Abstract
Rapid production of doubled haploids (DHs) through isolated microspore culture is an important and promising method for genetic study of alfalfa. To induce embryogenesis in alfalfa, isolated microspores were submitted to abiotic stresses during their initial culture, in order to stimulate them to form embryos and plantlets. ‘Baoding’ and ‘Zhongmu No 1’ alfalfa cultivars supported reproducible and reliable proliferation response irrespective of any stress treatment of microspores. The microspore developmental stage for isolated microspore culture was studied and we found that uninucleate microspores were best to initiate culture. Exposure of microspores to appropriate low temperature or heat shock stresses were able to increase the efficiency of embryogenesis. The most effective low-temperature treatment was 4 °C for 24 h and the frequency of plantlets induction was 20.0%. The most effective heat shock treatment was 32 °C for 2 d and the frequency of plantlets induction was 14.17%. The analysis of ploidy level performed by flow cytometer revealed that the majority of 278 regenerated plantlets were haploid (65.83%) or doubled haploid (33.81%). This is the first report of haploid production in alfalfa through isolated microspore culture.
Similar content being viewed by others
Introduction
Development of haploid plants through isolated microspore culture is an efficient method that are useful in genetic studies. The haploid plants have the gametic chromosome number (n) and when the chromosome number of a haploid have undergone spontaneous or induced chromosome duplication by chromosome doubling agents, it is termed as a doubled haploid (DH) with chromosome number 2n. For basic research the haploids have many important advantages. Haploids provide beneficial tools for genome research which aim to elucidate the structure function and evolution of past and present genomes. Doubled haploids (DHs) are genetically normal and it provides a unique opportunity to screen gametophytic variation caused by recombination and segregation during meiosis. Even the recessive variations can express itself in the absence of their dominant counterparts in such DHs plants. Isolated microspore culture and anther culture are the two major methods used for haploid production1. The regenerated plants produced by anther culture are composed of mixoploids and it is difficult and laborious to detect ploidy of the plants. The more advantageous method is regenerating plants by isolated microspore culture because haploids or DHs are produced. Isolated microspore culture has recently been used more frequently in many species, e.g., tobacco2, wheat3, barley4, canola5, rice6, cabbage7, pepper8, and maize9, for which efficient induction protocols are available10.
Alfalfa (Medicago sativa L.) is one of the most important legume forage which plays a vital role in livestock production. According to the statistics of the National Alfalfa & Forage Alliance, an estimated 32 million ha of alfalfa were grown annually in the world11. Success in the production of haploid plants from tetraploid alfalfa was achieved by crossing tetraploids (4X) with a diploid (2X) pollen parent to yield haploid progeny12,13. Further work by Zagorska et al.14,15 demonstrated conclusively that haploid plants could also be generated from alfalfa through anther cultures. In their studies, anthers were pretreated for four days at 4 °C and treated with 1 Gy gamma radiation prior to culture. Of 100 regenerants, a total of only four haploid plants were produced. The frequency of haploid production in alfalfa by the 4X-2X cross and anther culture methods is very low. Efficient microspore embryogenesis in culture is successfully induced by the application of abiotic stress and other pretreatments in a number of species16, including legumes17. Therefore, rapid production of haploid plants through microspore culture can be an important and promising method for genetic study of alfalfa18; however, haploid plants have not been obtained in alfalfa via isolated microspore culture.
The genotype, growth conditions of donor plants, the developmental stage of microspores, culture medium, and different stress treatments have been reported to influence the efficiency of embryogenesis in isolated microspore culture10,17,19. The microspore developmental stage has been extensively studied with late uninucleate and early binucleate microspores being most responsive17,20,21,22. The positive effects of modifying the osmotic pressure of the medium on microspore embryogenesis have been observed previously in many species, including legumes17. Short heat shock treatments (32 °C for 24–72 h) during the early stage of isolated microspore culture have been required to induce microspore embryogenesis in Brassica 23,24. Exposure of microspores to a low temperature (4 °C for 24–48 h) preceding the heat shock treatment reportedly promotes microspores embryogenesis25,26. Furthermore, the additions of a high level of sugar17, activated charcoal27,28 and colchicine29 in the induction medium were found to increase microspore embryogenesis. In conclusion, apart from selection of a genotype with embryogenic capacity and a suitable microspore development stage, it is important for microspores to be subject to the action of a single stress or several kinds of stress to induce embryos and plant formation22.
Against this background, the objectives of this study were to test the effects of genotype, microspore development stage, low temperature, and heat shock on microspore embryogenesis in alfalfa and to establish an efficient protocol for haploid production in alfalfa using isolated microspore culture.
Results
Microspore embryogenesis
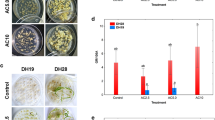
Flower buds (Fig. 1A) were harvested from young inflorescences. Then isolated microspores were cultured in NLN-13 liquid medium. Initial development of the microspores in culture was visible as an enlargement of the cells within 5–8 days of culture. Some of these swollen microspores (Fig. 1B) continued apparent gametophytic development whereas other moderately swollen ones showed sporophytic cell division, forming multicellular embryogenic clusters after approximately 15 days in culture. Well-developed embryos visible to the naked eye were formed after approximately 21 days of culture (Fig. 1C). Embryos with two or three leaves were placed on solid B5 medium. Plantlets with four or five leaves (Fig. 1E) were transferred to soil and acclimatized in a greenhouse.
Development of ‘Baoding’ alfalfa microspores in culture. (A) Flower buds at different developmental stages. (B) Swollen microspores after cell division, the arrows in B indicate these are swollen microspores. (C) Embryos of various sizes after 21 days of culture. (D) Callus formation stage. (E) Plantlets on B5 medium. (F) Uninucleate microspores, the arrows in F indicate these are uninucleate microspores.
Effect of genotype on alfalfa microspore embryogenesis
A range of genotypes was tested during our experiments. Significant variation in microspore embryogenesis among genotypes was found (Table 1). Initial microspore division was observed for all genotypes at different frequencies, and resulted in the formation of microcalluses. For some genotypes of alfalfa, growth was arrested very early. Ultimately, out of the 10 genotypes studied, only ‘Baoding’ and ‘Zhongmu No. 1’ alfalfa (Table 1) proliferated first to calluses (Fig. 1D) and then to plantlets (Fig. 1E). The greatest frequency of plantlet induction was found in ‘Baoding’ alfalfa and the frequency of plantlets induction was 7.5% (Table 1). The others remained recalcitrant, whatever the medium, treatment or culture conditions, indicating that genotype is the main factor governing androgenetic capacity in these species. A high frequency of plantlet formation was achieved for ‘Baoding’ alfalfa in microspore culture, therefore this genotype was used as plant material in the following experiments.
Effect of microspore developmental stage on alfalfa microspore embryogenesis
The developmental stage of microspores (tetrad, early uninucleate, late uninucleate, and binucleate stages) was assessed for improving embryo yield in isolated microspore culture. The best embryogenic development was from uninucleate microspores (Fig. 1F; Table 2) when flower buds were 6.02–6.20 mm long and 1.50–1.72 mm wide (Table 3). Earlier stages contained mainly tetrads that did not survive in culture, while at later stages microspores were already unable to shift from gametophytic to sporophytic development required for proliferation conductive to the ultimate formation of embryos.
Effect of low-temperature treatment on microspore embryogenesis
The effect of low-temperature treatment applied to isolated microspores on embryogenesis of ‘Baoding’ alfalfa is displayed in Table 4. The best proliferation responses were obtained with isolated microspores that were cultured at 4 °C for 24 h in the dark. After treatment with this temperature, the frequency of plantlets induction was 20.0%, whereas the frequency of plantlets induction was 10.0% in the control treatment combination. Isolated microspores that had been subjected to cold stress at 4 °C for 24 h showed increased viability during the culture. However, it was observed that cold treatment for more than 24 h had no positive effect on the percentage of microspore division.
Effect of heat shock stress on microspore embryogenesis
Performance of ‘Baoding’ alfalfa in isolated microspore culture after heat shock stress treatment at 32 °C is shown in Table 5. We found that an appropriate heat shock stress treatment had a positive effect on isolated microspore cultures. The most effective stimulant was the use of 32 °C for 2 d. From isolated microspores treated with a heat shock stress for 2 d, the frequency of plantlets induction was 14.17%, whereas by establishing isolated microspore culture without the heat shock stress treatment, the frequency of plantlets induction was 7.5%.
Analysis of ploidy level
In total, 278 plantlets, obtained through isolated microspore culture, were assessed for their ploidy level. The representative results on flow cytometric DNA analysis of cells in microspore-derived plantlets were shown in Fig. 2. Ploidy level of the plantlets was recorded in Table 6. The majority of plants were haploid and doubled haploid, with 65.83% haploid and 33.81% doubled haploid. In the microspore-derived populations, in addition to haploid and double haploid, there was a low frequency of higher ploidy. One octoploid plant (0.36%) was found in the population.
Discussion
Under certain conditions, microspores can shift development from gametophytic to sporophytic to form embryos and plantlets30. However, most of legume species are highly recalcitrant to microspore embryogenesis. Difficulties were posed by the generally small anther size and relatively low numbers of microspores per anther in legume crops31. In the present study, various efforts have been made to induce microspore embryogenesis in alfalfa. To the best of our knowledge, this is the first report of microspore-derived plantlet formation in alfalfa. The haploid and DHs plants obtained in our study are promising for applications in genetic studies. In the study, only 277 haploid and DH plants were produced from thousands of flower buds from ‘Baoding’ alfalfa indicating the necessity to improve the protocol for haploid production in alfalfa. Further work is under way to improve microspore responses and extend them to other genotypes.
To establish an efficient protocol for microspore embryogenesis in alfalfa, the effects of genotype, stage of microspore development, low-temperature treatment, and heat shock stress on microspore culture were studied in the present study. Of the 10 genotypes, only the microspores of ‘Baoding’ and ‘Zhongmu No. 1’ alfalfa proliferated to yield calluses and plantlets when microspores were not exposed to any specific treatment, indicating that genotype is crucial for microspore response. Results also demonstrated that ‘Baoding’ alfalfa is a highly responsive cultivar in isolated microspore culture and thus it can be used as experimental material in future studies. Additionally, the greatest amount microspore-derived plantlet formation in ‘Baoding’ alfalfa were observed when microspores were in the early and late uninucleate develemental stages. Low temperature and heat shock stresses had positive effects on plantlet formation in culture. The most effective stimulant was exposure to 4 °C for 24 h or 32 °C for 2 d, the frequency of plantlets induction was 20.0% and 14.17%, respectively. Apart from the above-mentioned parameters, addition of active carbon to medium16, kinds of medium14,18, culture conditions18, pyramiding electrostimulation17, and osmotic shock17, alone or in combination14, can also affect the efficiency of embryogenesis in microspore culture of alfalfa. Therefore, the effect of these parameters on induction of microspore embryogenesis in alfalfa will be studied in future studies.
The present study centered on a single factor influencing haploid production efficiency via inducing microspore embryogenesis in alfalfa and low temperature treatment is more efficient than heat shock stress. Pyramiding some stress agents is a promising way forward to trigger the switch of isolated microspores from the gametophytic to sporophytic developmental pathway in extremely recalcitrant genotypes30. A combination of osmotic pressure and electric shock significantly improved responses from isolated microspores of some legume species and yielded calluses17. Yuan et al.32 reported that the conjunction of cold pretreatment and heat shock stress, as compared to a single stress treatment alone, increased microspore embryogenesis in broccoli. Further efforts will be designed to improve microspore responses by pyramiding different stress agents.
Materials and Methods
Plant materials
Ten different genotypes of alfalfa, ‘Zhongmu No. 1’, ‘Zhongmu No. 6’, ‘Gongnong No. 1’, ‘Caoyuan No. 2’, ‘Baoding’, ‘Aohan’, ‘Longdong’, ‘WL323’, ‘Golden Empress’, and ‘Sanditi’, were used as donor materials. They were obtained from different seed companies. The donor plants were grown in plastic pots in horticultural peat substrate (one plant per pot) in a greenhouse. The alfalfa plants started to flower 9–10 weeks after sowing. Forty flower buds of appropriate size for microspore culture were collected per genotype and three replicates were undertaken.
Isolation and culture of microspores
For all genotypes, flower buds containing microspores at appropriate developmental stages were surface-sterilized in 75% ethanol for 30 s, followed by 10 min in 10% (v/v) bleach, and finally, five sterilized water rinses. Microspores were isolated by crushing the flower buds in B5 liquid medium (pH 5.8)33, with three successive steps of suspension and centrifugation (100 × g, 5 min each) to eliminate debris remaining after serial sieving through 40 um filters. During the whole isolation procedure all liquids and centrifugation were kept at 4 °C. The microspore concentration was determined with a hemocytometer. After counting, the microspores were finally suspended at a density of approximately 1 × 105 microspores ml−1 in NLN-13 liquid medium34 and incubated for culture in 60 mm × 15 mm Petri dishes (5 ml per dish). We added 100 ul of activated charcoal (1%) to the medium. The dishes were sealed with Parafilm. Then different stress treatments were applied in the dark, and thereafter cultures were maintained at 25 °C under dark conditions. When calluses reached a minimum diameter of 5 mm, they were transferred onto the induction medium as reported elsewhere for Medicago truncatula 35. Thus, embryogenesis proceeded through the typical successive stages (i.e. globular, heart, torpedo and cotyledonary) of somatic embryo induction, expression, growth and ultimate conversion to plants. Converted embryos with two or three leaves were placed on solid B5 medium33. Plantlets with four or five leaves were transferred to soil and acclimatized in a greenhouse.
Cytological analysis
During the culture of microspores, morphological characteristics and developmental pathways of microspores were observed by fluorescence microscopy. The microspores were collected by centrifugation, stained with 4′,6-diamidino, 2-phenylindole (DAPI) and observed under a Olympus SZX10 fluorescence microscope (Olympus, Japan). The determination of morphological characteristics and developmental pathways was conducted following the procedure described by Barinova et al.36.
Determination of the developmental stage of microspores
Flower buds were harvested from their identification to the opening of petals. Microspores isolated from flower buds at the various developmental stages (tetrad, early uninucleate, late uninucleate, and binucleate stages) were cultured and the optimum developmental stage for proliferation from microspores was identified. Prior to culturing, the stage of microspores was checked under microscope. This was performed by staining with 4% acetocarmine (W/V) in 50% acetic acid (v/v)37. Forty buds from each stage were collected and the lengths of the buds were measured.
Low temperature and heat shock treatments
At the optimum stage of microspore development, they were isolated from flower buds following the procedures outlined previously. Then they were cultured at 4 °C in the dark for 0, 1, 2, 3, 4, 5, 6 d or, alternatively, they were heat-shocked at 32 °C for 0, 3, 6, 12, 24, 48 h. Thereafter, cultures were maintained at 25 °C in the dark. The experiment was conducted with three replicates and each replicate consisted of forty buds. The number of plantlets obtained from isolated microspore cultures were counted after six weeks.
Determination of ploidy
Ploidy level was determined by a flow cytometer and the determination was carried out according to the procedures of Galbraith38. Approximately 20 mg of young leaves were crumbled with a razor blade in 0.1 M citric acid containing 0.5% Tween 20. After adding fluorochrome 4′,6-diamidino-2-phenylindole DAPI at 0.1 mg mL−1, samples were filtered through a 50-μm nylon filter. Measurements were done on a flow cytometer (BD FACSCalibur, USA) using a linear scale. Ploidy level was assessed using tetraploid alfalfa genotype as control.
Data analysis
All experiments were repeated three times, each including at least 10 replicate dishes (6 cm in diameter). The data were analyzed using ANOVA with mean separation by Duncan’s or LSD Multiple Range Test at a 95% confidence level.
Change history
13 September 2019
This paper has been retracted.
References
Asif, M. Androgenesis: A Fascinating Doubled Haploid Production Process in Progress and Opportunities of Doubled Haploid Production (ed. Asif, M.) 7–44 (New York, Dordrecht, London, Springer Cham Heidelberg, 2013).
Oliveira, E. D. Optimization of doubled haploid production in burley tobacco (Nicotiana tabacum L.). [doctoral theses]. [Lexington (Kentucky)]: University of Kentucky (2016).
Cistué, L. et al. Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep. 25, 257–264 (2006).
Kasha, K. J. et al. An improved in vitro technique for isolated microspore culture of barley. Euphytica 120, 379–385 (2001).
Chuong, P. V. & Beversdorf, W. D. High frequency embryogenesis through isolated microspore culture in Brasscica napus L. and B. Carinata braun. Plant Sci. 39, 219–226 (1985).
Raina, S. K. & Irfan, S. T. High-frequency embryogenesis and plantlet regeneration from isolated microspores of indica rice. Plant Cell Rep. 17, 957–962 (1998).
Rudolf, K., Bohanec, B. & Hansen, M. Microspore culture of white cabbage, Brassica oleracea var. capitata L.: Genetic improvement of non-responsive cultivars and effect of genome doubling agents. Plant Breeding 118, 237–241 (2010).
Lantos, C. et al. Improvement of isolated microspore culture of pepper (Capsicum annuum L.) via co-culture with ovary tissues of pepper or wheat. Plant Cell Tiss. Org. 97, 285–293 (2009).
Gaillard, A., Vergne, P. & Beckert, M. Optimization of maize microspore isolation and culture conditions for reliable plant regeneration. Plant Cell Rep. 10, 55–58 (1991).
Shariatpanahi, M. E. & Ahmadi, B. Isolated Microspore Culture and Its Applications in Plant Breeding and Genetics in Plant Tissue Culture: Propagation, Conservation and Crop Improvement, (eds Anis, M. & Ahmad, N.) 487–507 (Singapore, Springer, 2016).
NAFA (the National Alfalfa & Forage Alliance). Data from: the National Alfalfa & Forage Alliance, http://www.alfalfa.org/ (2016).
Bingham, E. T. Haploids from cultivated alfalfa, Medicago sativa L. Nature 221, 865–866 (1969).
Bingham, E. T. Isolated of haploids of tetraploid alfalfa. Crop Sci. 15, 889 (1971).
Zagorska, N. & Dimitrov, B. Induced androgenesis in alfalfa (Medicago sativa L.). Plant Cell Rep. 14, 249–252 (1995).
Zagorska, N., Dimitrov, B., Gadeva, P. & Robeva, P. Regeneration and characterization of plants obtained from anther cultures in Medicago sativa L. In Vitro. Cell. Dev-Pl. 33, 107–110 (1997).
Islam, S. M. S. & Tuteja, N. Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci. 182, 134–144 (2012).
Ochatt, S. et al. Abiotic stress enhances androgenesis from isolated microspores of some legume species (Fabaceae). J. Plant Physiol. 166, 1314–1328 (2009).
Croser, J. S. et al. Toward doubled haploid production in the Fabaceae: progress, constraints, and opportunities. Crit. Rev. Plant Sci. 25, 139–157 (2006).
Wędzony, M. et al. Progress in Doubled Haploid Technology in Higher Plants in Advances in Haploid Production in Higher Plants (eds. Touraev, A., Forster, B. P., & Jain S. M.) 1–33 (Netherlands, Springer, 2009).
Bhowmik, P., Dirpaul, J., Polowick, P. & Ferrie, A. M. R. A high throughput Brassica napus microspore culture system: influence of percoll gradient separation and bud selection on embryogenesis. Plant Cell Tiss. Org. 106, 359–362 (2011).
Grewal, R. K. et al. Doubled-haploid production in chickpea (Cicer arietinum L.): role of stress treatments. Plant Cell Rep. 28, 1289–1299 (2009).
Ribalta, F. M., Croser, J. S. & Ochatt, S. J. Flow cytometry enables identification of sporophytic eliciting stress treatments in gametic cells. J. Plant Physiol. 169, 104–110 (2012).
Keller, W. A. & Armstrong, K. C. Stimulation of embryogenesis and haploid production in Brasscia campestris anther culture by elevated temperature treatment. Theor. Appl. Genet. 55, 65–67 (1979).
Zoriniants, S., Tashpulatov, A. S., Heberle-Bors, E. & Touraev, A. The Role of Stress in the Induction of Haploid Microspore Embryogenesis in Haploids in Crop Improvement II-Biotechnology in Agriculture and Forestry (eds Don Palmer, C., Keller, W. A. & Kasha K. J.) 35–52 (Berlin, Springer, 2005).
Gu, H. H., Hagberg, P. & Zhou, W. J. Cold pretreatment enhances microspore embryogenesis in oilseed rape (Brassica napus L.). Plant Growth Regul. 42, 137–143 (2004).
Kiszczak, W., Kowalska, U., Kapuścińska, A., Burian, M. & Górecka, K. Effect of low temperature on in vitro androgenesis of carrot (Daucus carota L.). In Vitro Cell. Dev-Pl, https://doi.org/10.1007/s11627-015-9665-1 (2015).
Lichter, R. Efficient yield of embryoids by culture of isolated microspores of different Brassicaceae species. Plant Breeding 100, 320–322 (1989).
Zhou, W. J., Hagberg, P. & Tang, G. X. Increasing embryogenesis and doubling efficiency by immediate colchicine treatment of isolated microaspores in spring Brasscia napus. Euphytica 128, 27–34 (2002).
Leskovšek, L., Jakše, M. & Bohanec, B. Doubled haploid production in rocket (Eruca sativa Mill.) through isolated microspore culture. Plant Cell Tiss. Org. 93, 181–189 (2008).
Maraschino, S. F., de Priester, W., Spaink, H. P. & Wang, M. Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J. Exp. Bot. 56, 1711–1726 (2005).
Maluszynski, M., Kasha, K. J. & Szarejko, I. Published Doubled Haploid Protocols in Plant Species in Doubled Haploid Production in Crop Plants (eds. Maluszynski, M., Kasha, K. J., Forster, B. P. & Szarejko I.) 309–335 (Dordrecht, Kluwer Academic Publishers, 2003).
Yuan, S. X. et al. Effect of combined cold pretreatment and heat shock on microspore cultures in broccoli. Plant Breeding 130, 80–85 (2011).
Gamborg, O. L., Miller, R. A. & Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50, 151–158 (1968).
Lichter, R. Induction of haploid plants from isolated pollen of Brassica napus. Zeitschrift für Pflanzenphysiologie 105, 427–434 (1982).
Ochatt, S., Muilu, R. & Ribalta, F. Cell morphometry and osmolarity as early indicators of the onset of embryogenesis from cell suspension cultures of grain legumes and model systems. Plant Biosyst. 142, 480–486 (2008).
Barinova, I. et al. Regulation of developmental pathways in cultured microspores of tobacco and snapdragon by medium pH. Planta 219, 141–146 (2004).
Last, D. I. & Brettell, R. I. Embryo yield in wheat anther culture is influenced by the choice of sugar in the culture medium. Plant Cell Rep. 9, 14–16 (1990).
Galbraith, D. W. Flow Cytometric Analysis of the Cell Cycle in Laboratory Procedures and Their Applications (ed. VasilI. K.) 765–777 (Massachusetts, Academic Press, 1984).
Acknowledgements
This work was supported by Beijing Natural Science Foundation (6184047), the Modern Agricultural Industry Technology Research System (CARS34), the Fundamental Research Funds for Central Non-profit Scientific Institution (2018-YWF-YB-4), and the Agricultural Science and Technology Innovation Program (ASTIP-IAS10) of China.
Author information
Authors and Affiliations
Contributions
D.Y. and Z.W. designed the experiments. D.Y., J.S. and Y.S. performed the experiments, analyzed the data, and wrote the manuscript; Z.T. and T.Z. performed the experiments. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted by the authors because it was submitted without the knowledge or consent of the last author.
All authors agreed to the retraction.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yi, D., Sun, J., Su, Y. et al. RETRACTED ARTICLE: Doubled haploid production in alfalfa (Medicago sativa L.) through isolated microspore culture. Sci Rep 9, 9458 (2019). https://doi.org/10.1038/s41598-019-45946-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-45946-x
This article is cited by
-
Techniques and advantages of microspore culture for crop improvement
Plant Growth Regulation (2025)