Osteopontin facilitates tumor metastasis by regulating epithelial-mesenchymal plasticity. Cell Death Dis. 2016;7:e2564.
Gu Y, Taifour T, Bui T, Zuo D, Pacis A, Poirier A, et al. Osteopontin is a therapeutic target that drives breast cancer recurrence. Nat Commun. 2024;15:9174.
Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–6.
Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285:38923–32.
Dheilly E, Battistello E, Katanayeva N, Sungalee S, Michaux J, Duns G, et al. Cathepsin S regulates antigen processing and T cell activity in non-hodgkin lymphoma. Cancer Cell. 2020;37:674–89.e612.
Yang M, Liu J, Shao J, Qin Y, Ji Q, Zhang X, et al. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol Cancer. 2014;13:43.
Xiao M, Deng Y, Guo H, Ren Z, He Y, Ren X, et al. Single-cell and spatial transcriptomics profile the interaction of SPP1(+) macrophages and FAP(+) fibroblasts in non-small cell lung cancer. Transl Lung Cancer Res. 2025;14:2646–69.
Yang F, Akhtar MN, Zhang D, El-Mayta R, Shin J, Dorsey JF, et al. An immunosuppressive vascular niche drives macrophage polarization and immunotherapy resistance in glioblastoma. Sci Adv. 2024;10:eadj4678.
Klement JD, Paschall AV, Redd PS, Ibrahim ML, Lu C, Yang D, et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 2018;128:5549–60.
Shojaei F, Scott N, Kang X, Lappin PB, Fitzgerald AA, Karlicek S, et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012;31:26.
De Muynck K, Heyerick L, De Ponti FF, Vanderborght B, Meese T, Van Campenhout S, et al. Osteopontin characterizes bile duct-associated macrophages and correlates with liver fibrosis severity in primary sclerosing cholangitis. Hepatology. 2024;79:269–88.
Dai J, Li B, Shi J, Peng L, Zhang D, Qian W, et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol Immunother. 2010;59:355–66.
Boumans MJ, Houbiers JG, Verschueren P, Ishikura H, Westhovens R, Brouwer E, et al. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study. Ann Rheum Dis. 2012;71:180–5.
Lu C, Liu Z, Klement JD, Yang D, Merting AD, Poschel D, et al. WDR5-H3K4me3 epigenetic axis regulates OPN expression to compensate PD-L1 function to promote pancreatic cancer immune escape. J Immunother Cancer 2021;9:e002624.
Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, et al. Low-dose anti-angiogenic therapy sensitizes breast cancer to PD-1 blockade. Clin Cancer Res. 2020;26:1712–24.
Acknowledgements
We are grateful for the support from the Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital. We thank the technical services provided by the “Bioimaging Core Facility” of the National Core Facility for Biopharmaceuticals, National Science and Technology Council, Taiwan, as well as the support from the Core Research Laboratory, College of Medicine, National Cheng Kung University. We thank the National Center for Biomodels (NCB), NIAR, Taiwan, for technical support in contract breeding and testing services. This study received funding from the Taiwan National Science and Technology Council grant NSTC 112-2311-B-006-004-MY3.
Author information
Authors and Affiliations
Contributions
YCW conceived and supervised the project. YEY, IYK, and HL performed bioinformatic analyses of scRNA-seq data, including data visualization, interpretation, and experimental design support. YEY, YAL, LLL, and WTK contributed to experimental design and data validation. YEY and YAL provided clinical samples. YEY and YCW wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All experiments using mice were approved by the animal ethics committee of National Cheng Kung University and complied with all relevant ethical guidelines (#112062). All lung cancer samples were conducted in accordance with the requirements of Research Center of Clinical Medicine, National Cheng Kung University Hospital. The use of clinical samples was approved by the institutional review board of the hospital with the ethical number # A-ER-111-517.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, YE., Lin, YA., Ling, LL. et al. Rab37-mediated OPN secretion enriches SPP1+ macrophages through autocrine–paracrine signaling to drive lung tumor progression. Oncogenesis 15, 4 (2026). https://doi.org/10.1038/s41389-026-00596-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41389-026-00596-3