Abstract
The escalating environmental changes have exacerbated the physiological suffering and economic burden caused by allergic diseases worldwide. Bifidobacterium bifidum (B. bifidum), due to its positive regulatory effects on the gut microenvironment, is considered a promising strategy for the prevention and treatment of allergies. Our study utilized the ovalbumin (OVA)-induced allergy mouse model to explore the anti-allergic potential of the self-screened strain B. bifidum FB3-12 (FB3-12) via intestinal intervention. The results demonstrated that a three-week FB3-12 treatment effectively suppressed the levels of immune markers, including OVA-specific immunoglobulin E (OVA-sIgE), mast cell protease-1 (Mcpt-1), and histamine (HIS) in the serum of allergic mice, and restored the Th2/Th1 immune response imbalance in the spleen. Furthermore, compared to the OVA group, FB3-12 intervention ameliorated intestinal damage and inflammation, significantly increasing the relative expression of Mucin-2 and tight junction proteins. Analysis of the gut microbiota profile revealed a distinct shift in the enterotype of OVA-challenged mice, characterized by a decreased Firmicutes/Bacteroidetes (F/B) ratio and a marked increase in the relative abundance of Akkermansia. FB3-12 intervention significantly enriched beneficial genera such as Lactobacillus and Colidextribacter, and upregulated the levels of short-chain fatty acids (SCFAs) and associated G protein-coupled receptors (GPRs) in the intestine. These findings underscore the therapeutic potential and application value of FB3-12 in alleviating allergic diseases through modulation of the gut microbiome.

Similar content being viewed by others
Introduction
According to the World Health Organization (WHO), over 10% of the global population is currently afflicted with allergic diseases, and the number of affected individuals continues to rise rapidly1,2. Allergic diseases have become one of the most prevalent chronic conditions and a significant global health concern. Allergy is a rapid, abnormal response triggered by re-exposure to harmless substances (allergens), involving systemic immune system dysregulation and dysfunction, which can lead to a reduced quality of life and even an increased risk of mortality3. Against the backdrop of rapid societal development, in addition to genetic factors, complex environmental conditions, excessive hygiene practices, a trend toward monotonous high-sugar and high-fat diets, and high levels of psychological stress have all contributed to the exacerbation of allergic diseases4. Given the ubiquity of various inhalant, ingesting, and contact allergens that are difficult to avoid in daily life completely, and the risk of relapse after discontinuation of allergen-specific immunotherapy, there is an urgent need to explore novel anti-allergic therapies that can alleviate allergic symptoms effectively5,6,7.
Numerous studies have revealed that individuals with allergies exhibit distinct gut microbial profiles compared to healthy populations, characterized primarily by a significant reduction in the context of a diverse community of beneficial bacteria such as Lactobacillus and Bifidobacterium, alongside an increase in Proteobacteria8,9,10. Concurrently, the microbiota-gut-immune axis has been emphasized in recent research, suggesting that the restoration of gut microbial dysbiosis may be closely associated with the alleviation of various diseases, including allergic disorders11,12,13. For instance, dietary intervention with a formula with Lactobacillus rhamnosus LGG supplementation was shown to facilitate the development of tolerance in infants suffering from cow’s milk allergy (CMA)14. Bifidobacterium infantis has been demonstrated to alleviate shrimp tropomyosin-induced allergy through the induction of regulatory T cells and modulation of gut microbiota via tolerance-dependent dendritic cells15. Research by Shi et al. indicated that Bifidobacterium lactis Probio-M8 mitigated food allergy (FA) by regulating the Th1/Th2 response and modulating gut microbiota16. These studies suggested that probiotic interventions aimed at promoting gut homeostasis could be considered an effective strategy for alleviating exacerbated allergic symptoms.
Clinical trials and experimental studies have provided preliminary evidence for Bifidobacterium bifidum’s (B. bifidum) anti-allergic properties. Wei et al. demonstrated that long-term intervention (6 months) with B. bifidum TMC3115 significantly reduced clinical allergy scores and serum immunoglobulin levels in infants with cow’s milk protein allergy (CMPA), concurrently improving gut microbiota composition through increased probiotic genera abundance and decreased pathobiont prevalence17. Complementary murine research by Kim et al. revealed that B. bifidum BGN4 administration suppressed mast cell degranulation and attenuated dermatitis severity scores18. Besides, Helene et al. demonstrated that B. bifidum NCC453 significantly diminished airway hyperresponsiveness (AHR), pulmonary inflammation, and Bet v 1-specific Th2 responses in birch pollen-sensitized BALB/c mice19. While these studies establish the therapeutic potential of specific B. bifidum strains in ameliorating allergic diseases, the anti-allergic efficacy of B. bifidum intervention through the gut requires further validation; the underlying molecular mechanisms remain to be fully elucidated, and the investigation of host–microbiota–immune crosstalk is still insufficient.
In this study, we validated the potential anti-allergic effects of B. bifidum FB3-12 treatment via the gut in an ovalbumin (OVA)-induced mouse model of allergy. We further investigated the probiotic benefits of the commensal strain FB3-12, which are mediated through the reinforcement of intestinal barrier function and the optimization of gut microbiota composition and metabolites, such as short-chain fatty acids (SCFAs). Our findings highlight the efficacy of the probiotic strain FB3-12 in attenuating allergic responses and aim to provide novel theoretical evidence for its application in the prevention and treatment of allergic diseases.
Results
FB3-12 mitigates OVA-induced allergy symptoms in mice
To investigate the impact of B. bifidum FB3-12 on allergic diseases, we employed the commonly used allergen ovalbumin (OVA) and a three-time sensitization protocol to establish an allergic mouse model. Subsequently, an intervention with a dose of 109 CFU/d of FB-13 was administered to the sensitized mice, as illustrated in Fig. 1A. Throughout the intervention period, we monitored the body weight changes of three groups of mice. Notably, pronounced differences in body weight were observed between the CON group and the FB3-12 group compared to the OVA group, starting from day 21 and day 28, respectively (Fig. 1B). Upon completion of the intervention, the OVA group exhibited a significantly higher increase both in body weight and spleen weight (Fig. 1C, D). The spleen index, as shown in Fig. 1E, showed a marked elevation, indicating an enhanced immune response in the allergic mice. Correspondingly, the OVA-induced allergy caused physiological and behavioral alterations in the OVA group mice, including body swelling, ear scratching, prolonged immobility, and mild diarrhea. However, the FB3-12 intervention effectively ameliorated these allergic symptoms (Fig. 1F).
A Schematic overview of the Balb/c mice intervention experiment design. B Body weight in the intervention period. C Weight gain. D Spleen weight. E Spleen index (spleen index = spleen weight/body weight). F Allergy symptom scores. Statistical analysis was performed by the Kruskal–Wallis test. Data are shown as the mean ± SD (n = 6).
Effect of FB3-12 intervention on allergy-related immune markers in mice
We subsequently aimed to determine the impact of the OVA challenge on the levels of peripheral allergy-related indicators. As illustrated in Fig. 2A–C, compared with the unstimulated CON group, the levels of OVA-sIgE, Mcpt-1, and HIS in the serum of OVA group mice were significantly elevated, which may account for the pruritus experienced by the mice. However, a 3-week intervention with FB3-12 restored these serum markers to normal levels. Additionally, given that the serum levels of IgG1 and the IgG1/IgG2a ratio were significantly elevated in the OVA group mice than in the other two groups (Fig. 2D–F), which are associated with Th1/Th2 cell responses, we further quantified key transcription factors and cytokines in the mice spleen. It was found that FB3-12 intervention significantly reduced the levels of GATA3 and IL-4 while increasing the levels of T-bet compared to the OVA group (Fig. 2G, H). These findings suggest that the FB3-12 intervention ameliorated the allergy-relevant immune indicators in allergic mice, potentially exerting its anti-allergic effects primarily through modulating Th2-type immune responses.
A–E Serum concentrations of OVA-specific immunoglobulin E (OVA-sIgE), mast cell protease-1 (Mcpt-1), histamine (HIS), immunoglobulin G1 (IgG1), and IgG2a. F IgG1/ IgG2a. G, H Relative mRNA expression of key transcription factors and related cytokines of Th2 and Th1 cells in the spleens. Data are shown as the mean ± SD (n = 6).
FB3-12 alleviates intestinal barrier dysfunction and inflammation in mice
The impairment of intestinal barrier function renders the submucosal immune system more susceptible to allergen exposure. To evaluate the effect of FB3-12 on intestinal barrier integrity, H&E staining was initially employed to reveal the histopathological conditions. As shown in Fig. 3A, FB3-12 intervention was observed to significantly ameliorate the aggregation of lymphocytes and crypt structural alterations in the colon of allergic mice, resulting in a lower histological score (Fig. 3B). Figure 3C displays the goblet cell mucins stained purple-red by PAS, which were markedly reduced in the colon of OVA-treated mice. However, FB3-12 intervention resulted in a marked increase in goblet cell count in the crypts as well as the expression level of Mucin-2 (Fig. 3D, E). The relative mRNA expression levels of intestinal tight junction proteins, including ZO-1, Occludin, and Claudin-1, were significantly elevated in the FB3-12 group compared with the OVA group (Fig. 3F). Additionally, after a three-week intervention with FB3-12, the levels of pro-inflammatory cytokines IL-6 and IL-1β and the antimicrobial lectin RegⅢ-γ were significantly improved in allergic mice (Fig. 3G, H). Collectively, these results demonstrate that FB3-12 exerts a notable effect on enhancing colonic barrier function and suppressing inflammatory levels in OVA-induced allergic mice.
A Representative photomicrograph of hematoxylin and eosin (H&E)-stained colon sections. B Histology score of the colon. Statistical analysis was performed by the Kruskal–Wallis test. C Representative photomicrographs of periodic acid-schiff (PAS)-stained colon sections. D Goblet cell count. E, F Relative mRNA expression of Mucin-2 and tight junction proteins. G Relative mRNA expression of IL-1, IL-6, and TNF-α. H Relative mRNA expression of Reg Ⅲ-γ. Data are shown as the mean ± SD (n = 6).
FB3-12 modulates the gut microbiota structure of allergy mice
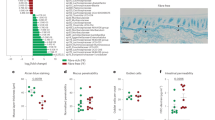
To further explore the probiotic benefits of FB3-12 intervention in the gut microenvironment of allergic mice, we utilized 16S rRNA sequencing to characterize the structural differences in the gut microbiota among different groups of mice. As shown in the Venn diagram in Fig. 4A, high-quality sequences clustered at a 97% similarity threshold indicated that the FB3-12 group possessed the highest number of operational taxonomic units (OTUs). Additionally, FB3-12 reversed the decline in the Simpson index, a measure of α-diversity, caused by OVA sensitization and partially restored the structural shifts in the gut microbiota of allergic mice, as demonstrated by principal coordinates analysis (PCoA) (Fig. 4B, C). In addition, Chao1 and Shannon indices provided a more comprehensive view of the α-diversity, further reinforcing the conclusion that FB3-12 selectively modulates gut microbiota composition in OVA-challenged mice (Fig. S1A). To clarify the microbial composition, we presented the top 10 annotated phyla and top 15 genera in terms of relative abundance using bar charts (Fig. 4D, E). The two dominant phyla, Firmicutes and Bacteroidetes, exhibited significant differences among the three groups of mice, with FB3-12 intervention significantly increasing the abundance of Firmicutes and the Firmicutes/Bacteroidetes (F/B) ratio (Fig. 4F). Moreover, at the phylum level, FB3-12 suppressed the enrichment of Verrucomicrobiota, which was significantly elevated due to OVA sensitization (Fig. 4G). More specifically, LEfSe analysis revealed that the genus Akkermansia (Verrucomicrobiota) was significantly enriched in the OVA group, while the genera Lactobacillus (Firmicutes) and Colidextribacter (Firmicutes) were enriched in the FB3-12 intervention group (Fig. 4H). In addition, we observed that FB3-12 promoted the abundance of Oscillospiraceae (Firmicutes), Ruminococcaceae (Firmicutes), and Bifidobacterium (Actinobacteriota) (Fig. S1B), and inhibited Enterorhabdus (Actinobacteria) (Fig. 4I). Together, our data show that FB3-12 intervention reshaped the gut microbiota ecology in allergic mice.
A Venn diagrams. B Alpha diversity was presented as the ACE and Simpson index. C PCoA on the OUT level. D Top 10 annotated phyla. E Top 15 annotated genera. F, G Phylum-level relative abundance of microbial taxa with significant differences. H LEfSe analysis. I Genus-level relative abundance of microbial taxa with significant differences. Data are shown as the mean ± SD (n = 6).
Effect of FB3-12 on microbial SCFA metabolism in allergic mice
To assess the impact of alterations in gut microbiota composition on their functional capacities, we quantified the levels of SCFAs in the feces of mice from each group using high-performance gas chromatography. As depicted in Fig. 5A, FB3-12 intervention significantly elevated the concentrations of propionate and butyrate in the mice’s gut compared with the other two groups. Furthermore, the OVA challenge led to a decrease in the relative mRNA expression levels of SCFA-related receptors GPR41 and GPR43 in the colons of allergic mice. However, this reduction was significantly ameliorated by a 3-week continuous intervention with FB3-12, while no significant differences were detected in the levels of GPR109A among different treatments (Fig. 5B). Therefore, the FB3-12 intervention not only remodeled the gut microbiota composition in allergic mice, but also correlated with increased fecal SCFA concentrations and intestinal GPR expression, implying enhanced microbial metabolic activity.
Correlations between gut microbiota and allergic parameters
To identify specific bacterial genera that may modulate allergic immune responses within the altered microbiota, a Spearman correlation analysis was conducted to examine the associations between taxonomic abundance and allergy-related indicators (Fig. 6). The genera Enterorhabdus and Muribaculaceae showed a significant positive relation to indicators associated with the development of allergic diseases. Conversely, Lactobacillus and Ligilactobacillus exhibited substantial positive correlations with indicators related to the recovery of immune responses in allergic mice, suggesting their potential beneficial roles in mediating the alleviation of OVA-induced allergic diseases.
Discussion
The unavoidable exposure to allergens and the resultant spectrum of adverse symptoms (e.g., edema, dyspnea, pruritus, and diarrhea) pose significant challenges for allergy sufferers worldwide, with rapidly changing environmental conditions further exacerbating the issue20. Previous observational studies have revealed distinct gut microbiota compositions in allergic individuals compared to healthy populations, and early-life microbial colonization dominated by Bifidobacterium has been shown to reduce the risk of allergic diseases significantly21,22. Probiotics with strong colonization capabilities were demonstrated to enhance the expression of tight junction proteins in intestinal epithelial cells (IECs) and modulate inflammatory signaling pathways, such as nuclear factor-κB (NF-κB)23,24. Additionally, probiotics and their metabolites can directly influence immune homeostasis by regulating the responses of submucosal immune cells25. Consequently, probiotic intervention is considered an effective strategy for modulating the gut microenvironment to mitigate the progression of allergic diseases. B.bifidum FB3-12, utilized in our study, was isolated from the feces of a healthy breastfed infant with a secretory (Se+) mother. Given that extensive research evidence consistently demonstrates a strong correlation among high human milk oligosaccharide (HMO) intake, bifidobacterial colonization dominance, and reduced allergy risk11,26, we selected this multi-HMO-utilizing probiotic strain as a distinctive candidate for allergy intervention.
In susceptible individuals, the allergen OVA is captured and processed by antigen-presenting cells (APCs) and subsequently presented to T cells, thereby activating a Th2-biased immune response. This process triggers a type I hypersensitivity reaction mediated by specific IgE, leading to the release of inflammatory mediators such as HIS from mast cells27,28. Consequently, following the final challenge, allergic mice exhibited pronounced allergic symptoms, including systemic edema and scratching behaviors. We further observed that the OVA challenge induced significant dysregulation of peripheral immune markers in mice, characterized by an imbalance in immunoglobulin secretion, heightened activity of Mcpt-1, and excessive HIS expression. However, a 3-week intervention with FB3-12 (1 × 109 CFU/mouse/day) effectively suppressed these allergic physiological changes, providing preliminary evidence of FB3-12’s anti-allergic potential. Furthermore, by assessing key transcription factors and cytokines of Th1 and Th2 cells in the spleen, the largest immune organ in mice, we found that FB3-12 intervention significantly alleviated the Th2/Th1 imbalance, thereby inhibiting the elevated IgG1/IgG2a ratio in serum expressed by B cells. Besides live bacteria, Meng Luo et al. previously reported that the exopolysaccharide (EPS) of Bifidobacterium breve WBBR04 could enhance intestinal barrier integrity by adhering to the intestinal lining and restoring serum IgE and IgG levels29.
Evidence has suggested that the gut microbiota exerts a pivotal influence in regulating intestinal barrier function and shaping immune maturation and tolerance. Disruption of the stability of the gut microbiota may further lead to dysregulation of barrier integrity and the submucosal immune system, potentially preceding the development of allergic diseases30,31. Given that the strict anaerobe B. bifidum predominantly colonizes and enriches in the colon, our study primarily investigated the effects of FB3-12 on the colonic microenvironment in OVA-induced allergic mice. Histopathological evaluation of mouse colon sections intuitively revealed that FB3-12 intervention reduced inflammatory cell infiltration, restored intestinal villi damage, and led to a significant rise in the number of goblet cells in the crypts. Additionally, the expression levels of Mucin-2 and tight junction proteins were markedly elevated in the colons of probiotic-treated mice. Previous studies have shown that TJ proteins form a complex network of transmembrane and scaffolding proteins that directly dictate paracellular permeability32. The alterations, including defective tight junction barrier, reduced mucin levels, and aberrant antimicrobial peptide changes, caused by OVA-sensitization may contribute to leak-flux diarrhea and heightened antigen translocation. The translocated antigens into the lamina propria trigger inflammation involving circulating and resident immune cells33. Consequently, we observed significant upregulation of pro-inflammatory cytokines in the colonic mucosa, which was significantly ameliorated by FB3-12 intervention. The reduction in intestinal permeability helps limit the entry of allergens into the circulatory system, thereby mitigating excessive immune responses and reducing the risk of inflammation, particularly in the context of FA34. For instance, compounds such as fucoxanthin, β-carotene, and oleuropein have been shown to alleviate OVA-induced FA by enhancing intestinal epithelial barrier function35,36,37. These findings suggested that FB3-12 may modulate susceptibility to allergens, thereby alleviating allergic symptoms by promoting intestinal barrier integrity.
It is well-established that allergic diseases are often accompanied by alterations in enterotypes38. In our study, a reduction in the F/B ratio was observed in the intestines of mice following OVA sensitization, a phenomenon also reported in previous studies utilizing the OVA-induced allergy model39,40. FB3-12 intervention significantly increased the abundance of several genera belonging to the phylum Firmicutes, promoting a more stable gut microbiota profile in mice. Interestingly, we found that FB3-12 treatment markedly enhanced the proliferation of Lactobacillus, providing new evidence for cross-feeding interactions between Bifidobacterium and Lactobacillus. This stable symbiotic relationship and evolutionary mechanism hold significant implications for gut health and immune regulation41. Moreover, studies have shown that Lactobacillus loses its dominant position in the gut microbiota of piglets following soybean allergy, and the anti-allergic properties of Lactobacillus have been repeatedly documented42,43. Nishiyama et al. revealed that B. bifidum leverages extracellular sialidase (SiaBb2), which degrades HMOs and mucins, to enhance mucosal adhesion and colonization competitiveness44. Bifidobacteria use their enzymes and oligosaccharide transport systems to consume host glycans, establishing stable gut colonization and facilitating cross-feeding interactions with other commensals45. For instance, Nogacka et al. investigated the 2′-fucosyllactose (2′-FL)-based metabolic interactions between B. bifidum IPLA20048 and Lactobacillus gasseri (L. gasseri) IPLA20136. They found that B. bifidum IPLA20048, through cross-feeding on extracellularly degraded products such as galactose, fucose, and lactose from 2′-FL, promoted the proliferation of L. gasseri IPLA20136. Moreover, the research by Frédéric Moens et al. demonstrated that under inulin-type fructan (ITF) conditions, the proliferation of butyrate-producing bacterial strains relies on the cross-feeding of monosaccharides and lactate from an ITF-degrading Lactobacillus strain and acetate produced by a Bifidobacterium strain46. Correspondingly, the genera Ruminococcus, Colidextribacter, and Oscillospiraceae, which have been reported to contribute to increased levels of SCFAs and positively influence immune homeostasis47,48,49, were significantly upregulated by FB3-12 intervention.
The optimized intestinal microbiota significantly increased the levels of the beneficial metabolite SCFAs in the allergic mice, collectively contributed by FB3-12 itself and the enriched commensals. SCFAs have been demonstrated to enter systemic circulation and modulate distant immune response by inhibiting histone deacetylase (HDAC) and/or activating GPRs, which upregulate tight junction protein expression and modulate immune homeostasis50,51,52. Among them, it has been recently reported that microbiota-derived butyrate can alleviate food allergy by repairing intestinal barrier integrity by suppressing ROS/Notch pathway53, and mitigate allergic disease asthma through inhibiting Tfh13-mediated IgE production54. Furthermore, while Akkermansia is widely reported for its health-promoting properties16,55, evidence reveals context-dependent detrimental effects in specific gastrointestinal environments56. Consistent with our findings, Shi et al. and Yan et al. reported that Akkermansia was enriched in OVA-challenged allergic mice and declined after prebiotic or probiotic intervention16,40. Parrish et al. demonstrated that under fiber-deprived conditions, the upregulation of A. muciniphila abundance and mucin-degrading activity potentially exacerbates food allergy through amplification of innate type 2 immune responses and oral tolerance disruption57. These studies indicate that the health impact of Akkermansia is context-dependent and can be detrimental in food allergy when the gut microenvironment is dysregulated and disrupted.
Recent studies have identified B. bifidum as a bacterial genus enriched in the gut microbiota of healthy individuals and long-lived elderly populations. It has been explored to improve dextran sulfate sodium (DSS)-induced colitis, alleviate estrogen deficiency-induced osteoporosis, prevent non-alcoholic steatohepatitis (NASH), and complement immune checkpoint inhibitors to mitigate tumor burden in mice58,59,60,61. However, research on the role of B. bifidum in mitigating allergic diseases still awaits further research. In our study, 3-week FB3-12 treatment significantly suppresses aberrant immune markers in the serum of OVA-induced allergic mice, including OVA-sIgE, HIS, Mcpt-1, and the IgG1/IgG2a ratio, while attenuating allergic symptoms. Our data demonstrate that B. bifidum FB3-12 intervention reverses OVA-induced microbiota dysbiosis and enriches beneficial commensals Lactobacillus and Colidextribacter. Furthermore, the restructured microbiota elevates SCFAs, activating GPRs expression, collectively upregulating tight junction proteins to reinforce intestinal barrier integrity, thereby restoring allergic immune tolerance and inhibiting Th2-biased immune responses in allergic mice. Our findings support the health benefits of B. bifidum FB3-12 in preventing allergic diseases and highlight its therapeutic potential and application value in targeting the gut microbiome to modulate systemic immune responses.
Methods
Bacterial culture
The Bifidobacterium bifidum FB3-12 strain (FB3-12) utilized in our study was obtained from the fecal sample of a healthy breastfed infant. It has undergone a safety evaluation and has been preserved at the Guangdong Microbial Culture Collection Center (GDMCC) in China (Accession Number: GDMCC No. 64013). FB3-12 was cultured anaerobically using modified MRS (de Man, Rogosa, and Sharpe) broth fortified with 0.05% (w/v) L-cysteine hydrochloride. After activation, the strain was subcultured to the third generation with a 2% inoculation rate. The strains were centrifuged at 6000 rpm for 5 min at 24 °C, washed twice with sterile PBS, and subsequently used for further experiments.
Animal experiments
Female Balb/c mice (6-week-old) from a specific pathogen-free (SPF) background were obtained from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed three per cage at the Laboratory Animal Center of Nankai University (Permission Number: SYKX 2019-0001). The mice were maintained under controlled conditions with a temperature of 22 ± 2 °C, relative humidity of 50 ± 15%, and a 12-h light/dark cycle. The mice were provided with a standard rodent diet and water ad libitum. The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Nankai University (Approval Number: 2022-SYDWLL-000163).
Following a 1-week acclimatization period, the mice were randomly divided into three groups (n = 6): the control group (CON), the ovalbumin (OVA)-induced allergic group (OVA), and the FB3-12 intervention group after sensitization (FB3-12). On days 0, 7, and 14, the mice were sensitized (i.p.) with OVA (Sigma Aldrich, America) at a final concentration of 0.25 μg/mL. For the CON group, the aforementioned treatment was replaced with 0.85% sterile saline. Following three consecutive weeks of oral gavage intervention with 1 × 109 CFU/mouse/day dosage of FB3-12, referring to previous studies62,63, the mice were challenged twice with concentrations of 0.25 μg/mL and 2.5 μg/mL, respectively, to enhance the immune response (Fig. 1A). The allergic symptoms of the mice were evaluated within 30 min post-challenge. The score criteria are detailed in Table S1. At the end of the experiment, after a 12-h fasting period, the mice were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) for blood collection. Subsequently, the mice were euthanized by cervical dislocation, and serum, spleen, and colon samples were collected for further analysis.
Serum biochemical assay
The levels of OVA-specific immunoglobulin E (OVA-sIgE), IgG1, IgG2a, mast cell protease-1 (Mcpt-1), and histamine (HIS) in mouse serum were measured using ELISA kits according to the manufacturer’s instructions provided by the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Histopathological observation
The distal colon tissues of the mice were collected and fixed in 4% paraformaldehyde for 24 h. Subsequently, the tissues underwent dehydration in a graded ethanol series, followed by paraffin embedding and sectioning (4 μm thick). The sections were then treated with hematoxylin and eosin (H&E) and Periodic Acid–Schiff (PAS) staining to facilitate morphological observation and pathological evaluation.
Quantitative Real-Time PCR (RT-qPCR)
Total mRNA was extracted from 100 to 200 mg of mouse spleen or colon tissue by homogenization and subsequently used as a template for reverse transcription to obtain cDNA. The expression levels of target genes were quantified using RT-qPCR. Specific primer sequences are provided in Table S2. Normalization of the raw data was performed using the 2−ΔΔCt method with β-actin as the internal reference gene64, and the relative mRNA expression levels in other groups were calculated with the CON group as the baseline.
Gut microbiota profiling
The methods employed in this study were adapted from our previous research65. Specifically, microbial DNA was extracted from mouse fecal samples, and the V3/V4 region of the 16S rRNA gene was targeted by universal primers for bacterial sequence amplification. Subsequent bioinformatics analyses, including species clustering, α-diversity analysis, principal coordinates analysis (PCoA), linear discriminant analysis effect size (LEfSe), and Spearman correlation analysis, were conducted on the NovoMagic Cloud Platform (Novogene Bioinformatics Technology Co., Ltd., Beijing, China).
SCFAs quantification
A standard curve was constructed using a standard mixture of acetic acid, propionic acid, butyric acid, and valeric acid at gradient concentrations. Following the methodology described by Wang et al.66, the supernatant of mouse fecal microbiota obtained after centrifugation was acidified with 10% sulfuric acid. Subsequently, the supernatant was extracted with ether, and the concentrations of SCFAs were determined using gas chromatography with the DB-FFAP capillary column (30 m × 0.25 mm × 0.25 μm, Agilent).
Statistical analysis
The experimental data were analyzed using Prism 8.0.1 (GraphPad Software, La Jolla, CA), with all results expressed as mean ± standard deviation (SD). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Additionally, the allergic symptom scores and histopathological scores were subjected to statistical analysis using the Kruskal-Wallis test. ∗p < 0.05 was considered statistically significant, and the letter-based significance marking method was applied in this study.
References
Agache, I. et al. Prioritizing research challenges and funding for allergy and asthma and the need for translational research—the European Strategic Forum on Allergic Diseases. Allergy 74, 2064–2076 (2019).
Sicherer, S. H. & Sampson, H. A. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immun. 141, 41–58 (2018).
Victoria, C. et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ. J. 13, 100472 (2020).
Wang, J. et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Tar. 8, 138 (2023).
Muraro, A. et al. Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy 72, 1006–1021 (2017).
Vazquez-Ortiz, M. & Turner, P. J. Improving the safety of oral immunotherapy for food allergy. Pediatr. Allergy Immunol. 27, 117–125 (2016).
Turner, P. J., Campbell, D. E., Boyle, R. J. & Levin, M. E. Primary Prevention Of Food Allergy: Translating Evidence From Clinical Trials To Population-based Recommendations. J. Allergy Clin. Immunol. 6, 367–375 (2018).
Jensen, C., Antonsen, M. F. & Lied, G. A. Gut microbiota and fecal microbiota transplantation in patients with food allergies: a systematic review. Microorganisms 10, 1904 (2022).
Bunyavanich, S. & Berin, M. C. Food allergy and the microbiome: current understandings and future directions. J. Allergy Clin. Immun. 144, 1468–1477 (2019).
Ryohei, S. et al. Neonatal gut microbiota and risk of developing food sensitization and allergy. J Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2024.10.029 (2024).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e3811 (2021).
Wang, Z. L. et al. The gut microbiome-immune axis as a target for nutrition-mediated modulation of food allergy. Trends Food Sci. Tech. 114, 116–132 (2021).
Bai, J. et al. Gut microbiota: a target for prebiotics and probiotics in the intervention and therapy of food allergy. Crit. Rev. Food Sci. 64, 3623–3637 (2024).
Canani, R. B. et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 10, 742–750 (2016).
Fu, L. L., Song, J. Y., Wang, C., Fu, S. J. & Wang, Y. B. Bifidobacterium infantis potentially alleviates shrimp tropomyosin-induced allergy by tolerogenic dendritic cell-dependent induction of regulatory T cells and alterations in gut microbiota. Front. Immunol. 8, 1536 (2017).
Shi, J. L. et al. Preventive effects of Bifidobacterium lactis Probio-M8 on ovalbumin-induced food allergy in mice. Food Sci. Hum. Well 13, 2346–2352 (2024).
Jing, W., Liu, Q. B. & Wang, W. Bifidobacterium bifidum TMC3115 ameliorates milk protein allergy in by affecting gut microbiota: a randomized double-blind control trial. J. Food Biochem. 44, e13489 (2020).
Kim, H., Kwack, K., Kim, D. Y. & Ji, G. E. Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol. Med. Microbiol. 45, 259–267 (2005).
Moussu, H. et al. Bifidobacterium bifidum NCC 453 promotes tolerance induction in murine models of sublingual immunotherapy. Int. Arch. Allergy Immunol. 158, 35–42 (2012).
Gu, S. M., Yang, D., Liu, C. L. & Xue, W. T. The role of probiotics in prevention and treatment of food allergy. Food Sci. Hum. Well 12, 681–690 (2023).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22, 1187–1191 (2016).
Tanaka, M. & Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 66, 515–522 (2017).
Xia, Y. J. et al. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 67, 103854 (2020).
Shao, H. M. et al. Novel perspective on the regulation of food allergy by probiotic: the potential of its structural components. Crit. Rev. Food Sci. 64, 172–186 (2024).
Yahfoufi, N., Mallet, J. F., Graham, E. & Matar, C. Role of probiotics and prebiotics in immunomodulation. Curr. Opin. Food Sci. 20, 82–91 (2018).
Zhang, T., Hu, M. M., Li, M. L., Li, C. C. & Miao, M. Effects of human milk oligosaccharides in infant health based on gut microbiota alteration. J. Agr. Food Chem. 71, 994–1001 (2023).
Boyce, J. A. et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J. Am. Acad. Dermatol. 64, 175–192 (2011).
Stone, K. D., Prussin, C. & Metcalfe, D. D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immun. 125, S73–S80 (2010).
Luo, M., Gan, M., Yu, X. M., Wu, X. L. & Xu, F. Study on the regulatory effects and mechanisms of action of bifidobacterial exopolysaccharides on anaphylaxes in mice. Int. J. Biol. Macromol. 165, 1447–1454 (2020).
Zhao, W., Ho, H. -e & Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 122, 276–282 (2019).
Rachid, R., Stephen-Victor, E. & Chatila, T. A. The microbial origins of food allergy. J. Allergy Clin. Immun. 147, 808–813 (2021).
Chelakkot, C., Ghim, J. & Ryu, S. H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 50, 1–9 (2018).
Lee, B., Moon, K. M. & Kim, C. Y. Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2645465 (2018).
Allaire, J. M. et al. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 40, 174–174 (2019).
Han, B., Ma, Y. & Liu, Y. X. Fucoxanthin prevents the ovalbumin-induced food allergic response by enhancing the intestinal epithelial barrier and regulating the intestinal flora. J. Agr. Food Chem. 70, 10229–10238 (2022).
Kuang, H. Y., Ma, Y. & Liu, Y. X. Protective effect of β-carotene on OVA-induced food allergy in mice by strengthening intestinal epithelial barrier function and regulating intestinal microflora. Food Funct. 13, 12330–12341 (2022).
Guo, Y. J. et al. Oleuropein prevents OVA-induced food allergy in mice by enhancing the intestinal epithelial barrier and remodeling the intestinal flora. Mol. Nutr. Food Res. 66, e2200455 (2022).
Liang, J. C., Zheng, B. D., Zhang, Y. & Zeng, H. L. Food allergy and gut microbiota. Trends Food Sci. Tech. 140, 104141 (2023).
Liu, Q. M. et al. Sulfated oligosaccharide of Gracilaria lemaneiformis protect against food allergic response in mice by up-regulating immunosuppression. Carbohydrate Polym. 230, 115567 (2020).
Yan, X. M. et al. Fructooligosaccharides protect against OVA-induced food allergy in mice by regulating the Th17/Treg cell balance using tryptophan metabolites. Food Funct. 12, 3191–3205 (2021).
Wang, G. et al. Microbiota-derived indoles alleviate intestinal inflammation and modulate microbiome by microbial cross-feeding. Microbiome. 12, 59 (2024).
Chang, M. N., Zhao, Y., Qin, G. X. & Zhang, X. D. Fructo-Oligosaccharide alleviates soybean-induced anaphylaxis in piglets by modulating gut microbes. Front. Microbiol. 9, 2769 (2018).
Huang, C. H., Lin, Y. C. & Jan, T. R. Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J. Funct. Foods 31, 44–51 (2017).
Nishiyama, K. et al. Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. Mbio 8, e00928–00917 (2017).
Xiao, M. F. et al. Cross-feeding of bifidobacteria promotes intestinal homeostasis: a lifelong perspective on the host health. Npj Biofilms Microbiomes. 10, 47 (2024).
Moens, F., Verce, M. & De Vuyst, L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 241, 225–236 (2017).
Bao, R. Y. et al. Fecal microbiome and metabolome differ in healthy and food-allergic twins. J. Clin. Invest. 131, e141935 (2021).
Liu, X. X. et al. Fucoidan ameliorated dextran sulfate sodium-induced ulcerative colitis by modulating gut microbiota and bile acid metabolism. J. Agr. Food Chem. 70, 14864–14876 (2022).
Yang, J. P. et al. Oscillospira-a candidate for the next-generation probiotics. Gut Microbes 13, 1987783 (2021).
Kim, C. H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol. Immunol. 20, 341–350 (2023).
Mukhopadhya, I. & Louis, P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-025-01183-w (2025).
Yang, W. J. & Cong, Y. Z. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 18, 866–877 (2021).
Shi, J. L. et al. Butyrate alleviates food allergy by improving intestinal barrier integrity through suppressing oxidative stress-mediated Notch signaling. Imeta 4, e70024 (2025).
Yu, B. C. et al. Microbiota-derived butyrate alleviates asthma via inhibiting Tfh13-mediated IgE production. Signal Transduct. Tar. 10, 181 (2025).
Cani, P. D., Depommier, C., Derrien, M., Everard, A. & de Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol Hepatol. 19, 625–637 (2022).
Luo, Y. H. et al. Rational consideration of Akkermansia muciniphila targeting intestinal health: advantages and challenges. NPJ Biofilms Microbiomes. 8, 81 (2022).
Parrish, A. et al. Akkermansia muciniphila exacerbates food allergy in fibre-deprived mice. Nat. Microbiol 8, 1863–1879 (2023).
Cui, Q. Y. et al. Bifidobacterium bifidum Ameliorates DSS-Induced Colitis in Mice by Regulating AHR/NRF2/NLRP3 inflammasome pathways through Indole-3-lactic acid production. J. Agr. Food Chem. 71, 1970–1981 (2023).
Zhang, J. C. et al. Bifidobacterium improves oestrogen-deficiency-induced osteoporosis in mice by modulating intestinal immunity. Food Funct. 15, 1840–1851 (2024).
Xu, J. Y. et al. Prophylactic treatment with Bacteroides uniformis and Bifidobacterium bifidum counteracts hepatic NK cell immune tolerance in nonalcoholic steatohepatitis induced by high fat diet. Gut Microbes 16, 2302065 (2024).
Lee, S. H. et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat. Microbiol. 6, 277–288 (2021).
Fang, Z. F. et al. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes. 14, 2044723 (2022).
Sharma, G. et al. A dietary commensal microbe enhances antitumor immunity by activating tumor macrophages to sequester iron. Nat. Immunol. 25, 790–801 (2024).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Li, A. et al. 2’-Fucosyllactose attenuates aging-related metabolic disorders through modulating gut microbiome-T cell axis. Aging Cell 24, e14343 (2024).
Wang, Y. et al. The protective effects of 2’-fucosyllactose against E. coli O157 infection are mediated by the regulation of gut microbiota and the inhibition of pathogen adhesion. Nutrients 12, 1284 (2020).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (grant No. 32302096).
Author information
Authors and Affiliations
Contributions
Ruixin Kou: Conducted experiments, collected, analyzed the data, and drafted the manuscript. Ang Li: Methodology. Miao Yu: Formal analysis. Jin Wang: Conceptualization and funding acquisition. Yaozhong Hu: Review editing and validation. Bowei Zhang: Data curation. Jingmin Liu: Software. Shuo Wang: Review editing, supervision, validation, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kou, R., Li, A., Yu, M. et al. Bifidobacterium bifidum FB3-12 attenuates ovalbumin-induced allergic diseases by enhancing intestinal barrier function and regulating gut microbiota. npj Sci Food 9, 227 (2025). https://doi.org/10.1038/s41538-025-00590-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00590-w