Abstract
Cardiovascular diseases (CVDs) remain a global health challenge, with programmed cell death (PCD) mechanisms like apoptosis and necroptosis playing key roles in the progression. Circular RNAs (circRNAs) have recently been recognized as crucial regulators of gene expression, especially in modulating PCD. In current researches, circRNA regulation of apoptosis is the most studied area, followed by autophagy and ferroptosis. Notably, the regulatory role of circRNAs in pyroptosis and necroptosis has also begun to attract attention. From a mechanistic perspective, circRNAs influence cellular processes through several modes of action, including miRNA sponging, protein interactions, and polypeptide translation. Manipulating circRNAs and their downstream targets through inhibition or overexpression offers versatile therapeutic options for CVD treatment. Continued investigation into circRNA-mediated mechanisms may enhance our understanding of CVD pathophysiology and underscore their potential as novel and promising therapeutic targets.
Similar content being viewed by others
Facts
CircRNAs regulate multiple types of programmed cell death (PCD) in cardiovascular diseases (CVDs), including apoptosis, autophagy, ferroptosis, pyroptosis, and necroptosis, with apoptosis being the most extensively studied.
CircRNAs primarily function as molecular sponges for miRNAs, influencing signaling pathways such as NF-κB, PI3K/AKT, and TGF-β1, thereby affecting cardiomyocyte survival and death.
CircRNAs have potential as diagnostic and prognostic biomarkers in CVDs due to their stability, specificity, and detectability in extracellular vesicles.
Recent studies suggest that circRNAs play an essential role in regulating ferroptosis and its interaction with autophagy, offering a novel target for therapeutic intervention in conditions such as myocardial infarction and ischemia/reperfusion injury.
Despite advancements, challenges remain in understanding circRNA biogenesis, standardizing circRNA nomenclature, and translating circRNA-based research into clinical applications.
Open Questions
What are the precise molecular mechanisms by which circRNAs regulate pyroptosis and necroptosis in CVDs, and how do these processes interact with other forms of PCD?
How can circRNA-based therapeutics be safely and effectively applied in clinical settings, minimizing off-target effects while maximizing therapeutic benefits?
How do circRNAs interact with other non-coding RNAs and protein networks to coordinate cell death regulation in CVDs?
Introduction
Cardiovascular disease (CVD), which encompasses systemic vascular diseases affecting the heart and brain, poses a significant threat to human health [1] due to its high mortality, disability rates, recurrence rates, and numerous complications [2]. Despite advancements in research aimed at improving patient outcomes, clinical morbidity and mortality rates continue to rise [3, 4]. Consequently, there is a growing focus on discovering new treatment methods for CVD.
Programmed cell death (PCD), including apoptosis [5,6,7,8,9,10,11,12,13], necroptosis [14,15,16], pyroptosis [17,18,19,20], ferroptosis [6, 21,22,23,24,25], and autophagy-related cell death [26,27,28,29], is considered a key player in various cellular processes [30]. The abnormal activation of PCD pathways is implicated in the pathogenesis of various CVDs, such as ischemia/reperfusion (I/R) injury, myocardial infarction (MI), cardiomyopathy [31, 32] and atherosclerosis [33]. Accordingly, regulating cell death represents a significant potential measure for treating CVDs, and the timely activation of PCD can reshape the structure and function of the heart after injury [34].
Circular RNA (circRNA) is a large class of animal RNA with regulatory effects and typically shows tissue/developmental stage-specific expression [35]. Most circRNAs exhibit a high degree of conservation, while their expression patterns within individuals display tissue-specific or developmental specificity [36,37,38]. CircRNAs can regulate gene expression by serving as microRNA (miRNA) sponges or as scaffolds to facilitate contact between two or more proteins, thus representing potential regulators of cellular function [39,40,41,42]. Aberrant expression of circRNAs has been observed in certain CVDs like atherosclerosis, heart failure (HF), MI, and cardiomyopathy [43,44,45,46,47,48]. Studies also show that circRNA plays a key role in regulating cardiomyocyte apoptosis [49].
This article reviews the role of circRNA in CVD by regulating cell death patterns. It explores the origins, categorization, defining features, and functional roles of circRNA, and examines the distinctive attributes of PCD and its pivotal significance in cardiovascular ailments. We highlight the immense potential, future outlook, and therapeutic relevance of circRNA in modulating PCD to address CVD.
Overview of CircRNA
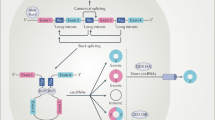
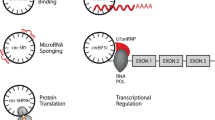
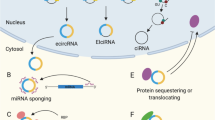
CircRNA was first discovered in 1976 in plant viruses and demonstrated by electron microscopy in 1979 [50, 51]. CircRNAs consist of a large class of noncoding RNAs whose formation is associated with a back-splicing process that covalently links a downstream splice donor site to an upstream splice acceptor site [52]. Current research has established that circRNAs are derived from the cleavage of pre-mRNA by the spliceosome or group I and group II ribozymes, and the competitive dynamic between the formation of linear RNA and circRNA during transcription in eukaryotic cells is also proved [53]. However, the precise molecular mechanisms underlying circRNA biogenesis remain incompletely understood [54]. The lariat-or-exon skipping model and the direct backsplicing model are two major mechanisms in canonical spliceosomes [55]. Most circRNAs are derived from known protein-coding genes through back-splicing events, identified as exonic circRNAs (ecircRNAs), intronic RNAs (ciRNAs), and exon-intron circRNAs (EIciRNAs) [56]. EcircRNA functions in the cytoplasm, while ciRNA and EIciRNA primarily operate within the nucleus [57]. CiRNA enhances the transcription rate of target genes by regulating the elongation activity of the RNA polymerase II complex [58], and EIciRNA is a crucial factor in influencing differential gene expression between cells [58,59,60]. Mitochondria-derived circular RNAs (mecciRNAs) from animals were newly discovered in 2020, and there remains significant potential for further exploration. These ciRNAs can exert their functions in the mitochondria, nucleus, and cytoplasm [61,62,63,64]. Some studies have also investigated tRNA intron circRNAs (tricRNAs) [65], interior circRNAs (i-circRNAs) [66], antisense circRNAs [67,68,69,70], and intergenic circRNAs [56, 71, 72]. In summary, circRNAs primarily function as molecular sponges that sequester specific miRNAs, thereby targeting and regulating mRNA translation. Additionally, circRNAs act as protein sponges, to serve as auxiliary or regulatory molecules for proteins, mRNA, and DNA. They can also serve as templates for translation and scaffolds for nuclear translocation [73,74,75]. They exhibit remarkable stability against RNase R digestion, and are abundantly expressed in various organisms, and demonstrate specificity to tissues, diseases, and developmental stages. These characteristics endow circRNAs with significant potential as biomarkers.
Circular RNAs modulate cell death in cardiovascular diseases
CircRNAs, attributable to their stability and ability to circulate via exosomes, have shown promise as potential biomarkers for diagnosing and monitoring various diseases. There has been growing interest in its role in CVD in recent years. Studies on apoptosis are relatively abundant, demonstrating that circRNAs modulate apoptosis through miRNA sponging and signaling pathways such as TGF-β1 and NF-κB, providing flexible therapeutic strategies. Investigations into autophagy have revealed that it frequently coexists with apoptosis in target cells, with circRNAs playing key roles in modulating these processes, suggesting therapeutic potential for myocardial cells. In terms of ferroptosis, researchers highlight its impact on cardiomyocyte death and cardiac function, with a growing focus on circRNA-regulated signaling pathways, particularly in animal models. Though studies regarding pyroptosis are still limited, emerging findings suggest that circRNAs’ involvement in its regulation shows promise for clinical treatment. Similarly, while necroptosis research remains in its early stages, the regulatory role of circRNAs is becoming more apparent, offering potential new therapeutic targets for CVDs (Fig. 1).
CircRNAs are non-coding RNAs formed via back-splicing, primarily through the lariat (exon-skipping) model or the direct back-splicing model. Based on their origin, circRNAs are categorized into EcircRNAs, ciRNAs, EIciRNAs, and mecciRNAs. They function through mechanisms such as miRNA sponging, regulation of pre-mRNA splicing, and protein scaffolding, with potential applications as therapeutic targets, therapeutic agents, and biomarkers.
Apoptosis
Apoptosis is directly induced by the intrinsic BCL-2 pathway and the extrinsic death receptor pathway, where apoptotic factors promote the activation of the caspase cascade, leading to protein cleavage and eventual cell destruction [76]. Apoptosis continues to be a focus in various fields, including CVDs, cancer, neurological disorders, and liver diseases [77,78,79,80,81,82]. The role of circular RNAs (circRNAs) in regulating apoptosis in CVDs has attracted significant attention [83], with numerous studies conducted on various cardiovascular conditions such as atherosclerosis, AMI, and myocardial I/R injury.
In the context of atherosclerosis-related circRNAs, circRSF1, circ_0007478, hsa_circ_0004831, circ_0026218, circLZIC, circ_0065149, circ_0030042, circHIPK3, circRNA-0024103, circ_0010283, hsa_circ_0001445, circ_0005699, among others, generally reduce apoptosis and mitigate ox-LDL-induced vascular endothelial cell damage by sponging miRNAs and processing downstream signals [84,85,86,87,88,89,90,91,92,93,94,95,96]. The upregulation of circNRG-1 has been demonstrated to alleviate apoptosis in atherosclerosis and hypertension model cells treated with ANGII, while circANKRD42 and circUBAC2 are believed to mitigate apoptosis in the context of atherosclerosis and MI through multiple signaling pathways, including the circUBAC2/hsa-miR-200b-3p/HIPK3 axis and circANKRD42/hsa-miR-324-5p/AP1G1 axis [97, 98]. Circ_0003645, circ_0004104, circANRIL, circTEX14, circTM7SF3, circ_USP36 (hsa_circ_0003204), circ_0029589, circMTO1, circ_0124644, circ_0005699, circUSP36, circ_0021155, circ_0006476 primarily function through miRNA sponging, but their increased levels exacerbate apoptosis in atherosclerosis model cells [7, 11, 48, 99,100,101,102,103,104,105,106,107,108].
Several studies have focused on the role of circRNAs in regulating apoptosis in AMI, involving circSAMD4A, circ_0060745, circRBMS1, circUSP39, and circ_0008842. These circRNAs primarily exert their effects by sponging miRNAs, except circ_0060745, which acts through the NF-κB pathway. When these circRNAs are upregulated, they enhance apoptosis and worsen AMI [109,110,111,112,113].
Myocardial I/R injury is another research hotspot. CircRbms1 has been shown to increase apoptosis and exacerbate hypoxia-induced cardiomyocyte injury through the miR-2355-3p/MST1 and miR‑742‑3p/FOXO1 axis [114, 115]. Other circRNAs involved include circ_0050908, circARPA1, circRNA Fbxl5, circ-0001380, circ_SMG6, and circHIPK3, which regulate apoptosis levels through various signaling pathways, primarily by sponging miRNAs, thereby influencing myocardial I/R injury [13, 116,117,118].
Beyond the aforementioned conditions, studies on circRNA regulation of apoptosis in CVDs such as abdominal aortic aneurysm, aortic aneurysm, atrial fibrillation, cancer therapy-related cardiovascular toxicity, coronary artery disease, and MI are summarized in the table (Table 1) [75, 103, 114, 116, 117, 119,120,121,122,123,124,125,126,127,128,129,130,131,132,133].
Currently, research on circRNA regulation of apoptosis in CVD treatment is relatively abundant. It is generally believed that circRNAs regulate apoptosis by sponging various corresponding miRNAs, as well as intervening in signaling pathways such as TGF-β1 and NF-κB. The inhibition or overexpression of specific circRNAs and their downstream molecular targets has different effects on apoptosis, offering flexible options in the clinical treatment of various CVDs (Fig. 2). This field has a solid research foundation and promising prospects for future studies.
CircRNAs influence apoptotic processes by interacting with specific miRNAs and modulating associated signaling cascades. Pro-apoptotic circRNAs intensify myocardial damage, while anti-apoptotic ones provide protective effects. The illustration highlights how various circRNAs contribute to the regulation of apoptosis during the progression of CVDs.
Autophagy
Autophagy refers to the process where autophagosomes encapsulate damaged proteins or organelles and transport them to lysosomes or vacuoles for degradation and recycling, typically to maintain homeostasis. Though, it is also implicated in cell death under certain circumstances [134]. Autophagy has garnered continuous attention in the fields of CVDs, cancer, neurodegenerative diseases, and metabolic disorders [135,136,137,138,139,140]. CircRNAs play a crucial role in regulating autophagy in CVDs [135, 141].
Studies have found that apoptosis and autophagy often occur simultaneously in the regulation of PCD in CVDs by circRNAs. CircRNA_101237 regulates IGF2BP3-dependent autophagy by sponging let-7a-5p, and the downregulation of IGF2BP3 resulting from circRNA_101237 downregulation reduces hypoxia/reoxygenation (H/R)-induced cardiomyocyte apoptosis and inhibits autophagy [140]. Ox-LDL-induced atherosclerosis remains a popular research focus. Circ_0002331 can enhance CCND2 activity to reduce autophagy and apoptosis, while circSQSTM1 exerts its effects through two pathways: one involves sponging miR-23b-3p, leading to increased Sirt1 expression, and the other enhances Sirt1 via the eIF4A3/FOXO1/Sirt1 axis [142, 143]. Knockdown of circPAN3 effectively alleviates autophagy and apoptosis, and improves cardiac function in MI mice via the miR-221/PTEN/AKT/PI3K pathway [144]. Circ-HIPK2 positively regulates ATG101 expression by sponging miR-485-5p, to accelerate apoptosis and cell death in myocardial oxidative damage induced by H2O2 [29]. Silencing circ_0010729 increases the viability of primary mouse cardiomyocytes and reduces OGD-induced myocardial cell injury by inhibiting apoptosis and autophagy through the miR-338-3p/CALM2 axis [145].
Research has proposed that circ-SIRT1 can promote autophagy in human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and H9c2 cardiomyocytes by activating SIRT1, thus mitigating Ang II-induced cardiac hypertrophy (CH), based on the finding that autophagy deficiency leads to CH [28].
In patients with coronary heart disease (CHD), hsa_circ_0030042 is significantly downregulated in peripheral blood. In ApoE−/− mice fed a high-fat diet, hsa_circ_0030042 counteracts eIF4A3-induced plaque instability and inhibits autophagy by sponging eIF4A3, preventing it from recruiting beclin1 and FOXO1 mRNA [27].
CircRNA ACR inhibits autophagy via the ACR-Pink1-FAM65B axis, where Pink1 suppresses autophagy and its downstream target FAM65B. When phosphorylated by Pink1, it also inhibits autophagy and cell death in the heart. ACR reduces myocardial I/R injury and MI area through this signaling pathway [146].
Several studies on circRNA regulation of CVDs through autophagy have noted that apoptosis and autophagy frequently coexist in target cells [147,148,149]. Furthermore, the exploration of circRNA regulatory mechanisms on autophagy and their downstream signals has advanced, particularly in deciphering molecular pathways associated with their miRNA-sponging functions (Fig. 3) [150,151,152,153]. The promotion or inhibition of autophagy by circRNAs may hold potential therapeutic value for myocardial cells in various CVDs.
CircRNAs modulate autophagic activity by acting as miRNA sponges and altering the activity of critical signaling pathways. The diagram depicts the roles of circRNAs in regulating three distinct forms of autophagy: (a) macroautophagy, (b) chaperone-mediated autophagy, and (c) microautophagy, within the context of CVDs.
Ferroptosis
Ferroptosis is a form of iron-dependent PCD in which the accumulation of lipid peroxides and reactive oxygen species generated by iron metabolism leads to lipid membrane damage and cell failure [154]. Ferroptosis continues to attract attention in the fields of CVDs, tumors, inflammation, kidney damage, etc. [155,156,157,158,159,160]. Circular RNA regulates ferroptosis and plays an important role in CVD [161, 162].
The link between autophagy and ferroptosis is gaining attention in the study of the molecular mechanisms by which circRNAs regulate CVDs. CircRNA1615 regulates the expression of LRP6 by sponging miR-152-3p, thereby preventing LRP6-mediated autophagy-associated ferroptosis in cardiomyocytes through the miRNA152-3p/LRP6 molecular axis, ultimately controlling the pathological process of MI [24].
CircPIK3C2A promotes ferroptosis in AIC-treated H9c2 cells by sponging miR-31-5p, which upregulates TFRC, thereby exacerbating I/R injury [163]. Circ_0091761 enhances ferroptosis and reduces cell viability in H9c2 cells under simulated heart failure conditions through the miR-335-3p/ASCL4 axis and the TFRC axis [164]. Similarly, circ_005077 aggravates the adverse cardiac effects in various myocardial lipotoxicity models by enhancing ferroptosis, but its effects are not mediated by sponging miRNA. Instead, it upregulates CyPA and downregulates p47PHOX [165].
Overexpression of circRNA FEACR can suppress H/R-induced ferroptosis, inhibit MI, and improve cardiac function. This effect is mediated through the circRNA FEACR-induced NAMPT-Sirt1-FOXO1-FTH1 signaling axis. FEACR and its downstream factors can be considered novel targets for mitigating ferroptosis-related MI in ischemic heart disease [25].
In a pathological CH model using transverse aortic constriction (TAC) mice, circCmss1 was significantly increased in normal TAC mice but decreased in NSD2−/− TAC mice. CircCmss1 interacts with the transcription factor EIF4A3 to induce the expression of transferrin receptor 1 (TfR1), thereby activating ferroptosis in cardiomyocytes [166].
The potential circRNA target for heart failure, circSnx12, can act as an endogenous sponge binding to miR-224-5p and regulating the miRNA binding site in the 3’UTR region of FTH1. Knockdown of circSnx12 increases ferroptosis, which is associated with mitochondrial abnormalities and myocardial cell death, exacerbating heart failure [167].
The role of circRNA-regulated ferroptosis in CVDs focuses on affecting cardiac function through the induction of cell death in cardiomyocytes and other cells. Current research frequently employs animal models of corresponding CVDs. The combined regulation of autophagy and ferroptosis by circRNAs is also receiving significant attention. Molecular information in this process, such as signaling axes involved in circRNA-regulated ferroptosis in CVDs, is gradually being elucidated, offering substantial targeted therapeutic potential for CVDs (Fig. 4).
Pyroptosis
Cell pyroptosis is a form of PCD associated with inflammatory responses [168]. Pyroptosis can be triggered by oxidative stress, hyperglycemia, inflammation, and other stimuli [169]. It is dependent on caspase-1 and is characterized by the release of large amounts of pro-inflammatory factors, making it a form of apoptosis specific to single cells [170]. Pyroptosis continues to receive widespread attention in the fields of CVDs, cancer, and metabolic diseases [171,172,173,174]. Its role in disease mechanisms, particularly through causing inflammation and tissue damage, is especially emphasized. Pyroptosis plays a crucial role in CVDs [175]. Currently, research on circRNA regulation of pyroptosis in the field of CVDs is relatively limited, primarily focusing on atherosclerosis, diabetic cardiomyopathy, heart failure, I/R injury, and MI.
In atherosclerosis mouse models and HUVECs treated with ox-LDL, upregulation of circ-USP9× has been observed. Circ-USP9× promotes ox-LDL-induced pyroptosis in HUVECs by binding to EIF4A3 and enhancing GSDMD stability in the cytoplasm. Conversely, the knockdown of circ-USP9× inhibits ox-LDL-induced pyroptosis in HUVECs [176]. CircRNA DICAR is considered an important endogenous regulator of diabetic cardiomyopathy and cardiomyocyte pyroptosis, functioning through DICAR-VCP-Med12 degradation. Studies have found that DICAR-deficient (DICAR+/−) mice exhibit spontaneous cardiac dysfunction and abnormal cardiomyocyte morphology, and DICAR knockout also enhances diabetic cardiomyocyte pyroptosis. Clinical samples have shown that DICAR expression in the circulating blood of diabetic patients is lower than in healthy controls [46].
The sponging function of circRNAs is significant in regulating pyroptosis. In the context of heart failure, circ-0006332 has been found to exacerbate pyroptosis and apoptosis, while also worsening cardiac dysfunction and myocardial fibrosis. This effect is mediated by sponging miR-143, leading to the upregulation of TLR2 [177]. As mentioned earlier, knockdown of circPAN3 has been shown to effectively alleviate autophagy and apoptosis, while another study focused on the reduction of pyroptosis following circPAN3 knockdown under I/R injury conditions, achieved by decreasing its sponging of miR-29b-3p [178]. CircDGKZ, via the miR-345-5p/TLR4/NF-κB axis, reduces pyroptosis while increasing autophagy, thereby mitigating I/R injury [179]. In cardiomyocytes, miR-133a-3p inhibit NLRP3 inflammasome activation induced by MI, while circHelz acts as an endogenous sponge for miR-133a-3p, inhibiting its activity and enhancing pyroptosis. CircHelz can also directly trigger NLRP3 inflammasome-mediated pro-inflammatory responses, causing MI. Therefore, silencing circHelz holds potential therapeutic value for alleviating ischemic heart disease [180].
Although the number of studies on circRNA regulation of pyroptosis in CVD treatment is still limited, the potential regulatory mechanisms of circRNAs on pyroptosis have gradually been uncovered. CircRNAs may serve as candidate drugs for the clinical treatment of CVDs or as upstream molecular signals, demonstrating significant research potential (Fig. 5).
Cell pyroptosis is dependent on caspase-1 and is characterized by the release of large amounts of pro-inflammatory factors, with or without sponging miRNA. The figure highlights pro-pyroptotic circRNAs in the context of CVD progression like circ-0006332, circ-Helz and circ-USP9x, underscoring their therapeutic potential.
Necroptosis
Necroptosis is a pathway independent of caspase activation, characterized morphologically by distinctive plasma membrane rupture, and induces an inflammatory response [181, 182]. Necrosis continues to attract attention in the fields of CVDs, tumors, neurological disorders, renal injury, and other diseases [155, 160, 183,184,185,186,187]. At present, there is a relatively limited amount of research on circRNA regulation of necroptosis in the field of CVDs.
In H2O2-induced necroptosis of human aorta smooth muscle cells, an upregulation of circHIPK3 was observed. CircHIPK3 impairs mitochondrial energy production and induces cell death by acting on DRP1, but its mechanism of action on mitochondrial function is independent of DRP1 abundance. In vivo experiments revealed that the downregulation of circHIPK3 can regulate necroptosis and vulnerable plaque formation in ApoE−/− mice, making it a promising therapeutic target for atherosclerosis [188].
Mmu_circ_000338, a cardiac-necroptosis-associated circRNA, is observed to be downregulated in cardiomyocytes exposed to H/R and in the hearts of mice with I/R injury. Overexpression of CNEACR reduces necroptosis and improves cardiac function in I/R injured hearts. The CNEACR/HDAC7/Foxa2/RIPK3 axis involved in this process may serve as an effective target for mitigating MI caused by necroptosis in ischemic heart disease (Table 2) [189].
The research on the regulation of necroptosis by circRNAs in CVDs is still in its early stages, involving relatively few CVDs and circRNAs. However, necroptosis has garnered extensive research attention across various disease fields, including CVDs. The signaling pathways by which circRNAs regulate necroptosis are gradually being elucidated, and the application of circRNA regulation of necroptosis in CVDs could provide new therapeutic targets, presenting strong developmental prospects for this field (Fig. 6).
Necroptosis is a caspase-independent cell death pathway associated with membrane rupture and inflammation. The figure depicts circRNA-regulated necroptotic signaling, including DRP1-mediated mitochondrial dysfunction and the Foxa2/RIPK3 axis, emphasizing circRNAs as emerging modulators in CVD pathogenesis.
Conclusions and perspectives
CVDs remain a critical global health challenge, with PCD playing a pivotal role in their progression. CircRNAs have garnered considerable attention for their ability to regulate PCD through various signaling pathways, thereby influencing the development and progression of CVDs. Significant advancements in this area have been achieved. For example, Made et al. constructed a circRNA-miRNA-mRNA dysregulation network in patients with ischemic heart failure, identifying approximately 662 circRNA-miRNA-mRNA interactions in the heart, thus providing novel insights into the pathogenesis of CVDs [190]. Another study in 2023 highlighted the potential of detecting differential circRNA profiles in peripheral circulation via exosomes, underscoring their promise as biomarkers and therapeutic carriers for targeted CVD treatment [191].
Clinical approaches to improving CVD treatment by targeting PCD have already been widely applied. Drugs such as dapagliflozin, carvedilol, dexmedetomidine, simvastatin, nicorandil, and trimetazidine have demonstrated therapeutic effects through various signaling pathways [192,193,194,195,196,197]. These therapies are effective against conditions like AMI, I/RI and HF, as well as mitigating cardiotoxicity induced by chemotherapeutic agents with strong PCD-inducing capabilities, such as cisplatin and paclitaxel [198,199,200,201,202,203,204,205,206,207]. However, the range of available drugs remains limited, highlighting the urgent need to develop new therapeutic strategies for CVD management. Research on circRNAs in CVDs continues to expand, with a primary focus on their role in regulating apoptosis, followed by autophagy and ferroptosis. In contrast, the effects of circRNAs on pyroptosis and necroptosis remain underexplored. While the regulatory roles of circRNAs in various types of cell death during CVD events are significant, and mediated signaling pathways have been partially elucidated, the majority of findings have yet to be translated into clinical applications [208].
Several challenges hinder the clinical translation of circRNA research. First, the lack of a standardized nomenclature for circRNAs complicates communication among researchers [209], as the same circRNA may be referred to by different names in different studies. Second, circRNAs may also play roles in maintaining normal physiological functions, making circRNA-targeted therapies a double-edged sword [21]. Lastly, while the use of circRNAs as diagnostic biomarkers and therapeutic targets represents an emerging direction, their full potential remains to be explored [210].
In summary, the interplay between circRNAs and PCD in the context of CVDs has attracted increasing attention and made substantial progress. This promising field is poised for significant future advancements.
Data availability
All data generated or analyzed during this study are included in this article.
References
Teo KK, Rafiq T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can J Cardiol. 2021;37:733–43. https://doi.org/10.1016/j.cjca.2021.02.009.
Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. https://doi.org/10.1016/j.jacc.2017.04.052.
Damen JAAG, Hooft L, Schuit E, Debray TPA, Collins GS, Tzoulaki I, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416 https://doi.org/10.1136/bmj.i2416.
Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685–95. https://doi.org/10.1038/s41569-023-00877-z.
XUAN F, JIAN J. Epigallocatechin gallate exerts protective effects against myocardial ischemia/reperfusion injury through the PI3K/Akt pathway-mediated inhibition of apoptosis and the restoration of the autophagic flux. Int J Mol Med. 2016;38:328–36. https://doi.org/10.3892/ijmm.2016.2615.
Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052–9. https://doi.org/10.7150/thno.54113.
Kou L, Yang N, Dong B, Yang J, Song Y, Li Y, et al. Circular RNA testis-expressed 14 overexpression induces apoptosis and suppresses migration of ox-LDL-stimulated vascular smooth muscle cells via regulating the microRNA 6509-3p/thanatos-associated domain-containing apoptosis-associated protein 1 axis. Bioengineered. 2022;13:13150–61. https://doi.org/10.1080/21655979.2022.2070582.
Zhang Z, Li L, Shi H, Chen B, Li X, Zhang Y, et al. Role of Circular RNAs in Atherosclerosis through Regulation of Inflammation, Cell Proliferation, Migration, and Apoptosis: Focus on Atherosclerotic Cerebrovascular Disease. Medicine. 2023;59:1461 https://doi.org/10.3390/medicina59081461.
Huang L, Guo B, Yan J, Wei H, Liu S, Li Y. CircHSPG2 knockdown attenuates hypoxia-induced apoptosis, inflammation, and oxidative stress in human AC16 cardiomyocytes by regulating the miR-1184/MAP3K2 axis. Cell Stress Chaperones. 2023;28:177–90. https://doi.org/10.1007/s12192-023-01328-x.
Zhang P, Wang W, Li M. Circ_0010283/miR-377-3p/Cyclin D1 Axis Is Associated With Proliferation, Apoptosis, Migration, and Inflammation of Oxidized Low-density Lipoprotein-Stimulated Vascular Smooth Muscle Cells. J Cardiovasc Pharmacol. 2021;78:437–47.
Wang X, Bai M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc Disord. 2021;21:51 https://doi.org/10.1186/s12872-020-01800-x.
Zheng C, Niu H, Li M, Zhang H, Yang Z, Tian L, et al. Cyclic RNA hsa-circ-000595 regulates apoptosis of aortic smooth muscle cells. Mol Med Rep. 2015;12:6656–62. https://doi.org/10.3892/mmr.2015.4264.
Li D, You J, Mao C, Zhou E, Han Z, Zhang J, et al. Circular RNA Fbxl5 Regulates Cardiomyocyte Apoptosis During Ischemia Reperfusion Injury via Sponging microRNA-146a. J Inflamm Res. 2022;15:2539–50. https://doi.org/10.2147/JIR.S360129.
Oerlemans MIFJ, Koudstaal S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JPG. Targeting cell death in the reperfused heart: Pharmacological approaches for cardioprotection. Int J Cardiol. 2013;165:410–22. https://doi.org/10.1016/j.ijcard.2012.03.055.
Oerlemans MIFJ, Liu J, Arslan F, Ouden K, Middelaar BJ, Doevendans PA, et al. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia–reperfusion in vivo. Basic Res Cardiol. 2012;107:270 https://doi.org/10.1007/s00395-012-0270-8.
Wang J, Wang Y. Circular RNA cerebellar degeneration-related protein 1 antisense RNA (Circ-CDR1as) downregulation induced by dexmedetomidine treatment protects hippocampal neurons against hypoxia/reoxygenation injury through the microRNA-28-3p (miR-28-3p)/tumor necrosis factor receptor-associated factor-3 (TRAF3) axis. Bioengineered. 2021;12:10512–24. https://doi.org/10.1080/21655979.2021.1999369.
Ding S, Liu D, Wang L, Wang G, Zhu Y. Inhibiting MicroRNA-29a Protects Myocardial Ischemia-Reperfusion Injury by Targeting SIRT1 and Suppressing Oxidative Stress and NLRP3-Mediated Pyroptosis Pathway. J Pharmacol Exp Therapeutics. 2020;372:128–35. https://doi.org/10.1124/jpet.119.256982.
Lu Y, Lu Y, Meng J, Wang Z. Pyroptosis and Its Regulation in Diabetic Cardiomyopathy. Front Physiol. 2022;12:791848 https://doi.org/10.3389/fphys.2021.791848.
Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, et al. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun. 2016;471:423–9. https://doi.org/10.1016/j.bbrc.2016.02.065.
Sun Y, Chu S, Wang R, Xia R, Sun M, Gao Z, et al. Non-coding RNAs modulate pyroptosis in myocardial ischemia-reperfusion injury: A comprehensive review. Int J Biol Macromol. 2024;257:128558 https://doi.org/10.1016/j.ijbiomac.2023.128558.
Liu Y, Ding W, Wang J, Ao X, Xue J. Non-coding RNA-mediated modulation of ferroptosis in cardiovascular diseases. Biomed Pharmacother. 2023;164:114993 https://doi.org/10.1016/j.biopha.2023.114993.
Li W, Feng G, Gauthier JM, Lokshina I, Higashikubo R, Evans S, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Investig. 2019;129:2293–304. https://doi.org/10.1172/JCI126428.
Arabpour J, Rezaei K, Khojini JY, Razi S, Hayati MJ, Gheibihayat SM. The potential role and mechanism of circRNAs in Ferroptosis: A comprehensive review. Pathol Res Pr. 2024;255:155203 https://doi.org/10.1016/j.prp.2024.155203.
Li RL, Fan CH, Gong SY, Kang S. Effect and Mechanism of LRP6 on Cardiac Myocyte Ferroptosis in Myocardial Infarction. Oxid Med Cell Longev. 2021;2021:8963987 https://doi.org/10.1155/2021/8963987.
Ju J, Li XM, Zhao XM, Li FH, Wang SC, Wang K, et al. Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J Biomed Sci. 2023;30:45 https://doi.org/10.1186/s12929-023-00927-1.
Chen Y, Zhao Y, Mishra PK. Editorial: Autophagy-Mediated Cell Survival and Death in Disease Progression and Treatment. Front Cell Dev Biol. 2022;10:916347 https://doi.org/10.3389/fcell.2022.916347.
Yu F, Zhang Y, Wang Z, Gong W, Zhang C. Hsa_circ_0030042 regulates abnormal autophagy and protects atherosclerotic plaque stability by targeting eIF4A3. Theranostics. 2021;11:5404–17. https://doi.org/10.7150/thno.48389.
Wang W, Wang L, Yang M, Wu C, Lan R, Wang W, et al. Circ-SIRT1 inhibits cardiac hypertrophy via activating SIRT1 to promote autophagy. Cell Death Dis. 2021;12:1069 https://doi.org/10.1038/s41419-021-04059-y.
Zhou J, Li L, Hu H, Wu J, Chen H, Feng K, et al. Circ-HIPK2 Accelerates Cell Apoptosis and Autophagy in Myocardial Oxidative Injury by Sponging miR-485-5p and Targeting ATG101. J Cardiovasc Pharmacol. 2020;76:427–36.
Kari S, Subramanian K, Altomonte IA, Murugesan A, Yli-Harja O, Kandhavelu M. Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis. 2022;27:482–508. https://doi.org/10.1007/s10495-022-01735-y.
Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23:90–100. https://doi.org/10.1016/j.arr.2015.04.002.
Yang Z, Li C, Wang Y, Yang J, Yin Y, Liu M, et al. Melatonin attenuates chronic pain related myocardial ischemic susceptibility through inhibiting RIP3-MLKL/CaMKII dependent necroptosis. J Mol Cell Cardiol. 2018;125:185–94. https://doi.org/10.1016/j.yjmcc.2018.10.018.
Liu C, Jiang Z, Pan Z, Yang L. The Function, Regulation and Mechanism of Programmed Cell Death of Macrophages in Atherosclerosis. Front Cell Dev Biol. 2022;9:809516 https://doi.org/10.3389/fcell.2021.809516.
Zhou L, Sun J, Gu L, Wang S, Yang T, Wei T, et al. Programmed Cell Death: Complex Regulatory Networks in Cardiovascular Disease. Front Cell Dev Biol. 2021;9:794879 https://doi.org/10.3389/fcell.2021.794879.
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. https://doi.org/10.1038/nature11928.
Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-Type Specific Features of Circular RNA Expression. PLoS Genet. 2013;9:e1003777 https://doi.org/10.1371/journal.pgen.1003777.
Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57. https://doi.org/10.1261/rna.035667.112.
Misir S, Wu N, Yang BB. Specific expression and functions of circular RNAs. Cell Death Differ. 2022;29:481–91. https://doi.org/10.1038/s41418-022-00948-7.
Panni S, Lovering RC, Porras P, Orchard S. Non-coding RNA regulatory networks. Biochim Biophys Acta Gene Regul Mech. 2020;1863:194417 https://doi.org/10.1016/j.bbagrm.2019.194417.
Kameda S, Ohno H, Saito H. Synthetic circular RNA switches and circuits that control protein expression in mammalian cells. Nucleic Acids Res. 2023;51:e24–e24. https://doi.org/10.1093/nar/gkac1252.
Zhou W-Y, Cai Z-R, Liu J, Wang D-S, Ju H-Q, Xu R-H. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172 https://doi.org/10.1186/s12943-020-01286-3.
Huang A, Zheng H, Wu Z, Chen M, Huang Y. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10:3503–17. https://doi.org/10.7150/thno.42174.
Huang S, Li X, Zheng H, Si X, Li B, Wei G, et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation. 2019;139:2857–76. https://doi.org/10.1161/CIRCULATIONAHA.118.038361.
Zheng H, Huang S, Wei G, Sun Y, Li C, Si X, et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol Ther. 2022;30:3477–98. https://doi.org/10.1016/j.ymthe.2022.06.016.
van Heesch S, Witte F, Schneider-Lunitz V, Schulz JF, Adami E, Faber AB, et al. The Translational Landscape of the Human Heart. Cell. 2019;178:242–.e29. https://doi.org/10.1016/j.cell.2019.05.010.
Yuan Q, Sun Y, Yang F, Yan D, Shen M, Jin Z, et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Sig Transduct Target Ther. 2023;8:99 https://doi.org/10.1038/s41392-022-01306-2.
Wang K, Long B, Liu F, Wang J-X, Liu C-Y, Zhao B, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37:2602–11. https://doi.org/10.1093/eurheartj/ehv713.
Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W, et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7:12429 https://doi.org/10.1038/ncomms12429.
Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8:5855–69. https://doi.org/10.7150/thno.27285.
HSU M-T, COCA-PRADOS M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–40. https://doi.org/10.1038/280339a0.
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci. 1976;73:3852–6. https://doi.org/10.1073/pnas.73.11.3852.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91. https://doi.org/10.1038/s41576-019-0158-7.
Vicens Q, Westhof E. Biogenesis of Circular RNAs. Cell. 2014;159:13–4. https://doi.org/10.1016/j.cell.2014.09.005.
Nisar S, Bhat AA, Singh M, Karedath T, Rizwan A, Hashem S, et al. Insights Into the Role of CircRNAs: Biogenesis, Characterization, Functional, and Clinical Impact in Human Malignancies. Front Cell Dev Biol. 2021;9:617281 https://doi.org/10.3389/fcell.2021.617281.
Eger N, Schoppe L, Schuster S, Laufs U, Boeckel J-N. Circular RNA Splicing. Adv Exp Med Biol. 2018;1087:41–52. https://doi.org/10.1007/978-981-13-1426-1_4.
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94 https://doi.org/10.1186/s12943-017-0663-2.
Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H, et al. Circular Intronic Long Noncoding RNAs. Mol Cell. 2013;51:792–806. https://doi.org/10.1016/j.molcel.2013.08.017.
Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M, Calin GA. Going circular: history, present, and future of circRNAs in cancer. Oncogene. 2023;42:2783–800. https://doi.org/10.1038/s41388-023-02780-w.
Hu Q, Zhou T. EIciRNA‐mediated gene expression: tunability and bimodality. FEBS Lett. 2018;592:3460–71. https://doi.org/10.1002/1873-3468.13253.
Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. https://doi.org/10.1038/nsmb.2959.
Liu X, Wang X, Li J, Hu S, Deng Y, Yin H, et al. Identification of mecciRNAs and their roles in the mitochondrial entry of proteins. Sci China Life Sci. 2020;63:1429–49. https://doi.org/10.1007/s11427-020-1631-9.
Ren B, Guan M-X, Zhou T, Cai X, Shan G. Emerging functions of mitochondria-encoded noncoding RNAs. Trends Genet. 2023;39:125–39. https://doi.org/10.1016/j.tig.2022.08.004.
Liu X, Yang Y, Shan G. Identification and detection of mecciRNAs. Methods. 2021;196:147–52. https://doi.org/10.1016/j.ymeth.2021.02.006.
Liu B, Tian Y, He J, Gu Q, Jin B, Shen H, et al. The potential of mecciRNA in hepatic stellate cell to regulate progression of nonalcoholic hepatitis. J Transl Med. 2022;20:393 https://doi.org/10.1186/s12967-022-03595-1.
Schmidt CA, Matera AG. tRNA introns: Presence, processing, and purpose. WIREs RNA. 2020;11:1583 https://doi.org/10.1002/wrna.1583.
Liu X, Hu Z, Zhou J, Tian C, Tian G, He M, et al. Interior circular RNA. RNA Biol. 2020;17:87–97. https://doi.org/10.1080/15476286.2019.1669391.
Zhang J, Wang C, Jia C, Zhang Y, Qing X, Zhang Y, et al. The Role of Circular RNAs in the Physiology and Pathology of the Mammalian Ovary. Int J Mol Sci. 2022;23:15204 https://doi.org/10.3390/ijms232315204.
Ma J, Du WW, Zeng K, Wu N, Fang L, Lyu J, et al. An antisense circular RNA circSCRIB enhances cancer progression by suppressing parental gene splicing and translation. Mol Ther. 2021;29:2754–68. https://doi.org/10.1016/j.ymthe.2021.08.002.
Zhang H, Liu S, Li X, Yao L, Wu H, Baluška F, et al. An Antisense Circular RNA Regulates Expression of RuBisCO Small Subunit Genes in Arabidopsis. Front Plant Sci. 2021;12:665014 https://doi.org/10.3389/fpls.2021.665014.
Wu N, Li F, Yang W, Du WW, Awan FM, Zhang C, et al. Silencing mouse circular RNA circSlc8a1 by circular antisense cA-circSlc8a1 induces cardiac hepatopathy. Mol Ther. 2023;31:1688–704. https://doi.org/10.1016/j.ymthe.2022.10.005.
Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P, et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun. 2018;9:4475 https://doi.org/10.1038/s41467-018-06862-2.
Long F, Li L, Xie C, Ma M, Wu Z, Lu Z, et al. Intergenic CircRNA Circ_0007379 Inhibits Colorectal Cancer Progression by Modulating miR-320a Biogenesis in a KSRP-Dependent Manner. Int J Biol Sci. 2023;19:3781–803. https://doi.org/10.7150/ijbs.85063.
Wang Y, Wu C, Du Y, Li Z, Li M, Hou P, et al. Expanding uncapped translation and emerging function of circular RNA in carcinomas and noncarcinomas. Mol Cancer. 2022;21:13 https://doi.org/10.1186/s12943-021-01484-7.
Diallo LH, Tatin F, David F, Godet A-C, Zamora A, Prats A-C, et al. How are circRNAs translated by non-canonical initiation mechanisms? Biochimie. 2019;164:45–52. https://doi.org/10.1016/j.biochi.2019.06.015.
Zeng Y, Du WW, Wu Y, Yang Z, Awan FM, Li X, et al. A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics. 2017;7:3842–55. https://doi.org/10.7150/thno.19764.
Cavalcante GC, Schaan AP, Cabral GF, Santana-da-Silva MN, Pinto P, Vidal AF, et al. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int J Mol Sci. 2019;20:4133 https://doi.org/10.3390/ijms20174133.
Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–51. https://doi.org/10.1002/ana.410380604.
Lin X, Ouyang S, Zhi C, Li P, Tan X, Ma W, et al. Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch Biochem Biophys. 2022;715:109098 https://doi.org/10.1016/j.abb.2021.109098.
Sugiura R, Satoh R, Takasaki T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells. 2021;10:2509 https://doi.org/10.3390/cells10102509.
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8:603–19. https://doi.org/10.18632/aging.100934.
Xu X, Lai Y, Hua Z-C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39. https://doi.org/10.1042/BSR20180992.
Patel T, Gores GJ. Apoptosis and hepatobiliary disease. Hepatology. 1995;21:1725–41. https://doi.org/10.1002/hep.1840210635.
Chen C, Shen H, Huang Q, Li Q. The Circular RNA CDR1as Regulates the Proliferation and Apoptosis of Human Cardiomyocytes Through the miR-135a/HMOX1 and miR-135b/HMOX1 Axes. Genet Test Mol Biomark. 2020;24:537–48. https://doi.org/10.1089/gtmb.2020.0034.
Zhang X, Lu J, Zhang Q, Luo Q, Liu B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol Res. 2021;54:11 https://doi.org/10.1186/s40659-021-00335-5.
Guan Z, Lu R, Sun Y, Wang X, Yu C, Song T. Regulation of oxidized LDL-induced proliferation and migration in human vascular smooth muscle cells by a novel circ_0007478/miR-638/ROCK2 ceRNA network. Vasc Med. 2023;28:6–17. https://doi.org/10.1177/1358863X221137617.
Su G, Sun G, Lv J, Zhang W, Liu H, Tang Y, et al. Hsa_circ_0004831 downregulation is partially responsible for atorvastatinalleviated human umbilical vein endothelial cell injuries induced by ox-LDL through targeting the miR-182-5p/CXCL12 axis. BMC Cardiovasc Disord. 2021;21:221 https://doi.org/10.1186/s12872-021-01998-4.
Men X, Hu A, Xu T. CircLZIC regulates ox-LDL-induced HUVEC cell proliferation and apoptosis via Micro-330-5p/NOTCH2 axis in atherosclerosis. Clin Hemorheol Microcirc. 2024;87:115–27. https://doi.org/10.3233/CH-232063.
Yang L, Chen W, Li B, Hu Y, Lu H, Zhang P, et al. Circular RNA circ_0026218 Suppressed Atherosclerosis Progression via miR-338-3p/SIRT6 Axis. Biomed Res Int. 2023;2023:1–13. https://doi.org/10.1155/2023/5647758.
Cai Y, Xu L, Xu C, Wang Y, Fan C. Hsa_circ_0001445 inhibits ox-LDL-induced HUVECs inflammation, oxidative stress and apoptosis by regulating miRNA-640. Perfusion. 2022;37:86–94. https://doi.org/10.1177/0267659120979472.
Wei Z, Ran H, Yang C. CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sci. 2020;259:118241 https://doi.org/10.1016/j.lfs.2020.118241.
Feng Z, Zhu Y, Zhang J, Yang W, Chen Z, Li B. Hsa-circ_0010283 Regulates Oxidized Low-Density Lipoprotein-Induced Proliferation and Migration of Vascular Smooth Muscle Cells by Targeting the miR-133a-3p/Pregnancy-Associated Plasma Protein A Axis. Circ J. 2020;84:2259–69. https://doi.org/10.1253/circj.CJ-20-0345.
Kang L, Jia H, Huang B, Lu S, Chen Z, Shen J, et al. Identification of Differently Expressed mRNAs in Atherosclerosis Reveals CDK6 Is Regulated by circHIPK3/miR-637 Axis and Promotes Cell Growth in Human Vascular Smooth Muscle Cells. Front Genet. 2021;12:596169 https://doi.org/10.3389/fgene.2021.596169.
Yu L, Ma W, Song B, Wang S, Li X, Wang Z. Hsa_circ_0030042 Ameliorates Oxidized Low-Density Lipoprotein-Induced Endothelial Cell Injury via the MiR-616-3p/RFX7 Axis. Int Heart J. 2022;63:22–065. https://doi.org/10.1536/ihj.22-065.
Li D, Jin W, Sun L, Wu J, Hu H, Ma L. Circ_0065149 Alleviates Oxidized Low-Density Lipoprotein-Induced Apoptosis and Inflammation in Atherosclerosis by Targeting miR-330-5p. Front Genet. 2021;12:590633 https://doi.org/10.3389/fgene.2021.590633.
Cao X, Yang J, He L, Liu C. Circ_0005699 Expedites ox-LDL-Triggered Endothelial Cell Injury via Targeting miR-384/ASPH Axis. Cardiovasc Toxicol. 2024;24:1067–76. https://doi.org/10.1007/s12012-024-09889-8.
Tian Y, Zheng G, Xie H, Guo Y, Zeng H, Fu Y, et al. Study on the Mechanism of circRNA-0024103 Reducing Endothelial Cell Injury by Regulating miR-363/MMP-10. Contrast Media Mol Imaging. 2022;2022:1709325 https://doi.org/10.1155/2022/1709325.
Sun Y, Zhang S, Yue M, Li Y, Bi J, Liu H. Angiotensin II inhibits apoptosis of mouse aortic smooth muscle cells through regulating the circNRG-1/miR-193b-5p/NRG-1 axis. Cell Death Dis. 2019;10:362 https://doi.org/10.1038/s41419-019-1590-5.
Holme FA, Huse C, Kong XY, Broch K, Gullestad L, Anstensrud AK, et al. Circular RNA Profile in Atherosclerotic Disease: Regulation during ST-Elevated Myocardial Infarction. Int J Mol Sci. 2024;25:9014 https://doi.org/10.3390/ijms25169014.
Zhang C, Wang L, Shen Y. Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/TRIM14 axis in atherosclerosis. BMC Cardiovasc Disord. 2021;21:207 https://doi.org/10.1186/s12872-021-02012-7.
Zhang Y, Li W, Li H, Zhou M, Zhang J, Fu Y, et al. Circ_USP36 Silencing Attenuates Oxidized Low-Density Lipoprotein-Induced Dysfunction in Endothelial Cells in Atherosclerosis Through Mediating miR-197-3p/ROBO1 Axis. J Cardiovasc Pharm. 2021;78:e761–72. https://doi.org/10.1097/FJC.0000000000001124.
Yu H, Zhao L, Zhao Y, Fei J, Zhang W. Circular RNA circ_0029589 regulates proliferation, migration, invasion, and apoptosis in ox-LDL-stimulated VSMCs by regulating miR-424-5p/IGF2 axis. Vasc Pharm. 2020;135:106782 https://doi.org/10.1016/j.vph.2020.106782.
Wang G, Li Y, Liu Z, Ma X, Li M, Lu Q, et al. Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol Cell Biochem. 2020;471:51–61. https://doi.org/10.1007/s11010-020-03764-0.
Chen T, Li L, Ye B, Chen W, Zheng G, Xie H, et al. Knockdown of hsa_circ_0005699 attenuates inflammation and apoptosis induced by ox-LDL in human umbilical vein endothelial cells through regulation of the miR-450b-5p/NFKB1 axis. Mol Med Rep. 2022;26:290 https://doi.org/10.3892/mmr.2022.12806.
Qin M, Wang W, Zhou H, Wang X, Wang F, Wang H. Circular RNA circ_0003645 silencing alleviates inflammation and apoptosis via the NF-κB pathway in endothelial cells induced by oxLDL. Gene. 2020;755:144900 https://doi.org/10.1016/j.gene.2020.144900.
Cong L, Zhao L, Shi Y, Bai Y, Guo Z. Circ_0006476 modulates macrophage apoptosis through the miR-3074-5p/DLL4 axis: implications for Notch signalling pathway regulation in cardiovascular disease. Aging. 2024;16:11857–76. https://doi.org/10.18632/aging.206049.
Ji N, Wang Y, Gong X, Ni S, Zhang H. CircMTO1 inhibits ox-LDL-stimulated vascular smooth muscle cell proliferation and migration via regulating the miR-182-5p/RASA1 axis. Mol Med. 2021;27:73 https://doi.org/10.1186/s10020-021-00330-2.
Miao J, Wang B, Shao R, Wang Y. CircUSP36 knockdown alleviates oxidized low-density lipoprotein-induced cell injury and inflammatory responses in human umbilical vein endothelial cells via the miR-20a-5p/ROCK2 axis. Int J Mol Med. 2021;47. https://doi.org/10.3892/ijmm.2021.4873.
Lin J, Liu C, xu J, Li S, Dai D, Zhang L, et al. Circ_0021155 can participate in the phenotypic transformation of human vascular smooth muscle cells via the miR-4459/TRPM7 axis. Biochem Biophys Res Commun. 2022;630:133–42. https://doi.org/10.1016/j.bbrc.2022.08.065.
Zhai C, Qian G, Wu H, Pan H, Xie S, Sun Z, et al. Knockdown of circ_0060745 alleviates acute myocardial infarction by suppressing NF‐κB activation. J Cell Mol Med. 2020;24:12401–10. https://doi.org/10.1111/jcmm.15748.
Hu X, Ma R, Cao J, Du X, Cai X, Fan Y. CircSAMD4A aggravates H/R‐induced cardiomyocyte apoptosis and inflammatory response by sponging miR‐138‐5p. J Cell Mol Med. 2022;26:1776–84. https://doi.org/10.1111/jcmm.16093.
Zhang L, Wang M, Liao R, Han Q. Clinical Significance and Potential Mechanism of Circ_00008842 in Acute Myocardial Infarction. Int Heart J. 2024;65:24–009. https://doi.org/10.1536/ihj.24-009.
Wang J, Wang X, Cao M, Zhang L, Lin J. CircUSP39/miR-362-3p/TRAF3 Axis Mediates Hypoxia/ Reoxygenation-Induced Cardiomyocyte Oxidative Stress, Inflammation, and Apoptosis. Int Heart J. 2023;64:263–73. https://doi.org/10.1536/ihj.22-232.
Jin L, Zhang Y, Jiang Y, Tan M, Liu C. Circular RNA Rbms1 inhibited the development of myocardial ischemia reperfusion injury by regulating miR-92a/BCL2L11 signaling pathway. Bioengineered. 2022;13:3082–92. https://doi.org/10.1080/21655979.2022.2025696.
Liang Y, Jie H, Liu Q, Li C, Xiao R, Xing X, et al. Knockout of circRNA single stranded interacting protein 1 (circRBMS1) played a protective role in myocardial ischemia-reperfusion injury though inhibition of miR-2355-3p/Mammalian Sterile20-like kinase 1 (MST1) axis. Bioengineered. 2022;13:12726–37. https://doi.org/10.1080/21655979.2022.2068896.
Liu B, Guo K. CircRbms1 knockdown alleviates hypoxia-induced cardiomyocyte injury via regulating the miR-742-3p/FOXO1 axis. Cell Mol Biol Lett. 2022;27:31 https://doi.org/10.1186/s11658-022-00330-y.
Li X, Guo L, Wang J, Yang X. Pro-fibrotic and apoptotic activities of circARAP1 in myocardial ischemia–reperfusion injury. Eur J Med Res. 2023;28:84 https://doi.org/10.1186/s40001-023-01001-0.
Jin A, Zhang Q, Cheng H, Yang C, Wang X. Circ_0050908 up-regulates TRAF3 by sponging miR-324-5p to aggravate myocardial ischemia-reperfusion injury. Int Immunopharmacol. 2022;108:108740 https://doi.org/10.1016/j.intimp.2022.108740.
Wang L, Wang C, Sun Z, Du A, Shan F, Sun Z. Knockdown of Mmu-circ-0001380 Attenuates Myocardial Ischemia/Reperfusion Injury via Modulating miR-106b-5p/Phlpp2 Axis. J Cardiovasc Transl Res. 2023;16:1064–77. https://doi.org/10.1007/s12265-023-10383-9.
Yue J, Zhu T, Yang J, Si Y, Xu X, Fang Y, et al. CircCBFB-mediated miR-28-5p facilitates abdominal aortic aneurysm via LYPD3 and GRIA4. Life Sci. 2020;253:117533 https://doi.org/10.1016/j.lfs.2020.117533.
ZHENG C, NIU H, LI M, ZHANG H, YANG Z, TIAN L, et al. Cyclic RNA has-circ-000595 regulates apoptosis of aortic smooth muscle cells. Mol Med Rep. 2015;12:6656–62. https://doi.org/10.3892/mmr.2015.4264.
Shi Z, Ye S, Xiang Y, Wu D, Xu J, Yu J, et al. circFAT1(e2) Inhibits Cell Apoptosis and Facilitates Progression in Vascular Smooth Muscle Cells through miR-298/MYB Axis. Comput Math Methods Med. 2021;2021:1–11. https://doi.org/10.1155/2021/1922366.
Dai H, Zhao N, Zheng Y. CircLDLR Modulates the Proliferation and Apoptosis of Vascular Smooth Muscle Cells in Coronary Artery Disease Through miR-26-5p/KDM6A Axis. J Cardiovasc Pharm. 2022;80:132–9. https://doi.org/10.1097/FJC.0000000000001275.
Ji P, Song X, Lv Z. Knockdown of circ_0004104 Alleviates Oxidized Low-Density Lipoprotein-Induced Vascular Endothelial Cell Injury by Regulating miR-100/TNFAIP8 Axis. J Cardiovasc Pharm. 2021;78:269–79. https://doi.org/10.1097/FJC.0000000000001063.
Ye Q, Ju C, Ye Z, Tong J. Circ_ROBO2/miR-186-5p/TRIM14 axis regulates oxidized low-density lipoprotein-induced cardiac microvascular endothelial cell injury. Regen Ther. 2022;20:138–46. https://doi.org/10.1016/j.reth.2022.04.005.
Shan T-K, Yang T-T, Jing P, Bao Y-L, Zhou L-H, Zhu T, et al. Circular RNA IGF1R Promotes Cardiac Repair via Activating β-Catenin Signaling by Interacting with DDX5 in Mice after Ischemic Insults. Research. 2024;7:0451 https://doi.org/10.34133/research.0451.
Zhu Y, Zou C, Jia Y, Zhang H, Ma X, Zhang J. Knockdown of circular RNA circMAT2B reduces oxygen-glucose deprivation-induced inflammatory injury in H9c2 cells through up-regulating miR-133. Cell Cycle. 2020;19:2622–30. https://doi.org/10.1080/15384101.2020.1814025.
Wang S, Li L, Deng W, Jiang M. CircRNA MFACR Is Upregulated in Myocardial Infarction and Downregulates miR-125b to Promote Cardiomyocyte Apoptosis Induced by Hypoxia. J Cardiovasc Pharm. 2021;78:802–8. https://doi.org/10.1097/FJC.0000000000001123.
Zhang J, Tang Y, Zhang J, Wang J, He J, Zhang Z, et al. CircRNA ACAP2 Is Overexpressed in Myocardial Infarction and Promotes the Maturation of miR-532 to Induce the Apoptosis of Cardiomyocyte. J Cardiovasc Pharm. 2021;78:247–52. https://doi.org/10.1097/FJC.0000000000001065.
Luo C, Ling G, Lei B, Feng X, Xie X, Fang C, et al. Circular RNA PVT1 silencing prevents ischemia-reperfusion injury in rat by targeting microRNA-125b and microRNA-200a. J Mol Cell Cardiol. 2021;159:80–90. https://doi.org/10.1016/j.yjmcc.2021.05.019.
Bai M, Pan C-L, Jiang G-X, Zhang Y-M. CircRNA 010567 improves myocardial infarction rats through inhibiting TGF-β1. Eur Rev Med Pharm Sci. 2020;24:369–75. https://doi.org/10.26355/eurrev_202001_19935.
Bai M, Pan C-L, Jiang G-X, Zhang Y-M, Zhang Z. CircHIPK3 aggravates myocardial ischemia-reperfusion injury by binding to miRNA-124-3p. Eur Rev Med Pharm Sci. 2019;23:10107–14. https://doi.org/10.26355/eurrev_201911_19580.
Zhang L, Han B, Liu H, Wang J, Feng X, Sun W, et al. Circular RNA circACSL1 aggravated myocardial inflammation and myocardial injury by sponging miR-8055 and regulating MAPK14 expression. Cell Death Dis. 2021;12:487 https://doi.org/10.1038/s41419-021-03777-7.
Huang K, Huang D, Li Q, Zeng J, Qin T, Zhong J, et al. CircSorbs1 regulates myocardial regeneration and reduces cancer therapy-related cardiovascular toxicity through the Mir-99/GATA4 pathway. Discov Oncol. 2024;15:319 https://doi.org/10.1007/s12672-024-01075-0.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. https://doi.org/10.1002/path.2697.
Gao J, Chen X, Shan C, Wang Y, Li P, Shao K. Autophagy in cardiovascular diseases: role of noncoding RNAs. Mol Ther Nucleic Acids. 2021;23:101–18. https://doi.org/10.1016/j.omtn.2020.10.039.
Chen T, Tu S, Ding L, Jin M, Chen H, Zhou H. The role of autophagy in viral infections. J Biomed Sci. 2023;30:5 https://doi.org/10.1186/s12929-023-00899-2.
Mizushima N, Levine B. Autophagy in Human Diseases. N Engl J Med. 2020;383:1564–76. https://doi.org/10.1056/NEJMra2022774.
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42. https://doi.org/10.1038/nrc.2017.53.
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. EMBO J. 2021;40:108863 https://doi.org/10.15252/embj.2021108863.
Gan J, Yuan J, Liu Y, Lu Z, Xue Y, Shi L, et al. Circular RNA_101237 mediates anoxia/reoxygenation injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int J Mol Med. 2019. https://doi.org/10.3892/ijmm.2019.4441.
Yu Z, Huang Q, Zhang Q, Wu H, Zhong Z. CircRNAs open a new era in the study of cardiovascular disease (Review). Int J Mol Med. 2020;47:49–64. https://doi.org/10.3892/ijmm.2020.4792.
Chen Z, Wang R, Zhu Y, Huang Z, Yang X, Li Q, et al. A novel circular RNA, circSQSTM1, protects the endothelial function in atherosclerosis. Free Radic Biol Med. 2023;209:301–19. https://doi.org/10.1016/j.freeradbiomed.2023.10.398.
Chen F, Yu X. Circ_0002331 Interacts with ELAVL1 to Improve ox-LDL-Induced Vascular Endothelial Cell Dysfunction via Regulating CCND2 mRNA Stability. Cardiovasc Toxicol. 2024;24:625–36. https://doi.org/10.1007/s12012-024-09865-2.
Farazi MM, Rostamzadeh F, Jafarinejad-Farsangi S, Moazam Jazi M, Jafari E, Gharbi S. CircPAN3/miR-221/PTEN axis and apoptosis in myocardial Infarction: Quercetin’s regulatory effects. Gene. 2024;909:148316 https://doi.org/10.1016/j.gene.2024.148316.
Ma B, Zhao M, Guo Z. Circular RNA circ_0010729 Knockdown Attenuates Oxygen–Glucose Deprivation-Induced Human Cardiac Myocytes Injury by miR-338-3p/CALM2 Axis. J Cardiovasc Pharm. 2021;77:594–602. https://doi.org/10.1097/FJC.0000000000000988.
Zhou L-Y, Zhai M, Huang Y, Xu S, An T, Wang Y-H, et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/ FAM65B pathway. Cell Death Differ. 2019;26:1299–315. https://doi.org/10.1038/s41418-018-0206-4.
Wang H, Han J, Kong H, Ma C, Zhang X. The Emerging Role of m6A and Programmed Cell Death in Cardiovascular Diseases. Biomolecules. 2025;15:247 https://doi.org/10.3390/biom15020247.
Wu H, Lan Q, He Y-X, Xue J-Y, Liu H, Zou Y, et al. Programmed cardiomyocyte death in myocardial infarction. Apoptosis. 2025;30:597–615. https://doi.org/10.1007/s10495-025-02075-3.
Lee T-L, Shen W-C, Chen Y-C, Lai T-C, Lin S-R, Lin S-W, et al. Mir221- and Mir222 -enriched adsc-exosomes mitigate PM exposure-exacerbated cardiac ischemia-reperfusion injury through the modulation of the BNIP3-MAP1LC3B-BBC3/PUMA pathway. Autophagy. 2025;21:374–93. https://doi.org/10.1080/15548627.2024.2395799.
Li F, Long T-Y, Bi S-S, Sheikh SA, Zhang C-L. circPAN3 exerts a profibrotic role via sponging miR-221 through FoxO3/ATG7-activated autophagy in a rat model of myocardial infarction. Life Sci. 2020;257:118015 https://doi.org/10.1016/j.lfs.2020.118015.
Chen T, Wei Y, Kang J, Zhang D, Ye J, Sun X, et al. ADAR1‐HNRNPL‐Mediated CircCANX Decline Promotes Autophagy in Chronic Obstructive Pulmonary Disease. Adv Sci. 2025;2414211. https://doi.org/10.1002/advs.202414211.
Zhao Q, Cai D, Xu H, Gao Y, Zhang R, Zhou X, et al. o8G-modified circPLCE1 inhibits lung cancer progression via chaperone-mediated autophagy. Mol Cancer. 2025;24:82 https://doi.org/10.1186/s12943-025-02283-0.
Shao M, Jin M, Feizhou L, Ma X, Wei Z. Administration of hypoxic pretreated adipose-derived mesenchymal stem cell exosomes promotes spinal cord repair after injury via delivery of circ-Astn1 and activation of autophagy. Int Immunopharmacol. 2025;152:114324 https://doi.org/10.1016/j.intimp.2025.114324.
Zheng X, Jin X, Ye F, Liu X, Yu B, Li Z, et al. Ferroptosis: a novel regulated cell death participating in cellular stress response, radiotherapy, and immunotherapy. Exp Hematol Oncol. 2023;12:65 https://doi.org/10.1186/s40164-023-00427-w.
Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A. Ferroptosis and Necroptosis in the Kidney. Cell Chem Biol. 2020;27:448–62. https://doi.org/10.1016/j.chembiol.2020.03.016.
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108 https://doi.org/10.1016/j.biopha.2020.110108.
Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215–27. https://doi.org/10.1016/j.molcel.2022.03.022.
Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. https://doi.org/10.1038/s41571-020-00462-0.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. https://doi.org/10.1038/s41580-020-00324-8.
Khoury MK, Gupta K, Franco SR, Liu B. Necroptosis in the Pathophysiology of Disease. Am J Pathol. 2020;190:272–85. https://doi.org/10.1016/j.ajpath.2019.10.012.
Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharm Res. 2021;166:105466 https://doi.org/10.1016/j.phrs.2021.105466.
Li F, Li P-F, Hao X-D. Circular RNAs in ferroptosis: regulation mechanism and potential clinical application in disease. Front Pharm. 2023;14:1173040 https://doi.org/10.3389/fphar.2023.1173040.
Miao S, Yang L, Xu T, Liu Z, Zhang Y, Ding L, et al. A novel circPIK3C2A/miR‐31‐5p/TFRC axis drives ferroptosis and accelerates myocardial injury. MedComm. 2024;5:571 https://doi.org/10.1002/mco2.571.
Wei Q, Jiang M, Tang B, You L, Zhao L. Downregulation of circular RNA 00091761 protects against heart failure after myocardial infarction via microRNA-335-3p/ ASCL4 axis. Acta Biochim Pol. 2023;70:509–16. https://doi.org/10.18388/abp.2020_6404.
Ni X, Duan L, Bao Y, Li J, Zhang X, Jia D, et al. Circ_005077 accelerates myocardial lipotoxicity induced by high-fat diet via CyPA/p47PHOX mediated ferroptosis. Cardiovasc Diabetol. 2024;23:129 https://doi.org/10.1186/s12933-024-02204-3.
Xu Q, Liu J, Zhu R, Huang W, Huang H, Liu J, et al. NSD2 promotes pressure overload-induced cardiac hypertrophy via activating circCmiss1/TfR1/ferroptosis signaling. Life Sci. 2023;328:121873 https://doi.org/10.1016/j.lfs.2023.121873.
Zheng H, Shi L, Tong C, Liu Y, Hou M. circSnx12 Is Involved in Ferroptosis During Heart Failure by Targeting miR-224-5p. Front Cardiovasc Med. 2021;8:656093 https://doi.org/10.3389/fcvm.2021.656093.
Hu Q, Zhang T, Yi L, Zhou X, Mi M. Dihydromyricetin inhibits NLRP3 inflammasome‐dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. BioFactors. 2018;44:123–36. https://doi.org/10.1002/biof.1395.
Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, et al. Induction of Inflammasome-dependent Pyroptosis by Carbon Black Nanoparticles. J Biol Chem. 2011;286:21844–52. https://doi.org/10.1074/jbc.M111.238519.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. https://doi.org/10.1038/nature15514.
Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M, et al. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid Med Cell Longev. 2017;2017:1–17. https://doi.org/10.1155/2017/9743280.
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310–29. https://doi.org/10.7150/thno.71086.
Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11:492 https://doi.org/10.1002/ctm2.492.
Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595 https://doi.org/10.1016/j.biopha.2019.109595.
Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019;52:12563 https://doi.org/10.1111/cpr.12563.
Xu S, Ge Y, Wang X, Yin W, Zhu X, Wang J, et al. Circ-USP9X interacts with EIF4A3 to promote endothelial cell pyroptosis by regulating GSDMD stability in atherosclerosis. Clin Exp Hypertens. 2023;45:2186319 https://doi.org/10.1080/10641963.2023.2186319.
Zhang P, Liu Y, Zhan Y, Zou P, Cai X, Chen Y, et al. Circ-0006332 stimulates cardiomyocyte pyroptosis via the miR-143/TLR2 axis to promote doxorubicin-induced cardiac damage. Epigenetics. 2024;19:2380145 https://doi.org/10.1080/15592294.2024.2380145.
An L, Zhong Y, Tan J, Liu Y, Li A, Yang T, et al. Sevoflurane exerts protection against myocardial ischemia–reperfusion injury and pyroptosis through the circular RNA PAN3/microRNA-29b-3p/stromal cell-derived factor 4 axis. Int Immunopharmacol. 2023;120:110219 https://doi.org/10.1016/j.intimp.2023.110219.
Li S, Zhou Y, Li K, Liu L, Fang M, Gao H. Inhibition of circDGKZ ameliorates myocardial ischemia/reperfusion injury by targeting miR‐345‐5p/TLR4. ESC Heart Fail. 2024;11:2730–41. https://doi.org/10.1002/ehf2.14809.
Bian Y, Pang P, Li X, Yu S, Wang X, Liu K, et al. CircHelz activates NLRP3 inflammasome to promote myocardial injury by sponging miR-133a-3p in mouse ischemic heart. J Mol Cell Cardiol. 2021;158:128–39. https://doi.org/10.1016/j.yjmcc.2021.05.010.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–21. https://doi.org/10.1038/s41423-020-00630-3.
Galluzzi L, Kepp O, Chan FK-M, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol Mech Dis. 2017;12:103–30. https://doi.org/10.1146/annurev-pathol-052016-100247.
Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Sig Transduct Target Ther. 2022;7:196 https://doi.org/10.1038/s41392-022-01046-3.
Yan J, Wan P, Choksi S, Liu Z-G. Necroptosis and tumor progression. Trends Cancer. 2022;8:21–7. https://doi.org/10.1016/j.trecan.2021.09.003.
Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. https://doi.org/10.1038/nature14191.
Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174 https://doi.org/10.1186/s13045-022-01392-3.
Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19–33. https://doi.org/10.1038/s41583-018-0093-1.
Li X, Yang Y, Wang Z, Lin X, Fu X, He X, et al. CircHIPK3 targets DRP1 to mediate hydrogen peroxide-induced necroptosis of vascular smooth muscle cells and atherosclerotic vulnerable plaque formation. J Adv Res. 2025;69:329–41. https://doi.org/10.1016/j.jare.2024.04.011.
Gao X-Q, Liu C-Y, Zhang Y-H, Wang Y-H, Zhou L-Y, Li X-M, et al. The circRNA CNEACR regulates necroptosis of cardiomyocytes through Foxa2 suppression. Cell Death Differ. 2022;29:527–39. https://doi.org/10.1038/s41418-021-00872-2.
Madè A, Bibi A, Garcia-Manteiga JM, Tascini AS, Piella SN, Tikhomirov R, et al. circRNA-miRNA-mRNA Deregulated Network in Ischemic Heart Failure Patients. Cells. 2023;12:2578 https://doi.org/10.3390/cells12212578.
Zhang S, Yang Y, Lv X, Liu W, Zhu S, Wang Y, et al. Unraveling the Intricate Roles of Exosomes in Cardiovascular Diseases: A Comprehensive Review of Physiological Significance and Pathological Implications. Int J Mol Sci. 2023;24:15677 https://doi.org/10.3390/ijms242115677.
Zhou L, Liu X, Wang Z-Q, Li Y, Shi M-M, Xu Z, et al. Simvastatin Treatment Protects Myocardium in Noncoronary Artery Cardiac Surgery by Inhibiting Apoptosis Through miR-15a-5p Targeting. J Cardiovasc Pharm. 2018;72:176–85. https://doi.org/10.1097/FJC.0000000000000611.
Li Q, Li N, Cui H, Tian X, Jin C, Chen G, et al. Tongxinluo exerts protective effects via anti‐apoptotic and pro‐autophagic mechanisms by activating AMPK pathway in infarcted rat hearts. Exp Physiol. 2017;102:422–35. https://doi.org/10.1113/EP086192.
Peng Y, Guo M, Luo M, Lv D, Liao K, Luo S, et al. Dapagliflozin ameliorates myocardial infarction injury through AMPKα-dependent regulation of oxidative stress and apoptosis. Heliyon. 2024;10:e29160 https://doi.org/10.1016/j.heliyon.2024.e29160.
Cai Y, Zhou Y, Li Z, Xia P, ChenFu X, Shi A, et al. Non-coding RNAs in necroptosis, pyroptosis, and ferroptosis in cardiovascular diseases. Front Cardiovasc Med. 2022;9:909716 https://doi.org/10.3389/fcvm.2022.909716.
Shen Z, Shen A, Chen X, Wu X, Chu J, Cheng Y, et al. Huoxin pill attenuates myocardial infarction-induced apoptosis and fibrosis via suppression of p53 and TGF-β1/Smad2/3 pathways. Biomed Pharmacother. 2020;130:110618 https://doi.org/10.1016/j.biopha.2020.110618.
Luo X, Zhong Z, Chong A, Zhang W, Wu X. Function and Mechanism of Trimetazidine in Myocardial Infarction-Induced Myocardial Energy Metabolism Disorder Through the SIRT1–AMPK Pathway. Front Physiol. 2021;12:645041 https://doi.org/10.3389/fphys.2021.645041.
Liu X, Yao X, Chen L. Expanding roles of circRNAs in cardiovascular diseases. Noncoding RNA Res. 2024;9:429–36. https://doi.org/10.1016/j.ncrna.2024.02.001.
Oun R, Moussa YE, Wheate NJ. The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 2018;47:6645–53. https://doi.org/10.1039/c8dt00838h.
Johnstone TC, Suntharalingam K, Lippard SJ. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem Rev. 2016;116:3436–86. https://doi.org/10.1021/acs.chemrev.5b00597.
Mf NM, Arunachalam S, Sheikh A, Saraswathiamma D, Albawardi A, Al Marzooqi S, et al. α-Bisabolol: A Dietary Sesquiterpene that Attenuates Apoptotic and Nonapoptotic Cell Death Pathways by Regulating the Mitochondrial Biogenesis and Endoplasmic Reticulum Stress-Hippo Signaling Axis in Doxorubicin-Induced Acute Cardiotoxicity in Rats. ACS Pharm Transl Sci. 2024;7:2694–705. https://doi.org/10.1021/acsptsci.4c00108.
Zhang H, Wang Z, Liu Z, Du K, Lu X. Protective Effects of Dexazoxane on Rat Ferroptosis in Doxorubicin-Induced Cardiomyopathy Through Regulating HMGB1. Front Cardiovasc Med. 2021;8:685434 https://doi.org/10.3389/fcvm.2021.685434.
Shan Q, Li X, Zheng M, Lin X, Lu G, Su D, et al. Protective effects of dimethyl itaconate in mice acute cardiotoxicity induced by doxorubicin. Biochem Biophys Res Commun. 2019;517:538–44. https://doi.org/10.1016/j.bbrc.2019.07.046.
Son CO, Yoon JJ, Han BH, Kim HY, Lee YJ, Kang DG, et al. Sibjotang attenuates Doxorubicin‐Induced Cardiac Hypertrophy: Involvement of necroptosis. FASEB J. 2019;33. https://doi.org/10.1096/fasebj.2019.33.1_supplement.691.9.
Chen J, Qiu S, Liu Y, Sun W, Zhou T, Zhao L, et al. Ultrasound targeted microbubble destruction assisted exosomal delivery of siHmox1 effectively inhibits doxorubicin-induced cardiomyocyte ferroptosis. J Nanobiotechnol. 2024;22:531 https://doi.org/10.1186/s12951-024-02794-w.
Shu G, Chen K, Li J, Liu B, Chen X, Wang J, et al. Galangin alleviated Doxorubicin-induced cardiotoxicity by inhibiting ferroptosis through GSTP1/JNK pathway. Phytomedicine. 2024;134:155989 https://doi.org/10.1016/j.phymed.2024.155989.
Sarg NH, Hersi FH, Zaher DM, Hamouda AO, Ibrahim SI, El-Seedi HR, et al. Unveiling the therapeutic potential of Taxifolin in Cancer: From molecular mechanisms to immune modulation and synergistic combinations. Phytomedicine. 2024;133:155934 https://doi.org/10.1016/j.phymed.2024.155934.
Cai K, Jiang H, Zou Y, Song C, Cao K, Chen S, et al. Programmed death of cardiomyocytes in cardiovascular disease and new therapeutic approaches. Pharm Res. 2024;206:107281 https://doi.org/10.1016/j.phrs.2024.107281.
Long Q, Lv B, Jiang S, Lin J. The Landscape of Circular RNAs in Cardiovascular Diseases. Int J Mol Sci. 2023;24:4571 https://doi.org/10.3390/ijms24054571.
Liu X, Zhang Y, Zhou S, Dain L, Mei L, Zhu G. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Controlled Rel. 2022;348:84–94. https://doi.org/10.1016/j.jconrel.2022.05.043.
Acknowledgements
We are grateful to Shaoling Li from Tongji University for her support in reading and revising the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82204831, 82174120), the Natural Science Foundation of Shanghai (No. 21ZR1463100), the Program of Shanghai Academic Research Leader (22XD1423400), and the Shanghai Sailing Program (No. 22YF1448800).
Author information
Authors and Affiliations
Contributions
Runfang Pan: conceptualization, formal analysis, and writing the original draft. Chinying Koo: validation and project administration. Wenyuan Su: investigation and project administration. Qianhui You: investigation. Haidong Guo: writing - review and editing. Baonian Liu: writing - review, editing, and conceptualization. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, R., Koo, C., Su, W. et al. Circular RNAs modulate cell death in cardiovascular diseases. Cell Death Discov. 11, 214 (2025). https://doi.org/10.1038/s41420-025-02504-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-025-02504-x