Abstract
Tree longevity is thought to increase in growth-limiting, adverse environments, but a quantitative assessment of drivers of global variation in tree longevity is lacking. We assemble a global database of maximum longevity for 739 tree species and analyse associations between longevity and climate, soil, and species’ functional traits. Our results show two primary pathways towards long lifespans. The first is slow growth in resource-limited environments, consistent with the “adversity begets longevity” paradigm. The second pathway is through relief from abiotic constraints in productive environments. Despite notable exceptions, long-lived gymnosperms tend to follow the first path through slow growth in cold environments, whereas long-lived angiosperms tend to follow the second (“productivity”) path reaching maximum longevity generally in humid environments. For angiosperms, we identify two mechanisms for increased longevity under humid conditions. First, higher water availability increases species’ maximum tree height which is associated with greater longevities. Secondly, greater water availability increases stand density and inter-tree competition, limiting growth which may increase tree lifespan. The documented differences between gymnosperm and angiosperm longevity are likely rooted in intrinsic differences in hydraulic architecture that provide fitness advantages for gymnosperms under high abiotic stress, and for angiosperms under increased productivity or competition.
Similar content being viewed by others
Introduction
Trees are widely revered as the longest-living group of organisms on Earth, including some species that can live for thousands of years1. Their lifespans control the time that carbon resides in tree stems, the main carbon reservoir of trees, and are thus closely related to the total carbon storage of forests2. Tree lifespan is also a key ecological and demographic trait that defines a species’ life history strategy, including its position along the fast-slow growth spectrum3,4,5, and its sensitivity to environmental variability6,7. Thus, to understand forest dynamics, and its response to global change, it is imperative to shed light on the drivers of tree lifespan among species, and across the globe. These insights can support predictions of future responses to global change, for example, by providing Earth System Models with more realistic estimates of tree turnover8,9. Yet, we lack systematic analyses of the environmental and climatic drivers, and species’ functional traits, associated with tree longevity.
Recent studies have provided strong evidence for increasing longevity towards high latitudes and altitudes10,11, confirming early observations that some of the longest-lived conifer trees such as 5000 year old bristlecone pines and 2000 year old junipers are usually found near tree lines, and at the coldest and/or driest places. These observations led early dendrochronologists to conclude that “adversity begets longevity”12. However, multi-millennia old trees are not just found in cold environments with short growing seasons, or sites with limiting water availability. They also grow in some of the most productive and humid temperate forests (e.g., Sequoia, Sequoiadendron), in flooded swamp forests (e.g., Taxodium13), and in African savannas (e.g., Adansonia14). These contrasting observations demonstrate that our understanding of the global environmental controls on tree longevity remains limited.
In addition, large differences exist among species growing in the same community. In tropical forests, longevity varies from a few decades for pioneers15 to several centuries for other species16,17. These differences arise from the diversification of species along different axes of demographic variation. One of these axes is the well-established fast-slow continuum of life-history strategies shaped by trade-offs between growth and survival18. Greater investment towards growth and resource acquisition traits are expected to result in lower tree lifespans3,19. Consistent with this trade-off, previous analyses have shown that faster growth is negatively associated with species’ longevity at the global scale11,20. However, very few studies have directly related species’ longevity to their traits. Most investigations instead have focused on the association between traits and demographic rates, such as tree mortality21,22. For example, wood density and tree mortality have repeatedly been shown to be negatively correlated21,23,24, but studies on relationships with longevity are less common25. In addition, mortality rates are far from uniform across a tree’s life history26, and mean mortality rates thus need not be correlated with maximum longevity across species and sites. Consequently, it remains unclear to what degree functional traits can be used to predict species’ genotypic or phenotypic variation in longevity, and a systematic quantitative analysis on the roles of environment, climate, and plant functional traits on species longevity is needed to shed light on the controls on growth-longevity trade-offs. Such analyses will also enhance our understanding of how climate change may affect tree longevity, and thus forest dynamics. Whether environmental drivers associated with longer lifespans among species (i.e. related to their adaptive traits) – and the focus of this study - also drive longer lifespans within species across habitats (i.e. related to their phenotypic responses) is poorly understood but is beyond the scope of the present work.
Here, we compile a new database of tree longevity or maximum life expectancy of 739 tree species. This dataset is based primarily on tree ring records complemented with radiocarbon dating, historical age records and growth projections from short-lived species, and tree species from under-represented biomes to capture the full spectrum of life history strategies. While conifers from cold sites are well represented (Supplementary Fig. 1b), our new dataset contains many tropical and short-lived (mostly broadleaved) species not included in previous analyses11,27,28. We use this database to assess the association of species’ longevity with global environmental gradients and functional traits, and aim to answer the following questions: (1) How is longevity associated with temperature, precipitation, and soil characteristics? (2) Can species’ traits predict variation in species’ longevity? And, (3) What are the relative roles of environment, traits and species growth rates on tree longevity? Previous studies showed that angiosperms and gymnosperms differ strongly in their growth strategies29, photosynthetic capacity30, hydraulic traits31,32,33 and longevity1,10,11,34, which we confirm in our analysis of the full dataset. Given that these taxonomic groups diverged over 300 million years ago35 and that they differ in their global distribution36, we primarily analyse the results for these groups separately to avoid any phylogenetic bias in the results. This represents the first effort aimed at disentangling the effects of climate, environment and species’ functional traits on tree species longevity.
Results
General patterns of variation in tree longevity
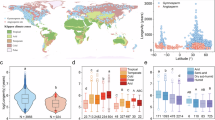
The oldest trees are typically found in mountain ranges at around 40° latitude such as the American Cordillera, Himalayas and Mediterranean mountains. However, trees over 1000 years old can also be found in tropical and subtropical regions (Supplementary Fig. 1a). We found that gymnosperm species live significantly longer (mean = 717 years, s.e. = 47 years) than angiosperm species (mean = 296, se = 12 years), and that there is a strong negative relationship between growth rate and longevity (Fig. 1a). When analysed by biome, we found that longevity increased from warm to cold climates (i.e., tropical <mediterranean <temperate mixed <boreal <temperate conifer biomes), and within (sub)tropical biomes from drier to wetter climates (dry grasslands and savannas & dry forest <moist forests, Fig. 1b). Species from tropical dry ecosystems (i.e., dry broadleaved forests, and grasslands, savannas and shrublands) had the lowest longevity, whereas coniferous temperate forest species displayed the highest longevity.
Relationship between species’ radial growth and tree longevity (a) and variation in tree longevity across biomes (b). Lines in the violin plots (a) and the box plots (b) show the median of the groups or biomes. The median and mean tree longevity was 204 years and 296 years (s.e. = 12 years) for angiosperms, and 534 and 717 (s.e. = 47 years) for gymnosperms. Both radial growth and maximum longevity were log10-transformed, and the lines in panel a corresponds to a linear regression. Box plots display the median (central line), upper and lower quartiles (box edges), and whiskers that extend to values within 1.5× interquartile range (IQR). Larger points beyond the whiskers are outliers.
Covariation of species’ longevity with climate and soil
The effects of climate on tree longevity, at the global scale, differed between angiosperm and gymnosperm species (Fig. 2, Supplementary Tables 1, 2). Temperature had a greater influence on gymnosperm longevity (Fig. 2a), while precipitation had a stronger effect on angiosperm longevity (Fig. 2b). Longevity of gymnosperm species increased towards colder climates (Fig. 2a) and for species growing closer to the sub-arctic and alpine tree lines (Fig. 2c). The effect of mean annual temperature on tree longevity is in almost equal measures due to decreased growing season temperature and a shortening of the growing season length in colder climates (see Supplementary Table 2). Angiosperm longevity increased with precipitation metrics and climate moisture index (CMI) that takes potential evapotranspiration into account (Supplementary Table 1). The strongest correlation was found with precipitation during the driest quarter of the year (Fig. 2b). These climate relationships are robust when excluding species with lower confidence in their longevity estimates (Supplementary Fig. 3, Supplementary Table 3c). We further find that gymnosperms show a decrease in longevity with increasing site productivity, whereas angiosperms show a weak positive relationship with site potential net primary productivity (Fig. 2d).
Variables include species’ mean annual temperature (a) and precipitation during the driest quarter (b), species’ mean proximity to the estimated tree line position (c), potential net primary productivity (d), and species’ functional traits including wood density (e), maximum species tree height (f), and leaf mass per area (g) and leaf nitrogen (h). Trend lines are fitted using a linear model. Statistics show the correlation coefficient (R) and significance levels (p) for linear regressions. Data were grouped by the two major taxonomic groups in the dataset, angiosperms and gymnosperms. Sample sizes are shown in Supplementary Table 1. Note that data for Leaf Mass per Area were log10 transformed. Phylogenetically controlled correlations using phylogenetic generalised linear models (PGLS, see methods) for all variables are shown in Table 2.
Soil cation exchange capacity (CEC) and soil pH showed bivariate relationships with tree longevity in both groups (Supplementary Tables 1, 2). However, these effects on longevity are confounded by covariation with climate at the global scale. For example, in the angiosperm dataset, soil pH strongly covaried with precipitation and its effect on longevity disappeared when controlling for the effects of precipitation, temperature and CEC. Similarly, we found that a negative global relationship between CEC and temperature partially obscured a negative, but weak effect of temperature on longevity in angiosperms (Supplementary Table 2). That is, increased longevity due to colder temperatures at higher latitudes was partially offset by decreased longevity due to greater CEC at higher latitudes. For gymnosperms, we find no direct effect of CEC or soil pH after accounting for the effects of temperature and precipitation (Supplementary Table 2).
Covariation of traits and tree longevity
Many of the studied traits showed significant bivariate relationships with longevity across the full dataset, including both angiosperms and gymnosperms (Supplementary Table 1). However, most of these relationships disappeared when the data were analysed separately for angiosperms and gymnosperms. Of all the studied traits, the only traits that remained strong predictors for longevity within gymnosperms and angiosperms, were species’ radial growth (Fig. 1a) and species’ maximum tree height (Fig. 2f). For gymnosperms, no other functional traits were significantly related to longevity. For angiosperms we found significant, but weak, effects of leaf nitrogen (Fig. 1h) and wood density (Fig. 1e). Our results remained largely robust even when excluding growth projections from our analysis although the relationship with wood density for angiosperms disappeared (Supplementary Fig. 4). Within the two groups, we found no effects of hydraulic traits (P50, hydraulic safety margin-HSM, conduit size, and density, Supplementary Table 1).
As plant trait variation at the global scale was influenced by climate, we used Structural Equation Modelling (SEM) to disentangle the direct and indirect effects of climate and traits on species’ longevity. We included in this analysis key climate variables, i.e., temperature and precipitation during the driest quarter of the year, and those traits that showed associations with longevity, i.e., radial growth, maximum tree height and wood density. Results of the SEM are shown in Fig. 3 with detailed statistics in Table 1. About two third of the effect of precipitation on longevity in angiosperms was due to its influence on tree height and growth, while one third was due to the direct effect of precipitation (Table 1). Higher precipitation was associated with greater maximum tree height and reduced tree radial growth, both contributing to increasing longevity in angiosperms (Fig. 3a, Table 1). Temperature had a modest direct negative effect on longevity, while indirect effects of temperature via wood density and radial growth cancelled each other out. For gymnosperms, all of the effect of temperature was due to its influence on growth (i.e., increased tree growth at warmer sites reducing longevity) while our analysis did not detect a direct effect of temperature (Fig. 3b, Table 1). We find no significant effect of precipitation on gymnosperm longevity.
Structural equation modelling (SEM) of the multivariate effects of climate (mean annual temperature and precipitation of the driest quarter) and traits (wood density-WD, mean radial growth, and maximum tree height), on species’ longevity for angiosperms (a) and gymnosperms (b). Solid lines represent significance influence (p < 0.05) with the numbers indicating the strength of the relationships. Blue indicates positive and red negative relationships. Model statistics and direct and indirect effects and sample sizes are shown in Table 1. Omission of non-significant variables and paths, resulted in a better fit for gymnosperms, but for comparison purposes we applied the same model to both groups.
Association with the global plant trait spectrum
To overcome the limitation of the limited amount of trait data available for each species, we also perform the analyses with a more complete set of imputed trait data from ref. 37. Despite the additional uncertainty of these infilling approaches, this approach provides a more complete perspective on the association of longevity with a wider range of traits commonly used in global plant trait analyses. These data showed that for angiosperms longevity was associated with plant economics traits such as wood density, specific leaf area (SLA), and leaf nitrogen, and with the orthogonal axis of plant stature traits including plant height, seed mass, and conduit density (Supplementary Fig. 8). In contrast, in gymnosperms, there was no association with the plant economic traits (SLA and wood density) and the association with tree height was contrary to that expected (i.e., greater tree height resulting in lower longevity).
Evaluation of phylogenetic effects
We find that both groups show significant phylogenetic signals in longevity variation (Pagel’s λ for Gymnosperms = 0.905, p < 0.001; Pagel’s λ for Angiosperms: 0.46, p < 0.001). We therefore checked whether our results are robust with regard to these phylogenetic relationships. To this end, we first repeated the correlation analysis for key variables shown in Fig. 2 whilst accounting for phylogenetic relationships between species and find that all remain significant with very little change in the correlation strength (see Table 2). The only correlations that switched to non-significant are those between angiosperm longevity and wood density (p = 0.069) and potential site productivity (p = 0.108). Secondly, we reanalysed the data using linear mixed effects models incorporating family as a random effect. This proves that relationships with climate hold up within families (i.e., standardized effect of temperature on gymnosperm longevity = −0.157, t-value = −7.23, p < 0.001, n = 205, standardized effect of precipitation during the driest quarter on angiosperm longevity = +0.107, t-value = 6.43, p < 0.001, n = 534). Finally, we reanalysed the results using genus level means of longevity and climate. It confirms again that longevity-climate relationships remain robust within both groups (Supplementary Fig. 8a, b).
Discussion
Our analyses of a greatly expanded and diverse dataset confirmed that gymnosperms live longer than angiosperms (mean = 744 vs. 296 years). This difference is larger than that reported in previous datasets1,10,11,34, likely because of the addition of a larger number of short-lived, angiosperm species. We also confirmed the existence of a strong negative relationship between radial growth and longevity (Fig. 1a), consistent with life history theory, which postulates that trees face allocation trade-offs3,4,18. We focus our discussion below on the new aspects of our study which includes 1) clear differences between angiosperms and gymnosperms in the association of longevity with climate independent of phylogeny, 2) insights in the ecological factors contributing to greater longevity for gymnosperms in cold climates (i.e., low growth) and for angiosperms in humid climates (i.e., increased tree height and competition), and 3) the existence of relatively weak effects of functional traits on tree longevity, once phylogenetic differences between angiosperms and gymnosperms are taken into account.
Association of tree longevity with climate
We found marked differences in climate-longevity relationships between gymnosperms and angiosperms. While gymnosperm longevity declines with increasing temperature, angiosperm longevity declines with increasing aridity. Previous research identified similar effects of temperature and precipitation on longevity when gymnosperm and angiosperm data were pooled10,11. Here, a database with greater replication allowed us to show that climate-longevity relationships differ between gymnosperms and angiosperms.
The negative effect of temperature on gymnosperm longevity has been known for a long time12, but the underlying cause of this relationship remained unclear. Our analysis suggests that all of the temperature effect is indirect due to the effect of temperature on radial growth (Fig. 3, Table 1). It is well known that in ecozones noted for their low temperatures and typically short growing season length38 and low nutrient supply rate39, conifers tend to have a constrained rate and duration of cambial cell division and expansion40,41, low leaf photosynthesis42, and low needle nitrogen43,44, as well as long needle lifespan43,44 (the latter two of which are known to co-vary with photosynthesis and growth rate)43,44. This, in turn, could prolong a trees’ lifespan, as longevity declines exponentially with radial growth (Fig. 1a). The simultaneous decrease in longevity and increase in growth with temperature is also consistent with the suggested control of metabolism and oxidative processes on longevity of tree stems (i.e., the rate of living (ROL)) theory of aging45, as metabolism is strongly controlled by temperature.
While there is an overall tendency for gymnosperm longevity to increase towards cold sites, we found that a subgroup of gymnosperms reached their optimum lifespans in productive, dense forest sites where growth is not restricted by abiotic limitations. These include large conifer species from taxa within the Araucariaceae, Podocarpaceae, Cupressaceae and some Pinaceae. The most famous of these are the redwoods of California (Sequoia, Sequoiadendron), Patagonian Alerce (Fitzroya), bald cypresses (Taxodium) and kauri (Agathis). A targeted analysis of this group revealed that variation in longevity for these gymnosperms is associated with maximum tree height and is not related to temperature or growth, in contrast to most other gymnosperms (Supplementary Fig. 6). This group instead seems to adopt a strategy of achieving old ages through sustained growth enabled by ample water availability and relatively large leaf area, which allows trees to reach large statures and outcompete other trees46. Additional features that allow these species to achieve old ages are the production of wood that is resistant to beetle and fungal infections47 and high fire resistance due to bark that can exceed 15 cm in Sequoia and 30 cm in Sequoiadendron48.
The effect of temperature on longevity in angiosperms is much weaker than for gymnosperms, with negative relationships emerging only after the influence of traits and other environmental factors are accounted for (Fig. 3, Table 1). An unexpected result of our study was a positive effect of precipitation on longevity in angiosperms (Figs. 2b, and 3). These results are not in line with the general idea that “adversity begets longevity”, and previous analyses have produced conflicting outcomes, finding both positive10,11 and negative effects27,34 of precipitation on longevity. While some of these discrepancies may be attributed to differences in datasets and their geographic extent (e.g., tree ring data only27,34 vs. more comprehensive datasets), or analytical approach (e.g., site-level27,34 vs. species-level analysis11), the positive association between longevity and precipitation across the largest global dataset of angiosperm species is clear. By accounting for indirect effects of precipitation on longevity through tree growth and traits, we show that the majority of the effect of precipitation is explained by the effects of precipitation on tree height and growth (Fig. 3). Increases in precipitation lead to greater maximum tree height49,50,51,52, which in turn is associated with tree longevity (Fig. 3). In addition, we found a weak indirect effect of precipitation on longevity through the effect of precipitation on growth (i.e., a negative relationship between radial growth and precipitation, see Supplementary Fig. 5b, Fig. 3). While this seems counterintuitive, it most likely reflects increases in competition for light in more productive, wetter sites at lower latitudes, where trees form taller and denser canopies and standing biomass is larger53,54. This increases competition for light, and restricts growth of trees in the understory55,56. Tree ring studies on both temperate and tropical angiosperms indeed show that trees can remain suppressed and survive in the understory for decades to centuries57,58,59, and radiocarbon and other dating techniques suggest that some trees may even reside in the understory of tropical moist forests for several centuries17,60. Trees in shaded environments have lower growth due to low light but are able to persist in such conditions due to both phenotypic and genotypic energy-saving shifts towards low carbon cost foliage with low respiration rates and often extended leaf life-spans61,62. These may represent important ecological processes that contribute to extending longevity of shade-tolerant species1. Finally, increasing precipitation could also prolong tree longevity directly by reducing the risk of drought-induced embolism, an important mortality mechanism in trees63. However, despite differences between angiosperms and gymnosperms, we do not detect strong relationships between longevity and hydraulic traits within these groups (P50, HSM, conduit size or density, Supplementary Table 1). This may point to a convergence of species’ hydraulic safety margins across the globe64, although other studies report that variation in hydraulic safety margins are a good predictor for drought related mortality63,65. More data on hydraulic properties are needed to fully explore these effects for a greater number of species.
Why do gymnosperms and angiosperms differ in their climate-longevity relationships?
The most likely cause for diverging climate-longevity relationships between angiosperms and gymnosperms is the intrinsic and phylogenetically determined differences in their hydraulic architecture, hydraulic safety margins, and ability to repair xylem embolisms, resulting in differences in resistance to climate stress32,64,66 and competitiveness29,67. Gymnosperm tracheids are, on average, narrower and shorter than angiosperms’ multicellular vessel conduits, which makes them less vulnerable to cavitation and embolism during drought or freeze-thaw events64,68. Gymnosperm tracheids also have bordered pits that provide greater embolism resistance33,69 which may allow gymnosperms to survive in harsh environments such as at cold sites and some semi-arid regions29,70.
Angiosperms are generally more efficient than gymnosperms at transporting water and have higher photosynthetic capacity30,67, making them more competitive in warm, wet, resource rich environments29,36. However, observed trade-offs between hydraulic conductance and safety from embolism in angiosperms limits height attainment, and thus tree lifespan, for this group in dry environments, whereas such trade-offs are weaker in gymnosperms51. These mechanisms may help explain why gymnosperm longevity was unaffected by precipitation, and why longevity responses to climate differ between gymnosperms and angiosperms. However, we acknowledge that our analysis is correlative and that climate explains only a modest amount of variation in longevity. Additional features that may explain some of the differences in response to climate, include a greater ability for gymnosperms to compartmentalise attacks by diseases, insects, and fungi25,71, and their greater allocation to defensive compounds and lignin concentrations72. Finally, differences in the climate response may also emerge from environmental filtering for different climates, as gymnosperms occur predominantly in environments that experience freezing during some parts of the year. In line with this we find that separation of the dataset into species occurring in freezing versus non-freezing environments results in similar relationships with temperature and precipitation as those observed for gymnosperms and angiosperms (Supplementary Fig. 9). These results show that more research is needed to assess interactions between environment and phylogeny, and to fully disentangle the relative roles of these various mechanisms in controlling tree survival and explain differences in associations between longevity and climate. Further investigations could also assess trait-longevity associations within ecosystems and biomes and explore to what degree longevity variations are related to external disturbances (e.g. fire, hurricanes).
Can functional traits predict tree longevity?
Based on plant economic theory we expected that allocation in resources towards one purpose, e.g. survival and maintenance, comes at the cost of investment in other functions, e.g., resource acquisition and growth73. Across the full dataset, we indeed found evidence for such trade-offs with greater longevity for species with safer hydraulic systems (i.e., higher HSM, lower P50, smaller conduits and higher conduit density), and species with slower, less acquisitive leaf traits (i.e., higher LMA, lower photosynthetic assimilation and nitrogen per mass) (see Supplementary Table 1). Leaf nitrogen was the single strongest predictor of longevity across all species, among all climate and trait variables (Supplementary Table 1). However, these global trait longevity associations were almost entirely driven by phylogenetically determined trait differences between the angiosperms and gymnosperms, as separate analysis for these groups showed fewer and relatively weak relationships.
Within gymnosperms, none of the plant economics traits were associated with longevity, while within angiosperms only wood density and leaf nitrogen were significantly related to longevity (Supplementary Table 1). Effects of wood density were weak however (Fig. 2e). These results are somewhat surprising, as wood density provides trees with mechanical strength74, protection from fungi and pathogens72, and resistance to drought embolism75, all of which affect tree survival, and were thus expected to exert strong control on tree longevity. The weak relationship between wood density and longevity is consistent with studies on tropical forests that found species’ wood density to be a relatively poor predictor for tree mortality (i.e., r2 ~ 0.2-0.4), while species’ mean radial growth rate was a better predictor23,24,76. The only study that previously related longevity with wood traits showed that wood density was weakly related to longevity for North American angiosperms, but not for gymnosperms, and that volumetric heat content (i.e., energy released upon burning) was a better predictor for tree longevity25. Volumetric heat content combines both structural and chemical investments, highlighting the strong role that chemical defence compounds may play in prolonging tree lifespan. These secondary compounds may be especially important for gymnosperms, as this group has slower wood decomposition rates than angiosperms despite a lower mean wood density72, and lacks a relationship between wood density and longevity (Fig. 2e). Apart from wood density, the only other economics trait that showed a significant relationship with longevity was leaf nitrogen for angiosperms, but the relationship was weak.
In contrast to the relatively poor within-group predictive power of traits associated with the fast-slow plant economic trait spectrum, species maximum height was significantly related to species’ longevity within both groups (Fig. 2f) and explained a larger, though still modest, share of variation. Maximum tree height integrates across many aspects of plant stature and is the second most important functional traits axis in trees, orthogonal to the fast-slow axis37,77,78. Large statured trees have greater exposure to wind gusts, which can increase the likelihood of stem breakage or windthrow79,80,81 and increased exposure to lightning82. Yet, at the global scale, the greater likelihood of damage or mortality posed by these factors is outweighed by the advantages conferred by greater maximum height. Large-statured species may reach greater tree ages by greater investment in growth in the adult phases allowing them to outcompete smaller statured species for light and survive longer21. This guild of trees has been described as long-lived pioneers, and occur in both tropical21 and temperate humid forests83. Examples of long-lived pioneers for temperate humid forests include the previously mentioned group of large conifer species (e.g., Sequoia, Sequoiadendron, Fitzroya, Agathis). Faster growth rates for some of these large, long-lived pioneers may also increase survival by reducing the exposure to some pathogens and herbivores, and by increasing bark thickness that provides protection from surface fires48,84. Interestingly, the longevity-height associations break down in those conifer species that usually grow in low competition sites where cold or dry conditions limits radial growth and where reproductive success does not depend on outcompeting other trees (Supplementary Fig. 6). Examples for this group are cold- and dry-adapted gymnosperms such as Pinus longaeva and several Juniperus species that reach old ages (>1000 yrs) with low maximum statures. These results are consistent with studies showing that the biggest trees are not always the oldest1,58,85, and contrasts strongly with the height-longevity associations for angiosperms (Fig. 2f).
In summary, our analyses showed that most species-specific traits provided relatively weak relationships with species’ tree longevity within both groups. This poor predictive power is not very different from the findings of other global studies on trait variation and species life history22, and could be caused by various factors86. First, we used species’ mean trait values while traits may vary strongly from site to site, or even within the site, for the same species86. Secondly, we mixed trait data from a large variety of ecosystems from across the globe, but traits are known to covary with global variation in soil and climate37,74,77. For most analyses, the use of species mean trait values should provide robust results due to limited species trait crossover across environments87, but we cannot discount that this broad approach may have contributed to obscuring the relationships between traits and longevity at a global scale. Finally, only a small number of species in our data set had a larger set of directly measured traits, which limited the power of our analyses. To overcome this problem we had to rely partially on imputed trait data37 to assess relationships with multiple traits at the same time (Supplementary Fig. 7). Future research should prioritize expanding databases to increase the coverage of the longevity and trait data and include additional traits relevant to longevity such as defensive compounds and bark properties.
While we could not fully assess trait-longevity associations for a larger range of traits, some differences emerged between angiosperms and gymnosperms. Angiosperm longevity was associated with the two dominant axes of trait variation, plant economics (i.e., fast-slow) and plant size (small-big). In contrast, for gymnosperms, there was only a relationship with plant size for a small subgroup, and almost no association with plant economics traits. These differences may be related to the greater variation in hydraulic and leaf morphological traits in angiosperms compared to gymnosperms. Evolution of a more flexible hydraulic architecture31,88 may have resulted in greater diversification of angiosperms along the fast-slow and small-large life history axes, allowing adaptation to different niches in relatively stable, but productive, competitive environments. This may have been further facilitated by faster demographic rates of angiosperms (i.e., more than two times shorter mean longevity), which itself may increase species diversification rates89. In contrast, gymnosperms predominantly seem to lack diversification along the fast-slow spectrum possibly because of their “slower” hydraulic systems that are more adapted to stress tolerances in low competition environments31,33,64.
Summary and outlook
We identified two fundamental pathways in which trees can become old. The first is slow growth driven by strong resource limitations, i.e. abiotic stress in the form of low temperature and/or poor soils, increasing longevity for a number of conifer species that confirms the long-established notion that “adversity begets longevity”. The second path to old ages involves the opposite conditions, i.e., relief from abiotic constraints at wet and productive sites. This second path is primarily found in angiosperm species, but also in a smaller group of gymnosperms growing in productive forests. In this productivity pathway increases in competition limits individual growth for trees in the understory and allows others to reach large statures, both of which are associated with greater longevity.
Differences in longevity among species are ultimately the outcome of long evolutionary divergence between species with specific adaptations arising to provide the fitness advantages conveyed by longevity in these very different environments. These two pathways to old ages in trees represent two extremes along a competition spectrum; low resource and low competition at one end, high resource and high competition at the other end. Indeed, these strategies are generally located within two corners of Grime’s “C-S-R” triangle90; old conifers at sites with adverse growing conditions (i.e., low temperature, short growing seasons, poor soils, etc.) would occupy the lower left-hand “S” corner representing extreme “stress tolerators” (low competition, extreme stress), whereas long-lived canopy and emergent trees in forests would occupy the upper “C” corner, representing the ultimate “competitors” (high competition, low stress) able to acquire an unequal share of the most limiting resource, light in the case of most productive forests.
Methods
Longevity database
We compiled a new extended database of tree species longevity, that includes a total of 739 species, 205 gymnosperms and 534 angiosperms. Species longevity was defined as the highest age of the above ground stem tissue recorded for a given species. While below ground tissues or genets may be older than our definition of stem longevity, we did not include these ages. As we focus here on variation in tree longevity, or tree maximum lifespan, between species, we ignored variation within species arising from differences in climate58, soil20 and disturbance frequency91. Age estimates were obtained from a mixture of tree ring records, radiocarbon dating, growth projections and historical records sourced from original databases and literature (see Supplementary Table 3a for key datasets). For most species (78%), the highest age estimates were based on tree ring counts. Where possible we recorded the mean radial growth increment for each species, calculated as the average ring width across all sites or obtained from reported mean ring width or diameter growth from literature. Original tree ring data were compiled from publicly available ITRDB data, National Forest Inventories, and data from several tree ring laboratories (see Supplementary Table 3a). In total, we used tree ring records from over 530.000 trees and over 125.000 sites. Maximum ages derived from tree ring records were only included if the field sampling indicated that large trees from natural forests were targeted in the field campaign. Studies were excluded if they focussed purely on even-aged plantations, non-natural systems, non-native species, or if they seemed to consist of partially recorded or artificially truncated time series. Our estimates do not necessarily represent the oldest estimates available, as popular media often reports exaggerated age estimates, which were avoided by relying only on scientific dating methods from traceable sources. In general, tree ring records with less than 10 series were excluded, but we accepted age estimates based on fewer samples if these can reasonably be believed to represent ages close to species’ maxima such as published on Oldlists for commonly sampled species (see Supplementary Table 3a). The final database was verified and checked by several authors on this article improving the database. Species names were checked against the World Flora Online (https://www.worldfloraonline.org/downloadData) using the R package WorldFlora, v1.14.592. We only included species listed on the GlobalTreeSearch93, or those that fit the definition of a tree agreed by IUCN’s Global Tree Specialist Groups (GTSG): “a woody plant with usually a single stem growing to a height of at least two metres or if multi-stemmed with then at least one vertical stem five centimetres in diameter at breast height.” This database presents a significant extension compared to previous studies (i.e., 438 species11, 237 species94, 246 species34, <200 species10,27).
To assess uncertainty on estimates of the maximum longevity for a species, we classified the age estimates for each species into four confidence categories according to the sampling methods, and number of sites and samples included (see Supplementary Table 3c for definitions). Estimates obtained from tree ring records containing less than 150 trees, or series, were classified as the lowest confidence (level 1). Radiocarbon dating on large trees, and growth projections or historical accounts were classified as moderate confidence (level 2). Tree ring records based on more than 150 samples and less than three sites, or those published on Oldlists for commonly sampled species were classified as high confidence (level 3), and tree ring records from more than 3 sites and more than 150 series as very high confidence (level 4). A relatively large portion of species longevity estimates (45%) fell into the lowest confidence category (Supplementary Table 3c). While this could potentially bias our results, we find no evidence for significant changes in the results when this category was excluded from our analysis (see e.g. Supplementary Fig. 3) and we thus chose to present the main results including the maximum number of species. We also note that a relatively large portion of samples (22%) came from growth projections and/or radiocarbon dating, which included important functional groups such as fast-growing tropical pioneer species and extremely old, slow-growing species that generally lack reliable tree ring dating. These age estimates based on projections using short-term growth and mortality rates are subject to several potential biases due to limited data availability, variation in growth and mortality with tree size, and trade-offs between growth and mortality within species that may be poorly captured. However, our results remained largely robust even when excluding growth projections from our analysis although the relationship with wood density for angiosperms disappeared (Supplementary Fig. 4). In all, this provides confidence in our conclusions.
Study sites are well distributed across the globe (see Supplementary Fig. 1a) and included species are from all biomes (Supplementary Fig. 1b). However, the database does not equally represent all biomes and groups. Tree ring data, in particular those from the ITRDB, are overrepresented by old, mostly gymnosperm species growing close to tree lines as these are most valuable for dendro-climate reconstructions95. As a result, there is a geographic bias towards mountain ranges around 40°N latitude such as the American Cordillera, Himalayas, Alps. Tree ring studies further underrepresent tropical trees. To overcome these two biases, we included previously compiled tropical tree ring data11, and explicitly searched for additional age records for common short-lived species to capture the full spectrum of life history strategies. We note that this resulted in a relatively large set of tropical species growing at temperatures >25 °C and somewhat unbalanced distribution of angiosperm species across the temperature axis (Fig. 1a), but we do not believe this influenced our results. We further note that our dataset sampled only a fraction (534) of the global estimated number of angiosperms species (>70.000)96. Filling this gap is a challenge, in particular for tropical angiosperms given the vast number of tropical species and their unreliable formation of tree rings. As a result, significant discussions remain regarding, for example, the longevity of slow-growing tropical shade tolerant species as radiocarbon dating in excess of 1000 years97 is still regarded uncertain17,98,99, and obtaining reliable estimates of tropical tree ages remains a major challenge. These shortcomings may raise questions as to what degree the small fraction of sampled angiosperms reflects the spectrum of life history strategies and could have led to biases in tree longevity estimates. To assess whether the life history strategies of the included species is different from the global diversity of strategies, we compared the range of wood densities and maximum tree height for our dataset to that available from global trait databases. This comparison included wood density as it is closely linked to life history variation along the fast-slow axis and other functional attributes of trees72,74,75 as well as tree height as a proxy for plant stature which is the second most important functional traits axis in trees37,77,78. This shows that both the average and range of wood density of our study are very similar to that of the globe for both angiosperm and gymnosperms (Supplementary Fig. 2a, b). It further shows very close agreement with tree height, although the functional trait dataset has a somewhat greater share of short statured trees for angiosperms (Supplementary Fig. 2c, d). This gives us confidence that our sample is a fair reflection of the global life history strategies of trees.
Environmental data
To assess the effects of climate, environment and soil properties on species’ longevity we extracted data from a variety of datasets. Firstly, climate and soil data were obtained, for most species, from the TreeGOER database (https://zenodo.org/records/10008994100. This database combines the Global Biodiversity Information Facility, GBIF, species occurrence data101 with 30-arc seconds resolution WorldClim 2.1102, aridity indices such as Climate Moisture Index (CMI)103, and SoilGrids 2.0 data104 to provide information on tree species’ mean climate and soil environments. For a small number of species (21 out of 739) not included in the TreeGOER database, we used the mean of the WorldClim 2.1 climate data from all sites for that species included in our database. In addition to standard climate and soil metrics, we also extracted growing season length and site level Net Primary Productivity (NPP) from CHELSA V2.1 dataset (https://chelsa-climate.org/downloads/)105, and calculated species means as the average across all sites included in our database.
For each species, we further calculated species mean proximity to the upper tree line, where tree growth is limited by cold temperatures. This was calculated as the mean of the difference between the actual elevation for each site and the potential upper tree line elevation for that sites estimated using an approximation of the relationship between latitude and potential tree line from Fig. 5 in Paulsen and Korner106. Lacking elevation site data were filled in using elevation data from WorldClim 2.1102.
Trait data
We extracted species trait data from various databases (see Supplementary Table 4). Maximum tree height was obtained from Tallo107, The Gymnosperm Database (www.conifers.org), and from Monumentaltrees.com. Wood density data came from the global wood density database108, CIRAD wood density109, complemented with several individual records provided by co-authors or accessed online. Conduit density and size were obtained from refs. 51,63 and 110, and P50 and hydraulic safety margins from the Xylem Functional Traits database63, complemented with unpublished tropical data (Sunny & Barua, 2024). Leaf traits were obtained from GLOPNet leaf economics dataset111, and the seedmass data from TRY plant trait database_ request No 30569112.
To include the maximum number of species and traits combinations in one single analysis, we extracted imputed trait data from ref. 37. These methods predict missing data using Bayesian hierarchical probabilistic matrix factorization algorithms (BHPMF) based on trait-trait correlations and trait variation (see ref. 37 for more information). We used all 17 plant functional traits of ref. 37 and log-transformed the data prior to analysis.
Analysis
We initially conducted a correlation analysis to evaluate the bivariate relationships between the log10-transformed longevity and various factors, including climate, environment, and traits. We then used multiple regression to assess the relative influence of climate variables, which co-vary at a global scale37. We used the Akaike Information Criteria (AIC) to select the set of environmental that best explained variation in longevity for angiosperms and gymnosperms modelled separately. We assessed collinearity between different variables by evaluating pairwise scatter plots between all variables and through the variance inflation factor (VIF). We avoided including closely correlated climate variables (e.g. growing season length and temperature, or temperature and proximity to tree line) within a single model and all VIF were lower than 5. To partition the contributions of different variables to explaining longevity, we used the lmg metric from the R package relaimpo, v2.2.7, as recommended by ref. 113.
We then assessed direct and indirect effects of climate and traits on maximum longevity using Structural Equation Models (SEMs). Previous studies showed that wood density114, leaf traits115 and tree height49,50 all vary along latitudinal gradients of climate and soil. SEM allowed separating direct climate and trait effects on longevity, from indirect effects of climate through climate’s effects on traits and radial growth. The included variables in this model were mean annual temperature, precipitation of the driest quarter, and wood density, radial growth (log10-transformed) and maximum tree height. We did not include other trait variables as these were usually available for much fewer species and/or showed poor bivariate relationships (see Supplementary Table 1). We used the R package lavaan, v0.6.19116 to fit the model, and report on conventional cutoff criteria117 to evaluate the model fit.
To obtain a more complete overview of trait – longevity relationships, we performed a principal component analysis using the R package FactoMineR, v2.12118 and 17 commonly used plant trait data from ref. 34. For this analysis all plant trait data were natural log-transformed. All analysis was performed in R 4.4.2119.
Evaluation of phylogenetic effects
Longevity is to some degree genetically controlled1, and evolutionary time between species and their phylogenetic relationships may thus affect our results. We therefore used phylogenetic relationships from ref. 120 to asses phylogenetic signals in both groups and re-analysed the main results accounting for phylogenetic relationships between species using phylogenetic generalised linear models (PGLS) from the R packages caper, v1.0.3121, phytools, v2.5.2122 and ape, v5.8.1123. We further checked whether climate effects on longevity could be driven by specific taxa selected for particular climates extremes. For example, the Pinaceae family contains some of the oldest species, and could be restricted to cold environments, driving the overall patterns. To check for these effects, we reanalysed the climate longevity relationships using linear mixed effects models from R package lme4124 incorporating family as a random effect. Finally, it could be argued that well represented genera (e.g. large genus of Pinus within the gymnosperms) could dominate the observed patterns. To check for this effect, we reanalysed the results using genus level means of longevity and climate.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data on species’ maximum longevity, traits, and climate that support the findings of this study are available from https://doi.org/10.6084/m9.figshare.29876984. Original raw tree ring data from the ITRDB can be downloaded from https://www.ncei.noaa.gov/products/paleoclimatology/tree-ring, and tropical tree ring data compilations from https://figshare.com/articles/dataset/Locoselli_et_al_2020_Global_tree-ring_analysis_reveals_rapid_decrease_in_tropical_tree_longevity_with_temperature_PNAS/13119842?file=25178405. Individual longevity records from following oldlists http://www.rmtrr.org/oldlist.htm, https://www.ldeo.columbia.edu/~adk/oldlisteast/, http://www.nativetreesociety.org/dendro/ents_maximum_ages.htm, https://www.oldgrowth.ca/oldtrees/. Tree height data can be downloaded from https://zenodo.org/record/6637599, and maximum height measurements were obtained from https://www.conifers.org and https://Monumentaltrees.com. Wood density data can be obtained from https://zenodo.org/records/13322441, and from https://doi.org/10.18167/DVN1/KRVF0E. Conduit density from https://doi.org/10.5061/dryad.1138, and conduit density, P50 and HSM from https://doi.org/10.5061/dryad.1138, and from https://doi.org/10.1126/sciadv.aav1332. Leaf traits from https://www.nature.com/articles/nature02403#Sec15, and seedmass data from https://www.try-db.org/TryWeb/dp.php, database request No 30569. Mean climate and soil data for a species were obtained from the TreeGOER database https://zenodo.org/records/10008994, and gridded climate and elevation data from https://www.worldclim.org/data/worldclim21.html, growing season length and site level Net Primary Productivity (NPP) from https://chelsa-climate.org/. Species occurrence data from https://doi.org/10.15468/dl.77gcvq.
References
Piovesan, G. & Biondi, F. On tree longevity. N. Phytologist 231, 1318–1337 (2021).
Körner, C. A matter of tree longevity. Science 355, 130–131 (2017).
Reich, P. B. The world-wide ‘fast–slow’plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Salguero-Gómez, R. Applications of the fast–slow continuum and reproductive strategy framework of plant life histories. N. Phytologist 213, 1618–1624 (2017).
Bialic-Murphy, L. et al. The pace of life for forest trees. Science 386, 92–98 (2024).
Morris, W. F. et al. Longevity can buffer plant and animal populations against changing climatic variability. Ecology 89, 19–25 (2008).
Chondol, T. et al. Habitat preferences and functional traits drive longevity in Himalayan high-mountain plants. Oikos 2023, e010073 (2023).
Friend, A. D. et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl. Acad. Sci. USA 111, 3280–3285 (2014).
Galbraith, D. et al. Residence times of woody biomass in tropical forests. Plant Ecol. Diversity 6, 139–157 (2013).
Liu, J. et al. Age and spatial distribution of the world’s oldest trees. Conserv. Biol. 36, e13907 (2022).
Locosselli, G. M. et al. Global tree-ring analysis reveals rapid decrease in tropical tree longevity with temperature. Proc. Natl. Acad. Sci. 117, 33358–33364 (2020).
Schulman, E. Longevity under Adversity in Conifers. Science 119, 396–399 (1954).
Stahle, D. et al. Longevity, climate sensitivity, and conservation status of wetland trees at Black River, North Carolina. Environ. Res. Commun. 1, 041002 (2019).
Patrut, A. et al. Radiocarbon dating of a very large African baobab. Tree Physiol. 27, 1569–1574 (2007).
Condit, R., Hubbel, S. P. & Foster, R. B. Identifying fast-growing native trees from the neotropics using data from a large, permanent census plot. Ecol. Manag. 62, 123–143 (1993).
Kurokawa, H., Yoshida, T., Nakamura, T., Lai, J. & Nakashizuka, T. The age of tropical rain-forest canopy species, Borneo ironwood (Eusideroxylon zwageri), determined by 14 C dating. J. Trop. Ecol. 19, 1–7 (2003).
Vieira, S. et al. Slow growth rates of Amazonian trees: Consequences for carbon cycling. Proc. Natl. Acad. Sci. USA 102, 18502–18507 (2005).
Stearns, S. C. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268 (1989).
Stephenson, N. L. et al. Causes and implications of the correlation between forest productivity and tree mortality rates. Ecol. Monogr. 81, 527–555 (2011).
Brienen, R. J. et al. Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat. Commun. 11, 4241 (2020).
Rüger, N. et al. Beyond the fast–slow continuum: demographic dimensions structuring a tropical tree community. Ecol. Lett. 21, 1075–1084 (2018).
Adler, P. B. et al. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. 111, 740–745 (2014).
Wright, S. J. et al. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91, 3664–3674 (2011).
Poorter, L. et al. Are functional traits good predictors of demographic rates? Evidence from five Neotropical forests. Ecology 89, 1908–1920 (2008).
Loehle, C. Tree Life-History Strategies - the Role of Defenses. Can. J. For. Res. 18, 209–222 (1988).
Lu, R. et al. The U-shaped pattern of size-dependent mortality and its correlated factors in a subtropical monsoon evergreen forest. J. Ecol. 109, 2421–2433 (2021).
Xu, C. & Liu, H. Hydraulic adaptability promotes tree life spans under climate dryness. Glob. Ecol. Biogeogr. 31, 51–61 (2022).
Liu, L. et al. Tropical tall forests are more sensitive and vulnerable to drought than short forests. Glob. Chang Biol. 28, 1583–1595 (2022).
Bond, W. The tortoise and the hare: ecology of angiosperm dominance and gymnosperm persistence. Biol. J. Linn. Soc. 36, 227–249 (1989).
Lusk, C. H., Wright, I. & Reich, P. B. Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. N. Phytologist 160, 329–336 (2003).
Brodribb, T. J. & Feild, T. S. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett. 13, 175–183 (2010).
Johnson, D. M., McCulloh, K. A., Woodruff, D. R. & Meinzer, F. C. Hydraulic safety margins and embolism reversal in stems and leaves: why are conifers and angiosperms so different?. Plant Sci. 195, 48–53 (2012).
Sperry, J. S., Hacke, U. G. & Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 93, 1490–1500 (2006).
Gao, J. et al. Climate-driven patterns of global tree longevity. Commun. Earth Environ. 6, 610 (2025).
Willis, K. J. & McElwain, J. C. The Evolution of Plants (Oxford University Press, USA, 2014).
Ma, H. et al. The global biogeography of tree leaf form and habit. Nat. Plants, 9, 1795–1809 (2023).
Joswig, J. S. et al. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evolution 6, 36–50 (2022).
Gao, S. et al. An earlier start of the thermal growing season enhances tree growth in cold humid areas but not in dry areas. Nat. Ecol. evolution 6, 397–404 (2022).
Reich, P. B. & Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. 101, 11001–11006 (2004).
Rossi, S., Deslauriers, A., Anfodillo, T. & Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 152, 1–12 (2007).
Björklund, J., Fonti, M. V., Fonti, P., Van den Bulcke, J. & von Arx, G. Cell wall dimensions reign supreme: cell wall composition is irrelevant for the temperature signal of latewood density/blue intensity in Scots pine. Dendrochronologia 65, 125785 (2021).
Berry, J. & Bjorkman, O. Photosynthetic response and adaptation to temperature in higher-plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 31, 491–543 (1980).
Reich, P. B., Walters, M. & Ellsworth, D. Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol. Monogr. 62, 365–392 (1992).
Reich, P. B., Rich, R. L., Lu, X., Wang, Y.-P. & Oleksyn, J. Biogeographic variation in evergreen conifer needle longevity and impacts on boreal forest carbon cycle projections. Proc. Natl. Acad. Sci. 111, 13703–13708 (2014).
Issartel, J. & Coiffard, C. Extreme longevity in trees: live slow, die old?. Oecologia 165, 1–5 (2011).
Sillett, S. C. et al. Comparative development of the four tallest conifer species. Ecol. Manag. 480, 118688 (2021).
Franceschi, V. R., Krokene, P., Christiansen, E. & Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. N. Phytol. 167, 353–376 (2005).
Sillett, S. C. et al. How do tree structure and old age affect growth potential of California redwoods?. Ecol. Monogr. 85, 181–212 (2015).
Scheffer, M. et al. A global climate niche for giant trees. Glob. Change Biol. 24, 2875–2883 (2018).
Moles, A. T. et al. Global patterns in plant height. J. Ecol. 97, 923–932 (2009).
Liu, H. et al. Hydraulic traits are coordinated with maximum plant height at the global scale. Sci. Adv. 5, eaav1332 (2019).
Klein, T., Randin, C. & Körner, C. Water availability predicts forest canopy height at the global scale. Ecol. Lett. 18, 1311–1320 (2015).
Madrigal-González, J. et al. Global patterns of tree density are contingent upon local determinants in the world’s natural forests. Commun. Biol. 6, 47 (2023).
Crowther, T. et al. Mapping tree density at a global scale. Nature 525, 201–205 (2015).
Brienen, R. et al. Paired analysis of tree ring width and carbon isotopes indicates when controls on tropical tree growth change from light to water limitations. Tree Physiol. 42, 1131–1148 (2022).
Canham, C. D. Suppression and release during canopy recruitment in Acer saccharum. Bull. Torre. Bot. Club 112, 145 (1985).
Brienen, R. J. W. & Zuidema, P. A. Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. J. Ecol. 94, 481–493 (2006).
Di Filippo, A. et al. The longevity of broadleaf deciduous trees in Northern Hemisphere temperate forests: insights from tree-ring series. Front. Ecol. Evol. 3, 46 (2015).
Pavlin, J. et al. Disturbance history is a key driver of tree life span in temperate primary forests. J. Vegetation Sci. 32, e13069 (2021).
Hubau, W. et al. The persistence of carbon in the African forest understory. Nat. Plants 5, 133–140 (2019).
Lusk, C. H. & Reich, P. B. Relationships of leaf dark respiration with light environment and tissue nitrogen content in juveniles of 11 cold-temperate tree species. Oecologia 123, 318–329 (2000).
Reich, P. B., Uhl, C., Walters, M. B., Prugh, L. & Ellsworth, D. S. Leaf demography and phenology in Amazonian rain forest: a census of 40 000 leaves of 23 tree species. Ecol. Monogr. 74, 3–23 (2004).
Sanchez-Martinez, P. et al. Increased hydraulic risk in assemblages of woody plant species predicts spatial patterns of drought-induced mortality. Nat. Ecol. Evol. 7, 1620–1632 (2023).
Choat, B. et al. Global convergence in the vulnerability of forests to drought. Nature 491, 752 (2012).
Tavares, J. V. et al. Basin-wide variation in tree hydraulic safety margins predicts the carbon balance of Amazon forests. Nature 617, 111–117 (2023).
McCulloh, K. et al. Moving water well: comparing hydraulic efficiency in twigs and trunks of coniferous, ring-porous, and diffuse-porous saplings from temperate and tropical forests. N. Phytol. 186, 439–450 (2010).
Brodribb, T. J., Feild, T. S. & Jordan, G. J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144, 1890–1898 (2007).
Carlquist, S. J. Ecological Strategies of Xylem Evolution (Univ of California Press, 1975).
Brodribb, T. & Hill, R. The importance of xylem constraints in the distribution of conifer species. N. Phytologist 143, 365–372 (1999).
Brodribb, T. J., Pittermann, J. & Coomes, D. A. Elegance versus speed: examining the competition between conifer and angiosperm trees. Int. J. Plant Sci. 173, 673–694 (2012).
Morris, H., Brodersen, C., Schwarze, F. W. & Jansen, S. The parenchyma of secondary xylem and its critical role in tree defense against fungal decay in relation to the CODIT model. Front. Plant Sci. 7, 1665 (2016).
Weedon, J. T. et al. Global meta-analysis of wood decomposition rates: a role for trait variation among tree species?. Ecol. Lett. 12, 45–56 (2009).
Herms, D. A. & Mattson, W. J. The dilemma of plants: to grow or defend. Q. Rev. Biol. 67, 283–335 (1992).
Chave, J. et al. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366 (2009).
Hacke, U. G., Sperry, J. S., Pockman, W. T., Davis, S. D. & McCulloh, K. A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126, 457–461 (2001).
Esquivel-Muelbert, A. et al. Tree mode of death and mortality risk factors across Amazon forests. Nat. Commun. 11, 5515 (2020).
Maynard, D. S. et al. Global relationships in tree functional traits. Nat. Commun. 13, 3185 (2022).
Diaz, S. et al. The global spectrum of plant form and function. Nature 529, 167–171 (2016).
Canham, C. D., Papaik, M. J. & Latty, E. F. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can. J. For. Res. 31, 1–10 (2001).
Rich, R. L., Frelich, L. E. & Reich, P. B. Wind-throw mortality in the southern boreal forest: Effects of species, diameter and stand age. J. Ecol. 95, 1261–1273 (2007).
Lee, C. A., Voelker, S., Holdo, R. M. & Muzika, R.-M. Tree architecture as a predictor of growth and mortality after an episode of red oak decline in the Ozark Highlands of Missouri, USA. Can. J. For. Res. 44, 1005–1012 (2014).
Gora, E. M. et al. A mechanistic and empirically supported lightning risk model for forest trees. J. Ecol. 108, 1956–1966 (2020).
Ishii, H. & Ford, E. D. Persistence of Pseudotsuga menziesii (Douglas-fir) in temperate coniferous forests of the Pacific Northwest Coast, USA. Folia Geobotanica 37, 63–69 (2002).
Gora, E. M. & Esquivel-Muelbert, A. Implications of size-dependent tree mortality for tropical forest carbon dynamics. Nat. Plants 7, 384–391 (2021).
Bigler, C. Trade-offs between growth rate, tree size and lifespan of mountain pine (Pinus montana) in the Swiss National Park. PloS One 11, e0150402 (2016).
Yang, J., Cao, M. & Swenson, N. G. Why functional traits do not predict tree demographic rates. Trends Ecol. Evol. 33, 326–336 (2018).
Hecking, M. J., Zukswert, J. M., Drake, J. E., Dovciak, M. & Burton, J. I. Montane temperate-boreal forests retain the leaf economic spectrum despite intraspecific variability. Front. For. Glob. Change 4, 754063 (2022).
Sperry, J. S. Evolution of water transport and xylem structure. Int. J. Plant Sci. 164, S115–S127 (2003).
Baker, T. R. et al. Fast demographic traits promote high diversification rates of Amazonian trees. Ecol. Lett. 17, 527–536 (2014).
Grime, J. P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Naturalist 111, 1169–1194 (1977).
Pavlin, J. et al. Pathways and drivers of canopy accession across primary temperate forests of Europe. Sci. Total Environ. 906, 167593 (2024).
Kindt, R. WorldFlora: an R package for exact and fuzzy matching of plant names against the World Flora Online taxonomic backbone data. Appl. Plant Sci. 8, e11388 (2020).
Beech, E., Rivers, M., Oldfield, S. & Smith, P. GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. Forestry 36, 454–489 (2017).
Biondi, F., Meko, D. M. & Piovesan, G. Maximum tree lifespans derived from public-domain dendrochronological data. Iscience 26, 106138 (2023).
Zhao, S. et al. The International Tree-Ring Data Bank (ITRDB) revisited: data availability and global ecological representativity. J. Biogeogr. 46, 355–368 (2019).
Cazzolla Gatti, R. et al. The number of tree species on Earth. Proc. Natl. Acad. Sci. 119, e2115329119 (2022).
Chambers, J. Q., Higuchi, N. & Schimel, J. P. Ancient trees in Amazonia. Nature 39, 135–136 (1998).
Worbes, M. & Junk, W. J. How old are tropical trees? The persistence of a myth. IAWA J. 20, 255–260 (1999).
Martinez-Ramos, M. & Alvarez-Buylla, E. R. How old are tropical rain forest trees?. Trends Plant Sci. 3, 400–405 (1998).
Kindt, R. TreeGOER: A database with globally observed environmental ranges for 48,129 tree species. Glob. Change Biol. 29, 6303–6318 (2023).
Gbif.org. GBIF occurrence download https://doi.org/10.15468/dl.77gcvq (2021).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Willmott, C. J. & Feddema, J. J. A more rational climatic moisture index. Professional Geographer 44, 84–88 (1992).
Poggio, L. et al. SoilGrids 2.0: producing soil information for the globe with quantified spatial uncertainty. Soil 7, 217–240 (2021).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 1–20 (2017).
Paulsen, J. & Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 124, 1–12 (2014).
Jucker, T. et al. Tallo: A global tree allometry and crown architecture database. Glob. Change Biol. 28, 5254–5268 (2022).
Zanne, A. E. et al. Data from: Towards a worldwide wood economics spectrum. Dryad Digital Repos. https://doi.org/10.5061/dryad.234 (2009).
Vieilledent, G. et al. New formula and conversion factor to compute basic wood density of tree species using a global wood technology database. Am. J. Bot. 105, 1653–1661 (2018).
Zanne, A. E. et al. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 97, 207–215 (2009).
Wright, I. J. et al. The worldwide leaf economics spectrum. Nature 428, 821–827 (2004).
Kattge, J. et al. TRY plant trait database - enhanced coverage and open access. Glob. Chang Biol. 26, 119–188 (2020).
Grömping, U. Relative importance for linear regression in R: the package relaimpo. J. Stat. Softw. 17, 1–27 (2007).
Swenson, N. G. & Enquist, B. J. Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. Am. J. Bot. 94, 451–459 (2007).
Maire, V. et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 24, 706–717 (2015).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Hu, L. T. & Bentler, P. M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 6, 1–55 (1999).
Lê, S., Josse, J. & Husson, F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008).
R_Core_Team. R: A Language and Environment for Statistical Computing (R_Core_Team, 2023).
Smith, S. A. & Brown, J. W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018).
Orme, D. et al. The caper package: comparative analysis of phylogenetics and evolution in R. R. Package Version 5, 1–36 (2013).
Revell, L. J. phytools 2.0: an updated R ecosystem for phylogenetic comparative methods (and other things). PeerJ 12, e16505 (2024).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, 48 (2015).
Acknowledgements
We acknowledge the contributors to International Tree-Ring Data Bank for making available raw tree-ring data, and we thank staff at the Direction des Inventaires Forestiers of the Ministère des Ressources naturelles et des Forêts du Québec for sharing tree-ring and sample plot data from the forest inventory program in Quebec, Canada. We further thank Ailene Ettinger, Gregory Peterson, Janneke Hille Ris Lambers, Jeremy Little, Jill Harvey, and Jordi Axelsons for contributing original data. This study was supported by the following grants; National Environmental Research Council grants NE/S008659/1 (R.B.), NE/N012542/1 (E.G.), and NE/R005079/1 (E.G., R.S.); FAPESP grants 12/50457-4, 2019/08783-0 (G.L., G.C.) and 17/5008-3 (G.L., G.C.); Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, grants 478503/2009 (G.L., G.C.), 311247/2021-0 (J.S.) and 441811/2020-5 (J.S.); CNPq/ FAPEAM, Fundação de Amparo à Pesquisa do Estado do Amazonas, grant number 01.02.016301.02630/2022-76 (J.S.); Czech Science Foundation research grants 24-12210 K (J.P. and M.S.) and 23-05272S (J.A., J.D., K.K., N.A., P.F., V.B.); Mobility Plus between the Czech Republic and Taiwan, NSTC-24-08 (J.A., J.D., K.K., N.A., P.F., V.B.); Czech Academy of Sciences long-term research development project No. RVO 67985939 (J.A., J.D., K.K., N.A., P.F., V.B.); Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number 9803 (R.J.D.); Academy of Finland, #339788 (S.H.); European Union, NextGenerationEU, Italian Ministry of University and Research under PNRR - M4C2-I1.4 Project code: CN00000033, Title: NBFC - National Biodiversity Future Center, CUP: J83C22000860007 (G.P.); Ministry of University and Research (MUR) via the Agritech National Research Centre, European Union Next-GenerationEU PNRR M4C2-I1.4 Project Code: CN00000022 (A.D.); Departments of Excellence (Law 232/2016) Project 2023-27 “Digital, Intelligent, Green and Sustainable (D.I.Ver.So)” (A.D.); National Science Foundation, Division of Environmental Biology, award #1945910 (N.P.); Directorate for Biological Sciences, Emerging Frontiers, award #1241870 (N.P.); Redes Federales de Alto Impacto, Bosque-Clima CN32 (L.L., R.V.); MSMT INTER-EXCELLENCE, # LUAUS24258 (J.D.), Estonian Research Council, grant PSG1044 (J.A.).
Author information
Authors and Affiliations
Contributions
R.B., G.L., S.K., E.G., and R.W. designed the study, R.B., R.W. and S.K. downloaded and compiled functional traits and ITRDB datasets, R.B., R.W. and S.K. analysed data, G.L. and S.K. compiled the tropical longevity datasets, M.M., D.B., R.S. and P.R. provided functional traits data, R.B., G.L., S.K., S.V., C.E., G.P. and N.P. revised and improved the longevity database, R.B., G.L., S.V., J.A., N.A., L.A., M.B., V.B., B.B., P.B., G.C., J.dR., J.V.D., A.D., J.D., L.D., C.E., P.F., H.G., S.H., S.K.l., K.K., D.L., S.L., L.L., T.N., J.P., N.P., G.P., C.R., D.S., J.S., J.D.S., D.S., M.S., R.V., L.W., and C.Z. contributed original longevity data, R.B. wrote the first draft of the manuscript and all authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Christian Korner, Ricardo Segovia, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brienen, R.J.W., Locosselli, G.M., Krottenthaler, S. et al. Contrasting pathways to tree longevity in gymnosperms and angiosperms. Nat Commun 17, 898 (2026). https://doi.org/10.1038/s41467-025-67619-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-67619-2