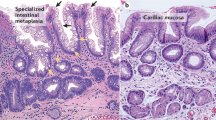
Abstract
The molecular underpinnings contributing to the onset of Barrett’s esophagus (BE) remain elusive. By studying familial clusters of the disease, here we identify a significant association between genetic variants in the V-set and Immunoglobulin Domain Containing 10 Like (VSIG10L) gene and BE predisposition. Using mammalian tissues and patient-derived organoids, we show VSIG10L is selectively expressed in the suprabasal squamous cells of the esophageal mucosa and is essential for epithelial maturation and homeostasis. Mice carrying human-orthologous germline mutations in Vsig10l exhibit loss of desmosomes, concomitant with disrupted epithelial differentiation programs, in the squamous mucosa. Upon long-term exposure to a bile acid (deoxycholate) supplemented diet, Vsig10l-mutant mice develop overt BE-like lesions in the forestomach. Furthermore, loss of esophageal VSIG10L expression is observed frequently in patients with chronic gastroesophageal reflux disease, a known risk factor for BE. Collectively, our study uncovers a fundamental link between VSIG10L, esophageal homeostasis, and BE predisposition.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the manuscript. Respective publicly-accessible databases (such as gnomAD and ExAC) were cited within the main text. Sharing of VSIG10L raw DNA sequencing data from familial subjects will be considered following formal written request by investigators to the corresponding author. All requests pertaining to the data in the current study will be processed in 3-5 weeks from the date of request. All data access requests will undergo in-depth review by University Hospitals Cleveland Medical Center Institutional Review Board (and by a genetic counselor) to ensure that distribution complies with ethical, institutional, and material-transfer guidelines, while ensuring strict confidentiality of the familial relationships. Source data are provided with this paper.
References
Stawinski, P. M., Dziadkowiec, K. N., Kuo, L. A., Echavarria, J., & Saligram, S. Barrett’s esophagus: an updated review. Diagnostics 13, 321 (2023).
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H. & Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 75, 10–45 (2025).
Rubenstein, J. H. & Shaheen, N. J. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 149, 302–317 e301 (2015).
Shaheen, N. J., Falk, G. W., Iyer, P. G. & Gerson, L. B. American College of G. ACG Clinical Guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 111, 30–50; quiz 51 (2016).
American Gastroenterological, A., Spechler, S. J., Sharma, P., Souza, R. F., Inadomi, J. M. & Shaheen, N. J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 140, 1084–1091 (2011).
Spechler, S. J. Clinical practice. Barrett’s Esophagus. N. Engl. J. Med. 346, 836–842 (2002).
Que, J., Garman, K. S., Souza, R. F. & Spechler, S. J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 157, 349–364 e341 (2019).
Chak, A. et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 51, 323–328 (2002).
Chak, A. et al. Identification of Barrett’s esophagus in relatives by endoscopic screening. Am. J. Gastroenterol. 99, 2107–2114 (2004).
Juhasz, A. et al. Prevalence of Barrett esophagus in first-degree relatives of patients with esophageal adenocarcinoma. J. Clin. Gastroenterol. 45, 867–871 (2011).
Sun, X. et al. Linkage and related analyses of Barrett’s esophagus and its associated adenocarcinomas. Mol. Genet Genom. Med 4, 407–419 (2016).
Sun, X. et al. Predicting Barrett’s esophagus in families: an esophagus translational research network (BETRNet) model fitting clinical data to a familial paradigm. Cancer Epidemiol. Biomark. Prev. 25, 727–735 (2016).
Sun, X. et al. A segregation analysis of Barrett’s esophagus and associated adenocarcinomas. Cancer Epidemiol. Biomark. Prev. 19, 666–674 (2010).
Fecteau, R. E. et al. Association between germline mutation in VSIG10L and familial barrett neoplasia. JAMA Oncol. 2, 1333–1339 (2016).
Chak, A. et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol. Biomark. Prev. 15, 1668–1673 (2006).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Harada, H. et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol. Cancer Res 1, 729–738 (2003).
Venkitachalam, S. et al. The ephrin B2 receptor tyrosine kinase is a regulator of proto-oncogene MYC and molecular programs central to Barrett’s neoplasia. Gastroenterology 163, 1228–1241 (2022).
Kowalczyk, A. P. & Green, K. J. Structure, function, and regulation of desmosomes. Prog. Mol. Biol. Transl. Sci. 116, 95–118 (2013).
Green, K. J. & Simpson, C. L. Desmosomes: new perspectives on a classic. J. Investig. Dermatol. 127, 2499–2515 (2007).
Dimitrova, N. et al. InFlo: a novel systems biology framework identifies cAMP-CREB1 axis as a key modulator of platinum resistance in ovarian cancer. Oncogene 36, 2472–2482 (2017).
Blum, A. E. et al. Systems biology analyses show hyperactivation of transforming growth factor-beta and JNK signaling pathways in esophageal cancer. Gastroenterology 156, 1761–1774 (2019).
Maslenkina K. et al. Signaling pathways in the pathogenesis of Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Mol. Sci. 24, 9304 (2023).
Fitzgerald, R. C. Molecular basis of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 55, 1810–1820 (2006).
Tobey, N. A., Carson, J. L., Alkiek, R. A. & Orlando, R. C. Dilated intercellular spaces: a morphological feature of acid reflux–damaged human esophageal epithelium. Gastroenterology 111, 1200–1205 (1996).
Souza, R. F. From reflux esophagitis to esophageal adenocarcinoma. Dig. Dis. 34, 483–490 (2016).
Masaoka, T. & Suzuki, H. Does bile reflux influence the progression of Barrett’s esophagus to adenocarcinoma? (Gastroenterology 2013;145:1300-1311). J. Neurogastroenterol. Motil. 20, 124–126 (2014).
Quante, M., Abrams, J. A., Lee, Y. & Wang, T. C. Barrett esophagus: what a mouse model can teach us about human disease. Cell Cycle 11, 4328–4338 (2012).
Prichard, D. O. et al. Deoxycholic acid promotes development of gastroesophageal reflux disease and Barrett’s oesophagus by modulating integrin-alphav trafficking. J. Cell Mol. Med 21, 3612–3625 (2017).
Morales, T. G. & Sampliner, R. E. Barrett’s esophagus: update on screening, surveillance, and treatment. Arch. Intern Med 159, 1411–1416 (1999).
Chen, Y. Y. et al. Significance of acid-mucin-positive nongoblet columnar cells in the distal esophagus and gastroesophageal junction. Hum. Pathol. 30, 1488–1495 (1999).
Goldblum, J. R. Barrett’s esophagus and Barrett’s-related dysplasia. Mod. Pathol. 16, 316–324 (2003).
Niv, Y. & Fass, R. The role of mucin in GERD and its complications. Nat. Rev. Gastroenterol. Hepatol. 9, 55–59 (2011).
Arul, G. S. et al. Mucin gene expression in Barrett’s oesophagus: an in situ hybridisation and immunohistochemical study. Gut 47, 753–761 (2000).
Warson, C. et al. Barrett’s esophagus is characterized by expression of gastric-type mucins (MUC5AC, MUC6) and TFF peptides (TFF1 and TFF2), but the risk of carcinoma development may be indicated by the intestinal-type mucin, MUC2. Hum. Pathol. 33, 660–668 (2002).
Paterson, A. L., Gehrung, M., Fitzgerald, R. C. & O’Donovan, M. Role of TFF3 as an adjunct in the diagnosis of Barrett’s esophagus using a minimally invasive esophageal sampling device-the cytosponge(TM). Diagn. Cytopathol. 48, 253–264 (2020).
Dunn, L. J., Jankowski, J. A. & Griffin, S. M. Trefoil factor expression in a human model of the early stages of Barrett’s esophagus. Dig. Dis. Sci. 60, 1187–1194 (2015).
Clemons, N. J. et al. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett’s esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1335–G1346 (2012).
Garman, K. S. Origin of Barrett’s epithelium: esophageal submucosal glands. Cell Mol. Gastroenterol. Hepatol. 4, 153–156 (2017).
Rhee, H. & Wang, D. H. Cellular origins of Barrett’s esophagus: the search continues. Curr. Gastroenterol. Rep. 20, 51 (2018).
Palles, C. et al. Polymorphisms near TBX5 and GDF7 are associated with increased risk for Barrett’s esophagus. Gastroenterology 148, 367–378 (2015).
Levine, D. M. et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nat. Genet. 45, 1487–1493 (2013).
Martinez-Uribe, O., Becker, T. C. & Garman, K. S. Promises and limitations of current models for understanding Barrett’s esophagus and esophageal adenocarcinoma. Cell Mol. Gastroenterol. Hepatol. 17, 1025–1038 (2024).
Kapoor, H., Lohani, K. R., Lee, T. H., Agrawal, D. K. & Mittal, S. K. Animal models of Barrett’s esophagus and esophageal adenocarcinoma-past, present, and future. Clin. Transl. Sci. 8, 841–847 (2015).
Macke, R. A. et al. Barrett’s esophagus and animal models. Ann. N. Y. Acad. Sci. 1232, 392–400 (2011).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Jiang, M. et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533 (2017).
Garman, K. S. et al. Genetic Defect in Submucosal Gland-Associated Caveolin-3: A New Paradigm in Esophageal Adenocarcinoma Risk. Gastroenterology 165, 1561–1564.e3 (2023).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18, e3000410 (2020).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20, 1297–1303 (2010).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Poplin, R. et al. Scaling accurate genetic variant discovery to tens of thousands of samples. bioRxiv 201178 (2018).
Ramos, A. H. et al. Oncotator: cancer variant annotation tool. Hum. Mutat. 36, E2423–E2429 (2015).
Karczewski, K. J. et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res 45, D840–D845 (2017).
Gudmundsson, S. et al. Variant interpretation using population databases: lessons from gnomAD. Hum. Mutat. 43, 1012–1030 (2022).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Kruger, L. et al. Ductular and proliferative response of esophageal submucosal glands in a porcine model of esophageal injury and repair. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G180–G191 (2017).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Drovdlic, C. M. et al. Demographic and phenotypic features of 70 families segregating Barrett’s oesophagus and oesophageal adenocarcinoma. J. Med. Genet. 40, 651–656 (2003).
Kalabis, J. et al. Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat. Protoc. 7, 235–246 (2012).
Ravillah, D. et al. Discovery and initial characterization of long intergenic noncoding RNAs associated with esophageal adenocarcinoma. Gastroenterology 165, 505–508.e507 (2023).
Fujioka, H., Tandler, B., Cohen, M., Koontz, D. & Hoppel, C. L. Multiple mitochondrial alterations in a case of myopathy. Ultrastruct. Pathol. 38, 204–210 (2014).
Fujioka, H. et al. Multiple muscle cell alterations in a case of encephalomyopathy. Ultrastruct. Pathol. 38, 13–25 (2014).
Jacob, A. et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488.e410 (2017).
Zhang, Y. et al. 3D modeling of esophageal development using human PSC-derived basal progenitors reveals a critical role for notch signaling. Cell Stem Cell 23, 516–529.e515 (2018).
Abbas, G., Pennathur, A., Keeley, S. B., Landreneau, R. J. & Luketich, J. D. Laser ablation therapies for Barrett’s esophagus. Semin Thorac. Cardiovasc. Surg. 17, 313–319 (2005).
Locke, G. R., Talley, N. J., Weaver, A. L. & Zinsmeister, A. R. A new questionnaire for gastroesophageal reflux disease. Mayo Clin. Proc. 69, 539–547 (1994).
Acknowledgements
This research was supported by Public Health Service (PHS) awards: P01 CA269019 (A.C., K.S.G., S.G.G., J.E.W., and K.G.); Case GI SPORE P50 CA150964 (K.G.), P30 CA043703 (K.G.); R01 DK118022 (K.S.G.); P30 DK034987 (K.S.G.); P30 CA014236 (S.G.G.); P30 DK097948 (A.C.); U01 CA271867 (A.C. and J.E.W.); The DeGregorio Family Foundation (K.G.), Torrey Coast Foundation GEMINI Network (K.G.), and The Gastric Cancer Foundation (K.G.). This work was also supported by the Animal Resource Center, Transgenic and Targeting core, Tissue Resources Core at the Case Western Reserve University, Molecular Genomics Core at Duke University, and Duke’s BioRepository & Precision Pathology Center (BRPC). We are grateful to the patients who opt to participate in our research. We thank our co-author, late Dr. Fujioka, who passed away recently, for his expert advice and help with electron microscopy studies that served as the foundation for understanding the biology of VSIG10L. We thank late Dr. Nathan A. Berger at Case Western Reserve University, who passed away recently, for expert scientific guidance and advice during the conceptualization phase of this study. We thank late Ms. Anne Baskin at Case Western Reserve University, who also passed away recently, for her technical assistance in animal husbandry. We thank Ms. Lakshmeswari Ravi for her technical input on the experimental methodologies used in this study. We thank the clinical gastroenterology providers who collect research samples from enrolled participants under their clinical care, especially Dr. Rahul Shimpi and Dr. Michael Feiler, as well as the Gastroenterology Clinical Research Unit staff at Duke. We thank Thomas C. Becker for performing lab-based assays in the Garman Lab to support this project.
Author information
Authors and Affiliations
Contributions
Study concept and design (D.R., A.C., and K.G.); Acquisition of data (D.R., A.M.K., B.U., R.G., W.B., Y.M., V.J., E.H., K.S.G., S.G.G., J.T.G., H.K., A.C., and K.G.); Analysis and interpretation of data (D.R., S.S., R.M.K., V.J., S.G.G., J.T.G., H.K., J.E.W., A.C., and K.G.); Drafting of the manuscript (D.R.); Critical revision of the manuscript (A.C. and K.G.); Statistical analysis (R.M.K.); Obtained funding (A.C. and K.G.); Administrative, technical, or material support (W.B., R.G., A.C., and K.G.); Study supervision (K.G.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ravillah, D., Singh, S., Katabathula, R.M. et al. VSIG10L is a major determinant of esophageal homeostasis and inherited predisposition to Barrett’s esophagus. Nat Commun (2026). https://doi.org/10.1038/s41467-026-68975-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-68975-3