Abstract
Separase is a well-conserved endopeptidase that facilitates sister chromatid separation at the metaphase-anaphase transition by cleaving cohesins. Beyond its role in chromosome segregation, Separase also participates in various biological processes, including chromatin organization and replication, centrosome disengagement and duplication, cytokinesis, and telomere capping. Here, we report that the loss of Drosophila separase (Sse) function induces significant changes in global protein expression and affects the protein levels of both A/C-type lamin C (LamC) and B-type lamin Dm0 (Dm0). We further demonstrate that SSE physically interacts with lamins and colocalizes with them at the nuclear envelope during interphase. Additionally, loss of SSE activity disrupts nuclear organization in larval muscles and impairs locomotion in adult flies as a consequence of misregulation of LamC levels. Notably, similar to SSE in flies, depletion of human separase (ESPL1) in SV40 fibroblasts leads to misshapen nuclei and increased levels of lamin A. Moreover, we show that ESPL1 interacts with lamin A in human fibroblasts, suggesting that the functional interaction between Separase and lamins is evolutionarily conserved across different organisms.
Similar content being viewed by others
Introduction
Separase is a conserved cysteine protease that facilitates sister chromatid separation at the metaphase-anaphase transition by cleaving the cohesin subunit Scc1/Rad21/Mcd1 [1]. For most of the cell cycle, separase is bound to its chaperone, securin, which inhibits its protease activity [2]. In addition to securin, separase is regulated through multiple mechanisms, including phosphorylation, autocleavage, and isomerization [2,3,4,5].
Beyond its role in chromatid cohesion resolution, separase participates in various cellular functions. It cleaves Kendrin, also known as pericentrin, a centrosomal scaffold protein required for recruiting centrosomal components, and is involved in proper spindle assembly and elongation in budding yeast [6,7,8]. Studies in Caenorhabditis elegans embryos have demonstrated that separase localizes to the ingressing furrow and midbody during cytokinesis, where it regulates RAB-11-positive vesicle trafficking at the cleavage furrow and midbody [9]. Moreover, separase is essential for double-strand break repair, where it locally cleaves cohesins to facilitate homology-directed repair, thereby preventing oncogenic transformation [10, 11].
The Drosophila genome contains a single separase-encoding gene, Sse. However, unlike separase in other model organisms, Drosophila SSE lacks the extensive N-terminal regulatory domain found in non-dipteran species. This region appears to have evolved into thr, a gene encoding the protein Thr, which binds to SSE and is essential for sister chromatid separation during mitosis [12, 13]. Previous studies on Drosophila separase mutants revealed that its inhibition leads to extensive endoreduplication in mitotic cells, consistent with its role in regulating sister chromatid cohesion [13]. Further research has shown that SSE is also required for chromosome separation, homolog and sister chromatid disjunction during male meiosis [14, 15], polar body formation in female meiosis [16], epithelial reorganization [17], and telomere capping—an evolutionarily conserved function also observed in human cells [18].
Lamins are type V intermediate filament proteins located on the inner surface of the nuclear envelope in metazoan cells, where they contribute to nuclear architecture and interact with chromatin. In addition to maintaining nuclear shape, lamins play key roles in nuclear genome organization, epigenetic histone mark maintenance and distribution, transcriptional regulation, and DNA synthesis [19,20,21,22,23]. Human lamins are classified into A- and B-types: A-type lamins (A, C and C2) arise from alternative splicing of the LMNA gene and are expressed in differentiating cells, whereas B-type lamins (lamins B1, B2, and B3) are encoded by separate genes and exhibit either ubiquitous expression (lamins B1 and B2) or are expressed only in spermatids (Lamin B3) [24, 25]. Mutations in nuclear lamin genes lead to a diverse group of human disorders known as laminopathies, which manifest in phenotypes such as premature aging, cardiomyopathy, neuropathy, and lipodystrophy [26].
In Drosophila, the single A-type lamin, lamin C (LamC), is encoded by the LamC gene, while the B-type lamin, lamin Dm0 (Dm0), is encoded by LamDm0. Dm0 is ubiquitously expressed, and its loss results in lethality at the pupal stage, with rare sterile escapers exhibiting locomotion impairments and reduced lifespan [27]. Drosophila LamC is expressed later in embryogenesis, and mutations in LamC are lethal, with rare survivors displaying severe muscle defects [20, 28]. Expression of human mutant pathogenic lamins A/C in Drosophila leads to muscle homeostasis defects, including alterations in nuclear shape, nuclear lamina component mislocalization, reduced larval mobility, and disruptions in the nucleus-cytoskeleton connection [28,29,30].
Here, we demonstrate for the first time that separase physically interacts with lamins in Drosophila and regulates their expression. Furthermore, the loss of separase affects nuclear morphology and impairs muscle function in Drosophila larvae and adults. Additionally, we show that the relationship between separase and A-type lamins is conserved in human cells, suggesting that this endopeptidase has evolved an evolutionarily conserved function as a lamin-binding factor and regulator.
Results
Sse regulates global protein expression and nuclear lamina component levels
Previous studies in Drosophila have shown that the loss of separase activity affects the expression and localization of several proteins required for chromatin organization and segregation at the post-translational level [14, 18]. While the SSE-dependent regulation of some of these factors was expected, as they are direct targets of SSE peptidase activity, the mechanism by which SSE influences the levels of other proteins remains unclear.
To further investigate the role of SSE in global protein expression, we performed mass spectrometry (MS) analysis on third instar larval brain extracts from the diplo-fused telomeres (dft) null allele of the Sse gene (Ssedft) and the Oregon-R (Or-R) strain as a wild-type control [18]. To distinguish protein expression changes specifically dependent on Sse function from those resulting from general mitotic defects, we simultaneously analyzed protein extracts from a trans-heteroallelic mutant combination of the diamond (dind) gene, an essential Drosophila gene required for mitosis [31]. dind mutant neuroblasts exhibit severe mitotic defects, including abnormalities in chromosome morphology and number, centrosome and mitotic spindle disorganization, chromosome segregation failure, and metaphase delay, all of which collectively impair mitotic progression.
After filtering for cell cycle regulators that were also differentially expressed in dind mutants, we identified 4622 proteins with altered expression in Ssedft mutants compared to controls. Of these, 402 were upregulated (log2FC > 0.4) and 482 were downregulated (log2FC < −0.4) (Supplementary Fig. 1A, B). Western blot analysis of two upregulated factors identified by MS, Hu-li tai shao (Hts), the Drosophila homolog of Adducin, and Pericardin (Prc), a heart collagen, confirmed a two-fold increase in their expression in Ssedft larval brain extracts compared to Or-R controls, validating our MS results (Supplementary Fig. 2A, B).
Functional enrichment analysis of the upregulated proteins revealed significant enrichment for factors involved in muscle system function (Fig. 1A and Supplementary Table 1). Conversely, as expected given the role of SSE in cell cycle regulation and its interactions with DNA, the downregulated proteins were enriched for factors involved in mitotic cell cycle regulation and chromatin organization (Supplementary Table 2). Consistent with our previous findings [18], we identified HP1a among the downregulated proteins, further validating our MS analysis in identifying protein modulation specifically dependent on SSE function.
A Bar plot displaying the top 10 enriched Gene Ontology (GO) categories among differentially expressed proteins. The x-axis represents the ratio of observed to expected proteins for each GO category (y-axis). Only categories with an FDR < 0.05 are shown. A complete list of differentially expressed proteins can be found in Supplementary Tables 1 and 2. B Interaction network analysis of proteins associated with the enriched GO terms “Muscle structure development” (green spheres) and “Chromatin Organization” (red spheres). A k-means clustering algorithm was applied to group proteins into distinct clusters. Dashed lines indicate interactions between proteins of different clusters. Known interactions retrieved from curated databases or experimental studies are depicted in light blue and light purple, whereas green, red, and blue lines represent predicted interactions based on genomic neighborhood co-occurrence, fusion events, and gene co-occurrence patterns across genomes, respectively. Yellow lines highlight proteins frequently mentioned together in literature, black lines indicate co-expression relationships, and violet lines denote protein homology.
An additional functional enrichment analysis (Supplementary Fig. 3 and Supplementary Tables 1 and 3) further confirmed the presence of proteins involved in muscle structure and fiber organization. These proteins are classified under the following Gene Ontology (GO) categories: Muscle System Process (GO:0003012), Muscle Structure Development (GO:0061061), and Supramolecular Fiber Organization (GO:0097435).
To explore potential functional correlations among differentially expressed proteins, we performed a network pathway enrichment analysis using the STRING tool (Fig. 1B). This analysis focused on proteins associated with the GO categories Muscle Structure Development (GO:0061061) and Chromatin Organization (GO:0006325), which represented the most significantly enriched categories for up- and down-regulated proteins, respectively. A k-means clustering algorithm was applied to the network to better distinguish distinct pathways. Interestingly, LamC emerged as a central node connecting these two pathways. On one hand, LamC functionally interacts with the Muscle LIM protein (Mlp84B), while on the other, it binds Dm0, highlighting a possible regulatory link between chromatin organization and muscle structure development.
SSE regulates nuclear lamin protein levels
To validate the MS data suggesting a functional relationship between separase (SSE) and lamins, we performed Western blot (WB) analysis on total protein extracts from the Ssedft mutant. Compared to the Or-R control strain, loss of SSE in mutant neuroblast cells resulted in a 60% reduction in Dm0 protein expression (Fig. 2A, C). Conversely, LamC protein levels showed a threefold increase in the same extracts (Fig. 2B, D).
Western blot analysis of total protein extracts from wild-type Oregon-R (Or-R) and Ssedft mutant (dft) third instar larval neuroblasts, probed with anti-Dm0 (A) and anti-LamC (B) antibodies. Anti-Actin antibody was used as a loading control. Quantification of Dm0 (C) and LamC (D) protein signals from western blot analysis. Data were collected from three independent experiments (*p < 0.05, **p < 0.01; Student’s t test). qPCR quantification of Dm0 (E) and LamC (F) mRNA expression. At least three independent experiments were conducted (*p < 0.05, **p < 0.01; Student’s t test). Western blot analysis of FLAG-tagged LamC expression in wild-type (Tg LamC) and Ssedft mutant (Tg LamC dft) backgrounds (G), with quantification of transgenic LamC expression levels (H). Three independent experiments were performed (*p < 0.05, **p < 0.01; Student’s t test). anti-Giotto (GIO) was used as a loading control. Immunofluorescence staining with anti-Dm0 (I) and anti-LamC (J) antibodies (green) in intact nuclei from Or-R and Ssedft third instar larval salivary glands. DAPI was used to stain DNA. Note the presence of LamC aggregates in the Ssedft nuclei (arrows). Quantification of Dm0 (K) and LamC (L) fluorescence intensity from salivary gland immunostaining (***p < 0.001; Student’s t test). M Immunostaining with anti-cleaved caspase Dcp-1 (red) in larval brains from untreated and IR-treated (10 Gy) Oregon-R controls, and from untreated (dft) and IR-treated (10 Gy dft) Ssedft mutant brains.
To determine whether the increase in LamC protein levels was due to transcriptional regulation, we performed qPCR analysis. Our results showed that LamC mRNA levels in Ssedft mutants were comparable to those in the Or-R control (Fig. 2F), suggesting that the increase in LamC occurs at the post-transcriptional level. In contrast, Dm0 transcript levels were significantly reduced in Ssedft mutants (Fig. 2E), indicating that SSE regulates lamins through distinct mechanisms.
Immunofluorescence analysis of salivary gland nuclei further confirmed the regulatory role of SSE on both Dm0 and LamC. SSE depletion led to a strong reduction in perinuclear Dm0 localization (Fig. 2I), whereas LamC staining was significantly increased (Fig. 2I–L). Interestingly, in addition to the overall LamC up-regulation, Ssedft mutant salivary gland nuclei exhibited extranuclear LamC aggregates that were absent in control cells. This suggests that LamC may form misfolded filaments in the absence of SSE. Furthermore, expression of a catalytically inactive SSE (SSEDH) in an Ssedft mutant background failed to completely restore wild-type LamC protein levels, suggesting that SSE-mediated LamC regulation requires its proteolytic activity (Supplementary Fig. 4).
To rule out the possibility that the increased LamC levels in Ssedft mutants resulted from a second-site mutation, we generated transgenic flies expressing N-terminal V5- and C-terminal Flag-tagged LamC under the control of the Drosophila tubulin promoter. WB and immunofluorescence analyses confirmed that transgenic LamC was correctly expressed, localized to the nuclear envelope, and functionally incorporated into the salivary gland nuclei. Furthermore, these flies were viable and fertile, suggesting that the molecular tags did not alter LamC function (Supplementary Fig. 5A, B). We then crossed V5-LamC-Flag transgenic flies with Ssedft mutants to analyze the transgenic LamC expression (using an anti-V5 antibody) in the absence of Sse. As expected, LamC levels were significantly higher in Ssedft; [V5-LamC-Flag] recombinants than in flies carrying only the transgene, further confirming SSE-mediated regulation of LamC (Fig. 2G, H).
SSE does not regulate LamC via apoptosis
Lamins are typically cleaved in a caspase-dependent manner during apoptosis to facilitate nuclear lamina disassembly [32, 33]. Recent studies have demonstrated that premature separase activation can induce Death in Mitosis, triggering separase-mediated cleavage of pro-survival factors MCL1 and BCL-X, leading to apoptosis [34].
Our MS analysis identified the apoptotic initiator caspase Dronc, along with the effector caspases Dcp-1 and Drice, as downregulated proteins in Ssedft mutants (Supplementary Table 2). This raised the question of whether the observed upregulation of LamC resulted from reduced apoptosis upon loss of SSE function.
To investigate this, we immunostained wild-type and Ssedft mutant neuroblasts with an anti-cleaved Dcp-1 antibody, which detects the 22 kDa active fragment of Dcp-1. In untreated control larval brains, cleaved Dcp-1 was almost undetectable, whereas Ionizing Radiation (IR)-treated cells showed a robust Dcp-1 signal, indicating apoptosis induction (Fig. 2M). Ssedft mutant brains exhibited basal levels of cleaved Dcp-1 staining, which significantly increased after IR treatment, suggesting that apoptosis still occurs in mutant cells. These findings rule out the possibility that LamC upregulation results from reduced apoptosis.
SSE physically interacts with lamins
To further investigate the relationship between SSE and nuclear lamins, we tested whether SSE physically interacts with lamins using GST pull-down assays. These experiments showed that bacterially purified GST-SSE, but not its interacting partner PIM, successfully precipitated both endogenous Dm0 and LamC proteins from total neuroblast extracts (Fig. 3A, B).
A GST pull-down assay using total protein extracts from wild-type third instar larval brains with GST, GSTPIM, and GSTSSE as baits. Interaction with Dm0 was assessed by western blot using an anti-Dm0 antibody. Input (IN) represents 1/10 of total protein extracts. B Western blot analysis with anti-LamC antibody following GST pull-down from brain extracts using GST, GSTPIM, and GSTSSE as baits. Input (IN) represents 1/10 of total protein extracts. The asterisk indicates a non-specific band. C Immunofluorescence staining of intact nuclei from Or-R salivary glands using anti-LamC (green) and anti-SSE (red) antibodies. Co-localization is observed as yellow staining in the merged images (c and f). DNA is counterstained with DAPI (gray in d, e, and f).
Finally, immunolocalization analysis of salivary gland nuclei revealed that SSE co-localizes with LamC at the nuclear envelope, further supporting a physical interaction between these two proteins (Fig. 3C).
However, despite our extensive efforts, we were unable to determine how SSE binding to LamC explains the accumulation of LamC upon SSE loss. We conducted several experiments to test whether LamC is a direct target of SSE peptidase activity, but these efforts did not yield conclusive results (See “Discussion”, and Supplementary Information).
Separase-mediated LAMC regulation is essential for muscle physiology
Since LamC homeostasis plays a crucial role in muscle physiology in Drosophila [29, 35, 36], we investigated whether SSE depletion affects muscle function and organization in both larvae and adults.
Locomotion analysis revealed that Ssedft mutant larvae exhibited a ~30% reduction in the number of peristaltic contractions per minute compared to controls, indicating that loss of SSE impairs larval mobility (Fig. 4A). This evidence was confirmed through Sse RNAi-mediated knockdown in muscle cells by using Mef2-GAL4 driver (Fig. 4B). Similarly, Sse RNAi adult flies showed a significant reduction in locomotion, as measured by the negative geotaxis assay in 3- and 10-days old flies (Fig. 4C, D). This finding confirms that Sse depletion affects muscle function in both larval and adult stages.
A Peristalsis count in Ssedft mutant larvae, showing a significant reduction in peristalsis per minute in SSE-depleted third instar larvae compared to Or-R controls (for each genotype n = 352; ****p < 0.0001; Student’s t test). B Larval locomotion analysis in individuals expressing UAS-Sse dsRNA through Mef2-GAL4 driver, both alone or in combination with heterozygous LamCLC58 mutation (****p < 0.0001; *p < 0.05 Student’s t test). C Climbing assay assessing locomotion in 3-day old flies after muscle-specific SSE depletion with or without heterozygous LamCLC58 mutation (***p < 0.001; *p < 0.05 Student’s t test). D Locomotion test in Mef2-GAL4 UAS-Sse RNAi flies performed 10 days after eclosion (***p < 0.001; *p < 0.05 Student’s t test). E Immunostaining of larval muscle fillets with anti-LamC (green) in control (Or-R) and Ssedft mutant larvae. Muscular fibers are counterstained with fluorescently labeled phalloidin (red). F Quantification of LamC signal intensity and nuclear circularity G from muscle fillet preparations (*p < 0.05, **p < 0.01; Student’s t test).
To further explore the molecular basis of these defects, we performed anti-LamC immunofluorescence on third instar larval body wall muscles, followed by phalloidin staining. This analysis revealed a significant increase in LamC levels at the muscle nuclear rim in Ssedft mutants compared to Or-R controls (Fig. 4E, F). Interestingly, we noticed that both larval and adult locomotion defects in Mef2-GAL4 Sse RNAi individuals were ameliorated when the RNAi flies were also heterozygotes for the LamCLC58 loss-of-function mutation (Fig. 4B, C, D). This suggests that locomotion defects in Ssedft mutants resulted from LamC accumulation at the nuclear periphery.
Additionally, we observed a reduction in nuclear circularity in Ssedft mutant muscle cells, compared to wild-type tissue (Fig. 4G). This suggests that separase plays a crucial role in maintaining proper nuclear morphology in muscle cells.
Separase-mediated nuclear lamina regulation is conserved in human cells
To determine whether the functional relationship between separase and lamin is conserved in human cells, we depleted human separase, Extra Spindle Pole Bodies-Like 1 (ESPL1), in SV40-fibroblasts using siRNAs and analyzed nuclear morphology and lamin regulation via immunofluorescence. In addition to endoreplication, depletion of ESPL1 resulted in the formation of misshapen nuclei (Supplementary Fig. 7), a phenotype previously observed in mammalian cells lacking separase [5, 37]. We also observed a statistically significant increase in lamin A levels in human SV40-fibroblasts (Fig. 5A). These results were further validated by WB blot analysis (Fig. 5B) and are consistent with the increased levels of LamC observed in Drosophila upon SSE depletion, suggesting that separase-dependent regulation of A-type lamins is evolutionarily conserved.
A Immunofluorescent staining of lamin A following ESPL1 knockdown in human cells. SV40-fibroblasts transfected with scrambled siRNAs (CTRL) or ESPL1-specific siRNAs were stained with a lamin A-specific antibody. Representative images and quantification of lamin A intensity per nucleus (mean ± SEM from five independent experiments) are shown (**p < 0.001; Student’s t test). B Western blot analysis of lamin A and ESPL1 protein levels following ESPL1 knockdown in human SV40-fibroblasts. Cell lysates from fibroblasts transfected with either ESPL1-specific siRNA (ESPL1 siRNA) or control scrambled siRNA (CTRL siRNA) for 48 h were probed with antibodies against lamin A, ESPL1, and ß-actin (loading control). Representative blots and quantification (mean ± SEM from three independent experiments) of lamin A and ESPL1 protein levels normalized to ß-actin are shown (*p < 0.05; Student’s t test).
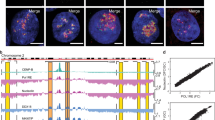
Next, we investigated whether the interaction between A-type lamins and separase, identified in Drosophila, is also conserved in human cells. To this end, we performed in situ Proximity Ligation Assay (PLA) experiments in human SV40-fibroblasts and human primary fibroblasts. This analysis confirmed that human separase interacts with lamin A in situ, as they were detected in close proximity (<40 nm) in both primary and transformed fibroblasts (Fig. 6A, B). Additionally, co-immunoprecipitation experiments in human SV40-fibroblasts using an anti-ESPL1 antibody reinforced that endogenous ESPL1 interacts with lamin A (Fig. 6C). To rule out the possibility that the lamin A-ESPL1 complex—both of which are also DNA-binding proteins [38, 39]—is bridged by DNA or RNA, we pretreated cellular lysates with benzonase nuclease, which degrades both nucleic acids, prior to immunoprecipitation. These experiments demonstrated that lamin A remains in complex with ESPL1 independently of DNA and RNA. Finally, reciprocal immunoprecipitation with an anti-lamin A antibody (Fig. 6D) confirmed that nuclear A-type lamins form a stable complex with human separase.
A Proximity ligation assay (PLA) using anti-lamin A (LamA) and anti-ESPL1 antibodies in human SV40-fibroblasts transfected with control siRNA (siCTRL) or ESPL1-specific siRNA (siESPL1). Non-transfected (NT) primary fibroblasts were also analyzed. Red dots indicate interactions between endogenous lamin A and ESPL1, with nuclei counterstained with DAPI (blue). Histograms depict the quantification of PLA dots per nucleus (***p < 0.0001; Student’s t test). Control PLA experiments were performed using a single primary antibody against either ESPL1 or lamin A alone. B Lamin A-ESPL1 PLA in human primary fibroblasts performed as described in (A). Co-immunoprecipitation assays for endogenous human ESPL1 and lamin A. SV40-fibroblast lysates (1 mg) pre-treated with benzonase were immunoprecipitated with 5 µg of either mouse anti-ESPL1 (C) or mouse anti-lamin A (D) antibodies. Western blot analysis was performed on precipitates (IP) and 30 µg of input lysates using specific antibodies against ESPL1 and lamin A. A mouse IgG control (5 µg) was included (IgG).
Collectively, our findings indicate that the interaction between lamin and separase is conserved from Drosophila to humans.
Discussion
We demonstrate that separase interacts with lamins and colocalizes at the nuclear envelope in Drosophila, playing a crucial role in regulating lamin levels in both flies and human cells. Depletion of separase results in increased lamin A levels and nuclear shape alterations across both species. Our findings suggest that separase, beyond its established non-canonical functions, is a genuine lamin-interacting protein involved in nuclear lamina maintenance. This conclusion is also supported by recent evidence of a physical interaction between Separase and Lamin B1 in human cells [40].
The observed effects of separase depletion on lamin regulation and nuclear morphology highlight its critical role in nuclear envelope integrity. Lamins are essential structural components of the nuclear lamina, and their misregulation has been linked to several pathological conditions, including laminopathies, premature aging disorders, and certain cancers. Our findings indicate that separase helps maintain nuclear homeostasis by modulating lamin levels, which in turn influences nuclear stiffness, chromatin organization, and gene expression. The fact that separase depletion leads to increased lamin A accumulation suggests a potential role in lamin turnover or degradation, possibly through direct cleavage or indirect regulation via proteasomal degradation pathways.
We also show that loss of separase affects nuclear morphology, inducing invaginations in Drosophila muscle cells, which subsequently leads to locomotion defects. Given that fruit flies serve as a model for human muscular dystrophies and laminopathies [28, 41], our findings highlight a conserved regulatory mechanism that may provide new insights into the etiology of lamin-associated muscle diseases. Muscle cells are particularly susceptible to lamin dysfunction, as the nuclear envelope must endure significant mechanical stress. Thus, separase may therefore contribute to nuclear resilience by regulating lamin composition and ensuring proper nuclear mechanics.
Interestingly, we found that separase depletion has differential effects on Dm0 and LamC levels: it decreases Dm0 while increasing LamC. This suggests distinct regulatory mechanisms at play. The reduction in Dm0 appears to be transcriptionally mediated, likely due to the role of separase in facilitating transcription factor accessibility through regulation of the cohesin complex [42]. This raises the possibility that separase positively regulates LamDm0 gene expression by modulating chromatin structure.
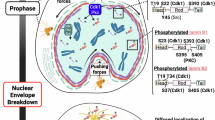
Conversely, the increase in LamC levels does not result from higher mRNA expression, suggesting a post-translational mode of regulation. One intriguing possibility is that separase directly cleaves LamC, as the Drosophila LamC sequence contains multiple putative separase cleavage sites (EXXR). If this is the case, separase might function in LamC turnover and processing to ensure a balanced nuclear lamina composition. In support of this idea, we detected several LamC bands in Oregon R extracts that disappeared in Sse mutant extracts, which instead showed a significant accumulation of full-length LamC (~75 kDa) (Supplementary Fig. 6). Our attempts to isolate LamC cleavage fragments by IP or detect them by IF were unsuccessful, leaving the precise nature of the role of separase in LamC regulation open for further investigation. However, our modeling analysis reveals strong structural conservation in the substrate recognition sites of human ESPL1 and Drosophila SSE, particularly their shared ability to bind the canonical “ExxR” cleavage motif found in Drosophila Lamin C and human Lamin A (Fig. 7A). AlphaFold3 (AF3) [43] structural models of ESPL1 and Sse bound to substrate peptides show that the arginine (P1) of the “ExxR” motif is coordinated by a conserved set of acidic and polar residues: Asp2070 and Thr1961 in ESPL1, and Asp539 and Ser428 in Sse. These residues form a negatively charged pocket that stabilizes the P1 arginine side chain and positions the scissile bond for cleavage. Additionally, both ESPL1 and SSE exhibit a positively charged cleft that interacts with the glutamate (P4) of the substrate, further anchoring the motif. The preservation of this electrostatic environment suggests a functionally conserved mechanism of substrate engagement, despite sequence divergence. These findings support the hypothesis that Lamin C and Lamin A are likely physiological targets of SSE and ESPL1, respectively (Fig. 7A).
A Predicted structures of the catalytic domains of Drosophila SSE (blue, residues 1–634) and human ESPL1(green, residues 1709–2120), shown in top and side views. The putative substrate-binding cleft is indicated (black ovals). Right panels depict close-up views of the electrostatic surface potential at the substrate-binding site, with the Lamin C/Lamin A EXXR motif in stick representation. B Model of Separase function in lamin regulation. In normal cells (left panel) Separase (red ovals), in addition to binding chromatin (not shown for clarity) localizes at the nuclear rim, where it interacts with A-type (green filaments) and B-type lamins (purple filaments). Upon Separase loss (right panel), B-type lamins are reduced, A-type lamins accumulate in the cytoplasm, forming aggregates (green rectangles), and nuclear membrane integrity is compromised, leading to invaginations and disrupted chromatin-membrane interactions.
Our MS analysis of Ssedft mutants confirmed the established roles of separase in cell cycle regulation and chromatin organization while uncovering an unprecedented function in Drosophila muscle homeostasis. While this could be an indirect consequence of lamin misregulation—given that lamins are critical for muscle development and nuclear integrity—it is also possible that separase has a more direct role in maintaining muscle function.
Notably, endopeptidase activity has been shown to be essential for generating micropeptides that regulate muscle contraction and heart function in Drosophila [44]. Given that separase itself is an endopeptidase, it may participate in a similar process, potentially by processing key muscle proteins or micropeptides involved in contraction dynamics. This raises exciting possibilities regarding the role of separase in muscle physiology and suggests that its activity extends beyond mitotic regulation into post-mitotic tissues. Further biochemical and genetic studies will be necessary to explore this hypothesis.
Our findings (summarized in Fig. 7B), reveal a novel regulatory axis linking separase, lamins, and nuclear organization which could have broad implications for understanding nuclear architecture-related diseases. Given that separase is evolutionarily conserved, its role in lamin regulation may extend to vertebrates, including humans. Future research should explore whether separase mutations contribute to laminopathies or other nuclear envelope-related disorders.
Materials and methods
Drosophila strains, transgenic lines, and crosses
The Ssedft mutant, the catalytic inactive separase UAS-6HA-Sse C497S expressing line (Dead Head, DH), the dind2870 and dind1332 mutant lines were described previously [18, 31]. LamCLC58 strain was a kind gift from Lori L. Wallrath. The UAS Sse RNAi (v29318), the 69B GAL4 and Mef2 GAL4 drivers (which express GAL4 in brain and muscle, respectively) were obtained from the Vienna Drosophila stock center. To perform Sse knockdown in heterozygous LamCLC58 genetic background, we created w; LamCLC58/CyOGFP; UAs Sse RNAi/TM6B strain by crossing w; Sco/CyOGFP; UAs Sse RNAi/UAS Sse RNAi with w; LamCLC58/CyOGFP; MKRS/TM6B flies. Muscle Sse depletion was achieved by crossing w; LamCLC58/CyOGFP; UAS Sse RNAi/TM6B with Kr/Mef 2 GAL4 CyO RFP; +/+ and LamCLC58/Mef 2 GAL4 CyO RFP; UAS Sse RNAi/+ flies were analyzed. To obtain flies expressing lamC with the N-terminal V5 and C-terminal Flag tags, synthetic V5lamCF coding sequence was cloned into pJZ4 vector (a pCASPER4 derivative) under the control of Drosophila tubulin promoter. Germline injection and transformation were carried out by the BestGene Company (Indiana, USA). To introduce recombinant V5-LamC-Flag encoding transgene in a Ssedft mutant genetic background, w; pJZ4 w+V5lamCFlag/pJZ4 w+V5lamCFlag (Ch.III) virgin females were crossed with w; Ssedft/TM6B males. w; pJZ4 w+V5lamCFlag/Ssedft trans-heterozygous F1 females were crossed with w; MKRS/TM6B males. Single F2 recombinant males were then crossed with w; MKRS/TM6B virgin females to establish w; w+pJZ4V5lamCFlag, Ssedft/TM6B recombinant lines. Ssedft mutation was scored through homozygous late-lethality and chromosome endoreduplication/telomere fusions, while w+pJZ4V5lamCFlag transgene was screened for w+ phenotype. All strains were maintained on standard Drosophila cornmeal medium at 25 °C.
Real-time PCR
Five third instar larvae RNA was extracted by using GENEzol Reagent (Geneaid, GZR100) following manual instructions as previously described [45]. One microgram of RNA for each sample was digested with 1 unit of DNase (DNase RNase-Free, Promega, M610C). RNA was reverse transcribed into cDNA with the 5x iScript™ Reverse Transcription Supermix kit (BIO-RAD, # 1708841) following the manual protocol. Expression of lamins was evaluated through Real-Time PCR with the SsoAdvanced Universal SYBR Green Supermix kit (BIO-RAD) and using the following forward and reverse primers:
RP49 forward 5′-ATCGGTTACGGATCGAACAA-3′
RP49 reverse 5′-GACAAT CTCCTTGCGCTTCT-3′
Dm0 forward 5′-TTCGAGGAAACGCGGAAGAA-3′
Dm0 reverse 5′-TGTCCTCGTAGAGGGACTGG-3′
LamC RA forward 5′-GCTTGAACGCCAAGCTACAG-3′
LamC RA reverse 5′-GCAACATCCTCTTTGTCGGC-3’
LamC RB forward 5′ -TGGATCTCGAAATTGCCGCT-3′
LamC RB reverse 5′-CAAGTGCCGCATGTGTTGTAT-3′
For each reaction, three technical replicates were prepared. Data from 7 and 11 reactions are shown for Dm0 and LamC, respectively.
GST-pulldown and co-immunoprecipitation
To obtain GST-SSE and GST-PIM, bacterially expressed GST fusion proteins were purified with glutathione sepharose beads from crude lysates by following manufacturer’s instructions. Protein extracts for GST-pulldown were obtained from third instar larval brains as previously described [46]. Tissues were lysed in an ice-cold buffer containing 20 mM Hepes KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 420 mM NaCl, 20 mM NaF, 10 mM Na3VO4, 10 mM BGP, 10 mM phenylmethyl sulfonyl fluoride, 0.1% NP40, and 1 protease inhibitor cocktail (Roche). Protein extracts were incubated with each GST fusion protein bound to sepharose beads for 1 h at 4 °C in incubation buffer containing 20 mM Hepes KOH, 20 mM NaF, and 0.8% NP40. After incubation, Sepharose-bound GST proteins were collected and washed with a solution containing 20 mM Hepes KOH, 20 mM NaF, and 0.8% NP40. For co-immunoprecipitation, whole cell extracts were obtained from human SV40-fibroblasts after lysing cells for 1 h in a lysis buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 0.5% NP40) supplemented with 1× complete protease inhibitor cocktail (Roche) and 1X phosphatase inhibitor cocktail (Roche), following by their centrifugation and the collection of supernatants containing protein extracts. Extracts were then treated with benzonase nuclease before co-immunoprecipitation using Dynabeads protein G kit (Life Technologies). 5 µg of antibody raised against ESPL1 (Novus) or lamin A (Abcam) were incubated with Dynabeads for 1 h at room temperature (RT). Normal mouse IgG (5 µg, Santa Cruz) was used as control. After washing with 0.05% Tween-20 in PBS, coupled beads were incubated with protein samples (0.5–1 mg) for 1.5 h at RT. After washing, protein precipitates were eluted in Laemmli buffer (2×) with 4% ß-mercaptoethanol. Protein precipitates were further resolved by SDS-PAGE as described above using mouse anti-separase antibody (6H6, Novus Biological) or mouse anti-lamin A (Ab8980, Abcam). Sixty micrograms of whole cell extracts were used as Input.
Locomotion assays
Analysis of larval locomotion was performed on third instar larvae placed on a 2% agar substrate. The number of peristaltic movements for each larva over 1 min was measured. Adult locomotion was evaluated through the Bang Sensitivity Test. After a mechanical stress, groups of 10 flies were pushed at the bottom of a cylinder, and their ability to reach a distance of 4 cm in 5 s from the bottom was evaluated as the percentage of flies that covered the distance over the total flies’ number. 11 and 10 groups from 3- and 10-day-old flies, respectively, were analyzed.
Cell culture and transfection
Human SV40-transformed fibroblasts (GM0639) and normal human diploid embryonic fibroblast (WI-38) (Coriell Cell Repositories, Camden, NJ) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco), 2 mM-Glutamine, 200 U/ml penicillin, and 200 mg/ml streptomycin (Sigma). For cell transfection, SV40-fibroblasts seeded on 6-plate wells (1.5 × 105 cells/well)-containing coverslips for immunofluorescent experiments- for 24 h were transfected with 20 nM siRNAs (smart-pool siRNAs designed against ESPL1 (Dharmacon) or a scrambled siRNA as control (Eurogenetec) using INTERFERin reagent (Polyplus). 48 h after transfection, cells were collected for WB analysis or fixed for immunofluorescence staining or PLA experiments.
In situ proximity ligation assay (PLA)
Cells plated on coverslips and fixed with ice-cold methanol (10 min), were blocked 1 h with 2% BSA/0.05% Tween 20 in PBS for 1 h, and incubated with a mouse primary antibody against ESPL1 (ab16170, Abcam) and a rabbit primary antibody against lamin A (1293, Sigma). Then, PLA was performed using the Duolink in situ PLA probes (anti-Mouse Minus and anti-Rabbit Plus, Sigma) and the Duolink in situ detection reagent red (Sigma) following the manufacturer’s instructions. Images were acquired with an SPE Leica DMRxA2 confocal microscope using a 63× objective lens and further analyzed with ImageJ software. Statistical analyses were performed with GraphPad PRISM (GraphPad Inc.) using unpaired 2-tailed Student’s t tests.
Cytology and immunofluorescence
For intact polytene nuclei preparation, third instar larvae salivary glands were dissected in a 0.7% NaCl and fixed in a drop of 1.8% formaldehyde and 40% acetic acid placed onto a cover slip. Samples were squashed gently and immediately frozen in liquid nitrogen. After flipping the coverslip, slides were raised in ice-cold Tris-buffered saline solution (TBS) for 10 min and subsequently permeabilized in TBS-Triton X-100 1% for 20 min. Slides were incubated with mouse anti-lamin Dm0 1:10 (ADL67.10-S Developmental Studies Hybridoma Bank), mouse anti-lamin C 1:10 (LC28.26-S Developmental Studies Hybridoma Bank), chicken anti-SSE 1:50 (16), and rabbit anti-V5 1:50 (abcam, ab15828) antibodies overnight at 4 °C in a wet chamber. After washing with TBS-Triton 0.05%, salivary gland preparations were incubated with anti-mouse TRITC 1:200 (Jackson Immuno Research), anti-Chicken IgG (HþL) 1:200 (Alexa Fluor 488 ab150169), or anti-Rabbit IgG Alexa FluorTM Plus 594 1:200 (Invitrogen, # A 11012) for 1 h at RT in a dark chamber. All slides were washed in TBS-Triton 0.05% and mounted with VECTASHIELD Antifade Mounting Medium with DAPI (Vector). Preparations were analyzed with a Zeiss AxioPlan epifluorescence microscope equipped with a cooled CCD camera (Photometrics). Images were pseudocoloured and merged with Adobe Photoshop CS4. For apoptosis analysis, third instar larvae were exposed to a γ-ray dose of 10 Gy from 137Cs sources using the Gammacell Exactor 40 (Nordion) provided at the Istituto Superiore di Sanità (ISS, Rome, Italy). At 8 h post-irradiation, larval brains were dissected and processed for anti-cleaved Drosophila Dcp1 staining as previously described [47]. For larval muscle fillets preparation, third instar larvae were dissected in PBS1x and fixed in a solution containing 4% paraformaldehyde and 4% sucrose for 1 h at RT. Tissues were washed three times with PBS and permeabilized with PBS-Triton 1% for 10 min. After two washes, the preparations were incubated with mouse anti-lamin C 1:10 (LC28.26-S Developmental Studies Hybridoma Bank) O.N. at 4 °C in a humid chamber. After two washes in PBS, the fillets were incubated with Alexa Fluor phalloidin 594 1:300 (Thermo Fischer Scientific) and with anti-mouse FITC 1:200 (Jackson Immuno Research) in PBS-Triton 0.1% BSA 2% for 30 min in the dark at RT. The fillets were washed three times and mounted with Vector.
For human fibroblasts staining, cells plated on coverslips and fixed in ice-cold Methanol (10 min) 48 h after transfection, were permeabilized with 0,5% Triton X-100 in PBS for 10 min, then blocked with 2% BSA/0.05% Tween 20 in PBS for 1 h. After the blocking step, cells were incubated with a mouse primary antibody against lamin A (ab8980, Abcam) for 1 h at RT. Cells were then incubated with an anti-mouse IgG AlexaFluor 488 (Life Technologies). DNA was counterstained with DAPI and coverslips were mounted onto slide with fluoromount mounting medium (Southern Biotech). Images were acquired using a leica DM5500B fluorescence microscope with a 63×-oil objective. Following acquisition, images were analyzed with ImageJ software. Statistical analyses were performed with PRISM using unpaired t-tests.
Western blot
Drosophila third instar larval brains were homogenized in SDS buffer (10 mM Tris (pH 7.5), 1% SDS, and 1X complete protease inhibitor cocktail (Roche). Protein samples were resolved by SDS-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membrane (Amersham). Membranes were blocked in 5% skim milk in PBS/0.1% Tween-20 for 45 min then probed with the indicated primary antibodies: mouse anti-lamin Dm0 1:500 (ADL67.10-S Developmental Studies Hybridoma Bank), mouse anti-lamin C 1:500 (LC28.26-S Developmental Studies Hybridoma Bank), anti-actin HRP conjugated (1:5000; Santa Cruz Biotechnology SC-1615), anti hts (AB528289 Developmental Studies Hybridoma Bank), anti-prc (EC11 Developmental Studies Hybridoma Bank), anti-FLAG HRP conjugated (1:3000; Sigma A8592), anti-Tubulin (1:100,000; Sigma T6199), anti-V5 HRP conjugated (1:4000; abcam 1325), anti-Giotto (1:5000; [48]). Primary antibodies were detected using sheep anti-mouse IgG HRP conjugated (1:5000 NA931V Amersham Biosciences) and donkey anti-rabbit IgG HRP conjugated (1:5000 NA934 Amersham Biosciences).
Human fibroblasts cells were homogenized in SDS buffer (10 mM Tris (pH 7.5), 1% SDS, 1X complete protease inhibitor cocktail (Roche) and phosphatase inhibitors cocktail 2 and 3 (Sigma). Protein samples were resolved by SDS-polyacrylamide gel electrophoresis on 4–12% NuPAGE Bis-Tris gradient gel or 3–8% Tris-acetate gel (Invitrogen) with MOPS or Tris-acetate running buffer (Invitrogen) and then, blotted onto on nitrocellulose membrane (Amersham). Membranes were blocked in 5% skim milk in PBS/0.1% Tween-20 for 45 min then probed with the indicated primary antibodies: ESPL1 (6H6, Novus Biologicals), lamin A (ab8980, Abam) and ß-actin (A2066, Sigma) as loading control. Primary antibodies were further detected using secondary fluorescent antibody (IR800 and IR700, Diagomics). Fluorescent signals were acquired with Odyssey imager (LI-COR Biosciences) and quantification was performed with Image Studio software (LI-COR Biosciences) or Image J software.
All WB images were assembled using the sections highlighted in the uncropped blots included in the Supplementary Figures.
Mass spectrometry
Proteins extract from third instar larvae brains were obtained through lysis in a solution containing 8 M Urea, 20 mM Hepes, pH 8, 1 mM PMSF, and protease inhibitor cocktail (Roche). Samples were digested with trypsin, followed by isobaric Tag for Relative and Absolute Quantitation labeling. After high pH reversed-phase liquid chromatography fractioning, peptides were subjected to tandem MS (Proteomics and Mass Spectrometry Facility, BRC, Cornell University). Proteins with at least two PSMs were considered for differential expression analysis. Differentially expressed proteins were detected with the t-test, and multiple tests adjusted p-values were calculated with the Benjamini-Hochberg method. GO enrichment analyses were performed using the WEB-based Gene Set Analysis Toolkit (WebGestalt) [49] over Biological Process, Molecular function, and Cellular Component GO categories, with the Over-Representation Analysis method based on the Hypergeometric test (Benjamini-Hochberg multiple test correction, FDR threshold: <0.05). All WebGestalt-based analyses used the protein-coding genome for Drosophila melanogaster as a background reference set. The enrichment ratio parameter was calculated as the number of observed entries divided by the number of expected entries from each GO category. Metabolic pathways and domains enrichment analyses were performed using the FlyEnrichr tool [50] on KEGG (2019) and InterPro (2019) databases, calculating p-values with the hypergeometric tests based on methods. A rank score (or z-score) is calculated to weigh the deviation from an expected rank. After functional enrichment analysis, a combined score is given, which represents a combination of p-value and z-score in the form of c = ln(p)*z. The STRING tool [51] was used to investigate protein networks among enriched GO categories. K-means algorithms were applied to the network to consider the distance matrix computed by STRING. Statistical analyses, plots, and consensus were computed using R software.
Separase-Lamin C/Lamin A interaction modeling
Modeling of the human ESPL1 (UniProt: Q14674)–lamin A (UniProt: P02545) and Drosophila SSE (UniProt: Q9VRN6)–LamC (UniProt: Q03427) complexes was performed using AlphaFold3 with default settings. For both species, the modeling focused on the cysteine protease domains of ESPL1 and SSE, in complex with target lamin subsequences containing the conserved “ExxR” cleavage motifs. Electrostatics calculations were performed using AMBER PyMOL plugin [52].
Statistical analysis
Statistical analyses were performed using GraphPad PRISM (GraphPad Inc.). Statistical significance of data was assessed by unpaired two-tailed Student’s t tests. P > 0.05 was considered not significant. Error bars represent standard error of the mean (SEM).
Data availability
Supporting data for this manuscript, including Tables with MS results, are available in the Supplementary Material and all other data are available upon request.
Change history
16 December 2026
The original online version of this article was revised: During the converting process figure 4 contains black squares. The figure has been updated with the corrected version.
28 January 2026
A Correction to this paper has been published: https://doi.org/10.1038/s41420-025-02938-3
References
Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–3.
Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7.
Gorr IH, Boos D, Stemmann O. Mutual inhibition of separase and Cdk1 by two-step complex formation. Mol Cell. 2005;19:135–41.
Hellmuth S, Rata S, Brown A, Heidmann S, Novak B, Stemmann O. Human chromosome segregation involves multi-layered regulation of separase by the peptidyl-prolyl-isomerase Pin1. Mol Cell. 2015;58:495–506.
Waizenegger I, Gimenez-Abian JF, Wernic D, Peters JM. Regulation of human separase by securin binding and autocleavage. Curr Biol. 2002;12:1368–78.
Baskerville C, Segal M, Reed SI. The protease activity of yeast separase (esp1) is required for anaphase spindle elongation independently of its role in cleavage of cohesin. Genetics. 2008;178:2361–72.
Lee K, Rhee K. Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle. 2012;11:2476–85.
Matsuo K, Ohsumi K, Iwabuchi M, Kawamata T, Ono Y, Takahashi M. Kendrin is a novel substrate for separase involved in the licensing of centriole duplication. Curr Biol. 2012;22:915–21.
Bembenek JN, White JG, Zheng Y. A role for separase in the regulation of RAB-11-positive vesicles at the cleavage furrow and midbody. Curr Biol. 2010;20:259–64.
Hellmuth S, Gutierrez-Caballero C, Llano E, Pendas AM, Stemmann O. Local activation of mammalian separase in interphase promotes double-strand break repair and prevents oncogenic transformation. EMBO J. 2018;37:e99184.
McAleenan A, Clemente-Blanco A, Cordon-Preciado V, Sen N, Esteras M, Jarmuz A, et al. Post-replicative repair involves separase-dependent removal of the kleisin subunit of cohesin. Nature. 2013;493:250–4.
Herzig A, Lehner CF, Heidmann S. Proteolytic cleavage of the THR subunit during anaphase limits Drosophila separase function. Genes Dev. 2002;16:2443–54.
Jager H, Herzig A, Lehner CF, Heidmann S. Drosophila separase is required for sister chromatid separation and binds to PIM and THR. Genes Dev. 2001;15:2572–84.
Blattner AC, Chaurasia S, McKee BD, Lehner CF. Separase is required for homolog and sister disjunction during Drosophila melanogaster male meiosis, but not for biorientation of sister centromeres. PLoS Genet. 2016;12:e1005996.
Weber J, Kabakci Z, Chaurasia S, Brunner E, Lehner CF. Chromosome separation during Drosophila male meiosis I requires separase-mediated cleavage of the homolog conjunction protein UNO. PLoS Genet. 2020;16:e1008928.
Guo Z, Batiha O, Bourouh M, Fifield E, Swan A. Role of Securin, Separase and Cohesins in female meiosis and polar body formation in Drosophila. J Cell Sci. 2016;129:531–42.
Pandey R, Heidmann S, Lehner CF. Epithelial re-organization and dynamics of progression through mitosis in Drosophila separase complex mutants. J Cell Sci. 2005;118:733–42.
Cipressa F, Morciano P, Bosso G, Mannini L, Galati A, Raffa GD, et al. A role for Separase in telomere protection. Nat Commun. 2016;7:10405.
Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–47.
Karoutas A, Akhtar A. Functional mechanisms and abnormalities of the nuclear lamina. Nat Cell Biol. 2021;23:116–26.
Wong X, Melendez-Perez AJ, Reddy KL. The nuclear lamina. Cold Spring Harb Perspect Biol. 2022;14:a040113.
Willaume S, Rass E, Fontanilla-Ramirez P, Moussa A, Wanschoor P, Bertrand P. A Link between replicative stress, lamin proteins, and inflammation. Genes. 2021;12:552.
Pennarun G, Picotto J, Etourneaud L, Redavid AR, Certain A, Gauthier LR, et al. Increase in lamin B1 promotes telomere instability by disrupting the shelterin complex in human cells. Nucleic Acids Res. 2021;49:9886–905.
Worman HJ. Nuclear lamins and laminopathies. J Pathol. 2012;226:316–25.
Elkhatib R, Longepied G, Paci M, Achard V, Grillo JM, Levy N, et al. Nuclear envelope remodelling during human spermiogenesis involves somatic B-type lamins and a spermatid-specific B3 lamin isoform. Mol Hum Reprod. 2015;21:225–36.
Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–75.
Lenz-Bohme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, et al. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Biol. 1997;137:1001–16.
Schulze SR, Curio-Penny B, Speese S, Dialynas G, Cryderman DE, McDonough CW, et al. A comparative study of Drosophila and human A-type lamins. PLoS One. 2009;4:e7564.
Shaw NM, Rios-Monterrosa JL, Fedorchak GR, Ketterer MR, Coombs GS, Lammerding J, et al. Effects of mutant lamins on nucleo-cytoskeletal coupling in Drosophila models of LMNA muscular dystrophy. Front Cell Dev Biol. 2022;10:934586.
Walker SG, Langland CJ, Viles J, Hecker LA, Wallrath LL. Drosophila models reveal properties of mutant lamins that give rise to distinct diseases. Cells. 2023;12:1142.
Graziadio L, Palumbo V, Cipressa F, Williams BC, Cenci G, Gatti M, et al. Phenotypic characterization of diamond (dind), a Drosophila gene required for multiple aspects of cell division. Chromosoma. 2018;127:489–504.
Takahashi A, Alnemri ES, Lazebnik YA, Fernandes-Alnemri T, Litwack G, Moir RD, et al. Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci USA. 1996;93:8395–8400.
Takahashi A, Goldschmidt-Clermont PJ, Alnemri ES, Fernandes-Alnemri T, Yoshizawa-Kumagaya K, Nakajima K, et al. Inhibition of ICE-related proteases (caspases) and nuclear apoptosis by phenylarsine oxide. Exp Cell Res. 1997;231:123–31.
Hellmuth S, Stemmann O. Separase-triggered apoptosis enforces minimal length of mitosis. Nature. 2020;580:542–7.
Dialynas G, Speese S, Budnik V, Geyer PK, Wallrath LL. The role of Drosophila lamin C in muscle function and gene expression. Development. 2010;137:3067–77.
Uchino R, Nonaka YK, Horigome T, Sugiyama S, Furukawa K. Loss of Drosophila A-type lamin C initially causes tendon abnormality, including disintegration of cytoskeleton and nuclear lamina in muscular defects. Dev Biol. 2013;373:216–27.
Wirth KG, Wutz G, Kudo NR, Desdouets C, Zetterberg A, Taghybeeglu S, et al. Separase: a universal trigger for sister chromatid disjunction but not chromosome cycle progression. J Cell Biol. 2006;172:847–60.
Shoeman RL, Traub P. The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem. 1990;265:9055–61.
Sun Y, Kucej M, Fan HY, Yu H, Sun QY, Zou H. Separase is recruited to mitotic chromosomes to dissolve sister chromatid cohesion in a DNA-dependent manner. Cell. 2009;137:123–32.
Picotto J, Cipressa F, Busso D, Cenci G, Bertrand P, Pennarun G. Lamin B1-dependent regulation of human separase in mitosis. bioRxiv. 2024. https://doi.org/10.1101/2024.11.28.625860.
Dialynas G, Flannery KM, Zirbel LN, Nagy PL, Mathews KD, Moore SA, et al. LMNA variants cause cytoplasmic distribution of nuclear pore proteins in Drosophila and human muscle. Hum Mol Genet. 2012;21:1544–56.
Perea-Resa C, Wattendorf L, Marzouk S, Blower MD. Cohesin: behind dynamic genome topology and gene expression reprogramming. Trends Cell Biol. 2021;31:760–73.
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500.
Dubinska-Magiera M, Zaremba-Czogalla M, Rzepecki R. Muscle development, regeneration and laminopathies: how lamins or lamina-associated proteins can contribute to muscle development, regeneration and disease. Cell Mol Life Sci. 2013;70:2713–41.
Bosso G, Cipressa F, Tullo L, Cenci G. Co-amplification of CBX3 with EGFR or RAC1 in human cancers corroborated by a conserved genetic interaction among the genes. Cell Death Discov. 2023;9:317.
Bosso G, Cipressa F, Moroni ML, Pennisi R, Albanesi J, Brandi V, et al. NBS1 interacts with HP1 to ensure genome integrity. Cell Death Dis. 2019;10:951.
Porrazzo A, Cipressa F, De Gregorio A, De Pitta C, Sales G, Ciapponi L, et al. Low-dose rate gamma-irradiation protects fruit fly chromosomes from double-strand breaks and telomere fusions by reducing the esi-RNA biogenesis factor Loquacious. Commun Biol. 2022;5:905.
Giansanti MG, Bonaccorsi S, Kurek R, Farkas RM, Dimitri P, Fuller MT, et al. The class I PITP Giotto is required for Drosophila cytokinesis. Curr Biol. 2006;16:195–201.
Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199–W205.
Kuleshov MV, Diaz JEL, Flamholz ZN, Keenan AB, Lachmann A, Wojciechowicz ML, et al. modEnrichr: a suite of gene set enrichment analysis tools for model organisms. Nucleic Acids Res. 2019;47:W183–W190.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613.
Jurrus E, Engel D, Star K, Monson K, Brandi J, Felberg LE, et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018;27:112–28.
Funding
This work was supported by a grant from AFM-Téléthon to GC and PB (N.21566), by Italy University and Research Ministry (MUR, N. 202227SYBW) and Istituto Pasteur Italia, Fondazione Cenci Bolognetti (Anna Tramontano Grant) to GC and by a grant from Italy Ministry of University and Research (PRIN, N 2022KKMTL9) to FC. PB’s laboratory is also supported by the Ligue Nationale Contre le Cancer (Haut-de-Seine committee), Association for Research against Cancer (Fondation ARC), AT Europe Association, INCA grant (PLBIO23-204-2023-179), Emergence Paris Cité, CEA Radiobiology Program and INSERM, Université Paris Cité, Université Paris-Saclay house funding (SGCSR unit).
Author information
Authors and Affiliations
Contributions
FC designed and performed all the experiments on Drosophila, supervised experiments, assembled the corresponding figures and wrote the manuscript. GP designed and performed all the experiments on human cells, designed the corresponding figures and wrote parts of the manuscript. GB, LT, SN, CDD, and CB performed experiments on Drosophila. GE supervised experiments and reviewed the manuscript. SR and AP analyzed the MS data and assembled the corresponding figures and data. MLG designed and supervised the MS experiments. PB designed the experiments, supervised, and reviewed the manuscript. GC designed and supervised the experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research did not involve human subjects and human material. All experiments involving Drosophila melanogaster were conducted in accordance with ethical guidelines and regulations for the use of invertebrate animals in research. Drosophila strains and crosses were maintained under standard laboratory conditions, with controlled temperature, humidity, and light cycles, and were provided with appropriate food and water. All experimental protocols were designed to adhere to the principles of the 3Rs (Replacement, Reduction, and Refinement) to ensure “humane” treatment of the flies.
Consent for publication
All authors commented on this version of the manuscript and approved the final manuscript. All authors have consented to the publication of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cipressa, F., Pennarun, G., Bosso, G. et al. An evolutionarily conserved role for separase in the regulation of nuclear lamins. Cell Death Discov. 11, 475 (2025). https://doi.org/10.1038/s41420-025-02758-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41420-025-02758-5