Abstract
Seasonal and pandemic influenza viruses are continuous threats to human health, requiring rapid development of vaccines to multiple evolving viral strains. RNA vaccine technologies have the adaptability and manufacturability to facilitate pandemic preparedness but have limited flexibility in their route of administration, reducing the ability to establish local protective immune responses such as respiratory mucosal immunity. Here, we describe monovalent and bivalent replicon vaccines against A/Vietnam/1203/2004 H5N1 and A/Anhui/PA-1/2013 H7N9. These replicon vaccines express either H5 or H7 hemagglutinin and are formulated with a nanostructured lipid carrier (NLC) that permits both intramuscular (IM) and intranasal (IN) dosing. In mice, IM vaccination established systemic humoral and cellular responses but no detectable mucosal response, while IN administration induced robust systemic and mucosal immunity. The replicon-NLC vaccines protected against morbidity and mortality in ferret challenge models, establishing this intranasally-administered replicon-NLC vaccine platform as a potential pandemic response tool.
Similar content being viewed by others
Introduction
Influenza virus is a significant threat to human health, with seasonal circulation and pandemic outbreaks resulting in 9.3–41 million illnesses, 100,000–710,000 excess hospitalizations, and 4900–51,000 deaths per year from 2010 to 2023 in the US alone1. Of particular concern are zoonotic crossover events of “pre-pandemic” influenza viral strains that harbor the potential to gain human-to-human transmissibility2. Avian influenza A viral strains have been detected in cows (H5N1), chickens (H5N2), and multiple avian species (H7N9), which increases the exposure risk to human hosts3,4. H5N1 influenza virus represents a particular concern given its current circulation in US bird and dairy cow populations3,5, its ability to infect multiple mammalian species6, and its potential pathogenicity in humans resulting in high mortality in Africa, Asia, and the Middle East7. As of January 2025, the CDC had confirmed 66 human H5N1 cases in the US across seven states, originating from cattle and poultry exposure sources8. This risk is emphasized by an H5N1-associated death in the US in January 20259. While not currently tracked as extensively as H5N1, H7N9 also presents a high potential risk due to its significant lethality in humans, up to 31.1%, from previous outbreaks10. An H7N9 outbreak was reported in a US domestic chicken flock in March 202511,12. Multiple properties of H7N9 influenza virus are of major concern, including the potential for animal-to-human transmission13, transmission by infectious respiratory particles14, and its ability to bind human sialic acid receptors15,16. Therefore, outbreaks of these variant strains pose a considerable risk for future pandemics.
Many vaccines have been developed to combat influenza17, the majority of which are multivariant live-attenuated (LAIV) or inactivated (IIV) vaccines against seasonal influenza viral strains that rely on traditional egg-based manufacturing methods. However, despite broad availability, vaccine effectiveness only ranges from 10 to 60% on a year-to-year basis and provides no protection against emerging pre-pandemic strains2. Moreover, given the lead time required to isolate, culture, and formulate vaccines against emerging viral strains, LAIVs and IIVs are susceptible to antigenic drift and are severely limited in pandemic response18.
RNA vaccine platforms overcome many of the limitations of traditional influenza vaccines by combining antigen flexibility with ease of manufacturing. As evidenced by the success of mRNA vaccines against SARS-CoV-2, which provided excellent protection against severe COVID-19, RNA-based vaccines exhibit properties necessary for pandemic preparedness and response, including establishment of robust immune responses. Recent work has also demonstrated their potential application in multivalent vaccine formulations19,20. However, there are major limitations with current RNA vaccines against respiratory pathogens, including their need for deep cold-chain storage21, their inability to prevent mild infection in respiratory tissues22,23 possibly due to lack of established mucosal immunity24, vaccine hesitancy25, and rapid waning of vaccine induced protective immunity26.
Intranasally administered vaccines are a promising tool for pandemic response. The ability to stimulate mucosal immunity at the site of natural respiratory viral infection may be key to improving vaccines’ ability to slow or halt a respiratory viral epidemic. In contrast to standard intramuscular (IM) vaccines, intranasal (IN) vaccines can stimulate mucosal immunity after local antigen exposure, with responses mediated by IgA antibodies and lung-resident memory T and B cells27,28, which can prevent early-stage infection and viral replication and shedding, thus minimizing chances for viral transmission29,30,31. Intranasal vaccination devices also permit self-administration, the avoidance of needles, easier storage and transport32, and simpler dosing of pediatric populations, thus increasing patient compliance and vaccine uptake. To date, the only FDA-approved IN vaccine is FluMist, a quadrivalent seasonal LAIV. Development of IN RNA vaccines would be a key tool for respiratory virus pandemic response, combining the mucosal immune stimulation of IN vaccines with the adaptability and production speed of RNA vaccines.
Here we report the application of a thermostable33,34 and intranasally-deliverable35 replicon-nanostructured lipid carrier (NLC) vaccine platform towards the development of highly effective monovalent and bivalent replicon-NLC vaccines against high-risk H5 and H7 influenza virus strains. We demonstrate the induction of both strong systemic and respiratory mucosal immune responses in mice that are unique to IN immunization. These vaccines also provide ferrets full protection from H5N1 and H7N9 viral-induced morbidity and mortality after high-dose challenge.
Results
Intranasal administration of a monovalent H5 replicon-NLC vaccine establishes dose-dependent systemic and mucosal immunity in mice
To establish proof-of-concept application of a replicon-NLC vaccine platform against high-risk avian influenza virus, we generated a Venezuelan equine encephalitis virus (VEEV)-based replicon (also known as self-amplifying RNA, or saRNA) expressing full-length H5 hemagglutinin (HA) derived from the prototypic A/Vietnam/1203/2004 H5N1 influenza viral strain (Supplementary Fig. 1). Vaccines were prepared by complexing H5-expressing replicon with NLC, followed by biophysical characterization of the resulting vaccine complex size, RNA dose, and RNA integrity (Supplementary Fig. 2A, B). This replicon-NLC vaccine formulation was previously optimized to allow both IM and IN delivery36.
We first verified the immunogenicity of this monovalent H5 replicon-NLC vaccine in mice when administered IN by droplets using a pipette and/or IM via injection. C57BL/6 mice were prime/boost vaccinated 21 days apart either IN/IN (1, 5, or 10 µg), IM/IM (5 µg), or IM-prime, IN-boost (IM/IN, 5 µg) to examine the effects of dose and administration route on immunogenicity (Fig. 1A). An alum-adjuvanted, inactivated whole virion influenza A/Vietnam/1203/2004 H5N1 vaccine37 (“Baxter,” BEI Resources #NR-12143) delivering 2.5 µg of HA protein was dosed IM/IM as a positive vaccine comparator, while 5 µg of NLC-delivered replicon expressing the non-immunogenic reporter protein secreted embryonic alkaline phosphatase (SEAP) dosed IN/IN served as a negative vector control. Of note, biodistribution studies (Supplementary Fig. 3) of mice dosed IN with 10 µg of replicon complexed with fluorescently-labeled NLC demonstrated that this method of administration delivers the vaccine throughout the upper and lower respiratory tract as well as the stomach (likely due to material being swallowed) as expected38. Reactogenicity was assessed by monitoring animal weights for 4 days post-vaccination (Supplementary Fig. 2C, D).
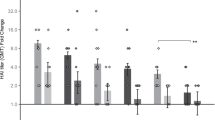
A Study design. Images for figure were obtained from Nicolás De Francesco/SciDraw (https://scidraw.io/) and Servier Medical Art (https://smart.servier.com/) under a CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/). Post-boost B serum H5-binding IgG titers, C serum H5 pseudovirus neutralization capacity (IC50), D splenic H5-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells, E H5-binding IgA titers in BAL samples, F H5-binding IgG titers in BAL samples, and G lung-resident (CD69+ CD103+) H5-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells. n = 6 for vector control group and n = 8 for experimental groups. All groups were sex balanced. Two statistical hypotheses were tested within each figure, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. B, E, F Statistical analyses performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. C Statistical analysis performed on log-transformed data using Kruskal–Wallis test with Dunn’s multiple comparisons. D, G Statistical analysis represents Kruskal–Wallis test with Dunn’s multiple comparisons. Data are presented as B, C geometric mean +/− geometric SD or E, F mean +/− SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated post-prime IgG and post-boost CD4+ and CD8+ T cell data in Supplementary Fig. 4. Source data are provided as a Source Data file.
All groups receiving at least 5 µg of H5 replicon-NLC developed equivalent serum H5-binding IgG and neutralizing antibody (nAb) titers, despite differences in administration route, indicating that IN vaccination with replicon-NLC establishes a robust systemic immune response comparable to IM or heterologous IM/IN immunization (Fig. 1B, C, post-boost; Supplementary Fig. 4A, post-prime). Additionally, regardless of administration route, 5 µg doses of H5 replicon-NLC induced responses comparable to those seen in the Baxter vaccine control group. Proportions of splenic H5-reactive activated (CD44+) CD4+ T cells and CD8+ T cells similarly increased in a dose-dependent manner following IN vaccination (Fig. 1D and Supplementary Fig. 4B, C). Comparable frequencies of polyfunctional CD4+ and CD8+ T cells were observed for IN/IN 10 µg, IM/IN 5 µg, or IM/IM 5 µg H5 replicon-NLC groups (p > 0.05), whereas the Baxter control H5N1 vaccine induced a negligible polyfunctional T cell response in the spleen. Notably, the heterologous IM/IN 5 µg regimen established splenic CD8+ T cell responses comparable to those induced by a double dose (10 µg) delivered IN (p > 0.05), indicating a potential advantage of a heterologous IM/IN prime/boost strategy. These data demonstrate that our H5 replicon-NLC vaccine, delivered either IN or IM, stimulates systemic humoral and cellular immune responses in mice at least comparable to the adjuvanted Baxter vaccine.
In addition to overcoming vaccine hesitancy due to fear of needles and the potential for self-administration, an IN vaccine may also be advantageous for inducing localized respiratory mucosal immunity at the site of infection. Strikingly, development of both H5-binding IgA in bronchoalveolar lavage (BAL) samples and lung-resident (CD69+ CD103+) polyfunctional CD8+ T cells was observed in animals receiving at least one IN vaccination (Fig. 1E, G), while little to no mucosal response was detected in either IM-only replicon-NLC or Baxter vaccinated animals. All vaccinated mice who developed significant serum IgG titers also developed detectable IgG in BAL (Fig. 1F), suggesting that even systemic IgG is detectable in BAL, while mucosal IgA in BAL is only induced by IN vaccination. Lung-resident (CD69+) polyfunctional CD4+ T cells and IFNγ+ CD8+ T cells followed a similar pattern (Supplementary Fig. 4D, E). These data demonstrate that homologous IN/IN and heterologous IM/IN administrations of our H5 replicon-NLC vaccine can establish both systemic and mucosal immune responses and that IN administration is necessary for the development of robust mucosal immunity.
Intranasal administration of a monovalent H7 replicon-NLC vaccine establishes dose-dependent systemic and mucosal immunity in mice
We next evaluated immunogenicity of a monovalent H7 replicon-NLC candidate. Similar to our H5 replicon-NLC vaccine, full-length H7 HA derived from A/Anhui/PA-1/2013 H7N9 influenza virus was engineered into a VEEV-based replicon (Supplementary Fig. 1), complexed with NLC, and characterized (Supplementary Fig. 5A, B). Mice were prime/boost immunized with H7 replicon-NLC via homologous (IN/IN or IM/IM) or heterologous (IM/IN) routes (Fig. 2A), and reactogenicity was assessed as above (Supplementary Fig. 5C, D). Unlike H5N1, no H7N9 vaccine comparator was readily available for this study.
A Study design. Images for figure were obtained from Nicolás De Francesco/SciDraw (https://scidraw.io/) and Servier Medical Art (https://smart.servier.com/) under a CC BY 4.0 license (https://creativecommons.org/licenses/by/4.0/). Post-boost B serum H7-binding IgG titers, C serum H7 pseudovirus neutralization capacity (IC50), D splenic H7-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells, E H7-binding IgA antibody titers in BAL samples, and F lung-resident (CD69+ CD103+) H7-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells. For all assays, n = 6 animals for vector control group and n = 8 for experimental groups. All groups were sex balanced. Two statistical hypotheses were tested within each figure, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. B, E Statistical analyses performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. C Statistical analysis performed on log-transformed data using Kruskal–Wallis test with Dunn’s multiple comparisons. D, F Statistical analysis represents Kruskal–Wallis test with Dunn’s multiple comparisons. Data are presented as B, C geometric mean +/− geometric SD or E mean +/− SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated post-prime IgG and post-boost CD4+ and CD8+ T cell data in Supplementary Fig. 6. Source data are provided as a Source Data file.
The H7 replicon-NLC vaccine induced H7-specific systemic and mucosal immune responses in similar patterns to those induced by the H5 vaccine. Strong H7-binding serum IgG titers were consistently observed in animals receiving doses of at least 5 µg of H7 replicon-NLC, regardless of administration route (Fig. 2B and Supplementary Fig. 6A). Immunization with 10 µg of H7 replicon-NLC IN/IN was required to induce serum neutralization equivalent to immunization with 5 µg dosed IM/IN or IM/IM (Fig. 2C), suggesting that any reduction in vaccine potency seen with IN administration, possibly due to lowering of effective dose delivered, may be overcome by a modest increase in nominal vaccine dose. The establishment of splenic antigen-reactive polyfunctional CD8+, polyfunctional CD4+, and IFNγ+ CD8+ T cells was likewise dose-dependent, with comparable responses induced by IN/IN 10 µg, IM/IN 5 µg, and IM/IM 5 µg vaccination schedules (p > 0.05) (Fig. 2D and Supplementary Fig. 6B, C).
Induction of respiratory mucosal immunity following H7 replicon-NLC vaccination was consistent with H5 replicon-NLC vaccination results. Development of H7-binding IgA in BAL samples strictly necessitated at least one IN immunization (Fig. 2E). Likewise, an IN/IN 10 µg dose established the highest mean frequency of lung-resident polyfunctional CD8+, polyfunctional CD4+, and IFNγ+ CD8+ T cells, while an IM/IN 5 µg dose established comparable responses at half the replicon-NLC dose (p > 0.05) (Fig. 2F and Supplementary Fig. 6D, E). As with the H5 replicon-NLC, no detectable mucosal IgA and low mucosal cellular immune responses were observed following IM-only administration of H7 replicon-NLC. Together, these data demonstrate that our IN H7 replicon-NLC vaccine formulation is immunogenic in mice, producing systemic and mucosal immune response patterns akin to those seen for the H5 replicon-NLC vaccine, thereby validating the use of this replicon-NLC vaccine platform for diverse influenza viral strains.
Intramuscular administration of a bivalent H5/H7 replicon-NLC vaccine induces strong systemic immunity without antigen competition, but no detectable mucosal immunity
Having confirmed the immunogenicity of the monovalent H5 and H7 replicon-NLC vaccines, we tested a bivalent H5/H7 replicon-NLC vaccine formulation for vaccine compatibility and antigenic competition in the context of IM immunization. Mice were prime/boost vaccinated IM/IM with 5 µg of monovalent H5 or H7 replicon-NLC vaccine, or 5 µg of each replicon-NLC vaccine combined by simple mixing (10 µg replicon total). Additional study groups were included to control for the total replicon and NLC content of the vaccines by admixing 5 µg of each monovalent replicon-NLC with 5 µg of the SEAP replicon-NLC (Supplementary Fig. 7). Systemic and mucosal humoral and cellular immune responses to both antigens were assessed as before after prime (Supplementary Figs. 8 and 9) and boost (Fig. 3 and Supplementary Figs. 8 and 9).
Post-boost serum A H5 and B H7-binding IgG titers, serum C H5 and D H7 pseudovirus neutralization capacity (IC50), splenic E H5- and F H7-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells, G H5- and H H7-binding IgA titers in BAL samples, and lung-resident (CD69+ CD103+) I H5- and J H7-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells. For all assays, n = 6 animals for vector control group, n = 8 for experimental groups. All groups were sex balanced. Two statistical hypotheses were tested within each figure, unless specifically listed, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. A, B, G, H Statistical analyses performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. C, D Statistical analysis performed on log-transformed data using Kruskal–Wallis test with Dunn’s multiple comparisons. E, F Statistical analysis performed using Kruskal–Wallis test with Dunn’s multiple comparisons (filled lines) or unpaired two-sided t-tests (dotted lines). I, J Statistical analysis performed using Kruskal–Wallis test with Dunn’s multiple comparisons (filled lines) or Mann–Whitney tests (dotted lines). Data are presented as A–D geometric mean +/− geometric SD or G, H mean +/− SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated post-prime and post-boost data in Supplementary Figs. 8 and 9. Source data are provided as a Source Data file.
We observed strong antigen-specific serum IgG and nAb responses against H5 or H7 antigens for both monovalent and bivalent replicon-NLC vaccines, with no indication of significant IgG cross-binding or cross-neutralization between the antigens (Fig. 3A–D and Supplementary Fig. 8A–F). Splenic cellular immune responses followed a similar pattern, with induction by both monovalent and bivalent replicon-NLC vaccines at notably high frequencies with respect to the overall T cell population (Fig. 3E, F and Supplementary Fig. 8G–L). Consistent with prior IM administrations, a limited mucosal immune response was induced by any IM-delivered vaccine, as seen by undetectable IgA titers in BAL samples and low frequencies (< 0.2%) of lung-resident antigen-reactive CD8+ T cells (Fig. 3G–J and Supplementary Fig. 9), underscoring the value of IN administration of replicon-NLC vaccines for generating localized respiratory mucosal immunity.
Intranasal administration of a bivalent H5/H7 replicon-NLC vaccine provides strong systemic and mucosal immunity against both viral targets
We next sought to confirm whether an intranasally-delivered bivalent H5/H7 replicon-NLC vaccine would elicit both systemic and mucosal immune responses against both viral targets. As such, we implemented an IN version of the IM bivalent study design and compared immunogenicity between bivalent and monovalent formulations and controls.
After prime/boost vaccination, we observed comparable humoral responses between the monovalent and bivalent replicon-NLC vaccines in terms of serum H5- or H7-binding IgG titers and pseudovirus neutralization, with no evidence of IgG cross-binding or cross-neutralization (Fig. 4A–D and Supplementary Fig. 10A–F). Splenic H5-specific and H7-specific polyfunctional CD8+ T cell responses were also comparable between monovalent and bivalent vaccines (Fig. 4E, F and Supplementary Fig. 10G–L), and consistent with previous studies (Figs. 1D and 2D). Like systemic responses, mucosal responses were comparable after monovalent or bivalent replicon-NLC vaccination. Strong, antigen-specific IgA responses were seen in BAL samples (Fig. 4G, H and Supplementary Fig. 11A, B), and similar frequencies of lung-resident antigen-responsive bifunctional (IFNγ+ TNFα+) T cells were detected, all with little evidence of cross-reactivity or inhibition by bivalent dosing (Fig. 4I, J and Supplementary Fig. 11C–H). These data verify that an IN/IN prime/boost regimen of the bivalent H5/H7 replicon-NLC vaccine stimulates excellent systemic and mucosal immunity without antigen interference or competition.
Post-boost serum A H5- and B H7-binding IgG titers, serum C H5 and D H7 pseudovirus neutralization capacity (IC50), splenic E H5- and F H7-reactive polyfunctional (IFNγ+ IL-2+ TNFα+) CD8+ T cells, G H5- and H H7-binding IgA antibody titers in BAL samples, and lung-resident (CD69+ CD103+) I H5- and J H7-reactive bifunctional (IFNγ+ TNFα+) CD8+ T cells. For all assays, n = 6 animals for vector control group, n = 8 for experimental groups. All groups were sex balanced. Two statistical hypotheses were tested within each figure, unless specifically listed, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. A, B, G, H Statistical analyses performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. C, D Statistical analysis performed on log-transformed data used Kruskal–Wallis test with Dunn’s multiple comparisons. E, F Statistical analysis performed using Kruskal–Wallis test with Dunn’s multiple comparisons (filled lines) or unpaired two-sided t-tests (dotted lines). I, J Statistical analysis performed using Kruskal–Wallis test with Dunn’s multiple comparisons (filled lines) or Mann–Whitney tests (dotted lines). Data are presented as A–D geometric mean +/− geometric SD or G, H mean +/− SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated post-prime and post-boost data in Supplementary Figs. 10 and 11. Source data are provided as a Source Data file.
Route of vaccination of a bivalent H5/H7 HA replicon-NLC vaccine influences mucosal but not systemic immunogenicity
Bivalent intramuscularly and intranasally administered replicon-NLC vaccines were observed to establish consistent systemic immune responses in mice; however, there were notable differences in the establishment of mucosal immunity between IM and IN vaccination. To optimize the induction of systemic and mucosal immunity by our vaccine, we tested permutations of heterologous vaccination regimens. Mice were vaccinated with 10 µg of the bivalent H5/H7 replicon vaccine (5 µg of each replicon) either through homologous (IM/IM and IN/IN) or heterologous (IM/IN and IN/IM) routes, and immune responses were assayed as above.
Consistent with the other monovalent and bivalent dosing studies, the route of administration did not result in significant differences in the systemic antibody response against either antigen after prime/boost vaccination (Fig. 5A–D and Supplementary Fig. 12A–F). Mucosal immunity, both humoral and cellular, still required IN vaccination either as part of a homologous or a heterologous vaccination regimen (Fig. 5E, F and Supplementary Fig. 13A, B). The IM/IN combination appeared to induce an optimal combination of both systemic and mucosal immune responses. Interestingly, the IN/IM combination resulted in higher spread or numbers of non-responders in systemic nAbs, IgA in BAL samples, and cellular responses (Fig. 5 and Supplementary Figs. 12 and 13). Thus, we proceeded to test IM/IN immunization further in efficacy models alongside the homologous IN/IN dosed vaccine candidate.
Post-boost serum A H5 and B H7 HA-specific IgG titers, serum C H5 and D H7 pseudovirus neutralization capacity (IC50), and E H5- and F H7-binding IgA antibody titers in BAL samples. For all assays, n = 6 animals for vector control group, n = 8 for experimental groups. All groups were sex balanced. Two statistical hypotheses were tested within each figure, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. A, B, E, F Statistical analyses performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. C, D Statistical analysis performed on log-transformed data used Kruskal–Wallis test with Dunn’s multiple comparisons. Data are presented as (A–D) geometric mean +/− geometric SD or E, F mean +/− SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated post-prime and post-boost data in Supplementary Figs. 12 and 13. Source data are provided as a Source Data file.
Monovalent H7 and bivalent H5/H7 replicon-NLC vaccines protect ferrets from virus-induced morbidity and promote rapid viral clearance in a nonlethal H7N9 ferret challenge model
We tested the protective efficacy of our IN/IN and IM/IN delivered H7 replicon-NLC vaccine candidates in an established sublethal high-dose H7N9 ferret challenge model. Sex-balanced groups of Fitch ferrets, n = 6 per group, were vaccinated either IN/IN or IM/IN with either 25 µg of monovalent H7 replicon-NLC or 50 µg (25 µg of each antigen) of bivalent H5/H7 replicon-NLC. Control groups included a prime/boost IN/IN dose of 50 µg SEAP replicon-NLC vector control or a prime/boost dose of 7.5 µg of H7 HA from the National Institute for Biological Standards and Control (NIBSC) unadjuvanted influenza antigen A/Anhui/1/2013 (NIBRG-268, NIBSC code 14/250) delivered IM in 250 µL. Three weeks after boost vaccination, ferrets were challenged intranasally with 1 × 106 pfu of A/Anhui/PA-1/2013 virus and observed for 14 days with regular body weight measurements, scoring of clinical disease, and nasal swabs for viral load measurements.
All animals survived throughout the 14-day challenge period (Fig. 6A), as expected for this non-lethal challenge model. Only vector control immunized animals developed higher average body temperatures from Days 3 to 5 post-challenge (Supplementary Fig. 14) and brief clinical signs of disease in a subset of animals noted 8 days post-challenge, which quickly resolved and was not seen in any H7 vaccinated animal (Fig. 6B). All H7 vaccinated animals were protected against weight loss relative to vector control immunized animals (Fig. 6C). Analysis of viral titers in nasal wash samples collected after challenge demonstrated that, while most animals across all study groups had similar viral loads 1 and 3 days post-challenge, reflecting equivalent challenge doses, by Day 5, viral loads in all H7 vaccinated animals were reduced to undetectable levels (Fig. 6D–F).
For all assays, n = 6 (3 male and 3 female) animals per group. A Animal survival after challenge with 1 × 106 pfu of intranasally-delivered A/Anhui/PA-1/2013. B Averaged clinical scores. C Percent change in body weight from date of challenge. Two statistical hypotheses were tested within each figure, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. D–F Viral load in nasal wash at Day D 1, E 3, and F 5 post-challenge. Statistical analysis performed on log-transformed data using one-way ANOVA with Dunnett’s multiple comparisons test. G H7-binding IgG antibody titers in pre-challenge ferret serum. H H7 pseudovirus neutralization capacity (IC50) of pre-challenge ferret serum. G Statistical analysis performed on log-transformed data used one-way ANOVA with Dunnett’s multiple comparisons test. H Statistical analysis performed on log-transformed data using the Kruskal–Wallis test with Dunn’s multiple comparisons. Data are presented as D–G mean +/− SD or H geometric mean +/− geometric SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, NIBSC NIBSC Influenza Antigen A/Anhui/1/2013 (H7N9), ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Associated body temperature data in Supplementary Fig. 14. Source data are provided as a Source Data file.
Post-boost, pre-challenge serology indicated that all replicon-NLC vaccine regimens induced H7-binding IgG responses measurably above the vector control group, with signal in the control group attributed to non-specific background signal (Fig. 6G). Of note, serum IgG and nAb titers induced by the replicon-NLC vaccines were comparable to those induced by the NIBSC H7N9 antigen (Fig. 6G, H). This challenge study thus demonstrated that 25 µg prime/boost doses of H7 or H5/H7 replicon-NLC vaccines, regardless of IN/IN or IM/IN administration, successfully protect ferrets from H7N9 challenge-induced morbidity.
Monovalent H5 and bivalent H5/H7 replicon-NLC vaccines completely protect ferrets from lethal H5N1 challenge
We then tested the protective efficacy of our IN/IN and IM/IN delivered H5 replicon-NLC vaccine candidates in an established lethal high-dose H5N1 ferret challenge model. Sex-balanced groups of Fitch ferrets, n = 6 per group, were vaccinated either IN/IN or IM/IN with either 25 µg of monovalent H5 replicon-NLC or 50 µg (25 µg of each antigen) of bivalent H5/H7 replicon-NLC. Control groups included a prime/boost IN/IN dose of 50 µg SEAP replicon-NLC vector control, or a prime/boost dose of 7.5 µg alum-adjuvanted Baxter H5N1 vaccine delivered IM. Three weeks after boost vaccination, ferrets were challenged intranasally with 1 × 106 pfu of A/Vietnam/1203/2004 virus and observed for 14 days with regular body weight measurements, scoring of clinical disease, and nasal swabs for viral load measurements.
All animals in the vector control replicon-NLC treated group succumbed to infection by 1 week post-challenge (Fig. 7A). One animal in the Baxter vaccinated group succumbed to infection 9 days after challenge. All animals vaccinated with H5 or H5/H7 replicon-NLC vaccines were fully protected from virus-induced mortality and from the development of clinical symptoms, weight loss, and severe fever (Fig. 7A–C and Supplementary Fig. 15A). Analysis of viral titers in nasal wash samples collected after challenge demonstrated that, while all animals had similar viral loads 1 day after infection, reflecting equivalent challenge doses, viral loads in all H5 vaccinated animals were significantly reduced by 3 days post-challenge relative to the vector control treated animals (Fig. 7D, E). Strikingly, no virus was detectable in the nasal wash of H5 vaccinated animals 5 days post-challenge, whereas vector control animals displayed viral titers equivalent to those 1 day post-challenge (Fig. 7F). Lung viral titers were evaluated upon necropsy after the animals either succumbed to infection or were euthanized, and these indicated that all deaths in the study were virus associated (Supplementary Fig. 15B, C).
For all assays, n = 6 (3 male and 3 female) animals per group. A Animal survival after challenge with 1 × 106 pfu of intranasally-delivered A/Vietnam/1203/2004. B Averaged clinical scores. C Percent change in body weight from date of challenge. Two statistical hypotheses were tested within each figure, with filled lines showing comparisons to the vector control and dotted lines representing tests between key experimental groups. D–F Viral load in nasal wash at Day D 1, E 3, and F 5 post-challenge. Statistical analysis performed on log-transformed data using one-way ANOVA with Dunnett’s (filled lines) or Tukey’s (dotted lines) multiple comparisons test. G H5-binding IgG antibody titers in pre-challenge ferret serum. H H5 pseudovirus neutralization capacity (IC50) of pre-challenge ferret serum. G Statistical analysis performed on log-transformed data used one-way ANOVA with Dunnett’s multiple comparisons test (filled lines and dotted lines). H Statistical analysis performed on log-transformed data using the Kruskal–Wallis test with Dunn’s multiple comparisons. Data are presented as D–G mean +/− SD or H geometric mean +/− geometric SD. IN Intranasal, IM Intramuscular, LLOD lower limit of detection, ns not significant. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Source data are provided as a Source Data file.
Post-boost, pre-challenge serology indicated the four replicon-NLC vaccine regimens successfully induced H5-binding IgG titers measurably above the vector control group, with signal in the control group attributed to non-specific background signal (Fig. 7G). Of note, the Baxter vaccine induced trending or significantly higher levels of H5-binding IgG and nAbs compared to the four IN/IN or IM/IN replicon-NLC vaccine groups (Fig. 7G, H). Despite replicon-NLC vaccines inducing a lower humoral response than the Baxter vaccine, replicon-NLC vaccines were completely protective, suggesting that serum antibody titers are insufficient correlates of protection for mucosal H5N1 influenza vaccines. In sum, this challenge study demonstrated that 25 µg prime/boost doses of the H5 or H5/H7 replicon-NLC vaccines, regardless of IN/IN or IM/IN administration, fully protect ferrets from H5N1 viral challenge-induced morbidity and mortality.
Discussion
The studies presented here demonstrate the immunogenicity and efficacy of IN- and IM/IN-administered replicon-NLC vaccines against two different pre-pandemic influenza viral strains. Vaccination with monovalent and bivalent replicon-NLC formulations induced robust systemic immune responses in mice against the cognate virus, with no signs of immune competition between antigens after bivalent dosing. Intranasal administration, either as a prime/boost or as a boost of a previous IM vaccination, added key mucosal IgA and lung-resident cellular immune responses that were not induced by IM injection of the replicon-NLC vaccines or the previously-authorized adjuvanted inactivated whole virion H5N1 Baxter vaccine. In ferrets, the H5 replicon-NLC vaccines demonstrated 100% efficacy, protecting animals against viral morbidity and mortality regardless of vaccine valency or route of administration.
Our results underscore the importance of administration route on the nature of immune response. Like other influenza vaccines, these replicon-NLC vaccines induced systemic humoral and cellular immune responses39,40,41. However, the mucosal immune responses to the intranasally administered replicon-NLC vaccines are unique relative to approved intramuscularly administered vaccines. When delivered intranasally, these replicon-NLC vaccines established robust local respiratory mucosal immune responses not seen following IM administration of either replicon-NLC or other influenza vaccines. The mucosal responses observed here, namely IgA in BAL samples and lung-resident (CD69+ and/or CD103+) antigen-specific CD4+ and CD8+ T cells, are associated with enhanced vaccine efficacy, control of early infection and transmission, and improved outcomes in the context of influenza viral infection41,42,43,44.
Several mRNA influenza vaccine candidates have been developed previously45,46,47; however, none has yet been demonstrated to support IN vaccination. This deficit may explain the inability of licensed SARS-CoV-2 mRNA vaccines to prevent mild infection in respiratory tissues22,23, possibly due to lack of established mucosal immunity24. The flexible mode of delivery demonstrated by this replicon-NLC vaccine platform is likely due to the unique properties of the NLC, which provides excellent RNA delivery while maintaining low reactogenicity33. Specifically, use of the commercially-available cationic lipid DOTAP in the NLC allows for ready complexing with the negatively-charged RNA backbone at a range of RNA sizes and concentrations, allowing for product flexibility. In complex with the positively-charged NLC, the RNA is effectively able to enter cells and is protected from nuclease-mediated degradation36. Notably for IN delivery, the positive charge exhibited by the replicon-NLC complex also allows for effective transport across the nasal mucosa. With this cationic lipid-based NLC composition, we identified optimal formulation ranges for IM delivery48 and IN delivery36—ranges that overlap and allow for the use of a single formulation for both delivery routes as demonstrated here. This ability to tune the immune profile by changing route of vaccine administration is a major advantage of this replicon-NLC platform as a pandemic response tool.
Notably, despite inducing lower serum IgG and nAb titers in ferrets relative to the intramuscularly delivered adjuvanted, inactivated whole virion vaccine, the H5-expressing replicon-NLC vaccines provide stellar protection of animals from H5N1 challenge. This equivalent or improved protection of the replicon-NLC vaccines despite lower serum antibody titers suggests that for mucosal influenza vaccines, serum antibody titers are insufficient correlates of protection. This is in contrast with the well-established standard of a 1:40 HA inhibition assay readout as an indicator of seroconversion and protection for traditional intramuscularly administered influenza vaccines. While there are indications that mucosal immune readouts can be correlated with early viral clearance of H5N1 influenza virus in ferrets49, reliable mucosal correlates of protection have been difficult to establish to date due to the difficulty of sample collection and determination of protective mucosal assay thresholds50,51. These challenges pose a barrier to optimal design of human clinical trials for mucosal vaccines. For this reason, further work examining potential mucosal correlates of protection in primate models is planned.
Despite the success of licensed IIVs and LAIVs, aspects of their manufacturing processes, such as reliance on egg- or cell-based virus production, are slow and can impair yearly efficacy, imposing limitations on successful seasonal and pandemic influenza response52. Antigenic drift over the 6-month lead time required for production and distribution can result in seasonal vaccine strain mismatch and an inability to rapidly develop vaccines for emerging strains of pandemic potential53. In contrast, nucleic acid vaccine platforms combine agile modification of antigen sequences with ease and rapidity of in vitro transcription-based manufacturing to allow for manufacture of vaccines targeting emerging strains in a fraction of the time required for IIVs and LAIVs54, as needed for pandemic preparedness and response. The replicon-NLC system is particularly amenable to rapid manufacturing and nimble adaptation to emerging viral strains. Unlike that of lipid nanoparticles used for other RNA vaccines, manufacture of the NLC RNA delivery component is straightforward and easily scalable, using standard emulsification equipment in place at most vaccine manufacturers worldwide. The NLC is additionally stable for long periods (> 1 year at refrigerated conditions) in the absence of RNA33, allowing for component stockpiling and last-minute changes in the identity of the RNA with which it is complexed in order to reflect circulating viral strains in real time.
While carefully designed and executed, these studies do have limitations. Pipet delivery of IN vaccines likely results in variability in delivered dose, which may contribute to the heterogeneity in immune response seen particularly for intranasally dosed animals. Our H5N1-targeting replicon-NLC vaccine expresses HA from the prototypic A/Vietnam/1203/2004 H5N1 influenza viral strain, rather than any of the panzootic highly-pathogenic avian H5N1 clade 2.3.4.4b strains circulating in US dairy cattle in 20243. This antigen selection was deliberate, intended to establish this IN vaccine technology with existing reagents and animal efficacy models, with the knowledge that nucleic acid vaccines allow for rapid reformulation against emerging antigen targets when required. While assessment of these replicon-NLC vaccines’ cross-protection against currently-circulating strains was not a part of this study, recent studies of cross-neutralizing antibodies induced by licensed H5N1 vaccines suggest some cross-protection against the current circulating strains may be expected, which may possibly be enhanced by respiratory mucosal immunity provided by IN dosing55,56. Finally, an additional weakness of the work presented here is that many of the conclusions from this study rely on the immune readouts after IN vaccine administration in mice and ferrets. As both of these animal models have substantially different respiratory structures and volumes than humans, this may result in differential vaccine material distribution relative to what would be seen in humans, emphasizing the need for testing in a non-human primate (NHP) model. Indeed, in mice, the 50 µL administration IN volume results in some vaccine delivery to the stomach, likely a result from swallowing some of the dose (Supplementary Fig. 338); this is nearly impossible to avoid in small rodent models given vaccine concentration and volume limitations. Thus, studies demonstrating induction of mucosal responses by IN vaccination should be repeated in NHPs and humans in order to accurately establish mucosal immune induction by a purely IN vaccine dose. We do note that the 250 µL delivery volume used in the ferret efficacy studies presented here has been previously demonstrated to stay in the head and nose of Fitch ferrets without delivery to the trachea or lungs57, thus demonstrating the efficacy of purely IN vaccination using this replicon-NLC system.
Future directions include formal toxicity and biodistribution testing studies for this vaccine candidate in animals, and rapid progression into a Phase 1 clinical trial to evaluate safety and immunogenicity of these intranasally-delivered replicon-NLC vaccines in humans. Simultaneously, detailed immunogenicity and efficacy studies in NHPs are planned, with immunological profiling to elucidate the mode of action of these intranasally-delivered vaccines in animals with similar nasal anatomy and immune responses to those of humans. A desired secondary outcome of this study is the identification of potential mucosal correlates of protection, as suggested by our ferret study outcomes. Similarly, compatibility and characterization studies of this product in nasal spray devices is necessary for enabling mid- and late-stage clinical trials and future vaccine commercialization. Finally, future work will include studies aimed at developing a greater mechanistic understanding of how mucosal immunity is induced by IN replicon-NLC immunization.
This vaccine platform is a powerful potential tool for pandemic preparedness and response efforts. Amenable to multiple routes of parenteral administration with an identical formulation, the replicon-NLC system can be flexibly used to combat pathogens of different natures (respiratory vs. mosquito-borne, etc.). Furthermore, when lyophilized, replicon-NLC vaccines are stable for at least 2 years at non-frozen temperatures33,34, thus demonstrating improved thermostability over other RNA vaccines and negating requirements for deep cold-chain storage and complex distribution21,58. Finally, the recent US FDA greenlighting of self-administration of the IN FluMist vaccine59 opens the door to a potential thermostable, self-administrable replicon vaccine as a pandemic response tool. If demonstrated to be safe and immunogenic in humans, these IN replicon-NLC influenza vaccines would represent a technology with key advantages over current influenza vaccines in terms of flexible mode of delivery, mucosal immune stimulation, agile antigen targeting, and desirable product profile. Such a product, with long-term thermostability and self-administration potential, could represent an invaluable tool in pandemic preparedness and response.
Methods
Experimental design
Details on sample size, timepoints, data inclusion, and selection of endpoints are listed in the animal-specific study protocols below. Group sizes, listed for each study, were based off power calculations that allowed for differentiation of dosing and formulation effects in immunogenicity assays below. All animal studies contained equal numbers of male and female animals in all groups to account for sex-related variability in vaccine responses. Animals were randomized between groups within sex. Studies were not blinded. Assays were run with technical replicates and internal controls to control for intra- and inter-assay variability. No exclusion or outlier criteria were utilized for these study data unless noted below. Sex-based analyses were not performed because disaggregating by sex would reduce power to unacceptable levels, but there were not significant differences in response by sex.
Replicon production
HA-expressing replicon template plasmids were created by subcloning gene blocks containing the influenza H5N1 HA (GenBank ID EU122404.1) or H7N9 HA (GenBank ID KF768181.1) antigens into a plasmid containing a 5′ UTR, a 3′ UTR, and non-structural proteins derived from the attenuated TC83 strain of VEEV (Supplementary Fig. 1, Supplementary Data—Plasmid Sequences). Replicon RNA was then produced using an in vitro transcription protocol and linearized DNA templates as previously described33,34,48. RNA was purified via AKTA using CaptoCore700 resin (Cytiva), then buffer exchanged and concentrated using a 500 kDa cut-off mPES hollow fiber tangential flow filter membrane (Repligen). For small-scale lots, RNA was lithium chloride precipitated followed by ethanol precipitation to remove proteins and residual reagents. Concentration and yield were determined using spectrophotometry and validated by RiboGreen assay. RNA integrity was validated by gel electrophoresis.
NLC production
The NLC was formulated as previously described33,34,48 as a solid/liquid oil core stabilized in an aqueous buffer by surfactants. Squalene (Sigma), sorbitan monostearate (Span 60; Spectrum Chemical Mfg. Corp.), DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride; Lipoid), and glyceryl trimyristate (Dynasan 114; Sigma) were emulsified with a 10 mM sodium citrate trihydrate (Spectrum Chemical Mfg. Corp.) buffer-polysorbate 80 (Tween 80; Millipore) aqueous phase at 7000 rpm using a high-speed laboratory emulsifier (Silverson Machines) followed by high-shear homogenization at 30,000 psi in a microfluidizer (Microfluidics M-110P). The end product was terminally filtered with a 0.22 µm polyethersulfone filter and stored at 2–8 °C.
Vaccine complexing and characterization
Monovalent vaccine and SEAP vector control material were prepared by diluting the replicon to 2× the final concentration of the complex, in a background of 10 mM sodium citrate pH 6.3 and 10% w/v sucrose. The NLC stock was similarly diluted to 2× the final NLC concentration in the same background buffer. These diluted components were combined 1:1 by volume via pipette mixing. The complexes were then incubated on ice for 30 min before storage at −80 °C. Complexed material was prepared at a nitrogen:phosphate (N:P) ratio of 8–10, indicating the ratio of nitrogen in the DOTAP component of the NLC to phosphate in the RNA backbone. Bivalent vaccines were prepared by simple pipette mixing of each monovalent vaccine 1:1 by volume.
The hydrodynamic diameter of the replicon-NLC complexes was determined by dynamic light scattering of 1:100 diluted sample in water using a Zetasizer Pro (Malvern) with a backscatter angle of 173°. Measurements were carried out at 25 °C with a dispersant and sample viscosity value of 0.8872 cP (water). Three repeat measurements per sample were collected and averaged.
Agarose gel electrophoresis was used to evaluate the replicon size and integrity after complexing. Complexed vaccine samples and replicon only controls were diluted to 40 ng/µL replicon in nuclease-free water, then extracted using phenol:chloroform:isoamyl alcohol (25:24:1). Each extracted sample was mixed with NorthernMax-Gly Sample Loading Dye (Invitrogen), heated, and loaded onto a 1 cm thick, 1% (w/v) agarose gel. The gels were run at 120 V for 45 min and imaged under UV lighting (BioRad ChemiDoc MP Imaging System). Gels contained an RNA ladder (Ambion) for size reference.
Mouse studies
Mouse studies were performed in accordance with national (NIH Guide for the Care and Use of Laboratory Animals) and institutional guidelines and approved by the Bloodworks Northwest Research Institute’s Institutional Animal Care and Use Committee (IACUC protocol 5389-01).
C57BL/6J mice (n = 8 per vaccine group, 6 per vector control group) sex balanced to account for sex-related variability in vaccine responses and between 6 and 8 weeks of age at study onset were obtained from The Jackson Laboratory (Harbor, ME). Body weights were collected up to 4 days post-vaccination to assess adverse effects. Animals were prime/boost immunized with replicon-NLC either IN (50 µL; 25 µL per nare) or IM (50 or 100 µL) 3 weeks between doses, and blood was collected retro-orbitally on isoflurane-sedated mice. Animals were euthanized using CO2 followed by cervical dislocation in accordance with the recommendation of the American Veterinary Medical Association, followed by collection of BAL, lung, and spleen tissues.
Data were collected at pre-defined weekly timepoints. Euthanasia criteria (20% weight loss, moribund state) were not met by any animals during the studies. Prospective data inclusion/exclusion criteria were based on animal condition before, during, and after vaccination; vector and/or naïve animal controls; and internal assay standards. Each study was conducted once, with key experimental group conditions repeated in subsequent studies.
Preparation of murine tissues into single-cell suspensions
Spleens were processed as previously described34. Briefly, spleens were individually homogenized by manual maceration through a 70 µm cell strainer. Red blood cells were lysed for 1 min with ACK Lysing Buffer (ThermoFisher) and quenched with RPMI medium. Samples were filtered through a 2 mL Acroprep filter plate and resuspended in RPMI medium with 10% FBS, and 1–2 × 106 cells/well were seeded in 96-well round-bottom plates in preparation for peptide stimulation.
Lungs were processed as previously described34. Briefly, lung tissue was dissociated via enzymatic digestion using Hanks’ Balanced Salt Solution supplemented with 10% Liberase (MilliporeSigma), 10% aminoguanidine, 0.1% KN-62, and 1.25% DNase. Lungs and enzymatic mix were added to a gentleMACS M tube (Miltenyi Biotec), run on a lung dissociation program in a gentleMACS Dissociator, incubated at 37 °C and 5% CO2 for 30 min, then run again on the gentleMACS Dissociator. The resulting slurry was washed with RPMI and then filtered through a 70 µm MACS SmartStrainer (Miltenyi Biotec). Cells were counted using a Guava easyCyte cytometer before plating in 96-well round-bottom plates at 1 × 106 cells/well in RPMI medium with 10% FBS in preparation for peptide stimulation.
Stimulation and intracellular staining of murine single-cell suspensions
H5 peptides (JPT Peptide Technologies #PM-INFA-HAIndo) used for stimulation comprised a pool of 140 peptides (15mers, 11 amino acid overlaps) derived from the full-length HA of influenza virus A/Indonesia/CDC835/2006(H5N1), which has a 96.64% sequence homology to the H5 HA from A/Vietnam/1203/2004 used in these studies. H7 peptides (custom ordered from GenScript) comprised a pool of 138 peptides (15mers, 11 amino acid overlaps) derived from the full-length HA sequence from A/Anhui/PA-1/2013.
Stimulation mixes consisted of RPMI media, 10% FBS, 50 μM 2-mercaptoethanol, α-CD28 (BD), brefeldin A (BioLegend), and one of three stimulation treatments: 0.26% dimethyl sulfoxide (DMSO) as a negative stimulation control, 0.2 µg/well (1 µg/mL) per peptide of H5 or H7 peptide pools in 0.26% DMSO, or phorbol myristate acetate-ionomycin positive stimulation solution.
Spleen and lung lymphocytes were stained for viability with Zombie Green (BioLegend #423112; 1:1000), then Fc receptors were blocked with CD16/CD32 antibody (Invitrogen #14-0161-82; 1:200). Cells were surface stained for mouse CD4 (APC-Cy7, BD Bioscience #552051; 1:250), CD8 (BV510, BD Biosciences #563068; 1:50), CD44 (PE-CF594, BD Biosciences #562464; 1:500), and CD154 (BV605, BD Bioscience #745242; 1:50). Lung cells were additionally stained for mouse CD69 (PE, BD Biosciences #561932; 1:33) and CD103 (BV711, Biolegend #121435; 1:20). After extracellular staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm (BD Biosciences #554714) and stained for intracellular cytokines with mouse TNFα (BV421, BioLegend #506328; 1:100), IL-2 (PE-Cy5, BioLegend #503824; 1:2000), IFNγ (PE-Cy7, BD Biosciences #557649; 1:400), IL-5 (APC, Biolegend #504306; 1:286), and IL-17A (AF700, BD Biosciences #560820; 1:100). Cells were run on a CytoFLEX flow cytometer (Beckton Dickson) and analyzed with FlowJo v.10 (BD Biosciences). Representative gating strategies are shown in Supplementary Figs. 16 and 17. Cells triple positive for TNFα, IL-2, and IFNγ were considered polyfunctional T cells. Of note, flow cytometry data in Fig. 4 excluded IL-2 (i.e., bifunctional instead of polyfunctional) due to poor data quality based on in-run standards. Samples with insufficient threshold events (< 50,000) collected per well and low viability (< 40% Zombie Green negative) were excluded from analysis.
Vaccine biodistribution imaging
Fluorescently labeled NLC was produced via the direct incorporation of a hydrophobic fluorescent dye into the NLC. The Cyanine5.5 (Cy5.5; Lumiprobe #3572-25mg) dye solution was prepared at a concentration of 1 mg/mL in DMSO and stored away from light at −20 °C until use. 100 µL of Cy5.5 dye solution was added to 1000 µL of prepared NLC (final dye concentration of 91 µg/mL Cy5.5), and the resultant solution was left to incubate at room temperature for 30 min. To remove unincorporated dye, the NLC-Cy5.5 solution was passed through two consecutive 15 mL desalting columns (ThermoFisher Scientific #89889) that had been pre-equilibrated with aqueous buffer (10 mM sodium citrate, 10% w/v sucrose, pH 6.3). The resulting NLC solution was visibly blue and highly fluorescent. Fluorescent replicon-NLC complexes were prepared by diluting the replicon to 2× the final desired concentration in 10 mM sodium citrate, pH 6.3, and 10% w/v sucrose. The fluorescently labeled NLC was similarly diluted to 2× the final desired concentration in the same buffer. These diluted components were combined 1:1 by volume via pipette mixing. The complexes were then incubated on ice for 30 min. Fluorescent replicon-NLC complexes were administered intranasally in a 50 µL volume to mice under anesthesia as described above. As a negative control, mice were administered sterile saline. Four hours post-administration, mice were euthanized and necropsied. To visualize the upper respiratory tract, mice were decapitated, and heads were bisected. To visualize the lower respiratory tract, rib cages were removed. Mice were then imaged using the Pearl Odyssey Imager (LI-COR) using both white light and at 700 nm to visualize the whole mouse as well as the fluorescent dye. All animals were imaged using the same parameters.
Ferret efficacy study
Ferret studies were performed in accordance with national (NIH Guide for the Care and Use of Laboratory Animals) and institutional guidelines for animal care of laboratory animals, approved by the Colorado State University IACUC (protocol 4228), and conducted as previously described60. Fitch ferrets (Mustela putorius fero), evenly balanced male and female to account for sex-related variability in vaccine responses, were purchased from Triple F Farms (Gillett, PA) at 3 months of age and confirmed to be serologically negative for influenza virus by microneutralization testing with H5N1-PR8 or H7N9 recombinant influenza virus prior to initiating immunization; sera for this testing were obtained prior to challenge by jugular venipuncture under ketamine-xylazine anesthesia. Vaccination with replicon-NLC complexes consisted of a prime dose of 25 µg given either IM or IN in 250 µL (125 µL per nare) to a cohort of 6 ferrets per group (3 male and 3 female). A subsequent boost dose of 25 µg replicon was delivered IN 21 days post-prime. The alum-adjuvanted inactivated whole virion H5N1 vaccine (“Baxter;” BEI Resources, NIAID, NIH: A/H5N1 Influenza Vaccine, Inactivated Whole Virion (A/Vietnam/1203/2004), Vero-Cell Derived, Adjuvanted, 15 Micrograms HA, NR-12143) was used as a positive control, while SEAP replicon was used as a negative control. Ferrets were regularly observed for symptoms and clinical scores after each vaccination and during the challenge phase. Clinical scores were defined as 0 = normal; 1 = possibly less active than normal OR not grooming as normal (questionably sick or mild illness); 2 = less active than normal when viewed in cage but appears nearly normal and responsive when handled (sick); 3 = reluctance to rise when viewed in cage and not normally responsive when handled by humans OR weight loss up to 15% of pre-challenge value; 4 = failure to move when stimulated by humans OR collapse when moving OR recumbency OR weight loss >20% of pre-challenge value OR manifestation of any neurologic signs (ataxia, head tilt, etc.) OR body temperature <100 °F; and 5 = found dead. For the challenge, ferrets were lightly anesthetized with ketamine-xylazine, and 0.5 mL containing 106 PFU of influenza A/Vietnam/1203/2004 H5N1 virus was instilled intranasally, split between nares. Following challenge, animals were observed for clinical scores, and temperatures were recorded at least once daily. The animals were weighed at the time of challenge and every day thereafter. Nasal washes were collected under ketamine-xylazine sedation on Days 1, 3, and 5 post-challenge. Lungs were collected after animals succumbed to infection or necropsy. Viral titers were quantified by plaque assay of serial dilution of nasal wash material or processed lung lysate applied to 6-well plates of confluent Madin-Darby canine kidney cells (BEI Resources #NR-2628).
Quantification of IgG and IgA binding titers by ELISA
Quantification of antigen-specific systemic IgG by ELISA was performed on serum as previously described34, and IgA and IgG ELISAs were performed on BAL samples. Plates were coated with either H5(H5N1)(A/Vietnam/1203/2004) obtained from Immune Technology (#IT-003-0051p) or H7N9 (A/Anhui/1/2013) HA from Sino Biological (#40103-V08H) followed by blocking for 1 h. Each serum or BAL sample was diluted 1:40 or 1:4, respectively, and then serially 1:2 to create a 14-point dilution curve for each sample. An anti-HA A/Vietnam/1203/04 influenza virus (VN04-8) antibody (Rockland Immunochemicals #200-301-976) was used as a positive control for H5-specific ELISAs. The Anti-H7N9 Hemagglutinin/HA Antibody (Sino Biological #11082-MM04) was used as a positive control for H7-specific ELISAs. The serially diluted samples were then transferred onto coated, washed plates followed by a 1-h incubation. For IgG ELISAs, antibodies were detected using the Anti-Mouse IgG (Fc specific)-Alkaline Phosphatase antibody (Sigma-Aldrich #A2429) or an anti-ferret HRP-conjugated antibody (Abcam #ab112770) at a 1:4000 dilution in blocking buffer. For IgA ELISAs, antibodies were detected using the Anti-Mouse IgA (α-chain specific)-Alkaline Phosphatase antibody (Sigma-Aldrich #A4937) at a 1:1000 dilution in blocking buffer. Plates were then developed using the appropriate reactive substrate. Sample concentrations were interpolated off the linear region of each sample dilution curve using the standard curve for absolute quantification of antibody titers, or endpoint titers were calculated by performing a least-squares fit of the OD values at each dilution to a four-parameter sigmoidal curve. LLODs were calculated by taking the slope of the linear portion of the four-point logistic standard curve and dividing that value by three times the standard deviation of the negative control samples.
Quantification of neutralizing antibodies by pseudovirus neutralization assay
Serum pseudovirus-neutralizing antibodies were quantified as previously described34 with the following modifications. Pseudovirus stocks were generated using HEK-293 cells (American Type Culture Collection #CRL-3216) that were cultured and co-transfected with the following plasmids: a plasmid containing a lentiviral backbone expressing luciferase and ZsGreen (BEI Resources #NR-52516), plasmids containing lentiviral helper genes (BEI Resources #NR-52517, NR-52518, and NR-52519), and a plasmid expressing either H5 A/Vietnam/1203/2004 HA or H7 A/Anhui/PA-1/2013 HA.
For the pseudovirus neutralization assay, each serum sample was serially diluted and mixed with a viral suspension that had a titer of 5 × 107 fluorescence units/mL. The solution was added to a confluent monolayer of HEK-293T cells plated at 1 × 104 cells/well. Plates were incubated at 37 °C and 5% CO2 for 3 days. Neutralization was measured via fluorescence in each well. The titers of nAbs in mouse or ferret sera were calculated based on the serum dilution that resulted in half the added virus being neutralized. Pseudovirus neutralization is presented as 50% inhibitory concentration (IC50), which was calculated using a set of virus-infected control wells (100% infectious signal) and media control wells (0% infectious signal), with the limit of detection set based on the reciprocal of the initial serum dilution.
The HEK-293T cell line was obtained directly from the American Type Culture Collection where it was authenticated by short tandem repeat profiling. HEK-293T cells were expanded after receipt and aliquots <4 passages stored cryopreserved in liquid nitrogen to enable consistency of cells used across the project. Individual cell cultures were passaged no more than 20 times from the original source vial prior to use in pseudovirus production or pseudovirus neutralization assay.
Statistical analysis
When possible, one-way, parametric statistical comparisons (one-way ANOVA) were performed to assess the statistical difference between groups in the in vitro and in vivo studies, with a p-value of 0.05 used as the cut-off. When the above analysis was not possible, such as when the standard deviation was statistically different between groups, a non-parametric analysis was used (Kruskal–Wallis), with a corresponding p-value of 0.05 as a cutoff. All statistical calculations were performed using GraphPad Prism v10.0. Log-transformed data (antibody titers, viral loads) are displayed with geometric mean and geometric SD, whereas untransformed data (flow cytometric data) show mean and SD.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Source Data file provided with this publication. AAHI’s replicon constructs and Nanostructured Lipid Carrier (NLC) are available for noncommercial research or scholarly purposes under the terms and conditions of an appropriate material transfer agreement. Correspondence and material requests should be addressed to the corresponding author. Source data are provided with this paper.
References
CDC. About Estimated Flu Burden. https://www.cdc.gov/flu-burden/php/about/index.html (2024).
Erbelding, E. J. et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 218, 347–354 (2018).
Burrough, E. R. et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg. Infect. Dis. 30, 1335–1343 (2024).
Abdelwhab, E. M. & Mettenleiter, T. C. Zoonotic animal influenza virus and potential mixing vessel hosts. Viruses 15, 980 (2023).
CDC. USDA reported H5N1 bird flu detections in US backyard and commercial poultry. Avian Influenza (Bird Flu) https://www.cdc.gov/bird-flu/situation-summary/data-map-commercial.html (2024).
Eisfeld, A. J. et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature 633, 426–432 (2024).
Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza, A. V. et al. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358, 261–273 (2008).
CDC. H5 bird flu: current situation. Avian Influenza (Bird Flu) https://www.cdc.gov/bird-flu/situation-summary/index.html (2025).
LDH reports first U.S. H5N1-related human death | La Dept. of Health. https://ldh.la.gov/news/H5N1-death.
Zheng, S. et al. Factors associated with fatality due to avian influenza A(H7N9) infection in China. Clin. Infect. Dis. 71, 128–132 (2020).
de La Hamaide, S. US Reports First Outbreak of Deadly H7N9 Bird Flu Since 2017 (Reuters, 2025).
Schnirring, L. H7N9 Avian Flu Strikes Mississippi Broiler Farm https://www.cidrap.umn.edu/avian-influenza-bird-flu/h7n9-avian-flu-strikes-mississippi-broiler-farm (CIDRAP Center for Infectious Disease Research & Policy Research and Innovation Office, University of Minnesota, Minneapolis, 2025).
Sanchez, J. L. et al. Respiratory infections in the U.S. military: recent experience and control. Clin. Microbiol. Rev. 28, 743–800 (2015).
Watanabe, T. et al. Characterization of H7N9 influenza A viruses isolated from humans. Nature 501, 551–555 (2013).
Zaraket, H. et al. Mammalian adaptation of influenza A(H7N9) virus is limited by a narrow genetic bottleneck. Nat. Commun. 6, 6553 (2015).
Xiong, X. et al. Receptor binding by an H7N9 influenza virus from humans. Nature 499, 496–499 (2013).
FDA. Vaccines licensed for use in the United States. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (2024).
Krammer, F. & Palese, P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 14, 167–182 (2015).
Plumb, I. D. et al. Effectiveness of a bivalent mRNA vaccine dose against symptomatic SARS-CoV-2 infection among U.S. Healthcare personnel, September 2022–May 2023. Vaccine 42, 2543–2552 (2024).
Kreier, F. Combined COVID–flu vaccines are coming: Moderna jab clears major test. Nature https://doi.org/10.1038/d41586-024-02121-1 (2024).
Markarian, J. Accelerating technology adoption to track the cold chain. BioPharm. Int. 34, 38–40 (2021).
Kissler, S. M. et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated persons. N. Engl. J. Med. 385, 2489–2491 (2021).
Garcia-Knight, M. et al. Infectious viral shedding of SARS-CoV-2 Delta following vaccination: a longitudinal cohort study. PLoS Pathog. 18, e1010802 (2022).
Toniolo, A., Maccari, G. & Camussi, G. mRNA technology and mucosal immunization. Vaccines 12, 670 (2024).
Freeman, D. et al. Injection fears and COVID-19 vaccine hesitancy. Psychol. Med. 53, 1185–1195 (2023).
Feikin, D. R. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 399, 924–944 (2022).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915.e19 (2022).
Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e13 (2020).
An, X. et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 24, 103037 (2021).
Li, X. F. et al. A highly immunogenic live-attenuated vaccine candidate prevents SARS-CoV-2 infection and transmission in hamsters. Innovation 3, 100221 (2022).
Dhama, K. et al. COVID-19 intranasal vaccines: current progress, advantages, prospects, and challenges. Hum. Vaccin. Immunother. 18, 2045853 (2022).
Gerhardt, A. et al. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 25, 205–214 (2022).
Voigt, E. A. et al. A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability. npj Vaccines 7, 1–13 (2022).
Jennewein, M. F. et al. Intranasal replicon SARS-CoV-2 vaccine produces protective respiratory and systemic immunity and prevents viral transmission. Mol. Ther. 33, 3286–3306 (2025).
McClary, W. D. et al. An intranasally- and intramuscularly-deliverable nanostructured lipid carrier-replicon RNA vaccine drives protective systemic and mucosal immunity. J. Control. Release. 385, 114054 (2025).
Ehrlich, H. J. et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N. Engl. J. Med. 358, 2573–2584 (2008).
Southam, D. S., Dolovich, M., O’Byrne, P. M. & Inman, M. D. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L833–L839 (2002).
Chivukula, S. et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. npj Vaccines 6, 1–15 (2021).
Hauguel, T. et al. Preclinical immunogenicity and safety of hemagglutinin-encoding modRNA influenza vaccines. npj Vaccines 9, 1–13 (2024).
Hoft, D. F. et al. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin. Vaccin. Immunol. 24, e00414–e00416 (2017).
Lin, Y., Hu, Z., Fu, Y.-X. & Peng, H. Mucosal vaccine development for respiratory viral infections. hLife 2, 50–63 (2024).
Carter, N. J. & Curran, M. P. Live attenuated influenza vaccine (FluMist®; FluenzTM). Drugs 71, 1591–1622 (2011).
Li, J., Arévalo, M. T., Chen, Y., Chen, S. & Zeng, M. T-cell-mediated cross-strain protective immunity elicited by prime–boost vaccination with a live attenuated influenza vaccine. Int. J. Infect. Dis. 27, 37–43 (2014).
Pardi, N. et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 9, 3361 (2018).
Bahl, K. et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 25, 1316–1327 (2017).
Kackos, C. M. et al. Seasonal quadrivalent mRNA vaccine prevents and mitigates influenza infection. npj Vaccines 8, 1–11 (2023).
Erasmus, J. H. et al. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol. Ther. 26, 2507–2522 (2018).
Wong, S.-S. et al. The immune correlates of protection for an avian influenza H5N1 vaccine in the ferret model using oil-in-water adjuvants. Sci. Rep. 7, 44727 (2017).
Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 19, 383–397 (2019).
Xu, H., Cai, L., Hufnagel, S. & Cui, Z. Intranasal vaccine: factors to consider in research and development. Int. J. Pharm. 609, 121180 (2021).
Gouma, S., Anderson, E. M. & Hensley, S. E. Challenges of making effective influenza vaccines. Annu. Rev. Virol. 7, 495–512 (2020).
Skowronski, D. M. et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS ONE 9, e92153 (2014).
Maruggi, G., Zhang, C., Li, J., Ulmer, J. B. & Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 27, 757–772 (2019).
Yegorov, S. et al. Inactivated and live-attenuated seasonal influenza vaccines boost broadly neutralizing antibodies in children. Cell Rep. Med. 3, 100509 (2022).
Sun, K., Ye, J., Perez, D. & Metzger, D. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J. Immunol. 186, 987–993 (2011).
Moore, I. N. et al. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. J. Virol. 88, 13879–13891 (2014).
Kar, S. et al. Oral and intranasal vaccines against SARS-CoV-2: current progress, prospects, advantages, and challenges. Immun. Inflamm. Dis. 10, e604 (2022).
Anderer, S. FDA greenlights nasal spray flu vaccine for use at home. JAMA https://doi.org/10.1001/jama.2024.21299 (2024).
Van Hoeven, N. et al. A formulated TLR7/8 agonist is a flexible, highly potent and effective adjuvant for pandemic influenza vaccines. Sci. Rep. 7, 46426 (2017).
Acknowledgements
The following reagent was obtained through BEI Resources, NIAID, NIH: A/H5N1 Influenza Vaccine, Inactivated Whole Virion (A/Vietnam/1203/2004), Vero-Cell Derived, Adjuvanted, 15 Micrograms HA, NR-12143. The Influenza Reagent, Influenza Antigen A/Anhui/1/2013 (H7N9) (NIBRG-268), NIBSC code 14/250, was obtained from NIBSC (Potters Bar, Hertfordshire, EN6 3QG). Images for figures were obtained from Clker.com under a CC0 1.0 license (https://creativecommons.org/publicdomain/zero/1.0/). We gratefully acknowledge Dr. Valerie Soza for her support in the preparation of this manuscript for publication and her editorial aid. This research was sponsored by the US Government under Other Transaction number W15QKN-16-9-1002 between the Medical CBRN Defense Consortium (of which Access to Advanced Health Institute is a member) and the Government. The US Government is authorized to reproduce and distribute reprints for Governmental purposes, notwithstanding any copyright notation thereon. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the US Government.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.A.V., C.C. Data curation: M.R.Y., D.N.K. Formal analysis: M.R.Y., M.A.D., D.N.K., M.F.J., E.A.V. Funding acquisition: C.C., A.G., R.A.B., E.A.V. Investigation: M.R.Y., D.N.K., M.F.J., E.L., J.S., S.B., N.C., E.M., S.R., C.P., D.S.B., R.M., P.F., J.B., A.T.H. Methodology: M.R.Y., D.N.K., E.L., S.B., N.C., D.S.B., W.D.M. Project administration: M.A.D., A.G., R.A.B., E.A.V. Resources: C.C., A.G., R.A.B., E.A.V., W.D.M. Supervision: E.M., A.G., R.A.B., E.A.V. Validation: M.R.Y., D.N.K., M.F.J. Visualization: M.R.Y., D.N.K. Writing–original draft: M.R.Y., M.A.D., E.A.V. Writing–review & editing: M.R.Y., M.A.D., D.N.K., M.F.J., E.L., J.S., S.B., N.C., E.M., S.R., C.P., D.S.B., W.D.M., R.M., P.F., J.B., C.C., A.T.H., A.G., R.A.B., E.A.V.
Corresponding author
Ethics declarations
Competing interests
A.G. and E.A.V. declare no Competing Non-Financial Interests but the following Competing Financial Interests. A.G. and E.A.V. are co-inventors on PCT patent application PCT/US21/40388, “Co-lyophilized RNA and Nanostructured Lipid Carrier,” and related national filings, as well as US provisional patent application 63/345,345, “Intranasal Administration of Thermostable RNA Vaccines,” and 63/144,169, “A thermostable, flexible RNA vaccine delivery platform for pandemic response.” All other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ykema, M.R., Davis, M.A., Kasal, D.N. et al. Intranasal replicon vaccine establishes mucosal immunity and protects against H5N1 and H7N9 influenza. Nat Commun 17, 434 (2026). https://doi.org/10.1038/s41467-025-64829-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64829-6