Abstract
Combination therapy in cancer treatment offers significant potential to overcome drug resistance, enhance efficacy, reduce toxicity, and expand drug indications. sorafenib, an FDA-approved multi-targeted kinase inhibitor, has demonstrated effectiveness across various cancers but currently lacks approved combination therapies. Recently, we identified LZX-2-73 as a promising drug candidate with potent anticancer activity, targeting the nuclear protein 1 (NUPR1), an emerging and promising target in cancer therapy. In this study, we report that the combination of the NUPR1 inhibitor LZX-2-73 with sorafenib produces strong synergistic anticancer effects in various cancer cell lines as well as in primary pancreatic ductal adenocarcinoma (PDAC) organoids. This combination significantly enhanced lactate dehydrogenase (LDH) release and caspase 3/7 activity, markedly induced ROS accumulation, reduced the reduced/oxidized glutathione ratio, and increased the accumulation of malondialdehyde (MDA) and lipid hydroperoxides. Collectively, the combination of LZX-2-73 and sorafenib led to a substantial increase in cell death due to massive oxidative stress. Additionally, in a pancreatic cancer xenograft mouse model, the combination of LZX-2-73 and sorafenib exhibited a synergistic anticancer effect, effectively inhibiting tumor growth. In summary, this study provides valuable insights into enhancing the anticancer activity of NUPR1 inhibitors through combination with sorafenib, offering a promising new avenue for cancer therapy and opening new indications.
Similar content being viewed by others
Introduction
In tumor therapy, the use of monotherapy often leads to the development of drug resistance over time. To address this challenge, combination therapy -using two or more drugs with different mechanisms of action- has proven highly effective. This approach not only helps to overcome drug resistance but also enhances therapeutic efficacy, reduces toxicity associated with high doses of a single drug, and broadens the range of conditions for which the drugs can be used [1, 2]. Common clinical combinations of antitumor drugs include the pairing of targeted anticancer agents with different mechanisms of action, combining targeted therapies with chemotherapy, integrating targeted therapies with immunotherapy, and combining immunotherapy with chemotherapy [3]. A notable example of such synergy is the combination of irinotecan and rabusertib (also known as LY260398), which has shown a powerful anticancer effect in KRAS-TP53 double-mutant colorectal cancer. Rabusertib, a selective checkpoint kinase 1/2 (CHEK1/2) inhibitor, is typically used to treat ovarian, non-small cell lung, and breast cancers [4, 5]. In the last few years, many clinical trials aimed to find synergistic effects in many types of cancers: loncastuximab tesirine with rituximab in patients with relapsed or refractory follicular lymphoma [6], inavolisib plus palbociclib-fulvestrant in PIK3CA-mutated advanced breast cancer [7], or Pembrolizumab plus lenvatinib in patients with pleural mesothelioma [8].
Sorafenib, a frontline clinical medication for HCC, inhibits tumor growth and microvasculature through antiproliferative, antiangiogenic, and/or proapoptotic effects by its multi-kinase-inhibitory activity, targeting the vascular endothelial growth factor receptors, the platelet-derived growth factor receptor, and FLT3, C-Kit, and B-RAF. However, this lack of specificity led to gastrointestinal adverse effects. In addition, sorafenib has a low bioavailability due to oral administration and fast metabolism [9]. Thus, enhancing the therapeutic effect of sorafenib, while minimizing its adverse effects, might represent a good therapeutic strategy. Original strategies, such as new nanodrug delivery systems, have implemented the activity of Sorafenib, in vivo and in vitro [10]; however, a phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib was performed, unfortunately, with no positive result [11].
Ferroptosis is an iron-dependent form of regulated cell death characterized by the accumulation of lethal lipid peroxides, distinct from apoptosis, necrosis, and other cell death modalities [12, 13]. This process is driven by oxidative stress and disrupted redox homeostasis, often involving the inactivation of the lipid repair enzyme glutathione peroxidase 4 (GPX4) or depletion of intracellular glutathione [14]. Since its formal identification in 2012, ferroptosis has garnered attention for its potential role in various pathologies, particularly in cancer, where tumor cells frequently exhibit altered iron metabolism and redox imbalance [15]. Notably, some cancer cells that are resistant to traditional forms of cell death appear susceptible to ferroptosis, making it an attractive therapeutic target [16]. Incorporating ferroptosis-inducing strategies into cancer treatment may overcome resistance mechanisms and improve outcomes in refractory tumors. Therefore, understanding the molecular underpinnings of ferroptosis is of growing importance in the context of cancer biology and therapy.
Nuclear protein 1 (NUPR1), also known as p8 and Com1, is emerging as a promising target for cancer therapy. This 82-amino acid chromatin-binding protein was first identified in 1997 in the exocrine pancreas during cellular injury caused by acute pancreatitis [17]. Subsequent research has revealed that NUPR1 is widely expressed in various cancer tissues, while it remains absent in healthy, unstressed cells. Notably, NUPR1 is overexpressed in several types of cancer cells, including pancreatic ductal adenocarcinoma (PDAC) cells [18, 19]. NUPR1 is implicated in numerous cancer-related processes, including cell cycle regulation, apoptosis [20, 21], cell migration, invasion, adhesion [22], metastasis [23], and DNA repair responses [24]. Gene inactivation of NUPR1 in different cancer cell lines, such as PDAC [22, 25], hepatocarcinoma [26], glioblastoma [27], multiple myeloma [28], osteosarcoma [29], and non-small lung cancer [30], has been shown to halt tumor growth. The role of NUPR1 in promoting the development and progression of PDAC has recently garnered significant attention, driving efforts to develop small-molecule inhibitors targeting this protein [31]. However, NUPR1 presents a unique challenge as it is an intrinsically disordered protein (IDP) without a stable three-dimensional structure [32,33,34]. This characteristic makes traditional structure-based high-throughput screening methods for small-molecule inhibitors unsuitable for NUPR1 [35, 36]. Despite these challenges, a combination of biochemical, biophysical, and biological techniques led to the identification of LZX-2-73, a promising NUPR1 inhibitor. LZX-2-73 demonstrates potent anticancer activity. In clinical practice, most patients with PDAC receive chemotherapy. Treating cancer cells with NUPR1 inhibitors leads to cell death through various processes, including apoptosis, necroptosis, and ferroptosis [35, 37]. This indicates that NUPR1 plays a protective role in helping cancer cells to increase resistance against stress and promoting survival against chemotherapy [38, 39]. In addition, previous studies have suggested a synergistic effect between sorafenib and NUPR1’s inhibition [26, 40], being highlighted as an important biomarker of sorafenib resistance [41, 42]. Therefore, a combination of sorafenib with LZX-2-73 might represent an interesting strategy for resistant tumors.
This study aimed to evaluate the combination of LZX-2-73 with classical chemotherapeutic agents to enhance their therapeutic efficacy. From all the drugs tested, sorafenib showed the best synergistic profile with LZX-2-73 in a wide range of cancers. The two drugs potentiate the oxidative stress, inducing massive cell death, offering a promising new approach for clinical combination therapy.
Material and methods
Experimental reagents and materials
Oxaliplatin, gemcitabine, paclitaxel, 5-FU, and SN38 were purchased from the Selleckchem company. Sorafenib was purchased from MedChemExpress. LZX-2-73 has been synthesized by AGVdiscovery (France). Z-VAD-FMK (Z-VAD), Necrostatin-1 (Nec-1), Ferrostatin-1 (Fer-1), N-acetyL-cysteine (NAC), were obtained from Merk.
Cell culture and patient-derived organoids culture
Human PDAC cells MIAPaCa-2, human colorectal cancer cells HT-29, human lung carcinoma cells H1299, human breast adenocarcinoma cells MCF-7 and MDA-MB-231, human primary glioblastoma cells U87, human hepatocellular carcinoma cells HepG2 and Hep3B were cultured in Dulbecco’s modified Eagle’s medium (DMEM) from Gibco supplemented with 10% fetal bovine serum (FBS) from Biosera. Human prostate cancer cells PC-3, human lung carcinoma cells H358, human Burkitt lymphoma cells Daudi, and human acute T cell leukemia cells Jurkat were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium from Gibco with 10% FBS. Cells were obtained from the American Type Culture Collection (ATCC, USA). Primary human PDAC cell lines were cultured in DMEM/F12 medium supplemented with 1.22 g/L nicotinamide, 5 g/L glucose, 5% Nu-Serum IV, 0.5% ITS+ Premix Universal Culture Supplement (containing insulin, human transferrin, and selenous acid), 1 μM dexamethasone, 10 ng/L cholera toxin, 50 nM 3,3′5-triiodo-L-thyronine, 25.2 mg/L bovine pituitary extract, and 20 μg/L epidermal growth factor. Cells were maintained at 37 °C with 5% CO2 in a humidified incubator.
Patient-derived organoids were obtained from a series of PDAC patients, including those with both resectable and unresectable tumors, as part of the PaCaOmics clinical trial NCT01692873. The organoids were cultured using Pancreatic Organoid Feeding Media (POFM), which consisted of Advanced DMEM/F12 with the addition of 10 mM HEPES, 1× Glutamax, and penicillin/streptomycin from Thermo Fisher. Supplements included 100 ng/ml Animal-Free Recombinant Human FGF10 and 50 ng/ml Animal-Free Recombinant Human EGF from Peprotech, 100 ng/ml Recombinant Human Noggin from Biotechne, 30% Wnt3a-conditioned medium, 10% RSPO1-conditioned medium, 10 nM human Gastrin 1, 10 mM Nicotinamide, 1.25 mM N-acetylcysteine from Sigma Aldrich, 1× B27 from Invitrogen, 500 nM A83-01, and 10.5 μM Y27632 from Tocris. The organoids were incubated at 37 °C in a 5% CO2 atmosphere, with the media being refreshed every 3–4 days.
Drug combination assay
Drug combination assays were conducted to assess the synergistic effects of LZX-2-73 in combination with SN38, oxaliplatin, gemcitabine, paclitaxel, 5-FU, or sorafenib on various cancer cell lines. Cells were exposed to different doses of LZX-2-73 and the aforementioned compounds using an 11 × 7 dose matrix that included concentrations above and below their IC50, as determined from previous studies. Initially, cancer cells were seeded into clear 96-well plates at a density of 5000 cells per well and cultured for 24 h. Serial dilutions from the main stock solutions of each compound were used to create the dosing matrix. The range concentration for each drug was: LZX-2-73 (2–10 µM), sorafenib (0.01–50 µM), SN38 (0.001–1000 µM), oxaliplatin (0.001–1000 µM), 5-FU (0.001–1000 µM), paclitaxel (0.001–60 µM), and gemcitabine (0.062–250 µM). After seeding for 24 h, the compounds were applied to the cells in triplicate and incubated for 72 h at 37 °C with 5% CO2. Following incubation, PrestoBlue™ Cell Viability Reagent from Thermo Fisher Scientific was added to each well, and the plates were incubated for an additional 2 h under the same conditions. Cell viability and inhibition were quantified using the Tristar LB941 plate reader. Untreated cells served as the control. The SynergyFinder software, version 3.0, was utilized to calculate synergy scores for the drug combinations based on the HSA reference model. A synergy score below −10 indicated antagonistic interactions, scores between −10 and 10 indicated additive interactions, and scores above 10 indicated synergistic interactions [43, 44].
Cell viability
Cell viability was determined using the PrestoBlue assay. Cells were seeded at 5000 cells per well in a 96-well plate, then treated the next day as indicated in 100 µL of medium. After 72 h of treatment, 10 µL of PrestoBlue (Thermo Fisher, A13262) was added to the medium and incubated for 2 h at 37 °C + 5% CO2. Then, 100 µL of medium was collected in a black plate. Cell viability and inhibition were quantified using the Tristar LB941 plate reader. Untreated cells served as the control.
Proliferation assay
Cultures were plated at 5000 cells per well in 96-well plates and allowed to incubate for 24 h to adhere to the surface. Cells were then treated with sorafenib, LZX-2-73, or a combination of both for 72 h. During the incubation period, cell growth was monitored using the Incucyte® S3 Live-Cell Analysis System (Sartorius, Germany), capturing phase contrast images every 4 h and quantifying culture confluence with the integrated confluence algorithm. All experiments were conducted in triplicate.
Organoid imaging
Organoids were seeded at 1000 cells/well in 96-well plates (surface: BIOFLOAT™, round base) and incubated for 2 days to form organoids. The following treatments were then applied for 72 h: PDAC056T organoids were treated with 12.5 µM sorafenib alone, 25 µM LZX-2-73 alone, or a combination of both; PDAC088T organoids were treated with 10 µM sorafenib alone, 25 µM LZX-2-73 alone, or a combination of both. Three days after drug treatment, the organoids were photographed using an EVOS cell imaging system (Invitrogen by Thermo Fisher Scientific). The diameter of the organoids was analyzed using ImageJ software.
LDH and caspase 3/7 activity measurement
Cultures were initially plated at 5000 cells per well in 96-well plates and allowed to incubate for 24 h to adhere to the surface. Cells were then treated with sorafenib, LZX-2-73, or a combination of both for 72 h. The LDH release and caspase 3/7 activity were measured using CytoTox-ONE™ and Caspase-Glo® 3/7 (Promega, France), respectively. For LDH and caspase 3/7 activity assays, data were normalized to the cell number.
Glutathione assay
The GSH/GSSG-Glo assay kit (V6611, Promega) was utilized according to the manufacturer’s instructions. Briefly, 5000 cells per well were plated in 96-well plates and left to grow overnight. The treatments are the same as those in the LDH release assay applied for 72 h, with each condition tested in triplicate. Measurements of total intracellular glutathione and oxidized glutathione (GSSG) were taken. To each well, the Luciferin Generation Reagent and Detection Reagent were added, mixed thoroughly, and the resulting luminescence was recorded using the Tristar multimode microplate reader. The concentrations of GSSG and total glutathione were determined using a standard curve and normalized to the cell count. The GSH/GSSG ratios were calculated with the formula: GSH/GSSG = [Total GSH - (2 × GSSG)]/GSSG.
Lipid peroxidation determination
The MDA lipid peroxidation assay kit (ab118970, ABCAM, Cambridge, UK) was employed following the manufacturer’s instructions. To measure MDA production in MIAPaCa-2 xenografts, 10 mg of tumor tissue was utilized. For cell experiments, 5 × 105 cells were seeded into 10-cm cell culture dishes and allowed to attach overnight. Treatments were performed with sorafenib, LZX-2-73, or a combination of both for 72 h and tested in triplicate. Tumor tissues and cells were collected, homogenized in a lysis solution, and centrifuged to obtain the supernatant. An equivalent amount of protein from each sample was mixed with the thiobarbituric acid solution and incubated at 95 °C for 1 h. Subsequently, the samples were cooled in an ice bath for 10 min to reach room temperature. Fluorescence measurements were acquired using a TECAN Infinite 96-plate reader with excitation/emission settings of 532/553 nm.
Cells were plated in 12-well plates at a density of 25,000 cells per well. The treatments are the same as those in the LDH release assay applied for 72 h, with each condition tested in triplicate. Subsequently, the cells were incubated in fresh medium containing 2 µM BODIPY™ 581/591 C11 (Invitrogen Molecular Probes, D3861) at 37 °C for 30 min. After incubation, the cells were washed twice with PBS. Finally, samples were imaged and analyzed with a Zeiss Axio Imager Z2 microscope.
GPx activity assay
GPx activity was assessed using a Glutathione Peroxidase Assay Kit (ab102530, ABCAM, Cambridge, UK). The assay relies on the GPx-catalyzed oxidation of glutathione (GSH) to glutathione disulfide (GSSG), which is then converted back to GSH by glutathione reductase using NADPH. The decrease in NADPH, reflected by its oxidation to NADP+, serves as an indicator of GPx activity. In summary, tumor tissues were homogenized using a Dounce homogenizer, and the supernatants were collected and kept on ice after centrifugation. Following the manufacturer’s instructions, the samples were mixed with the reaction reagent, and the optical density (OD) at 340 nm was measured. Cumene hydroperoxide solution was subsequently added to the samples. The enzymatic reaction was conducted in 96-well plates, and NADPH oxidation was monitored by measuring OD at 340 nm over 5 min at 25 °C using a FLUOstar Omega plate reader.
OXPHOS assay (Seahorse)
MIAPaCa-2 cells were plated at 100,000 cells/well in a 24-well plate (Seahorse) and incubated overnight. Then, cells were treated with 6 µM sorafenib and/or 6 µM LZX-2-73 for 6 h. The Oxygen Consumption Rate (OCR) (pmol O2/min) was measured using the Seahorse Bioscience XF24 Extracellular Flux Analyzer (Agilent) in response to 1 μM oligomycin, 0.25 μM carbonylcyanide p-(trifluoro-methoxy) phenylhydrazone (FCCP), 0.5 µM rotenone/Antimycine A (Millipore Sigma).
Catalase and SOD activity assay
MIAPaCa-2 cells were treated with 6 µM sorafenib and/or 6 µM LZX-2-73 for 24 h. Then, cells were collected and lysed in PBS with a sonicator. For the determination of catalase or SOD activity, we employed the Catalase (CAT) Activity Assay Kit (E-BC-K031-M) and Total Superoxide Dismutase (T-SOD) Activity Assay Kit (WST-1 Method) (E-BC-K020-M), respectively. For the determination, we followed the manufacturer’s instructions. For both kits, the absorbance was measured in a FLUOstar Omega microplate reader (BMG Labtech). For the normalization, protein level was determined by Pierce™ BCA Protein Assay (Thermo Fisher).
Western blotting
MiaPaCa-2 cells were treated with 6 µM sorafenib and/or 6 µM LZX-2-73 for 24 or 48 h. Then, cells were collected and lysed in RIPA buffer (0.5 M Tris-HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA). Then, 25 µg of each protein lysate was loaded in an acrylamide gel and transferred to a nitrocellulose membrane. The membranes were incubated with the primary antibody overnight at 4 °C and with an IgG-HRP conjugated secondary antibody for 1 h. For detection, we use the reagent ECL detection system (Millipore Corp., Bedford). The primary antibodies used in this study were: Survivin (1:1000, Cell Signaling 2808), NUPR1 (1:1000, homemade), B-RAF (1:1000, Cell Signaling 14814), phospho-B-RAF (1:1000, Cell Signaling 2696), NRF2 (Proteintech 16396-1-AP), NRF2 (phospho Ser40) (1:1000, ab76026), GPX4 (1:1000, Abcam 125066) and xCT/SLC7A11 (D2M7A) (1:1000, Cell Signaling 12691) and beta-Actin (1:15,000, Proteintech 81115-1-RR).
RT-qPCR
Total RNA was extracted from cells using the RNeasy kit (Qiagen), and cDNA was obtained by reverse transcription using the Go Script kit (Promega), according to the manufacturer’s instructions. Real-time quantitative PCR (RT-qPCR) was performed using the AriaMx system (Agilent). Primer sequences are listed below: Nupr1-F:5′-TAGAGACGGGACTGCG-3′; Nupr1-R: 5′-GCGTGTCTATTTATTGTTGC-3′; 36B4-F:5′-AATCCCTGACGCACCGCCGTGATG-3′; 36B4-R:5′-TGGGTTGTTTTCCAGGTGCCCTCG-3′.
ROS and mROS determination
Cells were seeded at 50,000 cells per well in 12-well plates, and treated the next day as indicated. After 24 h of treatment, the cells were detached with trypsin, collected, and rinsed with PBS. Subsequently, CellROX Green at 5 µM (Thermo Fisher C10444) or Mitosox Red at 5 µM (Thermo Fisher M36008) diluted in HBSS were added and incubated for 30 min at 37 °C + 5% CO2 in the dark. Excess probe was removed by washing the cells with HBSS, and Hoechst (1:2000) was added to stain dead cells. Fluorescence signals were measured using a flow cytometer (Cytoflex S). A minimum of 10,000 cells was analyzed for each condition. Data were analyzed using FlowJO software on median fluorescence level gated on live cells.
Xenografts development
Female Crl:NU(Ico)-Foxn1nu mice, aged 4 weeks, were obtained from Charles River Company. The mice were housed in the Experimental Animal House at the Centre de Cancérologie de Marseille, pôle Luminy, under specific pathogen-free conditions. All procedures followed laboratory animal care guidelines and ethical regulations. At 5 weeks old, the mice were subcutaneously inoculated with 10 million MIAPaCa-2 cells mixed with 50 µL of Matrigel (BD Pharmingen). When tumors reached a size of 200 mm³, the mice received daily intraperitoneal injections of either 5% DMSO in sunflower seed oil (vehicle), 25 mg/kg sorafenib, 10 mg/kg LZX-2-73, or a combination of 25 mg/kg sorafenib and 10 mg/kg LZX-2-73. The mice were weighed and tumor volumes measured with calipers twice a week, with tumor volumes calculated assuming an ellipsoid shape. After 28 days of treatment, the mice were sacrificed.
Hematoxylin and eosin staining
Serial 4 µm sections were cut from each paraffin-embedded tissue sample using a Leica Histocore Biocut (Leica, Germany). These sections were then stained with hematoxylin and eosin according to the manufacturer’s instructions using Leica Autostainer XL (Leica, Germany). Photomicrographs were captured using a ZEISS Axio Imager Z2 microscope (Zeiss, Germany).
In situ detection of apoptotic cells in tumor tissue
Serial 4 µm sections were prepared from each paraffin-embedded tumor sample using a Leica Histocore Biocut (Leica, Germany). Apoptotic cells were analyzed using the TUNEL Assay Kit - HRP-DAB (ab206386, Abcam) following the manufacturer’s protocol. Images of the sections were obtained with a ZEISS Axio Imager Z2 microscope (Zeiss, Germany).
Immunohistochemistry
Four-micrometer paraffin sections of tumors were incubated for half an hour at 65 °C and then rehydrated. Antigen retrieval was performed by incubating the slides in Citrate Buffer TRS pH 6 (Dako) at 96 °C for 20 min, followed by a 30-min incubation at room temperature to cool them down. Endogenous peroxidases were blocked using 3% H2O2 for 10 min, and the slides were rinsed three times with PBS. The slides were then incubated with monoclonal rabbit Anti-Human Ki-67 antibody (Abcam, 1:100) and rabbit polyclonal anti-4-Hydroxynonenal antibody (Abcam, 1:200) for 60 min at room temperature. After three washes in PBS, the slides were incubated with a biotinylated Goat anti-Rabbit Ig secondary antibody (Abcam, 1:400) for 30 min at room temperature. Following another three PBS washes, Streptavidin-HRP (Agilent; 1:500) was added and incubated for 30 min at room temperature. The staining was developed using diaminobenzidine (DAKO, Agilent) for 10 min at room temperature. The slides were rinsed in distilled water, counterstained with Mayer’s Hematoxylin for 30 s, and blued with 0.1% sodium bicarbonate solution for 3 min. Finally, the slides were dehydrated, cleared, and mounted with coverslips and permanent mounting medium. Images of the sections were captured using a ZEISS Axio Imager Z2 microscope (Zeiss, Germany).
Statistics
Statistical analyses were performed using one-way ANOVA, Dunnett’s test, or two-way ANOVA, Dunnett’s test. Results were presented as the mean ± SD from at least three independent experiments. A p-value of <0.05 was considered statistically significant.
Results
Evaluation of the synergistic anticancer activity of sorafenib and LZX-2-73
In this study, we assessed the anticancer efficacy of various clinically used chemotherapeutic agents -SN38, oxaliplatin, gemcitabine, paclitaxel, and 5-FU- along with the targeted drug sorafenib, in combination with the novel NUPR1 inhibitor LZX-2-73. This evaluation was performed across 15 cancer cell lines (both commercial and primary) using a dose-response matrix. The SynergyFinder tool was employed to calculate synergy scores for each drug combination (Table 1). Combinations such as paclitaxel and oxaliplatin with LZX-2-73 yielded synergy scores between −10 and 10, indicating predominantly additive effects. Notably, higher synergy scores were observed in cells with greater IC50 values upon treatment, particularly with sorafenib, suggesting that the combination could significantly enhance therapeutic efficacy. Among all tested agents, sorafenib demonstrated the most consistent and pronounced synergy across all cell lines. Consequently, we focused on further investigating the combinatory antitumoral effects of sorafenib and LZX-2-73. As shown in Fig. 1A, the combination exhibited strong synergistic activity in all tested cells within the dose-response matrices.
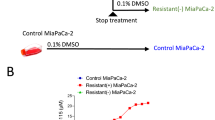
A HSA synergy score distribution plots for the combination treatment of LZX-2-73 with sorafenib. B Representative images after 72 h of treatment with sorafenib, LZX-2-73, or their combination to MIAPaCa-2, HT-29, MCF-7, HepG2, PDAC056T, or PDAC088T cells (left) and the quantification of cell confluency in the images (right) by the Incucyte device. Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (2-way ANOVA, Dunnett’s test) (n = 3), statistic shown on the last point of measurement, compared to combinatory treatment. C Representative images upon 72 h of treatment with sorafenib, LZX-2-73, or their combination to PDAC056T organoids and PDAC088T organoids (left) and the quantification of organoid diameters in the images (right). Data are shown as mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment) (n = 3).
For further analysis, we selected six cancer cell lines, MIAPaCa-2, HT-29, MCF-7, HepG2, PDAC056T, and PDAC088T- based on their highest synergy scores. Drug concentrations were predefined to optimize the synergistic interaction between sorafenib and LZX-2-73. To evaluate the impact on cell proliferation, we monitored cell confluency in real-time using the Incucyte device, comparing treatments with sorafenib alone, LZX-2-73 alone, and their combination. As shown in Fig. 1B, the combination treatment significantly suppressed cell proliferation in all six cancer cell lines compared to individual treatments, highlighting a potent synergistic effect. Additionally, Fig. 1C illustrates that the sorafenib and LZX-2-73 combination markedly reduced the diameter of primary PDAC organoids, demonstrating a strong inhibitory effect on organoid formation. In conclusion, our findings indicate that the combination of sorafenib and LZX-2-73 consistently outperforms conventional chemotherapeutic agents, exhibiting robust synergistic effects across all tested cancer models.
The combination of sorafenib and LZX-2-73 enhances cancer cell death
Building on our findings that the combination of sorafenib and LZX-2-73 exerts strong synergistic antitumor effects across multiple cancer types, we investigated the underlying mechanisms of cell death induced by this treatment. To achieve this, we measured lactate dehydrogenase (LDH) release and caspase 3/7 activity at various time points. Our results revealed that the combination treatment significantly increased LDH release compared to single-drug treatments across all tested cell lines (Fig. 2A, Supplementary Fig. 2A), indicating heightened membrane damage and cell death. Furthermore, caspase 3/7 activity was markedly elevated in the combination group, surpassing levels observed in the individual drug treatments (Fig. 2B, Supplementary Fig. 2B), suggesting enhanced apoptotic cell death. To gain deeper insight into the specific cell death mechanisms involved, we utilized various inhibitors, including Nec-1 (necroptosis inhibitor), Z-VAD-FMK (apoptosis inhibitor), Fer-1 (ferroptosis inhibitor), and NAC (antioxidant). Interestingly, treatment with Z-VAD-FMK and NAC partially rescued cell viability, indicating that the combinatory treatment induces both apoptosis and oxidative stress-driven cell death. These findings suggest that the anticancer activity of sorafenib and LZX-2-73 is mediated through the dual activation of apoptotic pathways and oxidative stress (Fig. 2C).
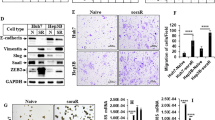
A LDH release and B caspase 3/7 activity were measured on MIAPaCa-2 cells, MCF-7 cells, and HepG2 cells, respectively. The cells were treated with single drugs and the drug combination for 48 h. Data are shown as mean ± SEM. Statistical significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment) (n = 3). C Viability of MIAPaCa-2 cells upon a 72-h treatment with sorafenib, LZX-2-73, or their combination, in the presence or not of several cell death inhibitors. D Expression of survivin, analyzed by western blot, in response to treatment with LZX-2-73 alone or in combination with sorafenib. Data are shown as mean ± SEM. Statistical significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 (1-way ANOVA, Dunnett’s test, compared combinatory treatment) (n = 4).
Regulation of survivin expression by LZX-2-73 and sorafenib
Survivin, a member of the inhibitor of apoptosis (IAP) protein family, plays a central role in tumor cell survival by suppressing apoptosis and regulating cell cycle progression [45]. Its aberrant expression has been associated with poor prognosis and therapy resistance in several cancers [46]. Previous studies have shown that NUPR1 can transcriptionally regulate genes involved in apoptosis and stress responses, including survivin, thereby promoting cancer cell survival [47]. In addition, Sorafenib has been reported to modulate survivin expression and reduce its levels under certain conditions [48]. These findings provide a biological rationale for investigating survivin expression in the context of NUPR1 inhibition and Sorafenib treatment.
To investigate this, we evaluated protein levels under different treatment conditions. Cells treated with Sorafenib exhibited a modest reduction in survivin expression compared to untreated controls, suggesting a limited effect of this multi-kinase inhibitor on anti-apoptotic signaling pathways involving survivin. In contrast, treatment with LZX-2-73, a selective inhibitor of NUPR1, led to a significantly greater decrease in survivin levels, indicating that NUPR1 activity contributes more directly to the regulation of this anti-apoptotic factor. Interestingly, combined treatment with LZX-2-73 and Sorafenib resulted in the most pronounced suppression of survivin expression observed among all conditions (Fig. 2D). These results support the hypothesis that NUPR1 inhibition not only promotes ferroptotic cell death, as previously reported [37], but also contributes to apoptotic signaling pathways. The downregulation of survivin likely plays a key role in this process, positioning survivin as a molecular link between ferroptosis and apoptosis in the context of NUPR1-targeted therapy.
Sorafenib and LZX-2-73 induce massive oxidative stress in tumor cells
Given our previous findings that antioxidants partially rescued cells from death following sorafenib and LZX-2-73 co-treatment, we further examined whether this combination therapy leads to reactive oxygen species (ROS) accumulation and its subsequent effects. To assess ROS levels, we measured intracellular ROS upon treatment. As shown in Fig. 3A, the combination treatment induced a substantial accumulation of ROS compared to sorafenib alone. To determine whether mitochondria were the primary source of ROS, we measured mitochondrial ROS levels. Although co-treatment resulted in a higher increase in mitochondrial ROS than the control, the levels were not significantly greater than those induced by sorafenib alone (Fig. 3B). This observation aligns with the fact that sorafenib itself causes considerable mitochondrial damage, as measured by the Seahorse assay (Fig. 3C), which was not further exacerbated by LZX-2-73. Cellular lipids, particularly those in the plasma membrane, are highly susceptible to peroxidation. To evaluate lipid peroxidation, we quantified malondialdehyde (MDA), a key byproduct of lipid oxidation. Compared to single-drug treatments, the combination therapy led to a significant increase in MDA accumulation across all tested cell lines (Fig. 3D, Supplementary Fig. 2A). Additionally, fluorescence microscopy using BODIPYTM 581/591 C11 staining confirmed a substantial rise in lipid hydroperoxides, along with increased levels of oxidized lipid peroxides in the combination treatment group compared to either drug alone (Fig. 3E, Supplementary Fig. 2B). Together, these findings demonstrate that the sorafenib and LZX-2-73 combination induces a pronounced accumulation of ROS, which in turn leads to extensive lipid peroxidation, contributing to the synergistic cell death observed in cancer cells.
ROS or mitochondrial ROS production was detected using CellROX (A) or MitoSOX (B), respectively, by flow cytometry analysis on MIAPaCa-2 cells incubated for 24 h with sorafenib, LZX-2-73, or their combination. Statistical significance is indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment). (n = 3). C OXPHOS metabolism, reflected by oxygen consumption rate (OCR) levels were measured on MIAPaCa-2 cells incubated for 24 h with sorafenib, LZX-2-73, or their combination Levels of lipid peroxidation, indicated by MDA levels (D) or by the oxidation of the BODIPY-C11 probe (E) (measured via fluorescence microscopy to monitor the oxidized and non-oxidized variants of lipid peroxides), were assessed in cells treated with sorafenib, LZX-2-73, or the combination of both drugs for 72 h. Statistical significance is indicated as follows: *p < 0.05, **p < 0.01, ****p < 0.0001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment) (n = 3). Scale bar: 50 μm.
Co-treatment with sorafenib and LZX-2-73 suppresses the oxidative stress response in tumor cells
Excessive ROS accumulation can trigger an oxidative stress response, activating transcription factors and mobilizing the cellular antioxidant defense system, including enzymatic and non-enzymatic antioxidants. Given the significant ROS buildup observed with sorafenib and LZX-2-73 co-treatment, we investigated its impact on the antioxidant response. The ratio of reduced glutathione (GSH) to oxidized glutathione disulfide (GSSG) is a critical determinant of a cell’s susceptibility to oxidative stress. To assess whether the combination treatment disrupts glutathione homeostasis, we measured intracellular GSSG levels and calculated the GSH/GSSG ratio. As shown in Fig. 4A and Supplementary Fig. 3A, co-treatment significantly reduced the GSH/GSSG ratio while markedly increasing GSSG levels across all tested cell models, indicating substantial reduced glutathione depletion and thus acute oxidative stress. Next, we examined the activity of key antioxidant enzymes. Catalase, a major ROS-detoxifying enzyme, was not significantly activated by the combination treatment (Fig. 4B), whereas superoxide dismutase (SOD) activity increased upon co-treatment (Fig. 4C), suggesting a partial compensatory response. To further investigate the oxidative stress response, we analyzed NRF2, the master regulator of antioxidant defense. Notably, phosphorylated NRF2 (p-NRF2 Ser40) levels were reduced following co-treatment (Fig. 4D), suggesting impaired NRF2 activation. Consistently, the expression of key NRF2 target proteins, including XCT (the functional cystine-glutamate antiporter involved in glutathione synthesis) and GPX4 (a crucial phospholipid hydroperoxidase that protects against lipid peroxidation and uses GSH as a substrate), was significantly downregulated at 24- and 48-h post-treatment (Fig. 4D). Collectively, these results demonstrate that sorafenib and LZX-2-73 co-treatment suppresses the oxidative stress response by depleting glutathione levels and downregulating essential antioxidant transcription factors and detoxification proteins.
A The ratio of GSH/GSSG was measured in MIAPaCa-2 cells, MCF-7 cells, and HepG2 cells treated with sorafenib, LZX-2-73, or the combination of the two drugs for 72 h. B Catalase and C SOD activity was measured in MIAPaCa-2 cells treated with sorafenib, LZX-2-7,3, or the combination of the two drugs for 24 h. D Western blot of phospho-NRF2, total NRF2, XCT, GPX4, and actin was performed in MIAPaCa-2 cells treated with sorafenib, LZX-2-7,3, or the combination of the two drugs for 24 or 48 h.
Synergistic antitumor effects of sorafenib and LZX-2-73 in a PDAC xenograft model
To further evaluate the synergistic antitumor effects of sorafenib and LZX-2-73 in vivo, we established a subcutaneous xenograft model by injecting MIAPaCa-2 cells into nude mice. As shown in Fig. 5A, monotherapy with either sorafenib or LZX-2-73 moderately inhibited tumor growth, whereas combination treatment resulted in near-complete suppression of tumor progression. To assess the impact on tumor proliferation, we performed immunohistochemical (IHC) analysis using an anti-Ki-67 antibody, a widely used proliferation marker. The results revealed a significant reduction in Ki-67-positive tumor cells in the combination group compared to the monotherapy groups (Fig. 5B), indicating a strong antiproliferative effect. Additionally, TUNEL staining confirmed a markedly higher incidence of apoptosis in tumors treated with the combination therapy compared to single-drug treatments (Fig. 5B). Given the role of oxidative stress in the observed tumor suppression, we measured lipid peroxidation markers in tumor tissues. Combination treatment significantly increased malondialdehyde (MDA) levels compared to monotherapy (Fig. 5C) and also elevated 4-hydroxynonenal (4-HNE) levels, two by-products of lipid peroxidation (Fig. 5D), indicating substantial lipid oxidative damage. Notably, although both sorafenib and LZX-2-73 individually induced a slight increase in glutathione peroxidase (GPx) activity, the combination treatment did not further enhance GPx activity (Fig. 5E), aligning with our in vitro findings of an impaired oxidative stress response that seems mainly due to a perturbation of the XCT/GSH/GPX4 axis. Importantly, no adverse effects were observed in treated mice throughout the experiment. All mice exhibited a slight but steady increase in body weight, with no significant differences between treatment groups (Supplementary Fig. 4A). Histological analysis of major organs (heart, liver, spleen, lungs, and kidneys) via hematoxylin and eosin (H&E) staining (Supplementary Fig. 4B) showed no evidence of tissue damage or pathological changes, indicating that the combination therapy was well tolerated. Overall, these findings provide strong in vivo evidence that the combination of sorafenib and LZX-2-73 exerts a potent synergistic anticancer effect, significantly inhibiting tumor growth through oxidative stress induction and apoptosis, while remaining non-toxic to healthy tissues.
Female Crl:NU(Ico)-Foxn1nu mice implanted with pancreatic cancer MIAPaCa-2 cell lines xenografts and treated daily with sorafenib (25 mg/kg), LZX-2-73 (10 mg/kg), or a combination of both drugs (25 mg/kg + 10 mg/kg) via intraperitoneal injection. A Tumor volume was measured twice per week. Data are shown as mean ± SEM. *p < 0.05, ****p < 0.0001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment). B Representative images of tissue sections following Ki-67 IHC staining and TUNEL assay from mice treated with sorafenib (25 mg/kg), LZX-2-73 (10 mg/kg), or their combination. Scale bar: 100 μm. C The levels of malondialdehyde (MDA) and (D) 4-Hydroxynonenal, and (E) the activity of glutathione peroxidase (GPx) were assessed in MIAPaCa-2 xenografted tumors following a 4-week treatment with sorafenib, LZX-2-73, or a combination of both. Statistical significance is indicated as follows: ns: not significant, ***p < 0.001 (1-way ANOVA, Dunnett’s test, compared to combinatory treatment). Scale bar: 100 μm.
Effect of LZX-2-73 and sorafenib on NUPR1 expression in MIAPaCa-2 cells
To evaluate the impact of sorafenib and LZX-2-73 on NUPR1 expression, we treated MIAPaCa-2 cells with sorafenib alone, LZX-2-73 alone, or a combination of both compounds. NUPR1 mRNA expression was assessed by RT-qPCR, and protein levels were analyzed by Western blotting. Data is presented in Supplementary Fig. 5A, B. Treatment with LZX-2-73 alone led to a significant upregulation of NUPR1 mRNA compared to control cells. This transcriptional activation was also maintained when LZX-2-73 was combined with sorafenib. In contrast, treatment with sorafenib alone had a minimal effect on NUPR1 mRNA levels. At the protein level, a modest increase in NUPR1 expression was observed following LZX-2-73 treatment, consistent with the transcriptional findings, although the magnitude of change was less pronounced. The combined treatment resulted in a slightly higher protein signal compared to either agent alone, but the difference did not reach statistical significance. These results indicate that LZX-2-73 is capable of inducing NUPR1 expression at the transcriptional level. This supports a potential role for LZX-2-73 in modulating stress-response pathways involving NUPR1 in pancreatic cancer cells.
We also investigated whether the combination of LZX-2-73 with sorafenib enhances the effect of sorafenib as a multi-kinase inhibitor. To this end, we evaluated a well-characterized target of sorafenib: the phosphorylation of B-RAF. MIAPaCa-2 cells were treated with sorafenib alone or in combination with LZX-2-73, and we observed that the presence of the NUPR1 inhibitor does not alter sorafenib’s activity on phosphorylatable substrates (Supplementary Fig. 5C). In conclusion, the synergistic antitumor effect observed between both compounds does not appear to result from a modification of sorafenib’s kinase-inhibitory capacity.
Discussion
Sorafenib is an oral multi-kinase inhibitor that targets tumor angiogenesis and reduces the nutrient supply to tumor cells, thereby inhibiting tumor proliferation and metastasis [49, 50]. In preclinical studies, sorafenib has demonstrated the ability to inhibit the growth of various human xenograft tumors, including renal cell carcinoma, ovarian cancer, PDAC, colon cancer, thyroid cancer, breast cancer, and melanoma [51,52,53,54]. The FDA has approved sorafenib as an oral anticancer medication for the treatment of HCC, renal cell carcinoma, and thyroid cancer [55]. While sorafenib is primarily approved as monotherapy for these cancers, ongoing clinical trials are evaluating its efficacy in combination with other drugs. Most of these trials are in phases I or II, with a predominant focus on HCC. Some trials have reported promising results, benefiting cancer patients; however, further research is necessary due to limited sample sizes [56, 57]. To date, no sorafenib combination regimen has received FDA approval, highlighting the need for the development of more effective combination therapies.
Recent research on sorafenib as an oxidative stress inducer in combination therapy has primarily focused on increasing the sensitivity of HCC to sorafenib. For example, the fatty acid synthase (FASN) inhibitor orlistat exhibits significant synergistic anti-HCC effects with sorafenib and can reverse sorafenib resistance. This effect is attributed to the solute carrier family 7 member 11 (SLC7A11, also called xCT System), which plays a critical role in sorafenib resistance. The downregulation of FASN activates ferroptosis triggered by SLC7A11, thereby overcoming sorafenib resistance [58]. Additionally, tiliroside, a natural flavonoid glycoside isolated from the oriental paperbush flower, has demonstrated synergistic anticancer activity in combination with sorafenib, enhancing HCC sensitivity to sorafenib. Tiliroside directly binds to and inhibits TANK-binding kinase 1 (TBK1), thereby suppressing the phosphorylation of serine 349 on sequestosome-1 (p62). This inhibition decreases the affinity of p62 for kelch-like ECH-associated protein 1 (Keap1), promoting the ubiquitination and degradation of nuclear factor erythroid 2-related factor 2 (Nrf2). Consequently, ferroptosis is induced, enhancing the sensitivity of HCC cells to sorafenib [59]. Nrf2 upregulation-induced ferroptosis resistance weakens the anticancer efficacy of sorafenib [60,61,62]. To address this issue, researchers have employed “click” chemistry-based multifunctional hyperbranched nanocarriers for the co-delivery of sorafenib and siNrf2. This approach overcomes Nrf2-mediated ferroptosis resistance and enhances sorafenib’s antitumor effects [63]. Interestingly, our results indicate that the combination of sorafenib and LZX-2-73 induces massive oxidative stress while reducing NRF2 activation. This suppression of NRF2 prevents the oxidative stress response, ultimately promoting cell death. Although sorafenib can induce ferroptosis in some models, it fails to do so across a wide range of models [64], suggesting diverse mechanisms of action primarily involving oxidative stress. Notably, when ferroptosis was inhibited in co-treated cells with LZX-2-73, Fer-1 failed to rescue cell viability, whereas antioxidants such as NAC successfully prevented cell death. Furthermore, sorafenib negatively impacts mitochondrial activity through oxidative and nitrosative stress [65], consistent with our observations. Sorafenib monotherapy already induces significant mitochondrial damage, which is not further exacerbated by co-treatment with LZX-2-73. Additionally, essential oxidative stress-response proteins, including xCT and GPX4, were downregulated upon combinatory treatment, implicating a severe and unresolved oxidative stress response that drives cell death through ROS accumulation.
NUPR1, a nuclear transcription factor, plays a crucial role in regulating the oxidative stress response [66]. For instance, Liu et al. identified NUPR1 as a key inhibitor of ferroptosis, demonstrating that it transcriptionally activates lipocalin-2 (LCN2), which significantly mitigates oxidative damage and suppresses ferroptosis in pancreatic cancer cells [67, 68]. Similarly, Zhang et al. showed that circPIAS1 confers resistance to ferroptosis in HCC cells by upregulating NUPR1. Moreover, Huang et al. found that the NUPR1 inhibitor ZZW-115 downregulates mitochondrial biogenesis regulator Mitochondrial Transcription Factor A (TFAM), thereby reducing the antioxidant capacity in pancreatic cancer cells [66]. Massive oxidative stress induction, including ferroptosis, leads to cell death, characterized by ROS accumulation, lipid peroxidation, decreased antioxidative enzyme activity (GPX, SOD, catalase), reduced cystine/glutamate antiporter (system xc-) function, and glutathione (GSH) depletion [13, 69, 70]. Our findings align with this phenomenon, as the combinatory treatment led to a significant disruption of the oxidative stress response. These results suggest that combining the NUPR1 inhibitor LZX-2-73 with sorafenib could yield synergistic anticancer effects via oxidative stress induction.
We demonstrated that NUPR1 is a promising target for the treatment of different types of cancers. However, due to the properties of this protein, the design of a clinically applicable pharmacological inhibitor has been a challenge. The most potent and best-studied inhibitor to date against NUPR1, the compound ZZW-115, has been delayed for clinical use due to its ability to interact with hERG receptors, a classic problem in the pharmaceutical industry [71]. This new NUPR1 inhibitor, LZX-2-73, has shown strong antitumor activity and no affinity for hERG receptors [72], making it a potentially safer drug agent for use in humans.
Although sorafenib can induce ferroptosis in certain cellular contexts, this effect is not consistent across all models, likely due to the activation of antioxidant defense mechanisms such as the NUPR1/LCN2 and NUPR1/NRF2 pathways. In our study, we observed that the combination of sorafenib with the NUPR1 inhibitor LZX-2-73 amplifies oxidative stress while disrupting these protective responses. Interestingly, although the molecular profile of co-treated cells suggests a ferroptosis-prone environment, the lack of rescue by the ferroptosis inhibitor Fer-1 indicates that cell death proceeds through a ferroptosis-independent, ROS-mediated mechanism. This suggests that targeting NUPR1 can sensitize cancer cells to oxidative damage induced by sorafenib, broadening its therapeutic potential. The central finding of this research underscores the potential of rational drug combinations to provide significant therapeutic benefits, particularly for patients with limited treatment options. Our study reveals that LZX-2-73 not only enhances sorafenib’s efficacy through synergistic interaction but also transforms sorafenib-resistant cancer cells into responsive ones. This discovery presents a promising therapeutic strategy for overcoming drug resistance in cancer treatment, a longstanding challenge in medical research.
In conclusion, our study demonstrates that combining the multi-target kinase inhibitor sorafenib with the novel, safe NUPR1 inhibitor LZX-2-73 produces significantly stronger synergistic effects than other chemotherapeutic agents. This potent combination therapy not only exhibits superior synergy in liver cancer but also extends its efficacy to pancreatic, prostate, colorectal, non-small cell lung cancer, breast cancer, leukemia, and glioblastoma. Our findings highlight that the sorafenib and LZX-2-73 combination markedly enhances cancer cell death, effectively inhibits tumor growth, boosts anticancer activity in vivo, and allows for a reduced monotherapy dosage. We believe this research could broaden sorafenib’s clinical application, expanding treatment options for cancer patients and offering new therapeutic alternatives for those in need.
Data availability
Data are available upon request.
References
Jaaks P, Coker EA, Vis DJ, Edwards O, Carpenter EF, Leto SM, et al. Effective drug combinations in breast, colon and pancreatic cancer cells. Nature. 2022;603:166–73.
Doroshow JH, Simon RM. On the design of combination cancer therapy. Cell. 2017;171:1476–8.
Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22:213–34.
Laquente B, Lopez-Martin J, Richards D, Illerhaus G, Chang DZ, Kim G, et al. A phase II study to evaluate LY2603618 in combination with gemcitabine in pancreatic cancer patients. BMC Cancer. 2017;17:137.
King C, Diaz H, Barnard D, Barda D, Clawson D, Blosser W, et al. Characterization and preclinical development of LY2603618: a selective and potent Chk1 inhibitor. Invest N Drugs. 2014;32:213–26.
Alderuccio JP, Alencar AJ, Schatz JH, Kuker RA, Pongas G, Reis IM, et al. Loncastuximab tesirine with rituximab in patients with relapsed or refractory follicular lymphoma: a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2025;12:e23–34.
Turner NC, Im S-A, Saura C, Juric D, Loibl S, Kalinsky K, et al. Inavolisib-based therapy in PIK3CA-mutated advanced breast cancer. N Engl J Med. 2024;391:1584–96.
Douma L-AH, Lalezari F, van der Noort V, de Vries JF, Monkhorst K, Smesseim I, et al. Pembrolizumab plus lenvatinib in second-line and third-line patients with pleural mesothelioma (PEMMELA): a single-arm phase 2 study. Lancet Oncol. 2023;24:1219–28.
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8.
Wu Y, Wang X, Li L, Wang M, Tian S, Song J, et al. Preparation of glutathione-regulated sorafenib targeted nanodrug delivery system and its antihepatocellular carcinoma activity. ACS Appl Mater Interfaces. 2024;16:65131–41.
El Dika I, Capanu M, Chou JF, Harding JJ, Ly M, Hrabovsky AD, et al. Phase II trial of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma after disease progression on sorafenib. Cancer Med. 2020;9:7453–9.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.
Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–49.
Nakamura T, Conrad M. Exploiting ferroptosis vulnerabilities in cancer. Nat Cell Biol. 2024;26:1407–19.
Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, et al. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997;272:32360–9.
Santofimia-Castaño P, Xia Y, Peng L, Velázquez-Campoy A, Abián O, Lan W, et al. Targeting the stress-induced protein NUPR1 to treat pancreatic adenocarcinoma. Cells. 2019;8:1453.
Murphy A, Costa M. Nuclear protein 1 imparts oncogenic potential and chemotherapeutic resistance in cancer. Cancer Lett. 2020;494:132–41.
Malicet C, Dagorn JC, Neira JL, Iovanna JL. p8 and prothymosin alpha: unity is strength. Cell Cycle. 2006;5:829–30.
Malicet C, Giroux V, Vasseur S, Dagorn JC, Neira JL, Iovanna JL. Regulation of apoptosis by the p8/prothymosin α complex. Proc Natl Acad Sci USA. 2006;103:2671–6.
Sandi MJ, Hamidi T, Malicet C, Cano C, Loncle C, Pierres A, et al. p8 Expression controls pancreatic cancer cell migration, invasion, adhesion, and tumorigenesis. J Cell Physiol. 2011;226:3442–51.
Ree AH, Pacheco MM, Tvermyr M, Fodstad Ø, Brentani MM. Expression of a novel factor, com1, in early tumor progression of breast cancer. Clin Cancer Res. 2000;6:1778–83.
Gironella M, Malicet C, Cano C, Sandi MJ, Hamidi T, Tauil RMN, et al. p8/nupr1 regulates DNA-repair activity after double-strand gamma irradiation-induced DNA damage. J Cell Physiol. 2009;221:594–602.
Palam LR, Gore J, Craven KE, Wilson JL, Korc M. Integrated stress response is critical for gemcitabine resistance in pancreatic ductal adenocarcinoma. Cell Death Dis. 2015;6:e1913.
Emma MR, Iovanna JL, Bachvarov D, Puleio R, Loria GR, Augello G, et al. NUPR1, a new target in liver cancer: implication in controlling cell growth, migration, invasion and sorafenib resistance. Cell Death Dis. 2016;7:e2269.
Li J, Ren S, Liu Y, Lian Z, Dong B, Yao Y, et al. Knockdown of NUPR1 inhibits the proliferation of glioblastoma cells via ERK1/2, p38 MAPK and caspase-3. J Neurooncol. 2017;132:15–26.
Zeng C, Yi B, Li X, Chen J. [Knockdown of nuclear protein 1 (NUPR1) gene inhibits proliferation and promotes apoptosis of human multiple myeloma U266 cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1240–6.
Zhou C, Xu J, Lin J, Lin R, Chen K, Kong J, et al. Long noncoding RNA FEZF1-AS1 promotes osteosarcoma progression by regulating the miR-4443/NUPR1 axis. Oncol Res. 2018;26:1335–43.
Guo X, Wang W, Hu J, Feng K, Pan Y, Zhang L, et al. Lentivirus-mediated RNAi knockdown of NUPR1 inhibits human nonsmall cell lung cancer growth in vitro and in vivo. Anat Rec. 2012;295:2114–21.
Cano CE, Hamidi T, Garcia MN, Grasso D, Loncle C, Garcia S, et al. Genetic inactivation of Nupr1 acts as a dominant suppressor event in a two-hit model of pancreatic carcinogenesis. Gut. 2014;63:984–95.
Santofimia-Castaño P, Rizzuti B, Abian O, Velázquez-Campoy A, Iovanna JL, Neira JL. Amphipathic helical peptides hamper protein-protein interactions of the intrinsically disordered chromatin nuclear protein 1 (NUPR1). Biochim Biophys Acta Gen Subj. 2018;1862:1283–95.
Santofimia-Castaño P, Rizzuti B, Pey ÁL, Soubeyran P, Vidal M, Urrutia R, et al. Intrinsically disordered chromatin protein NUPR1 binds to the C-terminal region of Polycomb RING1B. Proc Natl Acad Sci USA. 2017;114:E6332–41.
Neira JL, Rizzuti B, Iovanna JL. Determinants of the pKa values of ionizable residues in an intrinsically disordered protein. Arch Biochem Biophys. 2016;598:18–27.
Santofimia-Castaño P, Xia Y, Lan W, Zhou Z, Huang C, Peng L, et al. Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis. J Clin Invest. 2019;129:2500–13.
Santofimia-Castaño P, Rizzuti B, Xia Y, Abian O, Peng L, Velázquez-Campoy A, et al. Targeting intrinsically disordered proteins involved in cancer. Cell Mol Life Sci. 2020;77:1695–707.
Huang C, Santofimia-Castaño P, Liu X, Xia Y, Peng L, Gotorbe C, et al. NUPR1 inhibitor ZZW-115 induces ferroptosis in a mitochondria-dependent manner. Cell Death Discov. 2021;7:269.
Giroux V, Malicet C, Barthet M, Gironella M, Archange C, Dagorn J-C, et al. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res. 2006;12:235–41.
Santofimia-Castaño P, Fraunhoffer N, Liu X, Bessone IF, di Magliano MP, Audebert S, et al. Targeting NUPR1-dependent stress granules formation to induce synthetic lethality in KrasG12D-driven tumors. EMBO Mol Med. 2024;16:475–505.
Lan W, Santofimia-Castaño P, Xia Y, Zhou Z, Huang C, Fraunhoffer N, et al. Targeting NUPR1 with the small compound ZZW-115 is an efficient strategy to treat hepatocellular carcinoma. Cancer Lett. 2020;486:8–17.
Augello G, Emma MR, Azzolina A, Puleio R, Condorelli L, Cusimano A, et al. The NUPR1/p73 axis contributes to sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2021;519:250–62.
He W, Cheng F, Zheng B, Wang J, Zhao G, Yao Z, et al. NUPR1 is a novel potential biomarker and confers resistance to sorafenib in clear cell renal cell carcinoma by increasing stemness and targeting the PTEN/AKT/mTOR pathway. Aging. 2021;13:14015–38.
Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose–response matrix data. Bioinformatics. 2017;33:2413–5.
Yadav B, Wennerberg K, Aittokallio T, Tang J. Searching for drug synergy in complex dose–response landscapes using an interaction potency model. Comput Struct Biotechnol J. 2015;13:504–13.
LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–59.
Chen X, Duan N, Zhang C, Zhang W. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016;7:314–23.
Lee C-H, Lin Y-C, Chang Y-C, Chen P-C, Lin K-H, Yeh T-M, et al. NUPR1 contributes to endocrine therapy resistance by modulating BIRC5 expression and inducing luminal B-ERBB2+ subtype-like characteristics in estrogen receptor-positive breast cancer cells. J Cancer. 2025;16:1694–708.
Kim Y-S, Jin H-O, Seo S-K, Woo SH, Choe T-B, An S, et al. Sorafenib induces apoptotic cell death in human non-small cell lung cancer cells by down-regulating mammalian target of rapamycin (mTOR)-dependent survivin expression. Biochem Pharmacol. 2011;82:216–26.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–34.
Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Sorafenib. Profiles Drug Subst Excip Relat Methodol. 2019;44:239–66.
Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–9.
Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21.
Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today. 2005;41:773–84.
Patt Y, Rojas-Hernandez C, Fekrazad HM, Bansal P, Lee FC. Phase II trial of sorafenib in combination with capecitabine in patients with hepatocellular carcinoma: INST 08-20. Oncologist. 2017;22:1158–e116.
Fan G, Wei X, Xu X. Is the era of sorafenib over? A review of the literature. Ther Adv Med Oncol. 2020;12:1758835920927602.
Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L, et al. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Cancer Res. 2023;42:6.
Yang C, Lu T, Liu M, Yuan X, Li D, Zhang J, et al. Tiliroside targets TBK1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine. 2023;111:154668.
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84.
Fan Z, Wirth A-K, Chen D, Wruck CJ, Rauh M, Buchfelder M, et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371.
Roh J-L, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–62.
Tong R, Feng X, Sun J, Ling Z, Wang J, Li S, et al. Co-delivery of siNRF2 and sorafenib by a “click” dual functioned hyperbranched nanocarrier for synergistically inducing ferroptosis in hepatocellular carcinoma. Small. 2024;20:2307273.
Zheng J, Sato M, Mishima E, Sato H, Proneth B, Conrad M. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 2021;12:1–10.
Cruz-Ojeda P, de la, Navarro-Villarán E, Fuertes-Agudo M, Mata A, López-Lluch G, Navas P, et al. Peroxynitrite is involved in the mitochondrial dysfunction induced by sorafenib in liver cancer cells. Free Radic Biol Med. 2024;S0891-5849:01162–6.
Liu J, Song X, Kuang F, Zhang Q, Xie Y, Kang R, et al. NUPR1 is a critical repressor of ferroptosis. Nat Commun. 2021;12:647.
Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64:488–500.
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79.
Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19:1800311.
Lei P, Bai T, Sun Y Mechanisms of Ferroptosis And Relations With Regulated Cell Death: A Review. Front Physiol. 2019. https://doi.org/10.3389/fphys.2019.00139.
Garrido A, Lepailleur A, Mignani SM, Dallemagne P, Rochais C. hERG toxicity assessment: useful guidelines for drug design. Eur J Med Chem. 2020;195:112290.
Liu X, Jimenez-Alesanco A, Li Z, Rizzuti B, Neira JL, Estaras M, et al. Development of an efficient NUPR1 inhibitor with anticancer activity. Sci Rep. 2024;14:29515.
Funding
This work was supported by La Ligue Contre le Cancer, INCa, Canceropole PACA, and INSERM to JI; Foundation ARC to PSC; China Scholarship Council (to XL); Programme XU GUANGQI to JI. ME is supported by Foundation ARC.
Author information
Authors and Affiliations
Contributions
Xi Liu: Data curation, Formal analysis, Investigation, Writing – original draft, Matías Estaras: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Emma Cosialls: Data curation, Formal analysis, Investigation, Writing – review & editing, Ling Peng: Funding acquisition, Supervision, Writing – review & editing, Patricia Santofimia-Castaño: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing, Juan Iovanna: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
A patent has been filed containing part of this study: PCT filing number n° PCT/EP2025/053879, NUPR1 inhibitors for treating cancer. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Ethical approval was obtained from the Animal Experimentation Ethics Committee No. 014, under the authorization number APAFIS#40941-2023021415217230 v4.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the author(s) used ChatGPT in order to correct the grammar of the text. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Boris Zhivotovsky
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Estaras, M., Cosialls, E. et al. Synergistic antitumor activity of sorafenib and the NUPR1 inhibitor LZX-2-73 in multiple cancer models. Cell Death Dis 16, 839 (2025). https://doi.org/10.1038/s41419-025-08178-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41419-025-08178-8