Abstract
Advances in prostate cancer treatment have significantly improved survival, but quality of life for survivors remains an under-studied area of research. Androgen deprivation therapy (ADT) is a foundational treatment for advanced prostate cancer and is used as an adjuvant for prolonged periods in many high-risk, localized tumors. More than half of patients treated with ADT experience debilitating cognitive impairments in domains such as spatial learning and working memory. In this study, we investigated the effects of androgen deprivation on hippocampal-mediated cognition in rats. Vortioxetine, a multimodal antidepressant, has been shown to improve cognition in depressed patients. Thus, we also tested the potential efficacy of vortioxetine in restoring impaired cognition after ADT. We further investigated mechanisms that might contribute to these effects, measuring changes in the circuitry and gene expression within the dorsal hippocampus. ADT via surgical castration induced impairments in visuospatial cognition on the novel object location test and attenuated afferent-evoked local field potentials recorded in the CA1 region of the dorsal hippocampus. Chronic dietary administration of vortioxetine effectively reversed these deficits. Castration significantly altered gene expression in the hippocampus, whereas vortioxetine had little effect. Pathway analysis revealed that androgen depletion altered pathways related to synaptic plasticity. These results suggest that the hippocampus may be vulnerable to ADT, contributing to cognitive impairment in prostate cancer patients. Further, vortioxetine may be a candidate to improve cognition in patients who experience cognitive decline after androgen deprivation therapy for prostate cancer and may do so by restoring molecular and circuit-level plasticity-related mechanisms compromised by ADT.
Similar content being viewed by others
Introduction
Over 50% of patients treated with androgen deprivation therapy (ADT) for prostate cancer will experience significant cognitive decline [1, 2]. As of 2022, prostate cancer is the second most common type of cancer in American men, exceeded only by basal cell and squamous cell carcinoma [3]. Unfortunately in the last 10 years, the incidence of prostate cancer has nearly doubled [3]. Despite high prevalence rates, prostate cancer is relatively treatable, with a 98% 5-year survival rate when diagnosed across all stages [3]. With this increase in survival, there is a need to optimize the quality of life for prostate cancer survivors.
For advanced stages of prostate cancer as well as high-risk localized tumors, ADT is a mainstay treatment, most often using gonadotropin-releasing hormone (GnRH) agonists or antagonists, reducing testosterone to castration levels. Unfortunately, the debilitating cognitive side effects induced by ADT can negatively impact day-to-day activities and quality of life for many patients [1, 4]. The effects of ADT on cognition initially present in the first 6–12 months after beginning treatment, and increase in severity with duration of treatment [5]. Cognitive domains that are primarily affected include executive function, attention, and memory [1, 2]. Functional imaging studies in humans have revealed significant changes in brain activity after ADT. BOLD-fMRI revealed that the parietal-occipital region shows reduced activation during recall of spatial information [6]. Follow-up studies found these patients had impaired performance in working memory tasks [7]. Particularly, these findings suggest significant impairment of visuospatial memory, which implicates dysfunction of the hippocampus [8, 9]. Although evidence suggests that ADT can induce profound cognitive impairment, many of the studies include small sample sizes or are largely observational. Furthermore, the cognitive endpoints are often different between studies, making it difficult to identify cognitive domains impacted consistently after ADT. Even fewer studies have investigated mechanisms that underlie these effects. Such studies are necessary to identify potentially novel and effective therapeutic targets for the treatment of cognitive impairment after ADT.
Vortioxetine is approved as an antidepressant by the U.S. Food & Drug Administration. Vortioxetine is unique in that it improves cognition in depressed patients by comparison to other selective-serotonin reuptake inhibitors (SSRIs) such as duloxetine [10,11,12]. In a large, placebo-controlled randomized study of depressed patients, vortioxetine was shown to improve overall cognition and performance in several specific cognitive domains, including executive function, attention, and processing speed, as well as learning and memory [13]. These effects were independent of its antidepressant effects [12]. Previously, our lab investigated ADT-induced cognitive impairments associated with the medial prefrontal cortex in rats and found that androgen depletion by surgical castration induced significant deficits in set-shifting, a measure of cognitive flexibility [14]. We also showed that chronic treatment with vortioxetine improved cognitive flexibility and reversed the attenuated electrical responses evoked in the mPFC by stimulation of afferent input from the ventral hippocampus in castrated rats. An important observation was that castration specifically altered the response to ventral hippocampal input but did not attenuate responsivity to another afferent projection to the mPFC from the medial dorsal thalamus, suggesting that the hippocampus may also have been affected. As previous clinical studies have implicated dysfunction of the hippocampus after ADT, and rodent studies have shown that testosterone depletion can alter neuronal morphology in hippocampus [8, 9, 15,16,17], this brain area might be implicated in the deficits that manifest in ADT patients, but this remains to be investigated. Vortioxetine has been shown to increase excitatory transmission [18] and enhance maturation of spines and dendrites in the hippocampus [19]. Therefore, we hypothesized that vortioxetine may be efficacious in improving cognitive changes associated with hippocampal dysfunction after ADT by improving or reversing changes in activity-dependent neuroplasticity that is necessary for optimal cognitive function. Furthermore, synaptic plasticity can be influenced by changes in gene expression. As the androgen receptor is a transcription factor, castration may contribute to the detrimental effects on cognition by dysregulating gene expression in the hippocampus. Vortioxetine has been shown to enhance activity in the hippocampus [20]. Thus, we hypothesized that vortioxetine may reverse changes in plasticity-related processes in the hippocampus induced by ADT. As vortioxetine is already in use clinically, the results of this investigation may suggest vortioxetine as a promising candidate by which to improve quality of life for cancer survivors by mitigating cognitive impairment after ADT.
Methods
Animals
As prostate cancer only affects males, these studies used only male rats. Young adult male Sprague-Dawley rats (Envigo, USA) were ~60 days old and weighed 225–250 g upon arrival. Rats from a given cohort were randomly assigned to treatment groups. For ADT treatment, animals were surgically castrated or received sham surgery and were separated into single housing 7–10 days before initiating treatment with drug-infused diet on experimental day 0. For the sham surgery, animals were anesthetized with isoflurane, the surgical site was prepared as usual, and a small incision was made in the scrotum, which was then closed with surgical glue. After either surgery, animals received subcutaneous injections of 1 mg/kg meloxicam and 10 mg/kg enrofloxacin then returned to their home cage. All testing or tissue collection occurred on day 17 post diet initiation. Housing conditions were as follows: for the novel object location (NOL) test and microarray experiments, animals were housed on a 12/12 hr light cycle (lights on at 7:00 h); for electrophysiology experiments, animals were on a 14/10 hr light cycle (lights on at 7:00 h). All animals were provided access to water and food ad libitum. Experiments were conducted during the light phase, starting by 9:00 and completed by 17:00 h. Separate cohorts of animals were used for each experiment. At the end of the experiment, plasma testosterone levels were measured via ELISA (IBL Inc., Minneapolis, MN, Kit # IB79174, inter-assay coefficient of variability = 3.95%). Plasma testosterone levels in castrated rats were below the detection limit of the assay (0.066 ng/mL), compared to intact controls = 2.34 ng/mL. All procedures were approved by the University of Texas Health San Antonio Institutional Animal Care and Use Committee and complied with National Institutes of Health guidelines.
Vortioxetine treatment
Vortioxetine was obtained from H. Lundbeck A/S. Drug-infused chow containing 0.6 g vortioxetine per kg chow and control chow (Purina #5001) were prepared by Research Diets, Inc. Animals were placed on either control or vortioxetine diet beginning 7–10 days after castration and received drug for 17 days total. Free-feeding animals consumed about 7 g of food per 100 g body weight, corresponding to a dose of 40 mg/kg/day for an average 300 g rat. There were no differences in body weight between groups (castrated or sham-control rats receiving vortioxetine or control chow) at the time of testing, indicating no differences in food intake. This method of chronic vortioxetine administration has been well established by our group and others [14, 19, 21], and produces stable drug plasma levels within the range of therapeutic efficacy, with 60–95% occupancy at relevant drug targets in the brain [21].
Open field test
The open field test (OFT) was used to assess locomotor activity as a potential confound for object exploration. Secondarily, the OFT provides a measure of anxiety-like behavior, another potential confound. The open field test occurred on day 16 and also served as habituation for the NOL test. The testing apparatus was a white wooden arena (65 × 65 × 42 cm3), with the floor marked into 36 squares (6 × 6) and covered by plexiglass. One of the walls was marked with 10 horizontal blue stripes to provide spatial orientation. The test was performed under light conditions of 6 lux. Total time in the arena was 20 min. The first 5 min were scored manually for total number of line crosses and time in the center squares of the arena, as measures of locomotion and anxiety-like behavior, respectively. All tests were recorded by an overhead GoPro® camera, and videos were analyzed offline by a blinded experimenter.
NOL test
The NOL test was adapted from Barker & Warburton [22]. Testing (day 17) occurred 24 h after habitation (day 16). Animals were placed in the same testing apparatus as the OFT. Sample and test objects consisted of two identical Lego® figures (9.5 × 5.0 × 9.5 cm). The NOL test consisted of two phases. For the sample phase, two objects were placed in adjacent corners 10 cm from the walls and directly across from the striped wall. Animals were allowed 3 min to interact with both objects. After 3 min, the animals were removed from the arena and placed back in their home cage for a 5-min delay. During this time, one of the objects (the novel location object) was moved to an adjacent corner (nearest the striped wall) for the test phase. After the delay, the animals were returned to the arena for 3 min to complete the test phase. Interaction time with each object was measured, which included touching, sniffing, or facing the object within 2 cm. All objects and the arena were cleaned with 70% ethanol then water after each stage and between each animal. Interactions times (T) were scored for the familiar (f) and novel location objects (n) and calculated as a discrimination ratio [DR = (Tn−Tf)/(Tn+Tf)], in which values ranged from −1.0 to 1.0. Scores near zero indicate equal interaction time, thus a failure to recognize the new position of the object (i.e., a deficit of visuospatial memory). Scores closer to 1.0 indicate greater preference for the object in the novel location, hence better visuospatial memory of the object locations before they were moved.
Afferent-evoked local field potentials in the CA1 region of dorsal hippocampus
Procedures for recording afferent-evoked local field potentials were adapted from previous studies [14, 23, 24]. Responses were recorded in the CA1 region of dorsal hippocampus after stimulating the Schaeffer Collaterals. Recordings occurred on day 17, the same day as behavioral testing in the preceding experiment. Animals were anesthetized using chloral hydrate (400 mg/kg, i.p. supplemented 10% as needed through the duration of recording) then placed in a stereotaxic apparatus. Body temperature was maintained at 37 °C. A stainless steel bipolar twisted stimulating electrode was positioned in the Schaeffer Collaterals, angled 30° toward the midline (coordinates from bregma: AP −3.8, ML + 5.0, DV: 4.0–4.7 mm). A tungsten recording electrode was then placed vertically in the ipsilateral CA1 region of the dorsal hippocampus (AP −3.8, ML + 2.0, DV −3.0 mm). The signal was filtered with a low cutoff at 0.3 Hz, high cutoff at 1000 Hz, and sampling at 2000 Hz. Signal was then digitized using PowerLab (ADInstruments). The response of the CA1 region was recorded after a 10-min equilibration period. Stimulus pulses, delivered in the Schaeffer Collaterals, were increased in 100 μA increments (100–800 μA, 0.1 Hz, 260 μsec pulse width). Magnitude of evoked responses were measured from the first negative peak, occurring at ~5–8 msec latency, to the first positive peak, at 12–15 msec. Data are presented as a current-response curve, using the average of 30 responses recorded at each stimulation intensity. Electrode placement was confirmed histologically, and cases in which electrodes were located outside of the targeted regions were excluded from the study.
Whole genome microarray assessment of gene expression in the hippocampus
A separate cohort of animals treated identically to those described above but with no behavioral testing were sacrificed on day 17. The dorsal hippocampus was bluntly dissected on ice, and flash frozen in 2-methylbutane on dry ice. Samples were stored at −80 °C until use. Hemispheres were counterbalanced across experimental groups. Qiazol lysis buffer (Qiagen, Hilden, Germany) and Direct-zol miniprep RNA isolation kit (Zymo Research, Irvine, CA) were used to isolate RNA from the tissue. Samples with an RNA integrity number >7 were used for the assay, as determined using an Agilent Bioanalyzer (Agilent, Santa Clara, CA). mRNA expression patterns were evaluated using a whole genome microarray (Agilent G3 Rat GE 8 × 60 K v2 Microarray Kit, Agilent, Santa Clara, CA), as previously described [14]. Briefly, 100–200 ng of total RNA were used to create cyanine 3-labeled cRNA probes (Low Input Quick Amp Labeling kit, Agilent) that were hybridized to microarrays according to the manufacturer’s protocols (Agilent). For statistical analysis, differentially expressed genes (DEGs) were identified to test for main effects of castration and vortioxetine using the LIMMA R package (v3.46.0) [25], and utilizing Agilent Rat Microarray v2 gene annotation from NCBI/GEO (Platform GPL22145). DEGs were selected if they met criteria of adjusted p value < 0.01 and fold change (FC) > 2 or <−2. Gene Set Enrichment Analysis (GSEA; UC San Diego, San Diego, CA and the Broad Institute, Massachusetts Institute of Technology, Cambridge, MA) was performed by first selecting the top 60% most highly expressed genes (27,440 probes out of a total 45,738) and ranking them based on fold change (for a given comparison) to identify hallmark and KEGG pathways of interest [26, 27]. Significance was determined in GSEA by q < 0.05. Primary gene expression data files have been deposited in the Gene Expression Omnibus (GEO) database, accession number GSE236207.
Statistical analyses
On the day of an experiment, the investigator was blind to treatment condition. Behavioral data were analyzed by 2-way ANOVA to determine main effects and interactions of ADT and vortioxetine (Castration x Drug) using GraphPad Prism 9 (San Diego, CA). Holm-Sidak test was used to detect differences between experimental conditions. In electrophysiology experiments, stimulus-response curves were compared using an extra sum-of-squares F-test [28]. Group sizes for electrophysiology and behavioral testing were determined a priori by power analysis, with power = 0.80, estimated mean difference = 35%, standard deviation = 25%. Significance was determined at p < 0.05 (two-tailed), unless otherwise noted (i.e., in the gene expression analyses above). For the NOL, animals were excluded from in the study if they failed to interact with one object entirely in the sample phase, or failed to interact with at least one object in the test phase. This resulted in the exclusion of 10 rats, and was not specific to any one treatment condition.
Results
Experiment 1: effects of androgen deprivation and vortioxetine on anxiety and locomotion
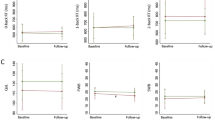
A total of 66 animals were used for open field testing. Measures included time in the center of the open arena and the number of line crosses, as measures of anxiety-like behavior and locomotion, respectively. There were no significant effects of castration (Fig. 1C, F1,62 = 0.1307, p = 0.71, n = 14–20/group) amongst any of the groups in line crosses, but there was a main effect of vortioxetine treatment (F1,62 = 4.528, p < 0.05). Vortioxetine increased locomotion overall, and this appeared to be driven primarily by an increase in non-castrated-control rats, although the pairwise comparison of intact controls receiving control diet and those receiving vortioxetine was not significant (p = 0.10). There were no significant effects of castration (Fig. 1D, F1,62 = 3.389, p = 0.07, n = 14–20/group) or drug treatment (F1,62 = 0.0578, p = 0.81) on time in the center of the arena.
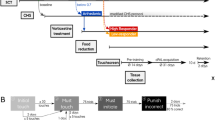
Timeline of experimental procedure for A behavior, and B electrophysiology and tissue collection. Separate cohorts of rats were used for each experiment. Vortioxetine was administered in the diet for 17 days through the day of testing for all experimental measures. C Vortioxetine overall increased the total number of line crosses as a measure of locomotion on the open field test (*p < 0.05, F1,62 = 4.528), but pairwise comparisons between groups were not significant. D There were no differences in anxiety-like behavior, measured as time in the center of the arena. E On the NOL test, there was a significant decrease in the discrimination ratio (DR), indicating an impairment in visuospatial memory in surgically castrated rats compared to intact-control rats (*p < 0.05, n = 11/group). When castrated rats were administered vortioxetine chronically through the diet, the DR was restored to a level comparable to intact rats (p < 0.05 castrated/control vs castrated/vortioxetine, n = 10–11/group).
Experiment 2: effects of androgen deprivation and vortioxetine on spatial memory
Fifty-four animals proceeded to the NOL test after habitation, as animals that failed to interact with both objects on the sample phase or at least one object during the test phase were excluded from analysis. Two-way ANOVA revealed significant main effects of castration (Fig. 1E, F1,50 = 6.658, p < 0.05, n = 11–17/group), vortioxetine treatment (F1,50 = 5.123, p < 0.05), and an interaction effect (castration x drug, F1,50 = 4.660, p < 0.05). Pairwise comparisons using the Holm-Sidak test revealed that surgical castration significantly decreased the discrimination ratio in rats receiving control diet (p < 0.05). The deficit in spatial memory was reversed by chronic vortioxetine treatment in castrated animals (p < 0.05).
Experiment 3: effects of androgen deprivation and vortioxetine on afferent-evoked response in the dorsal hippocampus
For recording in vivo afferent-evoked field potentials in the dorsal hippocampus of anesthetized animals, a total of 22 rats were used. Sum of squares F-test revealed that stimulus-response curves for evoked potentials recorded in the CA1 region in response to stimulation of the Schaeffer Collaterals were significantly altered after castration and vortioxetine treatment (F(9,164) = 6.710, p < 0.0001, n = 4–6/group, Fig. 2). Pairwise comparisons revealed that castrated rats receiving control diet had an attenuated response in the SC-CA1 pathway in comparison to intact-control rats (F(7,70) = 2.257, p < 0.05), and that vortioxetine reversed the attenuated responses in this pathway after castration (F(7,56) = 8.199, p < 0.0001).
A Pairwise comparison revealed that surgical castration reduced afferent-evoked responsivity compared to sham-control rats (*p < 0.05, n = 6/group). Vortioxetine treatment reversed these changes back to baseline levels (***p < 0.0001, castrated-control, n = 6 compared to castrated-vortioxetine, n = 4). B Representative trace of evoked response recorded in CA1 (arrow indicates stimulus artifact). C Placement of stimulating and recording electrodes in the Schaeffer Collaterals and CA1 region of dorsal hippocampus, respectively.
Experiment 4: effects of androgen deprivation and vortioxetine on gene expression in the hippocampus
For the microarray analysis, 24 animals were used. Genes were identified based on whether they were differentially expressed based on the background transcriptome. In the dorsal hippocampus, there were 279 genes differentially affected by castration (castrated/control—intact/control, p < 0.01, fold change≥ 2 or ≤−2). Of those genes, 165 were upregulated and 114 were downregulated (Fig. 3A). Vortioxetine affected fewer genes. In the main effect of vortioxetine (intact/vortioxetine—intact/control), 11 genes were affected; 4 genes were downregulated, and 7 genes upregulated (Fig. 3B). Similarly, 13 genes total were affected in the interaction of castration x vortioxetine (castrated/vortioxetine—castrated/control); 7 genes were upregulated and 6 were downregulated (Fig. 3C). Differentially expressed genes affected by castration are listed in Table 1, and genes affected by vortioxetine and in the interaction are listed in Table 2. GSEA was then used on the most highly expressed probes (27,440, or ~60% of the total 45,738 probes) rank-ordered by fold change, to determine how differentially expressed genes might be functionally related. Many of these pathways are associated with synaptic plasticity-related processes (Fig. 4A–C, q value < 0.05), such as PI3K/Akt/mTOR signaling. Within the KEGG pathways, several pathways were identified in the main effect of castration and vortioxetine treatments, but there were no pathways that reached significance in the interaction (Fig. 4D, E). There were several pathways affected consistently across conditions, including Long-Term Potentiation, Long-Term Depression, Axon Guidance, and MAPK Signaling.
Volcano plots depict differential gene expression in dHipp after castration and vortioxetine treatment. Dashed lines represent significance criteria: |FC| ≥ 2 and p < 0.01. Genes downregulated are denoted in blue and genes in red were upregulated. A In the main effect of castration, there were 279 genes that were differentially affected (165 were upregulated and 114 were downregulated). B In the main effect of vortioxetine treatment, 4 genes were downregulated and 7 genes upregulated. C Within the interaction of castration x vortioxetine, there were seven genes upregulated and 6 genes downregulated.
Pathways were identified by q value < 0.05 and ordered by normalized enrichment score. Hallmark pathways were identified in A the main effect of castration (castration-control vs. intact-control), B the main effect of vortioxetine (intact-vortioxetine vs. intact-control), and C the interaction of vortioxetine x castration (castration-vortioxetine vs. castration-control). KEGG pathways were identified in D the main effect of castration and E the main effect of vortioxetine. Pathways in the interaction did not meet the threshold for significance of q < 0.05.
Discussion
Quality of life is an essential consideration in the care of cancer patients. ADT produces significant impairments in cognitive domains associated with hippocampal function, specifically in spatial learning and memory, and these deficits can profoundly impact patients’ quality of life. As prostate cancer is most common in older men, these cognitive deficits can exacerbate other factors that may contribute to neurocognitive decline. Cognitive impairment not only impacts quality of life for cancer survivors, but can also affect their caregivers and families [29]. In prostate cancer patients, ADT increases the risk of Alzheimer’s disease and dementia [30, 31]. Therefore, finding ways to mitigate the detrimental effects of ADT on the brain may offer important advances to improve patients’ quality of life. Unfortunately, the brain mechanisms impacted by ADT, and the mechanisms by which cognition is disrupted have not been established. Furthermore, treatments for patients experiencing cognitive decline are largely ineffective. The purpose of this study was to investigate the cognitive decline associated with ADT, to understand the mechanisms that underlie the impairment, and to test a potentially novel therapeutic candidate.
To investigate the effects of ADT on cognition mediated by the hippocampus, we used the NOL test to assess visuospatial memory, as this test is specifically mediated by the dorsal hippocampus, and is largely unaffected by lesions in the prefrontal cortex [22]. Previous studies have shown that surgical castration induced deficits in spatial memory tasks such as the radial arm maze [32, 33], but chemical castration with bicalutamide and goserelin produced mixed results [32]. Likewise, not all types of spatial learning tasks are affected by castration [33]. This could be due to the involvement of brain regions other than the hippocampus in many of these tests, leading to inconsistent results. Therefore, we chose to use the NOL test, as this variation of the novel object paradigm is known to be specifically dependent on the hippocampus [22] and is therefore less subject to confounding effects by activity in other brain regions, such as the medial prefrontal cortex (mPFC), which we have shown previously to be dysfunctional after ADT [14]. In the current study, ADT impaired visuospatial memory on the NOL test, and vortioxetine reversed the deficit in castrated rats, restoring performance comparable to intact controls. This suggests that vortioxetine may be a potential therapeutic candidate to treat hippocampal-related cognitive decline associated with ADT in prostate cancer patients.
While the human literature on ADT-induced cognitive impairment is sparse, animal studies have provided insight to the effects of androgen depletion on brain processes. Androgens are known to broadly affect brain activity and cognition [34]. The AR is widely expressed in the brain, and regions such as the prefrontal cortex, thalamus, and hippocampus contain some of the highest densities [34]. Of note, the CA1 region of the hippocampus exhibits particularly high density of the AR, and this region is known to underlie visuospatial cognition [22, 35]. Castration with either surgical gonadectomy or administration of the AR antagonist, flutamide, in rats significantly reduced AR mRNA expression within this same region [35]. The hippocampus is therefore a crucial site for investigation of ADT-induced cognitive deficits. To further understand the mechanism by which ADT and vortioxetine treatment affected behavior, we investigated functional changes in responsivity to afferent input as a readout of synaptic efficacy within the dorsal hippocampus. Previously, we have shown that surgical castration disrupted the response of the mPFC to ventral hippocampal input but did not affect the afferent-evoked response to medial dorsal thalamic input to the mPFC [14]. Here, we found that castration attenuated responses evoked in the CA1 region of dorsal hippocampus by stimulation of the Schaeffer Collateral afferent, and these changes were reversed by chronic vortioxetine treatment. Together with our previous result, it is thus possible that the detrimental effects of ADT may originate primarily by disruption of local circuit function in the hippocampus. In the dorsal hippocampus, this may directly impair visuospatial cognition, whereas alterations in ventral hippocampal function may further disrupt executive processes modulated by its projection to the mPFC.
The androgen receptor is an important transcription factor in modulating plasticity in the brain [15,16,17, 36, 37]. Therefore, we assessed whole genome expression profiles using a microarray to identify possible changes at the transcriptome level. Castration had a significant effect on gene expression in the dorsal hippocampus. Of note, many of the affected genes contain androgen response elements, consistent with a direct effect on transcription that would be expected with depletion of testosterone. Genes with known androgen regulation, which were identified through the literature [38,39,40,41] are bolded in Tables 1 and 2. However, many of the affected genes did not contain androgen response elements, suggesting that they are likely downstream of directly androgen responsive genes. Several affected genes, such as Gabbr1, Got1, Kcnj4, and Rnd1, are highly expressed in the cortex and hippocampus and have been linked to regulation of neuronal function and cognition [42,43,44,45]. Genes such as Camk2a, Crebbp, and Grik4, which were also significantly affected, are important for neurotransmission and may be disrupted in neuropsychiatric conditions [46,47,48]. Therefore, the detrimental effects of ADT on cognition in the hippocampus may be attributable to changes in gene expression that disrupt neuronal plasticity necessary for optimal cognitive functioning. Further examination of these genes using gene set enrichment analysis revealed that gene expression changes were associated with signaling pathways that are known to participate in cognitive processes, such as PI3K/Akt/mTOR and JAK/STAT3 signaling. Changes consistent with this were seen in the KEGG pathways, as many synaptic plasticity-related pathways were identified in the castration and vortioxetine conditions. These signaling cascades affected by both vortioxetine and castration provide potential targets for future investigation.
The multimodal activity of vortioxetine provides this drug with distinctive qualities in comparison to other antidepressants and may underlie its unique capacity to enhance cognition. For example, vortioxetine is an antagonist at the 5-HT3 receptor, with high occupancy at therapeutic doses. Studies have shown that ondansetron, also a 5-HT3 antagonist, can enhance the antidepressant activity of SSRIs, improves cognition, and has anxiolytic effects on its own [49,50,51,52]. Certainly, the modulation of serotonin transmission is critically important to antidepressant action [53]. But antidepressants have also been shown to interact directly with the TrkB receptor [54], and the endogenous ligand for this receptor, brain derived neurotrophic factor (BDNF) is also important for cognition [55]. It is worth noting that in the KEGG analysis, the phosphatidylinositol signaling system was affected by both castration and vortioxetine treatment. However, in the context of cancer, it may be undesirable to target BDNF and TrkB directly by systemic treatment, as both are expressed in prostate tumors and their dysregulation can contribute to tumor progression [56, 57]. Furthermore, the Val66Met BDNF gene polymorphism is associated with neurocognitive alterations, particularly in executive function [57]. In clinical studies, Met/Met carriers seem to be protected from chemotherapy-induced cognitive decline [58] whereas Val/Met carriers were susceptible to impairments [59]. Thus, modulation of BDNF and TrkB signaling are important factors in cancer and cancer-therapy and may also be important to cognitive decline associated with cancer treatment. The multimodal pharmacological activity of vortioxetine targeting neurotransmitter receptors may selectively modulate, for instance, BDNF-related signaling pathways in the brain, including PI3 kinase-Akt-mTOR signaling, without promoting cancer peripherally, or interfering with the anti-cancer efficacy of ADT.
In contrast to a previous report that vortioxetine regulated gene expression in naïve animals [60], there were relatively few significant changes in gene expression in castrated animals after vortioxetine treatment. This discrepancy could be due to a few reasons. The study that showed vortioxetine regulated gene expression involved drug treatment for one month, and multiple doses were used, but not all doses produced similar effects on gene expression. Also, that study used a larger sample size, which provided more power to the analysis. Furthermore, it is important to note that changes in gene expression may occur transiently, at early stages of chronic antidepressant treatment. This has been reported in studies of antidepressant effects on serotonin transporter (SERT) mRNA expression using fluoxetine [61] and p-chlorophenylalanine [62]. Similarly, transient changes in gene expression may occur with vortioxetine treatment. It must also be recognized that either castration or vortioxetine could affect processes, including gene expression, in other brain regions that secondarily alter function of the hippocampus. For example, we have previously shown that cognitive flexibility mediated in the medial prefrontal cortex (mPFC) was impaired after surgical castration, with corresponding changes in gene expression in the mPFC [14]. The mPFC does not project directly to the dorsal hippocampus, but it could influence hippocampal function via, for example, a frontocortical-thalamic-hippocampal projection. Other brain regions that provide input to the hippocampus could also contribute to effects observed after castration or systemic vortioxetine treatment. Of course, the multiplicity of brain regions potentially affected by vortioxetine may be an advantage for therapeutic efficacy, as plasticity induced in many brain regions may contribute to beneficial effects on many behaviors impaired by androgen deprivation.
Nonetheless, the general lack of effects of vortioxetine on gene expression suggests that rather than reversing the detrimental effects of ADT on gene expression to rescue cognitive deficits, vortioxetine may instead initiate signaling processes in the brain that compensate for these changes. For example, chronic vortioxetine has been shown to increase phosphorylation of the GluA1 subunit of the AMPA receptor and of actin depolymerizing factor (ADF)/cofilin, without altering total protein levels [63]. Vortioxetine enhances long-term potentiation (LTP) in the hippocampus [63], which is dependent on synaptic plasticity and maturation of synaptic spines. The processes that underlie LTP require activity-dependent changes in plasticity-related signaling factors, such as the PI3K signal transduction pathway. Our enrichment analysis revealed pathways affected by both ADT and vortioxetine, including PI3K/MAPK/mTOR signaling, mitotic spindle, and unfolded protein response, which were upregulated. KEGG analysis also identified relevant pathways affected by both manipulations, e.g., long term potentiation and axon guidance. Given that PI3K and related signaling processes involved in plasticity were affected by both castration and vortioxetine, it may be a potential candidate for future investigation into mechanisms of ADT-induced cognitive impairment and effective therapeutic intervention.
There are some limitations to these initial experiments. One is that the animals in this study were healthy and did not have cancer. Cancer itself activates inflammatory and metabolic processes that can affect cognition [64, 65]. It can also produce symptoms of fatigue [66], which may exacerbate or contribute to cognitive impairment. The purpose in using cancer-free rats in these initial studies was to investigate mechanisms underlying the cognitive impairments induced by ADT without the potentially confounding effects of cancer pathophysiology. In our previous study, we showed that vortioxetine did not directly promote the growth of either androgen-dependent or -independent prostate cancer cells in vitro, and did not interfere with the anti-proliferative activity of the androgen-receptor antagonist enzalutamide [14]. Thus, future studies will test the potential influence of cancer on the cognitive impairments associated with ADT and the beneficial effects of vortioxetine using a syngeneic rat prostate cancer model. Secondly, we used surgical castration to produce testosterone depletion, which is less translationally relevant than other methods of chemical castration. However, this allowed us to isolate the effects of testosterone depletion alone on cognition, without the potentially confounding effects of chemical castration using a pharmacological intervention that may also have off-target effects. In ongoing experiments, we are replicating the effects of surgical castration using chemical castration with the GnRH antagonist degarelix, which is more translationally relevant.
In summary, the results of these experiments indicate that ADT alters synaptic efficacy in the hippocampus and compromises visuospatial cognition, providing a valid model of cognitive impairment after ADT that can be used to test the utility of potential therapeutic interventions to restore cognitive function and improve quality of life for cancer survivors. Further, our results suggest that vortioxetine, a novel FDA-approved multimodal antidepressant, may be one such candidate. Vortioxetine reversed deficits in hippocampal response and visuospatial cognition induced by ADT. Thus, vortioxetine, a relatively safe and well-tolerated drug, may have potential utility for treating cognitive impairment associated with ADT in prostate cancer survivors.
References
Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–47.
Nelson CJ, Lee JS, Gamboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer. Cancer. 2008;113:1097–106.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: Cancer J Clin. 2022;72:7–33.
Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003;170:1808–11.
Gonzalez BD, Jim HSL, Booth-Jones M, Small BJ, Sutton SK, Lin H-Y, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol. 2015;33:2021–7.
Cherrier MM, Borghesani PR, Shelton AL, Higano CS. Changes in neuronal activation patterns in response to androgen deprivation therapy: a pilot study. BMC Cancer. 2010;10:1.
Chao HH, Hu S, Ide JS, Uchio E, Zhang S, Rose M, et al. Effects of androgen deprivation on cerebral morphometry in prostate cancer patients—an exploratory study. PloS One. 2013;8:e72032.
Jamadar RJ, Winters MJ, Maki PM. Cognitive changes associated with ADT: a review of the literature. Asian J Androl. 2012;14:232–8.
Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53.
Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–23.
Mahableshwarkar AR, Zajecka J, Jacobson W, Chen Y, Keefe RSE. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40:2025–37.
McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The effects of vortioxetine on cognitive function in patients with major depressive disorder: A meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 2016;19:1–9.
McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17:1557–67.
Sharp AM, Lertphinyowong S, Yee SS, Paredes D, Gelfond J, Johnson-Pais TL, et al. Vortioxetine reverses medial prefrontal cortex-mediated cognitive deficits in male rats induced by castration as a model of androgen deprivation therapy for prostate cancer. Psychopharmacology (Berl). 2019;236:3183–95.
Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2008;53:638–46.
MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–65.
Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–92.
Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57.
Chen F, du Jardin KG, Waller JA, Sanchez C, Nyengaard JR, Wegener G. Vortioxetine promotes early changes in dendritic morphology compared to fluoxetine in rat hippocampus. Eur Neuropsychopharmacol. 2016;26:234–45.
Dale E, Zhang H, Leiser SC, Xiao Y, Lu D, Yang CR, et al. Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. J Psychopharmacol. 2014;28:891–902.
Wallace A, Pehrson AL, Sánchez C, Morilak DA. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int J Neuropsychopharmacol. 2014;17:1695–706.
Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–31.
Jett JD, Bulin SE, Hatherall LC, McCartney CM, Morilak DA. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience. 2017;346:284–97.
Li C, Maier DL, Cross B, Doherty JJ, Christian EP. Fimbria-fornix lesions compromise the induction of long-term potentiation at the Schaffer collateral-CA1 synapse in the rat in vivo. J Neurophysiol. 2005;93:3001–6.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genetics. 2003;34:267–73.
Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology. 2015;232:3123–33.
Meyer F, Zhang B, Gao X, Prigerson HG. Associations between cognitive impairment in advanced cancer patients and psychiatric disorders in their caregivers. Psycho-oncology. 2013;22:952–5.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol. 2016;34:566–71.
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association between androgen deprivation therapy and risk of dementia. JAMA oncology. 2017;3:49–55.
Shin MS, Chung KJ, Ko IG, Kim SH, Jin JJ, Kim SE, et al. Effects of surgical and chemical castration on spatial learning ability in relation to cell proliferation and apoptosis in hippocampus. Int Urol Nephrol. 2016;48:517–27.
Spritzer MD, Gill M, Weinberg A, Galea LAM. Castration differentially affects spatial working and reference memory in male rats. Arch Sex Behav. 2008;37:19–29.
Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95.
Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–21.
Hajszan T, MacLusky NJ, Johansen JA, Jordan CL, Leranth C. Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology. 2007;148:1963–7.
Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–65.
Rouillard AD, Gundersen GW, Fernandez NF, Wang Z, Monteiro CD, McDermott MG, et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database. 2016;2016:baw100.
Nelson PS, Clegg N, Arnold H, Ferguson C, Bonham M, White J, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci. 2002;99:11890–5.
Jiang F, Wang Z. Identification of androgen-responsive genes in the rat ventral prostate by complementary deoxyribonucleic acid subtraction and microarray. Endocrinology. 2003;144:1257–65.
Wilson S, Qi J, Filipp FV. Refinement of the androgen response element based on ChIP-Seq in androgen-insensitive and androgen-responsive prostate cancer cell lines. Sci Rep. 2016;6:32611.
Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–67.
Li K, Zheng Y, Wang X. The potential relationship between HIF-1α and amino acid metabolism after hypoxic ischemia and dual effects on neurons. Front Neurosci. 2021;15:676553.
Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11:187–91.
Perea G, Gómez R, Mederos S, Covelo A, Ballesteros JJ, Schlosser L, et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife. 2016;5:e20362.
Hirayama-Kurogi M, Takizawa Y, Kunii Y, Matsumoto J, Wada A, Hino M, et al. Downregulation of GNA13-ERK network in prefrontal cortex of schizophrenia brain identified by combined focused and targeted quantitative proteomics. J Proteomics. 2017;158:31–42.
Sharma N, Jadhav SP, Bapat SA. CREBBP re-arrangements affect protein function and lead to aberrant neuronal differentiation. Differentiation. 2010;79:218–31.
Shibata ACE, Ueda HH, Eto K, Onda M, Sato A, Ohba T, et al. Photoactivatable CaMKII induces synaptic plasticity in single synapses. Nat Commun. 2021;12:751.
Redrobe JP, Bourin M. Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol. 1997;325:129–35.
Gupta D, Radhakrishnan M, Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: Modulation of hypothalamic–pituitary–adrenocortical and brain serotonergic system. Pharmacol Biochem Behavior. 2014;124:129–36.
Roychoudhury M, Kulkarni SK. Antianxiety profile of ondansetron, a selective 5-HT3 antagonist, in a novel animal model. Methods Find Exp Clin Pharmacol. 1997;19:107–11.
du Jardin KG, Jensen JB, Sanchez C, Pehrson AL. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur Neuropsychopharmacol. 2014;24:160–71.
Delcourte S, Etievant A, Haddjeri N. Role of central serotonin and noradrenaline interactions in the antidepressants’ action: Electrophysiological and neurochemical evidence. Prog Brain Res. 2021;259:7–81.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.e19.
Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–50.
Li T, Yu Y, Song Y, Li X, Lan D, Zhang P, et al. Activation of BDNF/TrkB pathway promotes prostate cancer progression via induction of epithelial-mesenchymal transition and anoikis resistance. FASEB J. 2020;34:9087–101.
Toh YL, Ng T, Tan M, Tan A, Chan A. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: a systematic review. Brain Behav. 2018;8:e01009.
Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, Cheung YT, et al. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro Oncol. 2016;18:244–51.
Yap NY, Tan NYT, Tan CJ, Loh KW, Ng RCH, Ho HK, et al. Associations of plasma brain-derived neurotrophic factor (BDNF) and Val66Met polymorphism (rs6265) with long-term cancer-related cognitive impairment in survivors of breast cancer. Breast Cancer Res Treat. 2020;183:683–96.
du Jardin KG, Müller HK, Sanchez C, Wegener G, Elfving B. A single dose of vortioxetine, but not ketamine or fluoxetine, increases plasticity-related gene expression in the rat frontal cortex. Eur J Pharmacol. 2016;786:29–35.
Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology. 1996;15:515–22.
Rattray M, Baldessari S, Gobbi M, Mennini T, Samanin R, Bendotti C. p-Chlorphenylalanine changes serotonin transporter mRNA levels and expression of the gene product. J Neurochem. 1996;67:463–72.
Kugathasan P, Waller J, Westrich L, Abdourahman A, Tamm JA, Pehrson AL, et al. In vivo and in vitro effects of vortioxetine on molecules associated with neuroplasticity. J Psychopharmacol. 2017;31:365–76.
Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–86.
Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol. 1992;31:268–73.
Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74.
Acknowledgements
We thank Suphada Lertphinyowong for expert technical assistance. Statistical support was provided by Roman Fernandez and the Genome Sequencing Facility and Bioinformatics Core of the Mays Cancer Center, UT Health San Antonio.
Funding
This work was supported by research grant R01 CA224672 from the National Cancer Institute, National Institutes of Health; research grant RP180055 from the Cancer Prevention and Research Institute of Texas (CPRIT); CPRIT Research Training Award RP170345; the Quincy and Estine Lee Endowment Fund; and the Mays Cancer Center, UT Health San Antonio. Gene expression data were generated by the University of Texas Health, San Antonio Mays Cancer Center Genomics Shared Resource Facility (P30 CA054174 and the UT Health San Antonio Biospecimen and Translational Genomics Core; NIH Shared Instrument S10 grant 1S10OD021805-01; CPRIT Core Facility Award RP160732; NIH-NCATS 1UL TR002645-01). In-kind support was provided by H. Lundbeck A/S, who generously provided the drug-containing chow and control chow.
Author information
Authors and Affiliations
Contributions
AMV was involved in hypothesis generation, experimental design, and conduct, analysis, interpretation, and presentation of data, writing and revising the manuscript; YC performed the microarray data analysis, and prepared and uploaded data files to the GEO repository; JG consulted on experimental design and statistical analyses; TLJ assisted with sample preparation and conduct of the microarray; RJL, CR and IMT provided important intellectual contribution to hypothesis generation, data interpretation, and discussions of clinical relevance; DAM was involved in all aspects of hypothesis generation, experimental design, data analysis and interpretation, editing and revising the manuscript; and provided laboratory resources to support the experiments. All authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Competing interests
Vortioxetine was provided by H. Lundbeck A/S, which had no input into the design or conduct of the study, analysis or interpretation of the data, and no role in the decision to publish or in the writing of the manuscript. The authors declare no competing interests.
Compliance with ethical standards
The Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health, San Antonio, reviewed and approved all research procedures. All experiments were compliant with the ethical standards of the National Institutes of Health as specified in the Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vaiana, A.M., Chen, Y., Gelfond, J. et al. Effects of vortioxetine on hippocampal-related cognitive impairment induced in rats by androgen deprivation as a model of prostate cancer treatment. Transl Psychiatry 13, 307 (2023). https://doi.org/10.1038/s41398-023-02600-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41398-023-02600-5