Abstract
Haemodynamic management in critical care typically relies on static, population-based targets that overlook patient-specific physiology and the evolving nature of illness. We develop and validate a framework for real-time, personalised haemodynamic management using a time-dependent Cox model that integrates static and dynamic clinical data to predict survival probabilities and derive optimal heart rate and systolic blood pressure targets over time. Trained on the electronic Intensive Care Unit dataset and externally validated with Medical Information Mart for Intensive Care IV and Indiana University Health cohorts, the model demonstrates high predictive accuracy (c-index up to 0.931) and generalisability across diverse populations. Patients with heart rate and systolic blood pressure values closer to model-predicted targets exhibit significantly lower intensive care unit mortality than those aligned with fixed, population-based thresholds. Exploratory dose-response and propensity score–matched analyses confirm outcome relevance, while case studies illustrate feasibility in critical care settings. This personalised, dynamic approach—termed Haemodynamic Management by Time-Adaptive, Risk-Guided Estimation of Targets (HM-TARGET)—offers a scalable framework for precision haemodynamic management in critically ill patients. Prospective trials are warranted to evaluate clinical impact.
Similar content being viewed by others
Introduction
Intensive care units (ICUs) are critical for managing patients with severe illnesses or injuries, providing life-sustaining support to preserve organ function, prevent further physiological deterioration, and reduce mortality risks1,2. In the United States, ICU care is involved in approximately 20% of Medicare hospitalisations and 10% of commercially insured hospitalisations, and it is associated with disproportionately higher healthcare costs, longer hospital stays, and greater mortality compared to non-ICU admissions3. Globally, as the population ages, ICU admissions among elderly patients are rising at rates exceeding demographic shifts alone, driven further by the increasing burden of comorbidities4. These trends are expected to escalate demand for ICU services and associated healthcare expenditures4. Despite advances in critical care medicine, ICU mortality remains substantial: recent pooled estimates report approximately 24.5% ICU mortality and 36.7% 30-day mortality among critically ill patients aged 80 years or older in Europe5, and around 38% ICU mortality among critically ill cancer patients worldwide6. These statistics underscore the urgent need for continued innovation to improve ICU outcomes.
Among life-support measures in the ICU, circulatory support is central to patient management. Cardiovascular failure remains a major contributor to ICU mortality, with mortality rates exceeding 58% in patients with ventilator-associated lower respiratory tract infections—significantly higher than in those without cardiovascular failure (58% vs. 27%)7 and reaching 62% in patients with ICU-acquired pneumonia complicated by persistent cardiovascular failure8. In post-cardiac arrest patients, cardiovascular-related deaths account for approximately 30% of ICU mortality, with cardiac power output—a haemodynamic measure integrating blood flow and blood pressure—at 8 h emerging as an independent predictor of poor outcomes9. These findings highlight the critical importance of early recognition and management of cardiovascular compromise to improve ICU survival. Haemodynamic management, particularly the regulation of heart rate (HR) and blood pressure, remains a cornerstone of critical care. While advanced haemodynamic monitoring methods, such as central venous pressure and cardiac output measurements, are used selectively, continuous monitoring and management of HR and blood pressure underpin routine ICU practice10. HR is a key determinant of cardiac output, and both cardiac output and blood pressure are fundamental to maintaining tissue perfusion and oxygen delivery11,12. Extensive evidence links abnormalities in HR and blood pressure to adverse outcomes11,13,14,15,16,17,18, underscoring the essential role of optimal HR and blood pressure management in the critical care setting.
However, optimal management of heart rate and blood pressure remains challenging due to their inherently dynamic nature, varying significantly both between and within individuals over time. Current clinical practices predominantly rely on population-level guidelines, expert consensus, and clinician experience, often failing to account for individual variability11,12,19. While personalised blood pressure targets have been explored in specific contexts such as sepsis and perioperative care20,21,22, no existing frameworks address the simultaneous, dynamic, and real-time management of heart rate and blood pressure in critically ill patients. The cardiovascular system is regulated by a complex interplay between autonomic and hormonal mechanisms, including vasomotor control, chemoreflexes, sympathovagal balance, the renin-angiotensin system, and the actions of endogenous vasopressin, all of which are essential for maintaining circulatory homeostasis in health and critical illness23,24. The relationship between HR and blood pressure is complex and non-linear, reflecting dynamic interactions that critically influence organ function and clinical outcomes25.
To address this important gap, our study introduces a novel framework for dynamic, personalised haemodynamic management in ICU patients. Using a time-dependent Cox model, we quantify the mortality risk associated with varying HR and blood pressure combinations over time, incorporating individual patient characteristics and continuously evolving clinical data. This approach enables the generation of an HR-blood pressure-mortality map, which identifies the optimal HR and blood pressure targets associated with the lowest mortality risk or highest survival probability for each patient. By integrating the interdependent effects of HR and blood pressure, our methodology transcends traditional, static haemodynamic management paradigms.
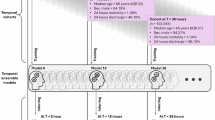
Our research advances the field through a two-step approach. First, we developed a robust time-dependent Cox model that incorporates demographic, baseline, and dynamic clinical variables to model the combined impact of HR and blood pressure on mortality26. Second, we leveraged this model to construct a dynamic heart rate–blood pressure–mortality map, providing actionable insights into optimal haemodynamic targets tailored to individual patients and specific ICU time points. This innovative framework, termed the Haemodynamic Management by Time-Adaptive, Risk-Guided Estimation of Targets (HM-TARGET) initiative, represents a paradigm shift in ICU care by transitioning from static, population-based standards to truly personalised haemodynamic management (Fig. 1). Through this study, we aim to not only fill an important knowledge gap but also lay the groundwork for improved outcomes in critically ill patients.
This figure depicts the three major components of the HM-TARGET framework. a A time-dependent Cox model was developed, integrating static factors (such as age, sex, and comorbidities) and dynamic clinical variables (such as HR, BP, respiratory status, and neurological assessments) to estimate instantaneous mortality risk in ICU patients. b At each specific time point after ICU admission, the HR–SBP combination associated with the lowest predicted mortality hazard was identified as the personalised haemodynamic target. c The clinical relevance of these personalised targets was explored through multiple analyses: a dose-response assessment relating deviations from personalised HR and SBP targets to ICU mortality; comparisons between patients whose haemodynamic values remained close to versus distant from their individualised targets; and head-to-head comparisons between alignment with personalised, dynamic targets and population-based, static thresholds. This framework demonstrates a real-time, patient-specific approach to guiding haemodynamic management in ICU settings. HM-TARGET Haemodynamic Management by Time-Adaptive, Risk-Guided Estimation of Targets, ICU intensive care unit, BMI body mass index, GCS Glasgow Coma Scale, HR heart rate, BP blood pressure, P/F ratio partial pressure of arterial oxygen to inspired oxygen fraction, RS respiratory support, RRT renal replacement therapy, SBP systolic blood pressure.
Results
Overview of HM-TARGET in critical care
HM-TARGET is a modelling framework designed to support personalised and real-time haemodynamic management in critically ill patients. By integrating static and dynamic clinical information through a time-dependent Cox model, HM-TARGET identifies individualised combinations of HR and systolic blood pressure (SBP) that correspond to the highest predicted survival probability at any given time point following ICU admission. This approach addresses a gap in critical care, where current haemodynamic targets are predominantly population-based and often fail to capture patient-specific trajectories.
The framework comprises four stages: (1) cohort definition and data preparation; (2) model development and evaluation; (3) derivation of personalised HR and SBP targets over time; and (4) preliminary validation of the clinical relevance of these targets using both statistical and case-based analyses.
We first curated three large datasets: the eICU Collaborative Research Database (n = 114,636)27, the Medical Information Mart for Intensive Care (MIMIC)-IV (n = 50,761)28, and a regional ICU dataset from Indiana University Health (IUH, n = 3839) (Supplementary Table 1). Only the last ICU admission was included for each patient to avoid duplication. Patients were excluded if they lacked sex, survival status, HR, or SBP data, or if vasopressor doses could not be standardised (i.e. norepinephrine equivalent). Variables were categorised into static (e.g. age, sex, comorbidities) and dynamic (e.g. hourly HR, SBP, vasopressor use, Glasgow Coma Scale) domains (Supplementary Table 2). Missing data were handled using different imputation or complete case methods (Supplementary Tables 3–7).
Using these data, we developed a time-dependent Cox model to estimate the instantaneous hazard of ICU mortality based on time-updated HR and SBP values, alongside interactions with clinically relevant covariates such as age, acute kidney injury, and vasopressor use. The interaction between HR and SBP was central to model performance and retained across all final models. Predictive accuracy was evaluated using the concordance index (c-index) and time-dependent area under the curve (AUC). The best-performing model achieved a c-index of 0.931 in eICU (training dataset), 0.906 (AUC 0.912) in MIMIC-IV (validation dataset), and 0.839 (AUC 0.843) in IUH (validation dataset), demonstrating robust generalisability across populations (Supplementary Tables 8–10).
Model diagnostics based on Schoenfeld residuals supported the proportional hazards assumption for static covariates (Supplementary Fig. 1). Residual analyses further confirmed the appropriateness of using natural cubic splines to model the HR–SBP interaction, as no systematic deviations were observed (Supplementary Fig. 2).
The performance of the model based on K-nearest neighbours (KNN)–imputed data was comparable to that of our current model, supporting the robustness of the imputation strategies used. Our current model slightly outperformed the KNN model on the MIMIC-IV dataset (n = 50,761), which had a larger and more diverse sample, whereas the KNN model performed better on the smaller IUH dataset (n = 3839). Given its superior performance on the larger dataset, we selected our current model for the HM-TARGET framework. In addition, we compared the results of our current model with those from complete-case analyses. Our model demonstrated superior performance across validation datasets, likely reflecting the limited sample sizes available under complete-case definitions. Nonetheless, these findings suggest that the imputation strategy used in our current model did not introduce substantial bias (Supplementary Table 9).
The trained model was then used to generate a time-varying two-dimensional surface that mapped HR and SBP values to survival probabilities at six representative post-admission time points (6, 12, 18, 24, 30, and 36 h). For each patient and timepoint, the optimal HR and SBP were defined as the values associated with the lowest estimated hazard. These personalised targets could be calculated for over 95% of patients at all time points (Supplementary Table 11), confirming the framework’s feasibility in routine ICU settings.
To evaluate clinical relevance, we performed a dose-response analysis in MIMIC-IV, stratifying patients based on the closeness of their actual HR and SBP values to model-predicted targets. Patients whose values remained consistently near the personalised targets had substantially lower ICU mortality. A clear monotonic trend in both mortality and odds ratios was observed across subgroups with different deviations, particularly for personalised targets (Fig. 2), suggesting strong prognostic alignment.
This figure depicts the association between haemodynamic deviation and ICU mortality using a dose-response framework. Analyses were based on the MIMIC-IV dataset and included patients who survived and remained in the ICU for at least 36 h post-admission. Two haemodynamic target sets were evaluated: personalised targets derived from the HM-TARGET time-dependent Cox model, and population-based fixed targets (HR of 80 bpm and SBP of 120 mmHg). a Patients were stratified into seven non-overlapping subgroups according to the number of sampled time points (6, 12, 18, 24, 30, and 36 h post-admission) at which their actual HR and SBP values fell within ±20% of the respective target values. Subgroup 0 included patients with no time points within range, and Subgroup 6 comprised patients whose values remained within range at all six time points. b, c The number of patients in each subgroup for personalised and population-based targets, respectively. d, e ICU mortality incidence across subgroups, showing a stepwise decrease in mortality with closer alignment to targets. f, g Odds ratios for mortality across subgroups, with Subgroup 6 serving as the reference, demonstrating a stronger association between closer adherence to personalised targets and reduced mortality risk compared with population-based targets. Source data are provided as a Source Data file. HR heart rate, SBP systolic blood pressure, ICU intensive care unit, HM-TARGET Haemodynamic Management by Time-Adaptive.
A separate propensity score-matched analysis further confirmed these associations. Patients whose HR and SBP values remained within ±20% of their personalised targets at three or more time points out of six time points sampled (6, 12, 18, 24, 30, and 36 hours post-ICU admission) had a significantly lower risk of mortality compared to those with fewer such alignments (odds ratio 0.443, 95% CI: 0.341–0.575; Fig. 3). A parallel analysis using population-based fixed targets yielded a higher odds ratio (0.511, 95% CI: 0.430–0.607; Fig. 3). To examine the comparative benefit of personalised versus population targets (i.e. dynamic vs static), a direct comparison of favourable subgroups (i.e. HR and SBP values remained within ±20% of target values at three or more time points out of six time points sampled) revealed a 40% relative risk reduction in favour of HM-TARGET-predicted targets (odds ratio 0.609, 95% CI: 0.369–0.997; Fig. 4).
This figure presents mortality comparisons between patients whose haemodynamic values remained closer to their targets and those with greater deviations based on MIMIC-IV data. The analysis included patients who survived and remained in the ICU for at least 36 h post-admission. a Patients were categorised according to alignment with two types of haemodynamic targets: personalised targets derived from the HM-TARGET time-dependent Cox model, and population-based fixed targets (HR of 80 bpm and SBP of 120 mmHg). Favourable deviations were defined as HR and SBP values within ±20% of the target at three or more of six sampled time points (6, 12, 18, 24, 30, and 36 h post-admission), while unfavourable deviations were defined as alignment at fewer than three time points. b For personalised targets, propensity score matching was performed to compare mortality risk between the favourable and unfavourable groups, using baseline static and dynamic covariates, excluding HR and SBP. c A parallel propensity score-matched analysis was conducted for population-based fixed targets using the same methodology. Odds ratios and 95% CIs were based on a two-sided Fisher’s exact test. This figure illustrates that closer alignment with haemodynamic targets—particularly personalised, dynamically derived targets—was associated with a lower risk of ICU mortality. HR heart rate, SBP systolic blood pressure, ICU intensive care unit, HM-TARGET Haemodynamic Management by Time-Adaptive, CI confidence interval, AKI acute kidney injury, P/F ratio partial pressure of arterial oxygen to inspired oxygen fraction, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, MSLD moderate or severe liver disease, AIDS acquired immunodeficiency syndrome, AMD absolute mean differences.
This figure assesses whether patients whose haemodynamic values aligned more closely with personalised HR and SBP targets had a lower mortality risk compared to those aligned with population-based fixed targets. Analyses were performed using the MIMIC-IV dataset and included patients who survived and remained in the ICU for at least 36 hours post-admission. a A head-to-head comparison was conducted between patients with favourable deviations relative to model-predicted personalised targets and those relative to population-based fixed targets (HR of 80 bpm and SBP of 120 mmHg). Favourable deviations were defined as HR and SBP values within ±20% of the respective targets at three or more of six sampled time points (6, 12, 18, 24, 30, and 36 h after ICU admission). Odds ratios and 95% CIs were based on a two-sided Fisher’s exact test. b To prevent confounding from patients meeting favourable criteria under both schemes, these overlapping patients were excluded. c Propensity score matching was then performed on the remaining unique patients in each group using baseline static and dynamic covariates, excluding HR and SBP. This figure demonstrates that closer alignment with personalised, dynamically derived haemodynamic targets was associated with a greater reduction in ICU mortality than alignment with population-based fixed thresholds. ICU intensive care unit, HR heart rate, SBP systolic blood pressure, CI confidence interval, AKI acute kidney injury, P/F ratio partial pressure of arterial oxygen to inspired oxygen fraction, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, MSLD moderate or severe liver disease, AIDS acquired immunodeficiency syndrome, AMD absolute mean differences.
Finally, illustrative patient-level cases demonstrated how HM-TARGET facilitates the visualisation of dynamic HR–SBP–mortality relationships over time. Survival contour maps and time series of actual versus predicted values revealed strong alignment in patients who survived and marked deviation in those who did not (Fig. 5, Supplementary Figs. 3–4).
This figure illustrates the application of the HM-TARGET framework in two patients with ischaemic stroke—one who survived and one who died. Both patients were selected from the MIMIC-IV dataset and survived and remained in the ICU for at least 48 h following ICU admission. a Summary of key clinical characteristics for both patients. b Two-dimensional dynamic colour contour maps of cumulative survival probability, depicting the relationship between actual HR and SBP values and model-predicted optimal targets over time. c Individual trajectories of cumulative survival probability during the ICU stay. For panels b and c, the actual HR, SBP, and GCS values, as well as the model-predicted HR and SBP targets, correspond to the six sampled time points (6, 12, 18, 24, 30, and 36 hours post-ICU admission). d, e Time-series plots showing actual versus predicted HR and SBP targets, along with GCS scores, for the deceased and surviving patients, respectively. In panels d and e, the values correspond to hourly measurements, with median values used when multiple data points were recorded within a single hour. The deceased patient’s SBP values consistently fell below the model-predicted target range, while the surviving patient’s SBP remained closely aligned with the predicted targets across the ICU course. These case studies exemplify the potential clinical relevance and feasibility of applying the HM-TARGET framework to guide real-time, personalised haemodynamic management in critical care. Source data are provided as a Source Data file. HM-TARGET Haemodynamic Management by Time-Adaptive, BMI body mass index, CCI Charlson Comorbidity Index, SOFA Sequential Organ Failure Assessment, HR heart rate, SBP systolic blood pressure, ICU intensive care unit, GCS Glasgow Coma Scale.
Together, these findings support HM-TARGET as a scalable, generalisable, and clinically meaningful framework for personalised haemodynamic management in critical care. Its ability to produce real-time, patient-specific targets represents a notable step towards precision medicine in the ICU.
Patient populations spanned diverse ICUs and clinical profiles
Supplementary Table 1 summarises the characteristics of the eICU, MIMIC-IV, and IUH datasets, highlighting the diversity and clinical relevance of the populations used for model development and validation. These datasets vary in geography, time period, and patient composition, enabling robust generalisability testing of the HM-TARGET framework.
The eICU dataset included 114,636 patients, primarily from 2014 to 2015 across over 200 hospitals in the United States, providing a broad representation of ICU care. The MIMIC-IV dataset contributed 50,761 patients, drawn from ICU admissions at a tertiary centre in Boston between 2008 and 2019, and served as a primary external validation cohort. The IUH dataset included 3,839 ICU patients treated between 2022 and 2024, reflecting a more contemporary regional cohort from Indiana.
ICU mortality rates differed significantly across cohorts, ranging from 4.8% in eICU to 27.5% in IUH, reflecting differences in patient acuity and institutional characteristics. Median ICU length of stay ranged from 38 h in eICU to 87 h in IUH. Median patient age was similar across datasets (65–66 years), though sex distribution varied: 53.9% male in eICU, 55.8% in MIMIC-IV, and 44.5% in IUH. Body mass index, ethnicity distribution, and comorbidity burden (e.g. Charlson Comorbidity Index scores) also varied meaningfully between datasets.
The severity of illness at ICU admission, captured by the worst Sequential Organ Failure Assessment score within 24 h, also differed: median scores were 5 in eICU, 3 in MIMIC-IV, and 4 in IUH. These differences underscore the heterogeneity of the datasets, supporting the robustness and transferability of HM-TARGET across varying ICU populations and practice settings.
Incorporating the HR–SBP interaction enhances model accuracy and supports personalised risk estimation
To optimise predictive performance, we systematically evaluated multiple model configurations that differed in their inclusion of interaction terms between key haemodynamic variables and relevant covariates. The best-performing model incorporated both the interaction between HR and SBP and their interactions with age, norepinephrine equivalent, and acute kidney injury. This comprehensive configuration demonstrated superior discrimination, with a c-index of 0.931 in the eICU development dataset, and 0.906 (AUC 0.912) and 0.839 (AUC 0.843) in the MIMIC-IV and IUH validation datasets, respectively (Supplementary Table 8).
A reduced model excluding the HR–SBP interaction but retaining the other interaction terms showed marked declines in accuracy, with c-index values falling to 0.914 (eICU), 0.878 (MIMIC-IV), and 0.763 (IUH). Comparison of model fit metrics further supported this difference, with the model including HR–SBP interaction demonstrating better performance (Akaike Information Criterion, 87499; Bayesian Information Criterion, 90421) compared to the model without it (Akaike Information Criterion, 89510; Bayesian Information Criterion, 90843) (P < 0.0001) (Supplementary Table 8). This underperformance underscores the central role of HR–SBP interplay in risk estimation across diverse ICU settings.
By contrast, a model that excluded the higher-order interactions with age, norepinephrine equivalent, and acute kidney injury—but retained the HR–SBP interaction—achieved c-index values of 0.928, 0.906, and 0.839 across eICU, MIMIC-IV, and IUH, respectively. These results suggest that while additional interaction terms may offer incremental benefit, the HR–SBP interaction alone accounts for the most critical variance in mortality prediction.
Taken together, these findings highlight the importance of modelling the dynamic interaction between HR and SBP, not only to improve predictive accuracy but also to enhance the biological and clinical plausibility of survival estimation. This interaction reflects well-established physiological principles, as HR and SBP jointly influence cardiac workload, perfusion pressure, and organ oxygenation. Its inclusion is, therefore, essential for real-time, individualised haemodynamic risk modelling.
HM-TARGET maintains strong predictive performance across clinically relevant subpopulations
The distribution of patients and ICU mortality rates across various demographic and clinical subgroups in the eICU, MIMIC-IV, and IUH datasets is summarised in Supplementary Table 12. Subpopulations were defined by age, sex, body mass index, Charlson Comorbidity Index score, and baseline illness severity based on the Sequential Organ Failure Assessment score. The HM-TARGET model demonstrated consistently high predictive accuracy across all subgroups in both MIMIC-IV and IUH cohorts, as detailed in Supplementary Table 13. C-index values were consistently above 0.82 across most strata, with area under the curve values ranging from 0.83 to 0.92. Model performance remained robust among younger and older patients, across both sexes and in those with varying degrees of comorbidity and illness severity. Notably, performance was preserved even in subgroups with elevated mortality risk, such as those with higher Sequential Organ Failure Assessment scores ( ≥ 5) or Charlson Comorbidity Index scores ( ≥2), highlighting that HM-TARGET captures clinically meaningful risk signals across diverse ICU populations and supports its application in personalised haemodynamic management across a broad clinical spectrum.
HM-TARGET enables real-time derivation of personalised HR and SBP targets with high feasibility
To assess the feasibility of generating individualised haemodynamic targets in real time, we applied the HM-TARGET model to a cohort of patients from the MIMIC-IV dataset who survived and remained in the ICU for at least 36 h post-admission. Using the trained model, optimal HR and SBP targets were calculated for each patient at six time points sampled: 6, 12, 18, 24, 30, and 36 h following ICU admission. These targets were derived by identifying the HR–SBP combinations associated with the lowest predicted mortality risk at each time point. The model exhibited high feasibility, successfully generating personalised targets for 95.06% to 96.89% of patients across these time points. A comparison between these predicted targets and the actual recorded HR and SBP values is presented in Supplementary Table 11. This high success rate underscores the model’s applicability across a wide spectrum of critically ill patients and supports its potential utility for dynamic, real-time, risk-guided haemodynamic management in ICU settings.
HM-TARGET–predicted personalised haemodynamic targets are associated with greater mortality reduction than population-based thresholds
To validate the clinical relevance of HM-TARGET–predicted haemodynamic targets, we applied the model to a cohort of MIMIC-IV patients who survived and remained in the ICU for at least 36 h. A dose-response analysis was performed by stratifying patients into seven subgroups based on the number of time points (6, 12, 18, 24, 30, and 36 h post-ICU admission) at which their actual HR and SBP values fell within ±20% of the model-predicted targets. Subgroup 0 included patients with no time points within range, while Subgroup 6 comprised those who consistently remained within range across all six time points (Fig. 2A). Patient counts declined progressively from Subgroup 0 to Subgroup 6 (Fig. 2B), suggesting that many patients did not maintain alignment with their personalised targets. This pattern was not observed with population-based fixed targets (Fig. 2C), indicating their relatively lower discriminatory capacity.
Mortality incidence followed a clear inverse gradient across subgroups, with the highest rates in Subgroup 0 and the lowest in Subgroup 6, for both personalised and population-based targets (Fig. 2D and E). Similarly, odds ratios declined in a stepwise manner across subgroups (Fig. 2F,G), reinforcing the association between closer alignment with targets and improved outcomes. Notably, the decline in odds ratios was steeper for personalised targets than for fixed targets, highlighting the superior predictive precision or stronger prognostic implications of HM-TARGET–derived estimates.
A separate propensity score-matched analysis further corroborated these findings. Patients were dichotomised into two groups: those with favourable deviations (i.e. actual HR and SBP values within ±20% of predicted targets at three or more time points) and those with unfavourable deviations (i.e. fewer than three time points within range). For personalised targets, the odds ratio for ICU mortality was 0.443 (95% CI: 0.341–0.575; Fig. 3), compared with 0.511 (95% CI: 0.430–0.607) for population-based fixed targets, suggesting that closer adherence to personalised targets may be associated with a greater reduction in mortality risk.
To directly compare the value of personalised versus population-based targets (i.e. dynamic vs static), we analysed patients who met favourable deviation criteria as specified above under either scheme. Among matched subgroups, patients whose haemodynamic values aligned with personalised dynamic targets had a 40% relative risk reduction compared to those aligned with population-based static targets (odds ratio 0.609, 95% CI: 0.369–0.997; Fig. 4). These findings underscore the prognostic utility of HM-TARGET–predicted HR and SBP targets and support their potential clinical application in real-time, individualised haemodynamic optimisation in ICU settings.
Case studies illustrate the survival relevance of adhering to HM-TARGET personalised haemodynamic targets
To illustrate the clinical interpretability and utility of HM-TARGET, we selected two patients with ischaemic stroke from the MIMIC-IV dataset—one who survived and one who died—for detailed visualisation and analysis. These case studies demonstrate how personalised, dynamic haemodynamic targets generated by HM-TARGET correspond to divergent clinical outcomes. Their clinical features are summarised in Fig. 5A.
We generated two-dimensional colour contour maps of cumulative survival probability to visualise the model-predicted risk landscapes over time (Fig. 5B). The patient who died showed a substantial and progressive decline in cumulative survival probability. In contrast, the surviving patient maintained a probability above 0.994 throughout the ICU stay, despite a gradual decrease, as projected by the time-dependent Cox model (Fig. 5C).
Notably, both patients had predicted optimal SBP targets around 150 mmHg, higher than standard thresholds—likely reflecting increased cerebral perfusion needs in the setting of acute stroke. However, the actual SBP of the deceased patient consistently remained below this personalised target (Fig. 5D). In contrast, the surviving patient’s SBP was more closely aligned with the predicted target across time points (Fig. 5E). These patterns underscore the clinical relevance of HM-TARGET–predicted targets, especially in conditions with context-specific perfusion demands.
Supplementary Fig. 3 presents additional case studies across age, sex, body mass index, and comorbidity strata, further supporting the model’s flexibility and individualised relevance. Supplementary Fig. 4 expands on these findings with cases across varied acute illness types, including sepsis, heart failure, and traumatic brain injury. Together, these visualisations reinforce the value of HM-TARGET in capturing patient-specific haemodynamic needs and guiding personalised management strategies.
Discussion
This study developed and validated a time-dependent Cox model that utilised dynamic, hourly data along with static baseline data while accounting for the interaction between HR and SBP in critically ill patients. By integrating these variables and their interplay, the model achieved strong predictive performance across diverse datasets and provided personalised HR and SBP targets tailored to individual patients. The preliminary validation demonstrated a significant association between closer alignment with these predicted, personalised, and dynamic targets and reduced mortality, underscoring their clinical importance. Furthermore, the feasibility of applying this personalised approach in real-world ICU settings highlights a meaningful advancement in haemodynamic management for critically ill patients.
HR and blood pressure management are essential components of care for critically ill patients11,13,14,15,16,17,18. However, conventional approaches often rely on fixed, population-based targets that fail to account for individual variability and the dynamic nature of critical illness. Luo et al. found that a low minimum HR after cardiac surgery in ICU patients was associated with poor prognosis29, while Wang et al. observed similar findings in ICU patients with myocardial infarction30. In contrast, Sandfort et al. reported that prolonged elevated HR was linked to decreased survival in a diverse cohort of ICU patients31. Studies by Zhou et al.32 and Yao et al.33 examined the relationship between mean HR and mortality in older sepsis patients and those with ischemic stroke and atrial fibrillation, respectively. While Maheshwari et al.16 and McGuigan et al.17 highlighted the adverse outcomes associated with hypotension and hypertension, their studies did not address the need for personalised, time-specific targets. Additionally, research by Asfar et al.19, Chen et al.34, and Khanna et al.18 focused on blood pressure thresholds in critical care and sepsis, but without considering the interplay between HR and SBP or incorporating dynamic modelling. Xiao et al. investigated the association between mortality and both HR and blood pressure; however, they treated HR and blood pressure as independent variables35. In contrast, our study proposes a novel approach by treating HR and SBP as interdependent variables, utilising dynamic time-series data to provide personalised, dynamic, and actionable insights. This approach bridges a critical gap in the literature and establishes a new standard for individualised haemodynamic management in critically ill patients.
While we used SBP to develop our model, we acknowledge that mean arterial pressure is more commonly used in clinical practice to guide vasopressor management and assess perfusion pressure. To reduce collinearity among blood pressure variables, we selected only one variable to represent blood pressure. SBP was chosen because models incorporating SBP demonstrated superior predictive performance compared to those using mean arterial pressure (Supplementary Table 10). Nonetheless, our open-source code enables clinicians and researchers to retrain the model using mean arterial pressure or other variables of interest, providing flexibility for adaptation to local practice patterns.
We included HR in the model for two main considerations. First, the interaction between HR and blood pressure plays a critical role in determining cardiovascular stress and organ perfusion, yet it has been largely overlooked in previous research11. By explicitly modelling this interaction, we substantially improved predictive performance and aligned the model more closely with the physiological complexity of critical illness. Therefore, the inclusion of HR in modelling may have enhanced the validity of blood pressure targets. Second, HR is often directly and indirectly influenced by blood pressure-targeted therapies, such as phenylephrine, which tends to decrease HR, and epinephrine, which can elevate it36. A combined target of HR and blood pressure may provide a useful reference for vasopressor selection. It is important to note that HR is less frequently a direct target of intervention, especially in the ICU setting, although evidence has suggested favourable outcomes associated with controlling HR within a targeted range13. Therefore, model-recommended HR targets should be interpreted primarily as reference values. They may inform care in select scenarios where HR optimisation could prevent harm, such as in the presence of haemodynamic instability or myocardial ischaemia, but they are not intended to prompt routine aggressive HR management in most patients.
We also recognise that the applicability of model-predicted targets depends on a patient’s physiological state and clinical context. Failure to achieve alignment with target values may reflect either (1) limitations in treatment options due to potential adverse effects (e.g., vasopressor toxicity) or (2) the severity of illness in patients who are nonresponsive to therapy. This interpretation is supported by our observation that vasopressor use progressively increased as deviations from HM-TARGET–predicted SBP targets widened (Fig. 6). These considerations underscore that model-derived targets are not intended as rigid prescriptions but rather serve as reference values to guide individualised care within clinically permissible and physiologically achievable contexts. They are likely to be most useful in patients who remain physiologically responsive to treatment but whose current management has not yet optimised haemodynamic alignment—thereby highlighting actionable opportunities to improve care.
This figure illustrates the distribution of HR and SBP deviations from haemodynamic targets and their association with vasopressor use, based on patients from the MIMIC-IV dataset who survived and remained in the ICU for at least 36 h following admission (n = 28,863). Deviations are expressed as percentages, calculated as the relative difference between actual HR and SBP values and their respective targets—either personalised model-predicted targets (a) or population-based fixed targets (b)—at 36 h post-ICU admission. Each point represents a patient, coloured by vasopressor use (red: vasopressor used; blue: no vasopressor use). Vasopressor use was defined as the administration of any agent from the following list, individually or in combination: norepinephrine, epinephrine, dopamine, phenylephrine, vasopressin, angiotensin II, terlipressin, methylene blue, metaraminol, hydroxocobalamin, or midodrine. Zero deviation lines for HR and SBP are labelled in panels a and b for clarity. The black square indicates the region corresponding to ±20% deviation from target values. c (personalised model-predicted targets) and d (population-based fixed targets) display patient counts across SBP deviation bins and the percentage of vasopressor use (black line) within each bin. Only SBP deviations are shown in panels c and d; HR deviations were not included. Source data are provided as a Source Data file. HR heart rate, SBP systolic blood pressure, ICU intensive care unit.
This study introduces key advancements in the management of critically ill patients by establishing and validating a time-dependent Cox model that generates dynamic survival predictions responsive to evolving ICU conditions. This real-time adaptability enhances both the precision and clinical relevance of the model’s predictions. Unlike population-based methods, our model provides individualised HR and SBP targets at specific time points during ICU admission, thereby addressing the limitations of static thresholds through real-world, real-time, and patient-specific applications. The preliminary validation using dose-response analyses and propensity score-matched comparisons demonstrated that closer alignment with these personalised targets was associated with reduced mortality. At the same time, case studies illustrated the model’s feasibility in guiding haemodynamic management at the bedside.
These findings have important clinical implications. By enabling real-time, individualised targets, the model supports a shift from population-based standards toward tailored care based on patient-specific needs. The ability to guide haemodynamic management dynamically represents a meaningful advance in personalised critical care. As a core component of the HM-TARGET initiative, this study highlights the value of large-scale data integration for developing clinically interpretable models and underscores the role of innovative modelling approaches in improving precision and outcomes in the ICU.
Future research should prioritise prospective randomised controlled trials to validate the clinical efficacy of personalised targets in improving patient outcomes. Expanding this approach to incorporate additional haemodynamic parameters and to encompass diverse patient populations will be essential for enhancing its applicability and generalisability. Additionally, developing user-friendly clinical decision support tools will be critical to facilitate seamless integration of the model into ICU workflows, enabling practical adoption and widespread implementation in real-world clinical settings.
This study has several limitations. The retrospective design, although leveraging large and diverse datasets, highlights the need for prospective randomised controlled trials to confirm whether adherence to model-derived targets improves patient outcomes. While the model was validated across datasets such as MIMIC-IV and IUH, further external validation is required to assess its generalisability to other populations and healthcare settings. Implementing the model into clinical workflows also presents practical challenges, including the need for robust infrastructure to support real-time data capture, processing, and decision-making.
The model’s current design does not account for certain clinical nuances, such as distinguishing between haemorrhagic and ischaemic stroke, which may affect target recommendations. Additionally, optimal HR and blood pressure targets could not be determined for certain time points in fewer than 5% of cases, reflecting areas for future improvement. The study’s focus on mortality as the primary outcome also limits its scope, as it does not consider other critical metrics such as organ function or long-term quality of life, which are essential for evaluating the broader impact of personalised haemodynamic management.
In addition to HR and blood pressure, other important haemodynamic variables, such as preload, contractility, stroke volume, cardiac output, and afterload (or systemic vascular resistance), are sometimes considered in the management of critically ill patients. Although these parameters are not routinely monitored in most ICU settings, their clinical relevance is well recognised, particularly in patients with complex cardiovascular conditions or when advanced haemodynamic monitoring is available11,12,37. While we would have welcomed the opportunity to incorporate these variables into our model, the large ICU datasets used in this study (eICU, MIMIC-IV, and IUH) did not consistently capture these parameters at sufficient resolution or frequency for dynamic modelling. We acknowledge this limitation and emphasise that future research should aim to incorporate more comprehensive haemodynamic data to further improve model fidelity and better capture the full scope of individualised haemodynamic decision-making in critical care.
In conclusion, this study establishes a novel, data-driven framework for personalised haemodynamic management in critically ill patients. Using a time-dependent Cox model that integrates dynamic, patient-specific data, we demonstrated that personalised, dynamic HR and SBP targets were associated with lower mortality and outperformed traditional population-based, static thresholds. Feasibility was confirmed through case studies that showed distinct alignment patterns between predicted targets and actual clinical data in patients with differing outcomes. These findings contribute to the broader objectives of the HM-TARGET initiative, which harnesses large-scale data to enable individualised therapeutic strategies. By aligning haemodynamic targets with each patient’s evolving physiological needs, this framework represents a meaningful step toward precision medicine in critical care. Future research should focus on prospective randomised trials to validate its clinical utility and explore its generalisability across diverse patient populations and healthcare settings.
Methods
This study aimed to develop a robust, data-driven framework for determining individualised, time-varying HR and blood pressure targets tailored to ICU patients. These targets were defined as the HR and blood pressure values associated with the highest predicted survival probability, accounting for patient-specific clinical characteristics. Personalised targets were estimated at specific time points during the ICU stay, enabling real-time haemodynamic guidance by identifying deviations from optimal values that may warrant intervention.
A two-step approach was employed (Fig. 1). First, a predictive model was developed using retrospective data to quantify the relationship between baseline and dynamic clinical variables and survival probability. Second, this model was used to calculate survival probabilities for various HR-SBP combinations at specific time points, generating an HR-SBP survival map. This map facilitates the identification of HR-SBP combinations associated with the highest survival probabilities, providing actionable insights for personalised haemodynamic management.
This framework, termed the HM-TARGET initiative, leverages population-level data to generate individualised targets. This retrospective study utilised data from three sources: the publicly available eICU and MIMIC-IV databases, and institutional data from Indiana University Health (IUH). The eICU and MIMIC-IV datasets are de-identified and publicly accessible under data use agreements; thus, IRB approval and informed consent were not required. The study was approved by the Indiana University Institutional Review Board (February 2, 2024; #22056), with a waiver of informed consent due to its retrospective design. The human data used in this study did not contain any identifiable private information, and none of the authors were involved in its collection. Additional details on the methodology are available in the Supplementary Information.
Datasets
This study utilised three distinct and complementary datasets to develop and validate the proposed framework. The eICU Collaborative Research Database (https://physionet.org/content/eicu-crd/2.0/) provided data from over 200,000 ICU admissions, representing approximately 150,000 unique patients across the United States during 2014 and 2015. This database aggregates electronic health records from more than 200 hospitals, offering a diverse and representative sample of ICU populations and enabling robust model development.
The second dataset, MIMIC-IV (https://physionet.org/content/mimiciv/2.2/), spans from 2008 to 2019 and includes de-identified health information from more than 50,000 patients admitted to ICUs at Beth Israel Deaconess Medical Center in Boston, Massachusetts. Comprising over 73,000 ICU stays, MIMIC-IV offers a rich source of longitudinal data, facilitating external validation of the developed model across a well-characterized patient cohort.
The third dataset consisted of ICU admissions from IUH hospitals between 2022 and 2024. As Indiana’s primary tertiary care provider, IUH serves the state’s most critically ill patients, offering a unique opportunity to evaluate the model’s performance in a distinct regional population. Additionally, this dataset reflects the post-COVID-19 healthcare landscape, providing valuable insights into contemporary ICU practices.
Together, these datasets encompass diverse populations, geographic regions, and timeframes, ensuring the development of a robust and generalisable framework for personalised haemodynamic management.
Participants
We included adult patients (aged ≥18 years) with known survival status, recorded sex, and available HR and SBP data. To minimise bias from repeated admissions, only the most recent ICU stay was analysed for patients with multiple ICU encounters. ICU stays were excluded if vasopressor doses were unavailable when vasopressors were administered, or if mechanical circulatory support was applied (Supplementary Fig. 5).
Input variables
The time-dependent Cox model incorporated a comprehensive set of static and dynamic variables to account for patient-specific characteristics and temporal changes during ICU stays (Supplementary Table 2). Demographic variables included age, sex, and body mass index. Comorbidities were assessed using the 17 conditions defined by the Charlson Comorbidity Index, represented as binary variables (present or absent) and treated as static variables. Dynamic variables captured evolving physiological status across circulatory, respiratory, neurological, renal, hepatic, and haematological systems. Circulatory status was evaluated using HR, SBP, and norepinephrine equivalent; SBP was selected over mean or diastolic arterial pressure to minimise collinearity. Respiratory function was assessed using arterial oxygen partial pressure to inspired oxygen fraction ratio and type of respiratory support. Neurological function was measured by the Glasgow Coma Scale. Renal function was indexed by acute kidney injury stage, hepatic function by serum bilirubin, and coagulation function by platelet count.
Sex and/or gender were considered based on the information available in each dataset. The eICU and MIMIC-IV datasets recorded gender, while the IUH dataset recorded biological sex. These variables were extracted from source medical records; however, the method of determination—whether by self-report or assignment—was not documented. As these datasets were collected approximately a decade ago, when distinctions between sex and gender were not routinely captured, we treated the recorded gender as sex for analytic purposes. Sex, defined as a binary variable (male/female), was included as a covariate in the model and used for stratification in subpopulation analyses. Disaggregated analyses by both sex and gender were not feasible due to the absence of consistently defined, individual-level data across datasets. This approach aligns with the Sex and Gender Equity in Research (SAGER) guidelines to the extent permitted by the available data.
Outcome
The primary outcome was ICU mortality, defined as a time-to-event variable. Time was measured from ICU admission until death. Patients who survived and were discharged were censored at the time of discharge.
Data preparation
Patient variables were categorised as static or dynamic (Supplementary Table 2). Static variables included demographic characteristics and comorbidities, while dynamic variables captured hourly physiological and treatment-related data during ICU stay. For quantitative variables with multiple recordings within a single hour, the median value was used.
There were no missing data for age or sex, as patients lacking either were excluded. Comorbidities were assumed absent if not explicitly recorded. Norepinephrine equivalent and respiratory support were considered absent if not documented for the hour in question. However, several variables—including body mass index, HR, SBP, Glasgow Coma Scale, arterial oxygen partial pressure to inspired oxygen fraction ratio, acute kidney injury stage, bilirubin, and platelet count—could contain missing values (Supplementary Table 3).
In the primary analysis, missing data were handled using variable-specific imputation strategies. Body mass index, as a static variable, was imputed using the cohort median. Hourly dynamic values were imputed using the most recent prior measurement or, if unavailable, the next recorded value38. For variables entirely missing throughout the ICU stay, clinically plausible constants or ranges were applied: the Glasgow Coma Scale was imputed as 15; the arterial oxygen partial pressure to inspired oxygen fraction ratio was randomly assigned a value between 350 and 360; bilirubin between 0.1 and 1.2 mg/dL; and platelet count between 150 and 450 × 10⁹/L. Patients without any recorded staging for acute kidney injury, a dynamic categorical variable, were assumed not to have acute kidney injury. HR and SBP did not require random assignments, as inclusion criteria ensured at least one measurement was available (Supplementary Table 4).
To assess robustness, we retrained the model after imputing missing values using K-nearest neighbours (KNN)39,40. For the static variable body mass index, we applied a univariate KNN approach: using age and sex as features, the model identified the five nearest patients (Euclidean distance, k = 5) with observed values and imputed missing values as the mean. For dynamic variables, including HR, SBP, Glasgow Coma Scale, the arterial oxygen partial pressure to inspired oxygen fraction ratio, acute kidney injury stage, total bilirubin, and platelet count, we used a two-stage KNN procedure. The first stage prioritised within-patient imputation to preserve short-term temporal trends. In the second stage, any remaining missing values were imputed using the five most clinically similar patients based on all other covariates. All features were standardised prior to distance calculation. This approach captures complex relationships between missing and available data (Supplementary Table 5).
We conducted sensitivity analyses based on two definitions of complete-case cohorts: (1) patients with at least one recorded value for each variable subject to potential missingness during the ICU stay (Supplementary Table 6), and (2) patients with complete hourly HR and SBP data across the entire ICU stay (Supplementary Table 7). In both analyses, missing values were addressed using the same variable-specific imputation strategies as in the primary analysis (Supplementary Table 4).
Time-dependent Cox model
This study employs a time-dependent Cox proportional hazards model to predict survival probabilities in critically ill patients. This model is well-suited for capturing the effects of variables that evolve over time, a critical requirement in ICU settings characterised by dynamic changes in patient conditions. The hazard function, \(\lambda \left(t\right)\), is defined as:
where \({\lambda }_{0}\left(t\right)\) represents the baseline hazard function, \(X\left(t\right)\) includes all time-dependent covariates except HR and SBP, and \(\beta\) denotes the coefficients of \(X\left(t\right)\). The combined effect of HR and SBP, \(f\left\{{{\rm{HR}}}\left(t\right),{{\rm{SBP}}}(t)\right\}\), is modelled nonparametrically as a surface approximated by cubic B-splines. The interaction between this combined effect and additional time-dependent covariates (Z(t)) is captured by \(g\left\{{{\rm{HR}}}\left(t\right),{{\rm{SBP}}}\left(t\right),Z\left(t\right)\right\}\), which is modelled by cubic B-splines similarly to f. We used natural splines to control the boundary variability. This flexible modelling framework dynamically updates risk estimates as covariate values change, enabling real-time identification of optimal HR and SBP targets.
The primary outcome of interest is time-to-mortality, measured from ICU admission to death. The model updates dynamically with each new measurement, providing instantaneous mortality risks and cumulative survival probabilities. This real-time adaptability is particularly advantageous in ICU care, where physiological variables exhibit significant temporal variation. By integrating dynamic changes in HR, SBP, and other critical parameters, the model offers detailed insights into their influence on survival probabilities, facilitating personalised patient management.
Model output
The model, organised on an hourly time grid, generated a hazard estimate at the end of each hour during a patient’s ICU stay. This hazard was converted into a conditional instantaneous survival probability, ranging from 0 to 1, for each hour. Cumulative survival probability was then calculated as the product of all instantaneous survival probabilities from the first hour to the hour of interest, providing a continuous measure of survival likelihood over time. This cumulative probability, also ranging from 0 to 1, offered a comprehensive and dynamic assessment of the patient’s prognosis throughout the ICU stay.
Sample size
The sample size was determined by the number of patients retained in the eICU, MIMIC-IV, and IUH datasets after applying the exclusion criteria outlined in this study’s design (see Results).
Model development and performance evaluation
The time-dependent Cox model was developed using the eICU dataset and validated externally with the MIMIC-IV and IUH datasets. Predictive performance was assessed using the concordance index (c-index) and time-dependent receiver operating characteristic (ROC) curves. Additional metrics, including sensitivity, specificity, and the area under the ROC curve (AUC), were calculated to evaluate the model’s discriminative ability.
We assessed the proportional hazards assumption for static covariates using scaled Schoenfeld residuals from a refitted model containing only time-constant variables. Graphical diagnostics showed no discernible trends and confirmed proportionality (Supplementary Fig. 1). Additionally, we evaluated the goodness-of-fit of the spline-based HR–SBP interaction model using martingale and deviance residual plots, which demonstrated appropriate calibration and no major model misspecification (Supplementary Fig. 2).
Subpopulation analyses and fairness
The model’s performance was evaluated across diverse subpopulations to ensure fairness and robustness. The dataset was stratified by age, sex, ethnicity (self-reported), body mass index, Charlson Comorbidity Index score, and Sequential Organ Failure Assessment score. Age groups were defined as younger than 65 years versus 65 years and older. Sex was categorised as male or female, while ethnic groups included Caucasians, African Americans, Latinos/Hispanics, and other ethnicities.
Additional stratifications included body mass index (less than 30 versus 30 or higher), Charlson Comorbidity Index score (less than 2 versus 2 or higher), and the worst Sequential Organ Failure Assessment score recorded within the first 24 h of ICU admission (less than 5 versus 5 or higher). These analyses assessed the model’s generalisability and ensured equitable performance across subpopulations.
Personalised HR and SBP targets for individual patients
This study aimed to achieve two objectives: (1) to develop a time-dependent Cox model for predicting survival probabilities in ICU patients using static and dynamic information, and (2) to identify personalised, optimal HR and SBP targets for individual patients at specific time points.
Two-dimensional colour contour maps, termed HR-SBP-survival maps, were created to visualise these optimal targets. These maps illustrate the relationship between HR, SBP, and cumulative survival probabilities at each hour during a patient’s ICU stay. Generated using the developed model, the maps highlight the HR and SBP combinations associated with the highest cumulative survival probabilities, providing an individualised representation of risk. Both predicted optimal HR and SBP targets and actual HR and SBP values were plotted on the contour maps to enable direct comparisons. Discrepancies between actual values and predicted targets signal potential needs for clinical intervention or further evaluation.
The HR-SBP-survival contour maps were constructed by interpolating a grid of points, resulting in quantile-defined levels that visually represent survival probabilities across HR and SBP combinations. These maps serve as a practical and actionable tool for guiding personalised haemodynamic management in critically ill patients.
Validation of personalised HR/SBP targets
The effectiveness of the HM-TARGET approach was validated by addressing the following clinical questions using a cohort of patients from the MIMIC-IV dataset who survived and remained in the ICU 36 h post-admission.
The first question assessed whether patients whose actual HR and SBP values were closer to their personalised targets had a lower mortality risk than those with greater deviations. Deviations were calculated as the absolute difference between actual values and predicted targets, divided by the target value and expressed as a percentage. A threshold of ±20% around the target was used to define alignment. We selected a cohort from the MIMIC-IV dataset, consisting of patients who survived and remained in the ICU for at least 36 h after admission. Deviations were evaluated at six time points: 6, 12, 18, 24, 30, and 36 h post-admission. Patients were stratified into seven subgroups (0–6) based on the number of time points at which their HR and SBP values fell within the ±20% targets. Subgroup 0 included patients with no aligned time points, while Subgroup 6 included those whose actual values remained within ±20% targets at all six time points. This stratification captured varying degrees of alignment with targets during the first 36 h of ICU stay. Outcomes were assessed using 24 h mortality, defined as death occurring between 36 and 60 h post-admission for this cohort of patients who had survived 36 h since ICU admission. Mortality incidence and odds ratios were calculated for each subgroup, with Subgroup 6 serving as the reference. This exploratory analysis enabled a dose-response assessment of the relationship between target alignment and clinical outcomes.
To further account for potential confounders, we performed a propensity score-matched analysis. Patients were categorised into two groups: those with favourable deviations—defined as HR and SBP values within ±20% of their personalised targets at three or more of the six evaluated time points—and those with unfavourable deviations, with fewer than three aligned time points. Matching was conducted using the model’s static and dynamic variables, excluding HR and SBP. For dynamic variables, median values from the 24- to 36 h post-admission window were used. Nearest neighbour matching with a calliper width of 0.1 was applied, allowing variable matching ratios to maximise cohort size. Odds ratios and 95% confidence intervals for 24 h mortality were calculated using a two-sided Fisher’s exact test to compare the two groups41. The definition of 24 h mortality followed the same criteria as described above.
We further evaluated whether patients whose HR and SBP values aligned more closely with personalised, dynamic targets derived from the time-dependent Cox model had better outcomes than those with population-based static targets. Mortality risk was compared between patients with favourable deviations relative to personalised targets and those with favourable deviations relative to population-based targets. Favourable deviations were defined using the same criteria described above. To account for potential overlap between groups, propensity score matching was performed using only patients uniquely assigned to each group. After matching, odds ratios and 95% confidence intervals were calculated using the methods described above to estimate the difference in mortality risk between the two approaches41.
Each of the three prespecified comparisons in the exploratory validation analyses addressed a distinct clinical question and was analysed independently, using separate propensity score-matched cohorts and a single p-value per comparison. These exploratory analyses were designed to assess the outcome relevance of HM-TARGET–predicted personalised targets rather than to evaluate a family of confirmatory hypotheses. Accordingly, no adjustments for multiple comparisons (e.g., Bonferroni or false discovery rate) were applied, as such corrections would likely be overly conservative and could obscure clinically meaningful patterns. In addition, stratified dose-response analyses were conducted descriptively to assess robustness and consistency in trends without inferential testing. We acknowledge that future confirmatory trials comparing HM-TARGET–guided care with standard practice will require appropriate statistical control for multiple comparisons.
Patient involvement and ethical considerations
This retrospective study did not involve direct patient participation in designing the research, defining outcomes, or planning recruitment. Patients were not consulted to interpret or report the results. All analyses were conducted in compliance with ethical standards, including strict adherence to data anonymisation and privacy protection. The study protocol was approved by the institutional review board, and the requirement for informed consent was waived due to the retrospective nature of the research.
Software and statistical environment
Data extraction, preprocessing, model development, and statistical analyses were conducted using R (version 4.3.1) and Python (version 3.11). In R, the following packages were employed: arrow (v15.0.1), caret (v6.0-94), cobalt (v4.5.5), dplyr (v1.1.4), ggplot2 (v3.5.0), lmtest (v0.9-40), MatchIt (v4.5.5), numDeriv (v2016.8-1.1), officer (v0.6.8), pROC (v1.18.5), psych (v2.4.6.26), rvg (v0.3.5), sandwich (v3.1-0), smd (v0.7.0), splines (v4.3.1), stringr (v1.5.1), survival (v3.5-8), tidyr (v1.3.1), tidyverse (v2.0.0), ggpubr (v0.6.0), and zoo (v1.8-12). These packages supported data cleaning, imputation, statistical modelling, survival analysis, and propensity score matching. Python was used primarily for SQL-based data extraction and additional data preprocessing, utilising pandas (v2.1.4), numpy (v1.26.4), matplotlib (v3.8.2), seaborn (v0.13.0), scikit-learn (v1.3.2), and psycopg2 (v2.9.10).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The eICU and MIMIC-IV data used in this study are available in the PhysioNet database under accession codes https://physionet.org/content/eicu-crd/2.0/ and https://physionet.org/content/mimiciv/2.2/. The processed data and code are available at Code Ocean under https://doi.org/10.24433/CO.0726324.v2. The IUH data are available under restricted access for privacy and institutional regulatory reasons, access can be obtained by reasonable request to the corresponding author (Lingzhong Meng; email: menglz@iu.edu) and is subject to Indiana University Health institutional approval. The raw Indiana University Health data are protected and are not available due to data privacy laws. Source data are provided with this paper.
Code availability
The code used to develop the time-dependent Cox model, personalised haemodynamic management targets, and other analyses is openly accessible in Code Ocean (https://doi.org/10.24433/CO.0726324.v2).
References
Vincent, J. L. et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir. Med. 2, 380–386 (2014).
Marshall, J. C. et al. What is an intensive care unit? A report of the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 37, 270–276 (2017).
Kannan, S., Giuriato, M. & Song, Z. Utilization and outcomes in U.S. ICU hospitalizations*. Crit. Care Med. 52, 1333–1343 (2024).
Jones, A., Toft-Petersen, A. P., Shankar-Hari, M., Harrison, D. A. & Rowan, K. M. Demographic shifts, case mix, activity, and outcome for elderly patients admitted to adult general ICUs in England, Wales, and Northern Ireland. Crit. Care Med. 48, 466–474 (2020).
Fronczek, J. et al. Short-term mortality of patients ≥80 years old admitted to European intensive care units: an international observational study. Br. J. Anaesth. 129, 58–66 (2022).
Nazer, L. H. et al. A systematic review and meta-analysis evaluating geographical variation in outcomes of cancer patients treated in ICUs. Crit. Care Explor 4, e0757 (2022).
Martin-Loeches, I. et al. The association of cardiovascular failure with treatment for ventilator-associated lower respiratory tract infection. Intensive Care Med. 45, 1753–1762 (2019).
Martin-Loeches, I. et al. Impact of cardiovascular failure in intensive careunit-acquired pneumonia: a single-center, prospective study. Antibiotics 10, 798 (2021).
Magni, F. et al. Cardiac power output is associated with cardiovascular related mortality in the ICU in post-cardiac arrest patients. Resuscitation 194, 110062 (2024).
Vincent, J. L. et al. Clinical review: update on hemodynamic monitoring - a consensus of 16. Crit. Care 15, 229 (2011).
Meng, L. Heterogeneous impact of hypotension on organ perfusion and outcomes: a narrative review. Br. J. Anaesth. 127, 845–861 (2021).
Meng, L. et al. Blood pressure targets in perioperative care: provisional considerations based on a comprehensive literature review. Hypertension 72, 806–817 (2018).
Morelli, A. et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 310, 1683–1691 (2013).
Nguyen, D., Kritek, P. A., Greco, S. A. & Prutkin, J. M. Bradycardia at the onset of pulseless electrical activity arrests in hospitalized patients is associated with improved survival to discharge. Heliyon 6, e03491 (2020).
Yong, J., Hibbert, P., Runciman, W. B. & Coventry, B. J. Bradycardia as an early warning sign for cardiac arrest during routine laparoscopic surgery. Int. J. Qual. Health Care 27, 473–478 (2015).
Maheshwari, K. et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 44, 857–867 (2018).
McGuigan, P. J. et al. The effect of blood pressure on mortality following out-of-hospital cardiac arrest: a retrospective cohort study of the United Kingdom Intensive Care National Audit and Research Centre database. Crit. Care 27, 4 (2023).
Khanna, A. K. et al. Association of systolic, diastolic, mean, and pulse pressure with morbidity and mortality in septic ICU patients: a nationwide observational study. Ann. Intensive Care 13, 9 (2023).
Asfar, P. et al. High versus low blood-pressure target in patients with septic shock. N. Engl. J. Med. 370, 1583–1593 (2014).
Simon, B. A. et al. Incorporating individual-level treatment effects and outcome preferences into personalized blood pressure target recommendations. J. Am. Heart Assoc. 13, e033995 (2024).
Nils, H. P. et al. Association of personalized blood pressure targets with hemorrhagic transformation and functional outcome after endovascular stroke therapy. JAMA Neurol. 76, 1256–1258 (2019).
Hong X., et al. Adaptive average arterial pressure control by multi-agent on-policy reinforcement learning. Sci Rep. 15, 679 (2024).
Dampney, R. A. L. Central neural control of the cardiovascular system: current perspectives. Adv. Physiol. Educ. 40, 283–296 (2016).
Miller, A. J. & Arnold, A. C. The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications. Clin. Autonomic Res. 29, 231–243 (2019).
Singh, V., Gupta, A., Sohal, J., Singh, A. & Bakshi, S. Age induced interactions between heart rate variability and systolic blood pressure variability using approximate entropy and recurrence quantification analysis: a multiscale cross correlation analysis. Phys. Eng. Sci. Med. 44, 497–510 (2021).
Therneau T. M., Grambsch P. M., Therneau T. M., Grambsch P. M. The Cox model. (Springer, 2000).
Pollard, T. J. et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data 5, 180178 (2018).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1 (2023).
Luo, C. et al. Minimum heart rate and mortality after cardiac surgery: retrospective analysis of the Multi-parameter Intelligent Monitoring in Intensive Care (MIMIC-III) database. Sci. Rep. 13, 2597 (2023).
Wang, J. et al. Minimum heart rate and mortality in critically ill myocardial infarction patients: an analysis of the MIMIC-III database. Ann. Transl. Med. 9, 496 (2021).
Sandfort, V., Johnson, A. E., Kunz, L. M., Vargas, J. D. & Rosing, D. R. Prolonged elevated heart rate and 90-day survival in acutely ill patients: data from the MIMIC-III database. J. Intensive Care Med. 34, 622–629 (2019).
Zhou, Q., Li, J., Miao, Y. & Li, N. Effect of mean heart rate on 30-day mortality in older patients with sepsis: data from the MIMIC-IV database. Am. J. Med. Sci. 369, 176–182 (2024).
Yao, S.-L., Chen, X.-W., Liu, J., Chen, X.-R. & Zhou, Y. Effect of mean heart rate on 30-day mortality in ischemic stroke with atrial fibrillation: data from the MIMIC-IV database. Front. Neurol. 13, 1017849 (2022).
Chen, Y., Luo, M., Cheng, Y., Huang, Y. & He, Q. A nomogram to predict prolonged stay of obesity patients with sepsis in ICU: relevancy for predictive, personalized, preventive, and participatory healthcare strategies. Front. public health 10, 944790 (2022).
Xiao, W. et al. The association of diastolic arterial pressure and heart rate with mortality in septic shock: a retrospective cohort study. Eur. J. Med. Res. 27, 285 (2022).
Meng, L. et al. Effects of phenylephrine on systemic and cerebral circulations in humans: a systematic review with mechanistic explanations. Anaesthesia 79, 71–85 (2024).
Han, J. et al. Care guided by tissue oxygenation and haemodynamic monitoring in off-pump coronary artery bypass grafting (Bottomline-CS): assessor blind, single centre, randomised controlled trial. Bmj 388, e082104 (2025).
Shao, J. & Zhong, B. Last observation carry-forward and last observation analysis. Stat. Med. 22, 2429–2441 (2003).
Beretta, L. & Santaniello, A. Nearest neighbor imputation algorithms: a critical evaluation. BMC Med. Inf. Decis. Mak. 16, 74 (2016).
Troyanskaya, O. et al. Missing value estimation methods for DNA microarrays. Bioinformatics 17, 520–525 (2001).
Fay, M. P. Two-sided exact tests and matching confidence intervals for discrete data. R. J. 2, 53 (2010).
Acknowledgements
We thank Rick V Tuason from Clinical Research Systems, Enterprise Analytics, Indiana University Health located in Indianapolis, Indiana, USA, for his help in preparing the Indiana University Health dataset. We thank the support provided by institutional and/or departmental sources. The images (ICU patient and physician symbols) in Fig. 1a and b were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
L.M. and D.S. conceived and supervised the project. L.M., J.S., Z.L., and H.T. designed the model and computational framework. L.M. and Y.S. designed the study and contributed to the initial drafting of the paper. Y.S., J.L., X.L., and G.M. collected and organized data. Y.S. and D.S. performed the statistical analysis. X.G., D.C.A., H.T., J.S., Z.L., and D.S. provided critical comments and reviewed the paper. All authors discussed the results and approved the final version before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Filippo Annoni and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Y., Li, J., Liu, X. et al. The HM-TARGET personalised real-time haemodynamic targets in critical care. Nat Commun 16, 7307 (2025). https://doi.org/10.1038/s41467-025-62527-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-62527-x