Abstract
Haemonchus contortus is a highly pathogenic nematode in small ruminants, causing major production losses and animal welfare concerns. With increasing anthelmintic resistance, vaccination offers a more sustainable control strategy. This study evaluated a novel glycoengineered vaccine produced in Hi5 insect cells in a randomized, controlled trial including Jura × Lacaune sheep. Animals received either Barbervax® (BVAX), the glycoengineered vaccine (GEA) composed of five antigens (H11, H11-1, H11-2, H11-4 and GA1), its non-glycoengineered counterpart (NEA), or served as controls. Over 16 weeks, clinical, immunological, and parasitological data were collected. GEA reduced fecal egg counts by 81.09% and worm burden by 25.36%, showing a lower degree of anemia compared to NEA and control. All vaccinated groups exhibited elevated IgG/IgE responses. The results underscore the importance of glycoengineering in achieving protective immunity against H. contortus, supporting the development of highly effective recombinant vaccines against this and other parasitic worms in the future.
Similar content being viewed by others
Introduction
Haemonchus contortus is the most pathogenic gastrointestinal nematode in small ruminants and South American camelids. Infection cause significant production losses (decreased weight gain and fertility as well as loss of young stock) and threatens animal welfare worldwide, especially in tropical and subtropical areas1,2,3,4,5,6. As a single worm ingests up to 50 μl of blood per day, even moderate infections can cause severe blood loss, resulting in anemia and hypoproteinemia. In severe cases, even death may occur5,7. Currently, parasite control relies primarily on the administration of anthelmintic drugs, which have several disadvantages. For instance, increasing prevalence of anthelminthic-resistant worms, long withdrawal periods for meat and milk and unwanted ecotoxicological effects8,9,10. Due to a lack of treatment response of resistant H. contortus, clinical disease will progress in affected hosts10,11,12,13.
In contrast, vaccination is a promising strategy to control the parasite in susceptible domestic animal species. In the past, a repertoire of potential vaccine targets, including somatic and secreted antigens, was identified and evaluated in in vivo trials14. Among the evaluated protein-based vaccine candidates, the H11 aminopeptidases and the H-gal-GP protease complex showed the highest efficacy15,16. Sheep vaccinated with either antigens showed significant reductions in fecal egg shedding of 72%-93% and of 68%-69% in worm burden17,18. Both H11 and H-gal-GP are “hidden” proteins, located in the intestine of adult worms, and are not naturally exposed to the host’s immune system during worm infection. Thus, a vaccine response to these antigens is not boosted by infection. Therefore, a repeated immunization is needed to maintain a protective effect during the grazing season19. H11 and H-gal-GP are major components of Barbervax®, which was licensed in Australia in 2014, but is available only in a few countries.
To meet local biosecurity regulations, Barbervax® is manufactured at the Albany Laboratory of the Department of Agriculture and Food in Western Australia from adult Haemonchus worms harvested from the stomach of donor sheep after slaughter on an industrial scale. One production step of Barbervax® involves mechanical disruption of the worms, by which 480 mg of antigen can be obtained from 90 g of worms20,21. For commercial-scale production, machines were implemented to open the stomachs and harvest the worms. The antigens are then isolated and formulated as a vaccine20. This approach is currently the only way to obtain adult worms for antigen preparation, as in vitro parasite culture systems for the propagation and multiplication of H. contortus are not yet been established22. Attempts to produce recombinant H11 vaccine antigens, using conventional expression platforms or the free-living nematode Caenorhabditis elegans, have been reported previously23,24,25,26. The resulting products were unable to reach a satisfactory efficacy regarding the reduction of worm burden and/or fecal egg count (FEC)14. H11 proteins are naturally glycosylated, carrying very distinct N-glycan structures compared to those found in mammals. Such glycan modifications are often considered crucial for maintaining the antigenicity25,27. To address this, a novel glycoengineered recombinant vaccine was designed that incorporates nematode-type glycan modifications and enhances solubility28. This novel recombinant vaccine contains five soluble proteins (H11, H11-1, H11-2, H11-4 and GA1) produced using Hi5 insect cells.
The objectives of the present study were to compare hematological and parasitological parameters between sheep vaccinated with the novel recombinant vaccines (GEA/NEA) or Barbervax® (BVAX), an unvaccinated/challenged positive (POS), and an uninfected, unvaccinated negative (NEG) control group. It was hypothesized that vaccination of sheep with the novel vaccine would result in reduced anemia, a significant decrease in fecal egg shedding and abomasal worm burden and an elevated concentration of specific immunoglobulins in comparison to the POS group. Furthermore, we expected results to be comparable or better than those seen in sheep of the BVAX group and that the level of protection was higher in sheep vaccinated with the glycoengineered form (GEA group) of the vaccine compared to the non-glycoengineered form (NEA group).
Results
Clinical assessment
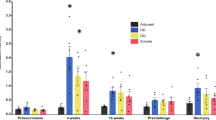
Following the challenge ( ≥ week 10), the clinical anemia scores (FAMACHA©) of the BVAX, GEA, NEA and NEG groups were lower than those of the POS group (p < 0.01). Mixed-model analysis revealed a mean FAMACHA© score of 2.42 for NEG (95%CI = 2.26; 2.59), 3.46 for POS (95%CI = 3.29; 3.63), 2.27 for BVAX (95%CI = 2.10; 2.44), 2.31 for GEA (95%CI = 2.14; 2.48) and 2.17 for NEA group (95%CI = 2.00; 2.33) (Fig. 1).
The five graphs show the FAMACHA© results for the NEG, the POS, the BVAX, GEA and NEA groups. Each column shows the relative percentage of the FAMACHA© score of the seven sheep within each group per study week. Black arrows highlight the 1st (week 1), 2nd (week 4) and 3rd (week 7) vaccination with either vaccine. The red arrow indicates the timepoint of challenge with 5000 L3 H. contortus (week 10).
In addition to the FAMACHA© score, changes in daily body weight gain (DBWG) were monitored. Prior to L3 challenge (week 1 to 9; 63 days in total) no differences in DBWG between the groups were observed among the groups, with average gains ranging from 95g to 222 g (Fig. 2; p = 0.87). After challenge ( ≥ week 10; 40 days in total), a significant difference in DBWG was detected between the BVAX and NEG groups, with BVAX sheep gaining less weight (Fig. 2; p = 0.02). All other groups showed a numerical but no statistical significant increase in DBWG. The NEG group gained a median of 213 g/d (min of 138 g, max of 238 g), the POS 150 g/d (min of 125 g, max of 188 g), the BVAX 138 g/d (min of 88 g, max of 138 g), the GEA 150 g/d (min of 113 g, max of 225 g) and the NEA 175 g/d (min of 100 g, max of 225 g).
Both GEA and Barbervax® improve the values of hematological parameters
Packed cell volume (PCV), erythrocytes per milliliter and hemoglobin concentrations were measured as indicators of potential blood loss and anemia. All three parameters were lowest in the POS group and highest in the NEG group after challenge (Fig. 3 and Table 1).
Each column shows the relative percentage of the PCV of the seven sheep within each group per study week. Black arrows highlight the 1st (week 1), 2nd (week 4) and 3r (week 7) vaccination with either vaccine. The red arrow indicates the timepoint of challenge with 5000 L3 H. contortus (week 10). Each column shows the relative percentage of the results of the PCV of the seven sheep within the group in study week 1 to study week 16. The black arrows highlight the 1st (week 1), 2nd (week 4) and 3rd (week 7) vaccination with either BVAX, GEA or NEA. The red arrows highlight week 10, when the sheep were challenged with 5000 L3 H. contortus.
Globulin and total protein concentrations were higher in the GEA group in comparison to the POS group depending on the time point after challenge (Fig. 4). The BVAX and GEA groups showed higher globulin concentrations (calculated as total protein minus albumin) than the NEA group throughout the entire period. Total protein levels did not differ between groups at weeks 8 (p = 0.08), week 9 (p = 0.06) and week 10 (p = 0.06). One week post challenge (week 11), the POS group showed lower total protein levels than the BVAX (p < 0.01), GEA (p < 0.01) and NEA (p = 0.02) groups. In week 12, there were no differences between the groups. In week 13, the POS group showed different total protein concentrations compared to the NEG (p < 0.01) and BVAX (p < 0.01) groups. The NEA group showed significantly lower total protein than the NEG group. In week 14, both BVAX and GEA groups had higher total protein concentrations than POS (p = 0.01). In week 15, BVAX, GEA and NEG groups maintained higher total protein concentrations than the POS (p = 0.01). Additionally, week 15 showed differences between POS and NEG (p = 0.04) and between BVAX and POS (p = 0.05).
The results showed statistically significant differences in total protein from week 10 onward (after challenge) between the GEA group (mean of 5.95 g/dl) and the POS group (mean of 5.58 g/dl), as well as between GEA and NEA (mean of 5.58 g/dl) (Table 2). Albumin concentrations did not differ between the BVAX and GEA groups. However, albumin concentration was higher in the BVAX group (mean of 3.76 g/dl) compared to the NEA group (mean of 3.52 g/dl) (p ≤ 0.05) (Table 2). No differences in albumin concentrations occurred at weeks 10 (p = 0.48) and 11 (p = 0.79). In week 12, the NEA group differed significantly from the NEG group (p = 0.02). In week 13, there was a difference between the NEA (p < 0.01) and GEA (p = 0.05) and the NEG, the POS and the NEG group (p = 0.01). There were no differences in week 14. In weeks 15 and 16, significant differences appeared between the POS and the NEG (p = 0.01 and p = 0.05, respectively), and between NEA and NEG group (p = 0.02 for both weeks).
The groups differed in the number of segmented neutrophils, eosinophils, basophils, and lymphocytes. Statistically significant differences appeared in the number of eosinophils, with the BVAX group showing the highest numbers throughout the trial. The relative distribution of the white blood cells per group over the period of 16 weeks is shown in Fig. 5. In week 1 (p = 0.37), week 2 (p = 0.07), week 3 (p = 0.24) and week 4 (p = 0.54), eosinophil percentage did not differ significantly between the five groups. Notably, all vaccinated groups exhibited a marked increase in eosinophils after the second vaccination (week 5 (p = 0.00), week 6 (p = 0.01), week 7 (p = 0.04), week 8 (p = 0.01). In week 9 (p = 0.08) and 10 (p = 0.43), eosinophil percentages again showed no differences. Two weeks after the challenge infection (week 12), eosinophil counts increased in the POS, BVAX and GEA groups but not in the NEA group. Statistical analysis revealed that the GEA group had the highest lymphocyte count (4679.40/µl), while the BVAX group had the highest eosinophil (507.17/µl) and basophil (52.75/µl) counts (Table 3).
Relative distribution of granulocytes of the five groups (for color code details see caption Fig. 1). The black arrows highlight the 1st (week 1), 2nd (week 4) and 3rd (week 7) vaccination with either BVAX, GEA or NEA. The red arrows highlight the week when the sheep were challenged with 5000 L3 H. contortus.
All vaccines show similar impact on clinical parameters after the 1st, 2nd, and 3rd vaccination
Body temperature was measured before vaccination, and with a mean of 39.37 °C ± 0.23 °C, there was no difference between the groups (p = 0.24). All sheep showed a bright and alert appearance and good appetite after vaccinations. After the second vaccination differences were seen on the day of vaccination (Table 4). At the day of the second vaccination one sheep vaccinated with GEA showed a body temperature of 39.6 °C 6 h after vaccination and 41.5 °C 8.5 h post vaccination and a slightly reduced demeanor.
Induration, lymph node enlargement, swelling and pain was recorded in all experimental groups with differing degrees after each vaccination (Supplementary Tables 1–4).
The glycoengineered vaccine reduces egg shedding and eggs per female worm
To evaluate vaccine efficacy against infections with H. contortus fecal egg counts (displayed as eggs per gram of feces = EpG) and worm burden were assessed. The reduction of egg shedding was 98.32% for BVAX, 81.09% for GEA and 32.72% for NEA in comparison to the POS group (Fig. 6). The BVAX group showed lower egg counts than the POS (p < 0.01) and the NEA groups (p = 0.02). Egg counts did not differ between the BVAX and GEA (p = 0.33). Although the GEA group exhibited numerically lower egg shedding than POS (p = 0.08) and the NEA (p = 1.0), these differences were not statistically significant. The median cumulative EpG values were 36,930 for POS, 620 for BVAX, 6,985 for GEA and 24,845 for NEA. The data on the egg shedding of the individual sheep is shown in Fig. 6.
In comparison to the POS, the highest worm burden reduction was observed in the BVAX (86.40%), whereas the GEA had a lower reduction (25.40%), and the NEA group showed no reduction. There were significant differences between BVAX and the GEA (p = 0.04), between the BVAX and the POS (p < 0.01) and BVAX and NEA group (p = 0.01). While the impact on the worm burden was not that pronounced, the number of eggs per female worm were significantly lower in GEA and BVAX groups compared to those of the POS group. There were differences between the POS and GEA (p < 0.01) and between the POS and the BVAX group (p < 0.01). GEA showed the overall lowest median value (7.98) (Fig. 7).
In addition to these parameters, a possible impact of the vaccine on the establishment rate of ingested larvae was investigated. NEA and GEA groups showed establishment rates of 48.44 and 41.90%, respectively, similar to or slightly below 44.86% observed in the POS group. However, none of these reached the low establishment rate seen in the BVAX group (10.28%).
The female: male ratio of recovered worms was 1.29 in the POS group (min of 0.78, max of 1.67), 0.59 in BVAX (min of 0.18, max of 1.02), 1.25 in GEA (min of 0.82, max of 2.10), and 1.24 in NEA (min of 0.94, max of 1.48). The number of eggs per female worm was lower in GEA and BVAX groups compared to those of the POS group (p < 0.01 for both comparisons) (Fig. 7).
Animals in the GEA-vaccinated group show a faster increase of parasite- and vaccine-specific serum immunoglobulins
To determine whether vaccination with the recombinant antigens GEA and NEA elicited specific antibodies in animals, sheep sera collected during the trial were examined by ELISA. ELISA data initially indicated that the total serum IgG and IgM antibodies did not change among the groups and during the trial (Fig. 8). Subsequently, serum samples were analyzed using ELISA plates coated with either soluble proteins of adult H. contortus or with vaccine antigens (indirect ELISA). Haemonchus-specific IgG antibodies were induced in the vaccine groups (BVAX, GEA, NEA; Fig. 9A). Vaccination with GEA and NEA led to a faster increase of IgG levels compared to sheep in the BVAX group, which showed a more gradual antibody increase. Animals in the BVAX group maintained the highest IgG levels of all vaccine groups after the third vaccination, in line with the assumption that BVAX contains more than the described antigens. As seen for other parameters, vaccination of animals with GEA led to a higher level of IgG antibodies compared to antibodies induced by NEA. This pattern was also observed for Haemonchus-specific IgE antibodies (Fig. 9B). No changes were detected for IgM antibodies in any group (Supplementary Fig. 3).
A, B show responses of Haemonchus-specific IgG and IgE antibodies, respectively. Assays were performed using plates coated with soluble proteins of adult parasites and combined sheep sera. C, D illustrate antigen-specific IgG and IgE responses in individual sheep. Vaccine-specific antibodies for the GEA and NEA groups were measured using plates coated with an equal amount of the corresponding vaccine antigens (marked with white arrows); the BVAX and negative and positive control groups were assayed using Barbervax®-coated plates. Data are plotted on different y-axis scales to better visualize the pattern of the different assays.
To assess the immune responses of individual animals, vaccine-specific antibodies were assessed using plates coated with either the corresponding vaccine antigens, whereas for the control groups (NEG and POS) Barbervax®-coated plates were used. The results indicated elevated levels of antigen-specific IgG (Fig. 9c) and IgE (Fig. 9d) antibodies in all three vaccine groups (BVAX, GEA, NEA). Although less pronounced in the BVAX group compared to GEA and NEA groups, IgG and IgE specific to the respective vaccine antigens were induced post vaccination but slowly tapering off during the trial. Analysis of IgA and IgM antibodies did not reveal differences between the vaccine groups and control group; vaccine-specific salivary IgA antibodies were also measured using antigen-coated plates. However, no obvious changes were observed (Supplementary Fig. 3).
Sera of GEA-vaccinated animals show a similar antigen-detection spectrum compared to sera of Barbervax®-vaccinated animals
In a further set of experiments, the antigen recognition patterns of GEA-induced IgGs were compared to those seen in sera of Barbervax®-induced IgG. The first experiment demonstrated that IgGs in sera of the BVAX and GEA groups recognized several protein targets with a broad molecular weight range (20–250 kDa) by Western-blotting. These proteins were not only present in adult-stage parasite but also in the infective L3 larvae (Fig. 10a; Supplementary Figs. 4 and 5). This suggested a broader antibody targeting upon infection. Haemonchus-specific IgG antibodies in the sera of the GEA vaccinated group were detectable after the 1st vaccination (week 4), presumably a few weeks earlier than the appearance of IgGs in sera of sheep vaccinated with Barbervax® (BVAX group).
contortus antigens in vaccinated sheep. A vaccination with either Barbervax® (BVAX) or GEA elevated IgG antibodies that bound to soluble proteins of L3 larvae (L3) and adult-stage (Ad) parasites. Parasite-specific IgG appeared earlier in GEA group than in BVAX group. B Cross-reactivities of IgG ware observed in both BVAX- and GEA-antisera; the comparison of Barbervax®-specific IgG (right panel) between the two groups suggested a similar binding pattern and intensity. C Deglycosylation of antigens using sodium metaperiodate (NaIO4) reduced Barbervax®-specific IgG targeting in both groups and completely abolished the recognition of GEA by BVAX IgG.
In addition to the appearance of IgGs, the binding spectrum of antibodies in animals vaccinated either with GEA or BVAX was compared. Antibodies in sera of animals vaccinated with GEA seemed to recognize many of the native antigens present in Barbervax® (Fig. 10b; Supplementary Figs. 6−8). Indeed, the IgG recognition patterns towards Barbervax® antigens displayed a comparable intensity and a similar molecular weight range. Similarly, sera from sheep vaccinated with Barbervax® showed comparable pattern recognition. Immunoblots were also performed on deglycosylated antigens, using Concanavalin A as a control to verify the completion of deglycosylation (Fig. 10c; Supplementary Figs. 9−11). IgG binding to the native antigens of Barbervax® was decreased after metaperiodate treatment in both groups, while the recognition of GEA by BVAX IgG was completely abolished, strongly suggesting a glycan-biased targeting manner. In contrast to Barbervax®-induced IgG in the BVAX group, the intensity of the GEA group IgG towards deglycosylated GEA did not change.
Pathohistological examination
The pathohistological examination showed a cell infiltration of the lamina propria mucosae of the sheep in the BVAX and the GEA group. One ileum and one abomasum sample are shown as a representative for respective group (Supplementary Fig. 1; Supplementary Fig. 2). The ileum of the sheep from the BVAX group showed a higher cell density in the lamina propria mucosae in comparison to the other study groups.
Discussion
The hematophagous parasite H. contortus is the most pathogenic trichostrongylid in small ruminants and South American camelids. Current control strategies primarily rely on the application of anthelmintic drugs; however, the prevalence of anthelminthic resistance is emerging worldwide8,9,10,12. Barbervax® has been the only commercialized vaccine against H. contortus, with high efficacy being reported in a number of trials in sheep, goats and alpacas18,29,30,31. Despite this, Barbervax® has two main drawbacks: First, it remains inaccessible in many countries due to biosecurity concerns, despite the fact that a safe, well-defined, and effective recombinant vaccine is urgently needed. Second, the vaccine relies on a constant need to harvest antigen mixtures from worms retrieved from the stomachs of slaughtered sheep, as it is currently practiced, which makes it less attractive to ensure adherence to 3 R guidelines. Thus, a vaccine containing defined antigens that can be produced cost-effectively at large scale in fermenters under controlled conditions, ensuring high hygiene and biosecurity standards, is urgently needed and would pave the way for the availability of vaccine doses year-round and worldwide. Suitable antigens have been indicated in studies using H11 and H-gal-GP antigens of the commercial vaccine Barbervax®. These studies further showed that these antigens are naturally glycosylated, carrying very distinct N-glycan structures compared to those found in mammals32,33. This glycosylation has made the production of recombinant forms of such antigens extremely challenging.
Previous studies using a combination of two recombinant H11 isoforms (antigen expressed in C. elegans; three injections with a saponin-based adjuvant; challenge with 5000 L3) reported no significant difference in fecal egg shedding, worm burden or worm female: male ratio in comparison to the adjuvant control group in Suffolk-cross lambs25. While this study showed promising methodological results, it also highlighted that correct glycosylation may play a far more important role than anticipated. Thus, the safety and efficacy of two recombinant vaccines produced in Hi5 insect cells, one in a glycoengineered form (GEA), the other in non-glycoengineered form (NEA), were evaluated in a randomized controlled vaccine trial in the present study. To the authors’ best knowledge, the presented animal trial is the first successful attempt to vaccinate sheep with an antigen cocktail consisting of glycoengineered recombinant proteins produced in insect cells against a parasitic worm. Overall, both GEA and NEA did not show any appreciable negative side-effects in vaccinated animals. Both vaccines showed significant impact on the parasite, with egg shedding in the GEA group was reduced by 81.09% and worm burden decreased by 25.36%. A minimum efficacy of > 65% is required to have a positive epidemiological effect to control haemonchosis (e.g., reduced pasture contamination with subsequently lower worm burdens)34, and the presented GEA vaccine fulfilled this requirement in the present experimental trial with a wide margin. Despite a reduction of egg shedding by 32.72% in the NEA group compared to the unvaccinated group, no significant reduction in the worm burden was observed in this group. The comparison between GEA and NEA groups strongly indicated that glycoengineering is essential for an improved immune response against H. contortus since the GEA group continuously showed a more favorable outcome in parasitological and hematological parameters compared to the NEA group. Our data therefore confirm that nematode-type glycan modifications are crucial in the production of an anti-Haemonchus vaccine. It needs to be highlighted that the statistical difference and the biological relevance of the findings in this study need to be considered and will require further investigation. For example, the significantly increased lymphocyte concentration in the GEA group in comparison to other groups needs to be further investigated.
According to the manufacturer’s instructions for Barbervax®, the local tissue reactions in the form of swelling at the injection site can last for up to 17 days35. In the present study, the injection site was assessed on daily basis for seven days after each vaccination. There was no difference between groups apart from a transient increase of body temperature following the second vaccination, which suggests that the insect cell-derived antigens are clinically safe. Haemonchus contortus is hematophagous and can cause severe anemia in hosts36. As anemia in infected sheep is often accompanied by nutrient loss, either directly by the worms or indirectly due to the damaged intestinal epithelial layers, weight loss/regain after challenge was compared. Before challenge, there were no differences in daily weight gain between the groups, while after challenge the BVAX group showed the lowest body weight gain, which was somewhat unexpected given that BVAX showed the highest reduction in worm burden/egg counts. This phenomenon can currently not be explained. Many sheep are kept for meat production, with body weight gain being a key performance indicator to measure the economic benefit of the farm. Thus, the differences in daily body weight gain suggest that is prudent to suggest that this parameter will be included in future vaccine trials as an important criterion. Even though individual feed intake was not measured, one can speculate that either daily dry matter intake was lower in the BVAX group (e.g., due to reduced appetite, abdominal pain) or that other mechanisms, such as an excessive immune reaction, caused the reduced daily weight gain. Indeed, vaccination against intestinal nematodes causes granulocyte infiltration in the mucosal layers. In previous works, during infection with trichostrongyloids a potent chemoattractant activity for ovine bone marrow-derived eosinophils was noted in vitro37. This activity was identified as a chemoattractant protein in whole worm extracts of third and fourth larval and adult stages of Teladorsagia circumcincta as well as adult stages of H. contortus. The resulting local eosinophil-mediated mucosal damage, comparable to that seen in asthmatic lungs, may provide a permissive local microenvironment for the parasite for increased attachment and feeding37,38. Overall, eosinophils have been reported to play a significant role in gastrointestinal nematode infection in sheep39. A negative association between blood eosinophils and FEC was observed in H. contortus infections40, and in vitro assays demonstrated that eosinophils may kill H. contortus larvae, thereby reducing parasite establishment41.
In the present study, pathohistological examination of the ileum of sheep in the BVAX group showed a higher density of cells in the lamina propria mucosae, compared to the other groups, which underscores this assumption. Additionally, the number of circulating eosinophils after challenge was higher in the BVAX group. It is therefore possible to speculate that the heightened immune response in the BVAX group, represented by higher concentrations of peripheral eosinophils and a higher cell density in the ileum, contributed to the reduced body weight gain. Furthermore, higher concentrations of eosinophils in the blood of sheep vaccinated with GEA compared to the blood of sheep vaccinated with NEA was observed. Following the 3rd vaccination the eosinophils did not increase as drastically in all three experimental groups compared to the increase seen after the 1st and the 2nd injection. While pre-activation of eosinophils by vaccination may be an essential part of the induction of a protective immune response, subsequently limiting worm establishment, this assumption needs to be corroborated by analyzing the cytokine and chemokine profile in sheep sera generated from the different groups.
In addition to eosinophil concentration in peripheral blood, we also analyzed further blood parameters related to worm infection. Packed cell volume and hemoglobin levels can decrease severely after H. contortus infection since the sheep may lose up to 0.2 to 0.6 liters of blood per day42. In the present study, sheep vaccinated with GEA showed a higher total protein (5.95 versus 5.58 g/dl) and packed cell volume (26.77 versus 26.11%) compared to sheep vaccinated with NEA. These results indicate a further benefit of GEA that seems to notably reduce blood losses through worm feeding. Even though the reduction in the worm burden in sheep vaccinated with GEA was only 25.36%, the PCV and Hb levels indicate that the remaining worms in GEA vaccinated animals were probably weakened. Whether this is part due to the impact of GEA on the integrity of the female worms, supported by the reduced number of female eggs per worm, remains to be followed up in detail in further trials. In this context, we also established whether the vaccine responses depended purely on secretory IgA, or whether a systemic adaptive immune response was involved. Our data suggested that IgG and IgE were the major isotypes that were elevated post-vaccination, post-infection, as well as post-challenges. There was a link between parasite-specific antibodies and efficacy, with higher IgG/IgE level being associated with lower EpG values and worm burdens. The overall antibody titers specific for Haemonchus in the GEA group were lower than those in the BVAX group over the trial period, which could be the cause of a lower efficacy in this group.
The low titer of vaccine-specific antibodies observed in the BVAX group might be due to the low antigen coating efficiency despite optimization efforts. Independent of potential technical issues, ELISA data suggested that antibody in sera of sheep in the GEA group and NEA group increased quickly in comparison to the BVAX group, which indicated that the recombinant H. contortus antigens in the GEA and NEA preparation may be more efficient in inducing antigen-specific antibody responses compared to those being in the native worm antigens in Barbervax®.
Western blot analysis confirmed this observation and further revealed an unexpectedly broad antibody recognition pattern where numerous proteins from both L3 larvae and adult parasites were recognized by vaccine-induced antisera. Notably, IgG antibodies in sera of Barbervax®-vaccinated animals exhibited a strong bias toward glycan moieties, which is in line with literature25. Similarly, anti-glycan IgG antibodies were detected in animals from the GEA group, alongside the ones specific to protein backbones. These findings implicate that the vaccine antigens, both the native and glycoengineered forms, can efficiently trigger antibody production in sheep that target a much broader range of glycoproteins than previously thought.
The novel glycoengineered vaccine GEA, comprising five glycoengineered recombinant antigens was tested for the first time in vivo in sheep and demonstrated that glycoengineering is essential to achieve a significant reduction in egg shedding and worm burden, and to improve hematological parameters. Although the overall efficacy of GEA at 100 µg/dose was lower than the commercial vaccine Barbervax®, the efficacy benchmark in this trial, animals in the GEA group exhibited greater body weight gain following challenge infection. Direct comparison, however, is complicated by the fact that Barbervax® contains native H11 and additional components of the H-gal-GP complex, whereas GEA consists of a defined cocktail of glycoengineered recombinant H11 isoforms. However, it opens the way for future development of highly effective recombinant vaccines against this and other parasitic worms. To enhance the effectiveness of GEA, further studies to investigate the most effective recombinant antigen isoforms, the vaccine dose as well as immunization schedules are needed. While we are aware that the small sample size of our study has its limitations, we still obtained some interesting and promising results. It is clear, though, that future trials should not only focus on vaccine formulations and adjuvant evaluation, but also include larger, more physiological and genetically diverse sample groups to improve the general applicability of the findings. Despite this, our study demonstrates the promising potential of glycoengineered recombinant antigens as effective substitutes for native worm-derived components in helminth vaccine development. The glycoengineering approach offers precise antigen composition, enhanced safety and scalable production, addressing key challenges in vaccine manufacturing. Furthermore, this strategy lays a valuable foundation for advancing helminth vaccine research and addressing the growing issue of AR. In conclusion, these findings mark a step toward the development of recombinant helminth vaccines, contributing to improved control of parasitic diseases.
Methods
Ethical considerations
The study was approved by the Ethics and Animal Welfare Committee of the University of Veterinary Medicine, Vienna in accordance with the University’s guidelines for Good Scientific Practice and authorized by the Austrian Federal Ministry of Education, Science and Research (vaccine trial: GZ:2023-0.734.950; H. contortus L3 production: GZ: 2022-0.599.404) in accordance with current legislation. The study was conducted according to the ARRIVE guidelines43.
Sample size calculation and randomization
The sample size calculation was based on the following assumptions: vaccine efficacy is assessed by evaluating the number of excreted eggs (eggs per gram (EpG) of feces; EpG) and the worm burden (number of worms in the abomasum). In the work by González-Sánchez et al.44) (publication Table 2), the standard deviation was approximately half of the mean of the total egg excretion, and the difference between the vaccinated and non-vaccinated groups was about 75%44. With a significance level of 5%, a power of 80%, and using a one-sided test procedure (vaccinated animals excrete fewer eggs per gram of feces), the calculated group size was seven animals per group. The sheep were allocated to each study group randomly (stratified randomization) depending on their body weight and breed using the “rand()” function in Microsoft Excel.
Sheep
The sheep were born between August and October 2023 (average weight of 26.7 kg; average age of 4 months) and raised on the same dairy sheep farm in the federal state of Lower Austria. The farm is run as a commercial organic dairy sheep farm with ~200 dairy sheep. The ewes were thoroughbred Lacaune and the three rams were thoroughbred Lacaune or Jura. Therefore, the lambs were Lacaune x Lacaune or Lacaune x Jura breed. The lambs were born and remained with their mothers for 14 days before being moved to a separate barn. The lambs did not have any access to pasture, only to a concrete outdoor loafing area. The farmer separated 39 male lambs for the animal trial and housed them according to the national regulations. All lambs were vaccinated against clostridial disease on the farm within the regular herd health management procedure using Miloxan® (Boehringer Ingelheim Animal Health France SCS, Lyon, France) with a dosage of 2 ml per sheep subcutaneously. They received the first immunization on October 25th, 2023, and the second one on November 28th, 2023.
Deworming strategy before the trial
The first deworming was carried out on December 15th, 2023, where the sheep received 0.2 mg/kg body weight (BW) of ivermectin (Ivomec® 10 mg/ml, Boehringer Ingelheim Animal Health) subcutaneously. At the farm of origin, the sheep were assessed, and the heaviest sheep was weighed and the dosage was chosen after that. Additionally, fecal samples were collected in September 2023 and examined for gastrointestinal nematodes, with negative results. At the day of arrival (January 2nd and 3rd, 2024) the sheep were treated orally with 5 mg/kg BW of fenbendazole (Panacur® 250 mg, Intervet GesmbH, Vienna), 7.5 mg/kg BW of netobimin (Hapadex® 50 mg/kg, Intervet GesmbH, Vienna) and 20 mg/kg BW of toltrazuril (Baycox® Multi 50 mg/ml, Bayer Animal Health GmbH, Leverkusen, Germany).
Vaccine efficacy trial in vivo
The randomized controlled vaccine trial was carried out at the Clinical Center for Ruminant and Camelid Medicine, University of Veterinary Medicine, Vienna, Austria. The sheep were housed in a separate building and divided into five groups of seven animals (Fig. 11). All groups received the same amount of hay (not weighed) twice daily and water was provided ad libitum. In total, four groups were challenged with 5,00 in-house produced H. contortus L3 larvae, an isolate from Boehringer Ingelheim Vetmedica GmbH, Kathrinenhof Research Center, Rohrdorf, Bavaria, Germany. A negative control group (NEG; no vaccination and no challenge) and a positive control group (POS; no vaccination and challenge) were included. The seven sheep in each experimental group were vaccinated three times subcutaneously either with 1 ml Barbervax® (BVAX; Wormvax Australia Pty Ltd., Albany, WA, Australia; imported from UK via Merlin Vet, Kelso, UK), or with 1 ml of the glycoengineered recombinant antigen cocktail (GEA,28) or with 1 ml of the non-glycoengineered recombinant antigen cocktail (NEA). Recombinant vaccines (GEA and NEA) were formulated by mixing 100 µg of insect cell (Hi5)-derived recombinant products (H11, H11-1, H11-2, H11-4 and GA1; 20 µg/antigen/dose) with 1 mg of Quil-A® adjuvant (InvivoGen, San Diego, CA, USA) in PBS solution. Sheep were challenged three weeks after the third vaccination using a 2 ml disposable plastic pipette (dosage 2 ml). Multiple samples, including fecal, blood and saliva samples, were collected from animals during the study period (Fig. 11). The sheep were held by one person and euthanized by a veterinarian using an intravenous dosage of 60 mg/kg BW of Pentobarbital-Natrium (Release®, 300 mg/ml, WDT, Garbsen, Germany). Auscultation of the heart and the lungs, and subsequently testing the absence of the cranial reflexes, was performed to verify the dead.
The sheep were kept in five groups with seven sheep in each group. The negative control group (NEG) received no vaccination and no challenge. The positive control (POS) and the experimental groups were challenged with 5000 H. contortus L3 (in week 10). The experimental groups were vaccinated three times subcutaneously with Barbervax® (BVAX), the novel glycoengineered vaccine (GEA) or the non-glycoengineered vaccine (NEA). Examinations, sampling dates and ELISA test dates are shown [Created in BioRender. Sajovitz-Grohmann, F. (2025) https://BioRender.com/pkpssk5].
The production of the glycoengineered recombinant antigen cocktail is patented under the intellectual property IP (European Patent Application No. EP24216456.4 entitled “Expression system for glycoengineered antigens” filed November 29, 2024). Detailed description of the production and in vitro characterization of the antigen cocktail is described by Adduci et al., 202528.
Clinical assessment and differential blood counts
The clinical assessment (e.g., body temperature, FAMACHA©) was carried out using a standardized protocol for physical examination45,46,47. Blood samples (EDTA, serum, Tempus™ Blood RNA Tubes [Thermo Fisher Scientific, Waltham, MA, USA]) were drawn from the external jugular vein at defined time points throughout the study period (Fig. 11). The sheep were weighed using Gallagher W0 digital scales (Gallagher Europe, Groningen, The Netherlands). Hematological parameters of whole blood sample, including leukocytes, erythrocytes, hemoglobin, and erythrocyte indices, were analyzed by flow cytometry (Advia® 2120i, Siemens, Erlangen, Germany). Total protein and albumin were measured by photometry (Cobas® pure C303, Roche Diagnostics International Ltd, Rotkreuz, Switzerland). During the whole study period, the vaccination was carried out after the clinical assessment and blood sample collection.
Assessment after vaccination and local injection site reaction
The 21 sheep from the BVAX, GEA and NEA groups underwent eight assessments following each vaccination. The local injection site was assessed on the day of vaccination (morning: vaccination; afternoon: first assessment), and then once daily until day 7 after vaccination, following the protocol from Stadler and coworkers, which was adapted for sheep48. The following signs of the injection site and the surrounding area were assessed: swelling (none; slight; severe), pain during palpation (yes/no), necrosis (none; slight; severe), tissue induration (none; firm; hard) and lymph node enlargement (none; slight; severe).
Preparation of soluble H. contortus proteins
Soluble proteins from adult H. contortus were extracted using a two-step homogenization process. Briefly, adult worms were resuspended in Tris-buffered saline (100 mM Tris-HCl, 100 mM NaCl, pH 7.4) supplemented with 0.02% sodium azide, 1% octylthioglucoside, and a 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The worms were first homogenized on ice for 2 min with intermittent pauses using a Turrax® dispersion tool (T10 basic, equipped with an S10N-10G-ST probe; IKA, Staufen, Germany). The homogenate was then transferred to an ice-cold Dounce homogenizer for manual homogenization. Resulted homogenate was centrifuged at 12,000 × g for 30 min at 4 °C. The supernatant was filtered through 0.22 µm sterile syringe filters and collected into sterile Falcon tubes. For larval (L3) parasites, due to the limited material, homogenization was performed in 1.5 ml tubes using disposable polypropylene pestles (Thermo Fisher Scientific). Protein concentration was measured using a Pierce™ Bradford Plus protein assay kit (Thermo Fisher Scientific).
Enzyme-linked immunosorbent assay (ELISA)
Native antigens of adult H. contortus and recombinant antigen cocktails (GEA and NEA) were diluted to a concentration of 2 μg/ml in coating buffer (200 mM carbonate/bicarbonate, pH 9.6). 96-well Nunc-Immuno™ MicroWell™ ELISA plates (Sigma-Aldrich) were coated overnight at 4 °C with 50 μl of antigens (100 ng/well). Post blocking with 2% BSA, sheep sera (dilution 1:100) were added to the wells and incubated at 37 °C for 1 h. Serum antibodies were probed with anti-sheep IgG (Sigma-Aldrich, dilution 1:10,000), IgE (Bio-Rad Laboratories Ges.m.b.H., Hercules, CA, USA; 1E7, dilution 1:10,000), IgM (Sigma-Aldrich; rabbit polyclonal, dilution 1:10,000) and IgA (Bio-Rad Laboratories Ges.m.b.H; rabbit polyclonal, dilution 1:1,000). Horseradish peroxidase(HRP)-conjugated secondary antibodies, anti-mouse IgG (Sigma-Aldrich, dilution 1:5,000) and anti-rabbit IgG (Sigma-Aldrich dilution 1:10,000), were used to react with tetramethylbenzidine-hydrogen peroxide (Thermo Fisher Scientific). Post addition of a reaction stopping buffer (Carl Roth GmbH + Co. KG, Karlsruhe, Germany; 0.5 M sulfuric acid), optical density at 450 nm (OD450) was measured using a microplate reader (FilterMax F5; Molecular Devices, San Jose, CA, USA). Total serum IgG and IgM were examined by performing direct ELISA using an anti-sheep antibody (stated above) to probe sheep sera (1:100 diluted) coated into ELISA plates.
Western blotting and metaperiodate treatment
Worm-derived and recombinant antigens were dissolved in SDS-loading dye, denatured at 95 °C and loaded on 12% SDS-PAGE gels. Precision Plus Protein™ Dual Color Standards (Bio-Rad Laboratories GesmbH) was used as protein ladder. Post electrophoresis at 200 V, proteins were transferred onto nitrocellulose membranes using a TransBlot® Turbo™ Transfer System (Bio-Rad Laboratories GesmbH). After blocking in 0.5% BSA, nitrocellulose membranes were incubated with sheep antisera, combined from the 7 sheep of the same group (dilution 1:200), then with a mouse anti-goat/sheep IgG (Sigma-Aldrich; dilution 1:10,000), and finally with an alkaline phosphatase (AP)-conjugated anti-mouse IgG (Sigma-Aldrich; Fc-specific; dilution 1:10,000). SIGMAFAST™ BCIP®/NBT (Sigma Aldrich) was used as the substrate for color development.
Protein deglycosylation was performed on-membrane using a protocol adapted from Roberts et al.25. Briefly, immunoblots were treated overnight at 4 °C with 10 mM sodium meta-periodate dissolved in 0.05 M sodium acetate, pH 4.5, 0.05 M sodium chloride buffer, prior to subsequent blocking and incubation steps. To monitor the deglycosylation efficiency, a treated blot was incubated with biotinylated Concanavalin A (Vector Laboratories, Newark, CA, USA; Conn A, dilution 1:250) in PBS buffer supplemented with 1 mM calcium chloride followed by a goat anti-biotin secondary antibody (Sigma-Aldrich; dilution 1:10,000).
Mini-FLOTAC method
Feces were sampled and analyzed individually by Mini-FLOTAC with a detection limit of five eggs per gram (EpG)49,50. Samples were weighed (5 g feces) and mixed with 45 ml of a saturated saline solution (density 1.18 g/ml) and sieved through a mesh (average mesh size: 1.3 mm) in a beaker afterwards. An equal distribution of the eggs was ensured by using a magnetic stirrer (IKA-COMBIMAG REO, Janke & Kunkel GmbH u. Co. KG, Staufen, Germany). Samples were quantitatively evaluated under the light microscope (Eclipse Ci-S, 100 x magnification, Nikon, Vienna, Austria). The EpG was calculated by multiplying the number of eggs counted in both chambers by 5 (multiplication factor for livestock feces)50.
Worm burden and worm sexing
The worm burden was evaluated after euthanizing the sheep. First, the abdominal cavity was opened, and the duodenum was ligated. The omasum was cut away to keep the content within the abomasum. Subsequently, the abomasum was removed from the gastro-intestinal bundle and put into a bucket. After opening the greater curvature, the content was transferred into a sieve with a mesh width of 150 µm and washed thoroughly. Subsequently, the entire abomasal content was transferred into a bucket for storage and further assessment. The abomasal mucosa as well as the entire abomasal content were inspected for adult worms under good light conditions. All worms were collected, separated by gender and counted within three days after sacrifice51,52.
Patho-histological examination
Abomasal and ileum tissues were collected for pathohistological examination postmortem. Briefly, abomasal tissue (location fundus region) and ileum tissue, in size of one square-centimeter, were taken immediately after opening the abdominal cavity using a scissor and forceps and fixed in 10% formalin (BiopSafe®, Vedbœk, Denmark) in a 20 ml container. Samples were collected from three sheep from each group. Subsequently, the samples were dehydrated in graded series of ethanol and embedded in paraffin wax. Thereafter, approximately 3 μm thick sections were stained with HE for further assessment.
Statistics
Descriptive and explorative statistical analysis was performed using Microsoft Excel 2010 (Microsoft, Washington, USA) and IMB SPSS® Statistics Version 29 (IBM, New York, USA). Descriptive statistics were carried out using mean, median, 25 and 75th percentile, minimum and maximum. An extreme outlier was defined as: >75th percentile plus 3 times the interquartile range (IQR). Extreme outliers are shown as asterisks in the boxplots and normal outliers are shown as circles. Normal distribution was tested using the Kolmogorov-Smirnov test. The daily weight gain in gram was calculated before (63 days) and after (40 days) challenge by using the body weight at the begin of the trial (2nd/3rd January), before challenge (27th March) and at the end of the trial (6th May). For the packed cell volume, thresholds were calculated using the 25, 50, and 75th percentile. The cumulative egg excretion was calculated by summarizing the egg excretions over time (syn. cumulative FEC). The EpG values per worm was calculated by dividing the cumulative EpG values by the number of female worms (no normal distribution). The female/male ratio was calculated by dividing female by male worms. The EpG values and worm counts were log transformed using the lg10 function in SPSS. All measures (EpG values, worm counts, blood values) were analyzed using a linear mixed model followed by a post hoc test (Sidak test) between the experimental groups. Group identification and sampling date were included as fixed values. Sampling date was included as repeated measure and sheep identification as subject. All missing values were excluded in the analysis. The Akaike’s information criterion (AIC) revealed 215.24 for the egg excretion. Comparison between groups without repeated measures was carried out using the Kruskal Wallis test ( > 2 samples) including the Bonferroni correction (e.g., worm burden, cumulative EpG), the Mann-Whitney U test (2 samples) or the Fishers exact test ( < 5 cases). For comparison between groups with a normal distribution a one-way ANOVA was carried out. For all tests the significance level was set at p ≤ 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
References
Coop, R. L. & Holmes, P. H. Nutrition and parasite interaction. Int. J. Parasitol. 26, 951–962 (1996).
Valderrábano, J., Delfa, R. & Uriarte, J. Effect of level of feed intake on the development of gastrointestinal parasitism in growing lambs. Vet. Parasitol. 104, 327–338 (2002).
Mavrot, F., Hertzberg, H. & Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: a systematic review and meta-analysis. Parasites Vectors 8, 557 (2015).
Charlier, J. et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Preventive Vet. Med. 182, 105103 (2020).
Besier, R. B., Kahn, L. P., Sargison, N. D. & van Wyk, J. A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 93, 95–143 (2016).
Edwards, E. E., Garner, B. C., Williamson, L. H., Storey, B. E. & Sakamoto, K. Pathology of Haemonchus contortus in New World camelids in the southeastern United States: a retrospective review. J. Vet. Diag. Investig. Publ. Am. Assoc. Vet. Lab. Diag. 28, 105–109 (2016).
Besier, R. B., Kahn, L. P., Sargison, N. D. & van Wyk, J. A. Diagnosis, treatment and management of Haemonchus contortus in small ruminants. Adv. Parasitol. 93, 181–238 (2016).
Charlier, J. et al. Anthelmintic resistance in ruminants: challenges and solutions. Adv. Parasitol. 115, 171–227 (2022).
Rose Vineer, H. et al. Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite 27, 69 (2020).
Untersweg, F. et al. Multispecific resistance of sheep trichostrongylids in Austria. Parasite 28, 50 (2021).
Jesse, F. F. A. et al. A veterinary clinical case of severe chronic Haemonchus contortus infection in a goat the clinical management of the case and pathology findings. Adv. Animal Vet. Sci 7, 507 (2019).
Hinney, B. et al. High rates of benzimidazole-resistance-associated alleles in Haemonchus contortus and detection of resistance against macrocyclic lactones in strongylids from German alpaca herds. Parasites Vectors 17, 296 (2024).
Jabbar, A., Campbell, A. J. D., Charles, J. A. & Gasser, R. B. First report of anthelmintic resistance in Haemonchus contortus in alpacas in Australia. Parasites Vectors 6, 243 (2013).
Adduci, I. et al. Haemonchosis in sheep and goats, control strategies and development of vaccines against Haemonchus contortus. Anim. Open Access J. MDPI 12, 12182339 (2022).
Smith, T. S., Munn, E. A., Graham, M., Tavernor, A. S. & Greenwood, C. A. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contortus. Int. J. Parasitol. 23, 271–280 (1993).
Smith, W. D., Smith, S. K. & Murray, J. M. Protection studies with integral membrane fractions of Haemonchus contortus. Parasite Immunol. 16, 231–241 (1994).
Smith, W. D., Newlands, G. F. J., Smith, S. K., Pettit, D. & Skuce, P. J. Metalloendopeptidases from the intestinal brush border of Haemonchus contortus as protective antigens for sheep. Parasite Immunol. 25, 313–323 (2003).
Bassetto, C. C. et al. Attempts to vaccinate ewes and their lambs against natural infection with Haemonchus contortus in a tropical environment. Int. J. Parasitol. 44, 1049–1054 (2014).
Bassetto, C. C. & Amarante, A. F. T. Vaccination of sheep and cattle against haemonchosis. J. Helminthol. 89, 517–525 (2015).
4c Design Ltd. Barbervax® Commercial Vaccine Production System. Available at (Submission Date: 2018).
Smith, W. Development of a commercial vaccine for Haemonchus contortus, the Barber’s Pole Worm, final report B.AHE.0068 (2014).
Nisbet, A. J., Meeusen, E. N., González, J. F. & Piedrafita, D. M. Immunity to Haemonchus contortus and Vaccine Development. Adv. Parasitol. 93, 353–396 (2016).
Newton, S. E. & Meeusen, E. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. 25, 283–296 (2003).
Reszka, N., Rijsewijk, F. A. M., Zelnik, V., Moskwa, B. & Bieńkowska-Szewczyk, K. Haemonchus contortus: characterization of the baculovirus expressed form of aminopeptidase H11. Exp. Parasitol. 117, 208–213 (2007).
Roberts, B. et al. Novel expression of Haemonchus contortus vaccine candidate aminopeptidase H11 using the free-living nematode Caenorhabditis elegans. Vet. Res. 44, 111 (2013).
Zhou, Q.-J. et al. Expression of Caenorhabditis elegans-expressed Trans-HPS, partial aminopeptidase H11 from Haemonchus contortus. Exp. Parasitol. 145, 87–98 (2014).
Wang, C. et al. H11-induced immunoprotection is predominantly linked to N-glycan moieties during Haemonchus contortus infection. Front. Immunol. 13, 1034820 (2022).
Adduci, I. et al. Glycoengineering of Nematode Antigens using Insect Cells: A Promising Approach for Producing Bioactive Vaccine Antigens of the Barberas Pole Worm Haemonchus contortus. BioRxiv 11, 646772 (2025).
Bassetto, C. C. et al. Trials with the Haemonchus vaccine, Barbervax®, in ewes and lambs in a tropical environment: nutrient supplementation improves protection in periparturient ewes. Vet. Parasitol. 264, 52–57 (2018).
Matos, A. F. I. M. D. et al. Attempt to control Haemonchus contortus in dairy goats with Barbervax®, a vaccine derived from the nematode gut membrane glycoproteins. Small Rumin. Res. 151, 1–4 (2017).
Teixeira, M. et al. Strategic vaccination of hair sheep against Haemonchus contortus. Parasitol. Res. 118, 2383–2388 (2019).
Haslam, S. M. et al. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J. Biol. Chem. 271, 30561–30570 (1996).
Geldhof, P. et al. Presence of the LDNF glycan on the host-protective H-gal-GP fraction from Haemonchus contortus. Parasite Immunol. 27, 55–60 (2005).
Broomfield, M. A., Doyle, E. K., Kahn, L. P., Smith, W. D. & Walkden-Brown, S. W. A simplified Barbervax® vaccination regimen in lambs to evoke immunological protection to Haemonchus contortus. Vet. Parasitol. 287, 109243 (2020).
Australian Pesticides and Veterinary Medicines Authority. Barbervax Barber’s Pole Worm Vaccine. APVMA Approval No: 90013/127053.
Besier, R. B., Kahn, L. P., Sargison, N. D. van Wyk, J. A. Chapter Six - Diagnosis, Treatment and Management of Haemonchus contortus in Small Ruminants. In Adv. Parasitology and Haemonchosis Past, Present and Future Trends, edited by R. B. Gasser and G. V. Samson-Himmelstjerna (Academic Press, 2016).
Wildblood, L. A. et al. Production of eosinophil chemoattractant activity by ovine gastrointestinal nematodes. Vet. Immunol. Immunopathol. 107, 57–65 (2005).
Vervelde, L. et al. Protection studies with recombinant excretory/secretory proteins of Haemonchus contortus. Parasite Immunol. 24, 189–201 (2002).
McRae, K. M., Stear, M. J., Good, B. & Keane, O. M. The host immune response to gastrointestinal nematode infection in sheep. Parasite Immunol. 37, 605–613 (2015).
Balic, A., Cunningham, C. P. & Meeusen, E. N. T. Eosinophil interactions with Haemonchus contortus larvae in the ovine gastrointestinal tract. Parasite Immunol. 28, 107–115 (2006).
Terefe, G. et al. In vitro pre-exposure of Haemonchus contortus L3 to blood eosinophils reduces their establishment potential in sheep. Vet. Res. 38, 647–654 (2007).
Flay, K. J., Hill, F. I. & Muguiro, D. H. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals Open Access J. MDPI 12, 12101238 (2022).
National Centre for the Replacement, Refinement & Reduction of Animals in Research. ARRIVE guidelines. Available at http://arriveguidelines.org/.
González-Sánchez, M. E., Cuquerella, M. & Alunda, J. M. Vaccination of lambs against Haemonchus contortus with the recombinant rHc23. Effect of adjuvant and antigen dose. PLOS One 13, e0193118 (2018).
Baumgartner, W. & Wittek, T. (eds.). Klinische Propädeutik der Haus- und Heimtiere. 9th ed. (Enke, 2017).
Larsen, J. W., Anderson, N., Vizard, A. L., Anderson, G. A. & Hoste, H. Diarrhoea in merino ewes during winter: association with trichostrongylid larvae. Aust. Vet. J. 71, 365–372 (1994).
Van Wyk, J. A. & Bath, G. F. The FAMACHA© system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33, 509–529 (2002).
Stadler, J. et al. Safety and immune responses after intradermal application of Porcilis PRRS in either the neck or the perianal region. PLOS One 13, e0203560 (2018).
Cringoli, G., Rinaldi, L., Maurelli, M. P. & Utzinger, J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 5, 503–515 (2010).
Cringoli, G. et al. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 12, 1723–1732 (2017).
Veglia, F. The Anatomy and Life History of the Haemonchus contortus (Rud.). Government Printer and Stationery Office: Pretoria, South Africa (1916).
Burden, D. J. et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.): Third edition of the guideline for evaluating efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet. Parasitol. 329, 110187 (2024).
Acknowledgements
We thank Drs Dietmar Hamel and Steffen Rehbein for sharing their expertise at multiple occasions during the whole project period. Special thanks go to the students who supported the whole team during the trial, especially Gudrun Gerstner, Judith Hauth, Vinzent Leonfellner, Roman Rupprechter, Christina Schroll, Vanessa Stadlmann, Regina Walch and Glenn Wang. Additionally, we thank Roman Peschke, Sonja Rohrer, Bärbel Ruttkowski, Maria Unterköfler, and Hugo Weidinger at the Institute of Parasitology who supported us in the laboratory and the Clinical Pathology Platform who performed the hematological examinations. Special thanks go to René Brunthaler who supported us in preparing the pathology images.
Author information
Authors and Affiliations
Contributions
S.Y., K.L., A.J., T.W., and D.W. conceived the project. S.Y., K.L., A.J., T.W., F.SG., I.A., B.H., and D.W. contributed to the design of the methodology. S.Y., K.L., F.SG., I.A., D.W., and A.T. carried out the formal analysis. S.Y., K.L., F.SG., I.A., J.E., J.Z., B.P., L.W., S.W., and B.H. performed the experiments and collected the data. A.J., T.W., B.H., S.Y., K.L., F.SG., I.A., S.W., and L.W. provided the resources in terms of labor resources, materials, reagents and analysis tools. F.S.G. and K.L. prepared the initial draft of the manuscript. F.S.G., K.L., and I.A. visualized the data. K.L., S.Y., T.W., A.J., B.H., and D.W. acted as supervisors. S.Y., K.L., F.S.G., and I.A. managed the project administration. S.Y., K.L., T.W., and A.J. acquired the funding. F.S.G., K.L., S.Y., A.J., D.W., T.W., I.A., B.H., L.W., B.P., S.W., J.Z., J.E., and A.T. reviewed and edited the manuscript and accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application related to this work has been filed at the European Patent Office (application No. EP24216456.4). All other authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sajovitz-Grohmann, F., Adduci, I., Werling, D. et al. Safety and efficacy of a novel glycoengineered recombinant vaccine candidate against Haemonchus contortus in sheep. npj Vaccines 10, 190 (2025). https://doi.org/10.1038/s41541-025-01249-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-025-01249-z