Abstract
Postoperative acute exacerbation of interstitial lung disease (AE-ILD) can be fatal in patients with lung cancer concomitant with ILD. We aimed to elucidate the predictive potential of high-mobility group box 1 (HMGB1), which is associated with the development and severity of lung injury, for evaluating the risk of this complication. We included 152 patients with lung cancer and ILD who underwent radical surgery between January 2011 and August 2019. We evaluated the preoperative levels of serum HMGB1 and its predictive potential for postoperative AE-ILD. Postoperative AE-ILD developed in 17 patients. Serum levels of HMGB1 were significantly higher in patients with postoperative AE-ILD than in those without (median [interquartile range]: 5.39 [3.29–11.70] ng/mL vs. 3.55 [2.07–5.62] ng/mL). Univariate and multivariate logistic regression analyses revealed that higher HMGB1 levels were significantly associated with the development of postoperative AE-ILD in entire studied patients (n = 152). In the subgroup analysis, higher HMGB1 levels were associated with a significantly increased risk of this complication in patients who underwent lobectomy (n = 77) than in those who underwent sublobar resection (n = 75). Serum HMGB1 could be a promising marker for evaluating the risk of postoperative AE-ILD, specifically in patients who underwent lobectomy.
Similar content being viewed by others
Introduction
High-mobility group box 1 (HMGB1) is a nuclear protein that is involved in DNA organization and the regulation of transcription1. HMGB1 is released passively during cellular necrosis by almost all cells. It is also secreted actively by immune cells, including monocytes, macrophages, and dendritic cells2. Circulatory HMGB1 binds to pattern recognition receptors on the cell surface, such as the receptor for advanced glycation end product (RAGE) and toll-like receptor (TLR). Their interactions result in the activation of pro-inflammatory responses associated with acute lung injury3,4. We have previously reported that serum levels of HMGB1 are significantly higher in patients with idiopathic pulmonary fibrosis (IPF) than in healthy subjects, and its higher levels at baseline are associated with the earlier development of acute exacerbation of IPF (AE-IPF)5. Additionally, when AE-IPF is developed, HMGB1 levels are further increased compared to those in IPF patients without AE5,6. Although the trigger for AE-IPF has not been sufficiently elucidated, these data suggest that higher levels of HMGB1 play a role in the development of lung injury superimposed with interstitial lung disease (ILD), including IPF.
Postoperative AE-ILD is a fatal complication in patients with lung cancer treated with radical surgery, especially when concomitant with ILD. Previous reports have shown that postoperative AE-ILD occurs in 9.3% of patients with lung cancer and ILD and has a mortality rate of 43.9%7. Although lobectomy, as a standard radical treatment, is generally associated with a higher risk of postoperative AE-ILD, compared to sublobar resection7, the pathophysiology of this fatal adverse event is still unclear. Several studies have demonstrated that HMGB1-induced lung injury is accelerated during surgery. For example, mechanical ventilation induces elevated expression of HMGB1 in lung tissue and bronchoalveolar lavage fluid (BALF), and anti-HMGB1 antibody can ameliorate ventilation-induced lung injury8,9. These data led us to speculate that HMGB1 is associated with the development of postoperative AE-ILD as well as AE-IPF, and thus we hypothesize that baseline levels of circulatory HMGB1 can be a predictive blood marker of this severe complication.
This study aimed to elucidate the association between serum levels of HMGB1 and the development of postoperative AE-ILD in patients with lung cancer concomitant with ILD. First, the association of baseline levels of serum HMGB1 with the incidence rate of postoperative AE-ILD was analyzed. Second, its association was separately evaluated in the subgroups treated with lobectomy and those treated with sublobar resection. Finally, a logistic regression model was used to identify the independent risks of this severe complication.
Results
Patient characteristics
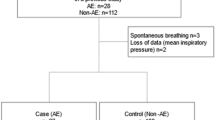
The subjects were 157 patients with lung cancer and ILD who underwent radical surgery and had blood samples collected before surgery. After excluding 5 patients who had another primary cancer or active autoimmune diseases, 152 patients with lung cancer and ILD were included in the analysis (Table 1). Of the 152 patients, 139 patients had idiopathic interstitial pneumonia and 13 patients had secondary ILD related to well-controlled autoimmune disease. In this study, operative time exhibited a positive correlation with bleeding volume (rs = 0.640, P < 0.001) (Figure not shown).
The patients with postoperative AE-ILD were male dominant and had significantly lower vital capacity (VC) (% predicted), lower forced expiratory volume in one second (FEV1) (% predicted), longer operative time, and greater bleeding volume than patients without postoperative AE-ILD (Table 1). Additionally, 1/17 patients with postoperative AE-ILD and 5/135 patients without were treated with pirfenidone before surgery. There was no association between pirfenidone and AE-ILD (P = 0.664). Only one patient with postoperative AE-ILD was treated with induction chemotherapy prior to surgery.
The association of baseline concentration of HMGB1 with postoperative AE-ILD and baseline characteristics
Overall, 17 patients developed postoperative AE-ILD. Serum levels of HMGB1 were significantly higher in patients with postoperative AE-ILD than in those without (median [IQR]: 5.39 [3.29–11.70] ng/mL vs. 3.55 [2.07–5.62] ng/mL, P = 0.031) (Fig. 1). Higher baseline levels of HMGB1 were significantly and independently associated with younger age, higher pack-year smoking history, and lower VC (Table 2).
Baseline levels of serum HMGB1. Serum levels of HMGB1 in patients with postoperative acute exacerbation of interstitial lung disease (AE-ILD) were higher than those in patients without (median [IQR]: 5.39 [3.29–11.70] ng/mL vs. 3.55 [2.07–5.62] ng/mL, P = 0.031). Boxes represent the 25th to 75th percentiles; solid lines within the boxes show the median values; whiskers represent the 10th and 90th percentiles; the circles represent outliers. * P < 0.05 using the Mann–Whitney U test. IQR, interquartile range.
The risk stratification of postoperative AE-ILD based on HMGB1
Receiver operating characteristic (ROC) curve analysis revealed that the optimal cut-off level of serum HMGB1 for predicting postoperative AE-ILD was 3.82 ng/mL (area under the curve [AUC] 0.661, specificity 51.9%, and sensitivity 76.5%). Serum levels of HMGB1 > 3.82 ng/mL was significantly associated with a higher incidence of postoperative AE-ILD in the entire population (Fig. 2a). To elucidate the influence of surgery as the trigger for postoperative AE-ILD, the incidence was evaluated separately by the type of surgical intervention: lobectomy and sublobar resection. Subgroup analysis revealed that the association was significant in the lobectomy group, but not in the sublobar resection group (Fig. 2b,c).
The incidence of postoperative AE-ILD based on HMGB1 and type of surgical intervention. The cut-off level for HMGB1 was 3.82 ng/mL. In the entire population, the incidence of postoperative acute exacerbation of interstitial lung disease (AE-ILD) in patients with HMGB1-high was significantly higher than that in patients with HMGB1-low (a). It was also significant in the subgroup treated with lobectomy (b), but not in those treated with sublobar resection (c). * P < 0.05 using Pearson’s chi-squared tests.
Univariate logistic regression analysis revealed that lower VC, lower FEV1, longer operative time, greater bleeding volume, and higher levels of serum HMGB1 were significantly associated with increased risk of postoperative AE-ILD (Table 3a). Because of the significant correlation between operative time and bleeding volume, they were separately included in the multivariate logistic regression analysis. In the first multivariate analysis, operative time and higher levels of serum HMGB1 were significantly associated with the development of postoperative AE-ILD in the entire population (n = 152). In the subgroup analysis, higher HMGB1 significantly increased the risk of this complication in the patients who underwent lobectomy (n = 77) (Table 3b). In the second multivariate analysis including bleeding volume, both higher HMGB1 and greater bleeding volume were significantly associated with an increased risk of AE-ILD in the entire population as well as in the subgroup of patients who underwent lobectomy (Table 3c).
To investigate this discrepancy based on lobectomy and sublobar resection, exploratory analysis revealed that the patient subgroup treated with lobectomy had a significantly younger age, higher DLco, larger primary tumor size, longer operative time, and greater bleeding volume than those treated with sublobar resection (Table 4). Among these five factors, operative time and bleeding volume were significantly different between the patients with and without postoperative AE-ILD (Table 1).
Operative time longer than 200 min (AUC 0.671, specificity 58.9%, and sensitivity 80.0%) was associated with a higher incidence of this fatal complication (25.0% vs. 6.9%, P = 0.005). When the operative time was co-analyzed with HMGB1, combination of “higher HMGB1 and longer operative time” was associated with the highest incidence of this complication compared to those with either HMGB1-high or operative time-long and those with neither (35.0%, 10.8%, and 3.4%, respectively) (Fig. 3a). Additionally, ROC curve analysis, which was used to predict postoperative AE-ILD using HMGB1 and operative time, provided an AUC of 0.728, which is higher than that calculated by each of HMGB1 and operative time (0.661 and 0.671, respectively).
The risk stratification based on HMGB1 and factors related to surgical invasiveness. The cut-off levels of operative time and bleeding volume were 200 min and 155 mL, respectively. When HMGB1 and operative time/bleeding volume were combined, the incidence of postoperative acute exacerbation of interstitial lung disease (AE-ILD) in patients with both HMGB1-high and operative time-long/bleeding volume-high was significantly higher than that in the other two groups (a,b). *P < 0.05, using Pearson’s chi-squared tests.
Bleeding volume greater than 155 mL (AUC 0.720; specificity 88.9%; sensitivity 52.9%) was also associated with a higher incidence of AE-ILD (32.0% vs. 7.1%, P < 0.001). When the bleeding volume was co-analyzed with HMGB1, a combination of “higher HMGB1 and higher bleeding volume” was associated with the highest incidence of AE-ILD (46.2%), compared to those with either HMGB1-high or bleeding volume-high (11.7%) and those with neither (3.2%) (Fig. 3b). Additionally, ROC curve analysis was used to predict postoperative AE-ILD using HMGB1 and bleeding volume, and the AUC of 0.750 was higher than that calculated by HMGB1 and operative time each (0.661 and 0.720, respectively).
Discussion
Postoperative AE-ILD is a fatal complication, and thus, it is important to increase its predictive accuracy before surgery. This study showed that higher levels of serum HMGB1 were significantly associated with a higher incidence of postoperative AE-ILD in patients with lung cancer concomitant with ILD, especially in patients who underwent lobectomy. Additionally, the significance of HMGB1 for predicting this fatal complication was also confirmed when adjusted for lower pulmonary function parameters, which are well-known risk factors for postoperative comorbidities. No blood markers for predicting postoperative AE-ILD caused by lung resection have been reported; therefore, this study showed that HMGB1 can be a promising biomarker for predicting postoperative AE-ILD in patients with lung cancer and ILD, especially in those treated with lobectomy.
This study is the first to show that higher levels of HMGB1 were significantly associated with a higher incidence of postoperative AE-ILD in patients with lung cancer and ILD. We and others previously reported that HMGB1 levels in serum and BALF in patients with IPF were significantly elevated compared to those in healthy subjects5,10. Additionally, circulatory levels of HMGB1 are elevated in patients with lung cancer11,12. Importantly, HMGB1 itself accelerates pro-inflammatory signaling by interacting with RAGE and TLR43,4, and intratracheal administration of HMGB1 induces acute lung injury13. This study also demonstrated that there was a negative correlation between HMGB1 and VC. These observations suggest that higher levels of circulating HMGB1 may accelerate underlying lung damage as well as indicate its severity. Thus, its higher levels can predict the development of postoperative AE-ILD.
The association of higher levels of serum HMGB1 with higher incidence of postoperative AE-ILD was significant only when the patients underwent lobectomy, which required longer operative time and greater bleeding volume, in this study. Additionally, this exploratory study showed that patients with both higher HMGB1 and longer operative time/higher bleeding volume had a significantly increased risk of postoperative AE-ILD than patients with either higher HMGB1 or longer operative time/higher bleeding volume and patients with neither. When the operative time increases, it requires a longer time for artificial ventilation, which increases the expression of HMGB1 in BALF and lung tissue in a tidal volume- and time-dependent manner8,9. Exposure to hyperoxia during artificial ventilation also promotes the increased expression of HMGB1 in a time-dependent manner14. This mechanical ventilation- and hyperoxia-induced lung injury can be attenuated by inhibition of HMGB18,14. Additionally, longer operative time was correlated with greater bleeding volume, which is also reported to increase the expression of HMGB1 in the plasma and lung15. These observations suggest that lobectomy, which requires longer operative time, might be associated with an accelerated inflammatory response via HMGB1 secretion. Thus, lobectomy results in the development of postoperative AE-ILD, especially in patients with higher levels of preoperative HMGB1.
Lobectomy is generally performed as a standard radical treatment for lung cancer, but it is also associated with higher postoperative morbidity of complications, especially when the patient has concurrent ILD16. Preventing this fatal complication is crucial to improve the long-term outcomes in patients with lung cancer and ILD who underwent surgical resection. According to this study, one potential preventive strategy is that patients with higher HMGB1 would be treated with sublobar resection because it generally requires shorter operative time than lobectomy. Another potential strategy is to use HMGB1-inhibitory drugs. Some existing drugs have been reported to inhibit HMGB1-induced inflammatory signaling by decreasing HMGB1 secretion and capturing HMGB117.
Another aspect of this study was to confirm the pathogenic association between AE-ILD caused by known triggers and increased HMGB1 levels. The diagnostic criteria of AE-IPF revised in 2016 have demonstrated two types of AE: idiopathic and triggered AE. Triggered AEs can be caused by surgery and drug toxicity. In line with this study showing the association between higher serum HMGB1 levels and a higher risk of postoperative AE, we have previously reported that higher levels of HMGB1 are associated with an earlier onset of cytotoxic chemotherapy-induced lung injury in patients with advanced lung cancer and ILD11. It should be noted that these two potential triggers, such as chemotherapy and surgery, have been reported to increase the expression of HMGB18,9,15,18. These data imply that the additional release of HMGB1 by triggers of AE promotes the development of triggered AE, especially when baseline levels of HMGB1 are high. Therefore, the combination of HMGB1 with invasiveness of surgery may be able to stratify the risk of AE-ILD with higher accuracy compared to HMGB1 alone. Further investigations are needed to elucidate the utility of serum HMGB1 as a predictive biomarker and molecular target for preventing triggered AE, including postoperative AE-ILD, in patients with lung cancer and ILD.
This study has several limitations. First, this was a single-institution study, and the number of patients with postoperative AE-ILD was relatively small. These factors may substantially impact the generalizability of the study findings. Multicenter studies with a larger sample size are needed to validate the predictive value and optimal cut-off levels of HMGB1. Second, the main source of circulating HMGB1 was not identified in this study. HMGB1 may be elevated not only by the presence of ILD, but also by lung cancer. We and others have reported that HMGB1 is elevated by the presence of as well as the progression of lung cancer11,12. However, this study revealed a significant association between HMGB1 and VC, but not with FEV1, DLco, primary tumor size, cancer stage, and fluorodeoxyglucose uptake in cancer. Further investigations are warranted to elucidate the associations between cancer-derived HMGB1 with its magnitude and influence on lung damage, and the development of postoperative AE-ILD.
In conclusion, higher levels of circulatory HMGB1 are associated with postoperative AE-ILD in patients with lung cancer and ILD. This might be more useful in patients treated with lobectomy as a standard radical surgery.
Methods
Study design and population
This retrospective cohort study consecutively included lung cancer patients with ILD who collected blood samples before surgery and underwent radical surgery for lung cancer between January 2011 and August 2019 at the Hiroshima University Hospital. The type of surgical intervention was determined as follows: sublobar resection was mainly performed in patients unable to tolerate lobectomy due to poor pulmonary function. Additionally, sublobar resection was considered for small (≤ 20 mm) tumors. Wedge resection or segmentectomy was performed for peripherally or centrally located tumors, respectively. The staging of lung cancer was according to the TNM Classification of Malignant Tumors, 8th edition19.
This study was approved by the Ethics Committee of Hiroshima University Hospital. All research was performed in accordance with the ethical standards established by the Helsinki Declaration of 1975. To research the risk of postoperative AE-ILD, all participants provided written informed consent for obtaining and using the blood samples before surgery (Gen-38). Additionally, an opt-out method was used to inform the patients or legally authorized representatives that serum levels of HMGB1 was measured by using the already gathered samples (E-1707).
Diagnostic criteria for ILD and postoperative AE-ILD
The diagnostic criteria for ILD were the presence of bilateral reticulation and consolidation or ground-glass attenuation on high-resolution computed tomography (HRCT). The HRCT pattern was classified according to the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association (ATS/ERS/JRS/ALAT) clinical practice guideline of IPF. It was classified as usual interstitial pneumonia (UIP) pattern, probable UIP pattern, indeterminate UIP pattern, and alternative diagnosis20.
Postoperative AE-ILD caused by lung resection was defined based on the criteria proposed by previous studies for the disease and the ATS/ERS/JRS/ALAT statement as follows: (i) acute worsening or development of dyspnea and oxygen desaturation; (ii) CT showing new ground-glass abnormality bilaterally and/or consolidation; (iii) no evidence of other alternative causes, such as pulmonary infection, cardiac failure, pulmonary embolism, and pneumothorax; and (iv) development of AE-ILD within 30 days after lung resection21,22,23.
Measurement of serum HMGB1 concentrations
Serum samples were collected before surgery at our hospital and stored at − 80 °C. Serum levels of HMGB1 were measured using commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions (HMGB1 ELISA Kit II [Shino-Test Corporation, Tokyo]).
Statistical analysis
Values are expressed as mean ± standard deviation or median (IQR) according to their distribution. Normally and non-normally distributed variables were evaluated using the t-test and Mann–Whitney U test, respectively. Pearson’s chi-squared tests were also conducted. Linear regression analysis was conducted to study the independent effects of baseline characteristics on serum HMGB1 levels. Logistic regression analysis was conducted to identify independent risk factors for the development of postoperative AE-ILD and to estimate the respective odds ratios and their 95% confidence intervals (CI). Confounders with a p-value < 0.05 according to the univariate analysis were included in the multivariate analysis. ROC curve analysis was performed to identify the optimal cut-off levels of serum HMGB1, operative time, and bleeding volume for predicting the development of postoperative AE-ILD. All statistical analyses were performed using JMP version 14.1.0 (SAS Institute Inc., Cary, NC, USA). A P-value of less than 0.05 was considered statistically significant.
Abbreviations
- AE:
-
Acute exacerbation
- ALAT:
-
Latin American Thoracic Association
- ATS:
-
American Thoracic Society
- AUC:
-
Area under the curve
- BALF:
-
Bronchoalveolar lavage fluid
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- DLco:
-
Diffusing capacity for carbon monoxide
- ERS:
-
European Respiratory Society
- FDG:
-
Fluorodeoxyglucose
- FEV1:
-
Forced expiratory volume in one second
- FVC:
-
Forced vital capacity
- HMGB1:
-
High-mobility group box 1
- HRCT:
-
High-resolution computed tomography
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IQR:
-
Interquartile range
- JRS:
-
Japanese Respiratory Society
- KL-6:
-
Krebs von den Lungen 6
- OR:
-
Odds ratio
- RAGE:
-
Receptor for advanced glycation end product
- ROC:
-
Receiver operating characteristic
- SUV:
-
Standard uptake value
- TLR:
-
Toll-like receptor
- UIP:
-
Usual interstitial pneumonia
- VC:
-
Vital capacity
References
Klune, J. R., Dhupar, R., Cardinal, J., Billiar, T. R. & Tsung, A. HMGB1: Endogenous danger signaling. Mol. Med. 14, 476–484. https://doi.org/10.2119/2008-00034.Klune (2008).
Ulloa, L. & Messmer, D. High-mobility group box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev. 17, 189–201. https://doi.org/10.1016/j.cytogfr.2006.01.003 (2006).
Deng, Y. et al. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS ONE 8, e64375. https://doi.org/10.1371/journal.pone.0064375 (2013).
Wu, X. et al. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-kappaB pathway. Mol. Cell. Biochem. 380, 249–257. https://doi.org/10.1007/s11010-013-1680-0 (2013).
Yamaguchi, K. et al. Serum high-mobility group box 1 is associated with the onset and severity of acute exacerbation of idiopathic pulmonary fibrosis. Respirology 25, 275–280. https://doi.org/10.1111/resp.13634 (2020).
Abe, S. et al. Reduction in serum high mobility group box-1 level by polymyxin B-immobilized fiber column in patients with idiopathic pulmonary fibrosis with acute exacerbation. Blood Purif. 32, 310–316. https://doi.org/10.1159/000330325 (2011).
Sato, T. et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J. Thorac. Cardiovasc. Surg. 147, 1604-1611.e1603. https://doi.org/10.1016/j.jtcvs.2013.09.050 (2014).
Ogawa, E. N. et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 174, 400–407. https://doi.org/10.1164/rccm.200605-699OC (2006).
van Zoelen, M. A. et al. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock 29, 441–445. https://doi.org/10.1097/SHK.0b013e318157eddd (2008).
Hamada, N. et al. The role of high mobility group box1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 39, 440–447. https://doi.org/10.1165/rcmb.2007-0330OC (2008).
Nakao, S. et al. Serum high-mobility group box 1 as a predictive marker for cytotoxic chemotherapy-induced lung injury in patients with lung cancer and interstitial lung disease. Respir. Med. 172, 106131. https://doi.org/10.1016/j.rmed.2020.106131 (2020).
Shang, G. H. et al. Serum high mobility group box protein 1 as a clinical marker for non-small cell lung cancer. Respir. Med. 103, 1949–1953. https://doi.org/10.1016/j.rmed.2009.05.019 (2009).
Ueno, H. et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am. J. Respir. Crit. Care Med. 170, 1310–1316. https://doi.org/10.1164/rccm.200402-188OC (2004).
Entezari, M. et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2, 314–322. https://doi.org/10.1016/j.redox.2014.01.013 (2014).
Kim, J. Y. et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. 288, L958-965. https://doi.org/10.1152/ajplung.00359.2004 (2005).
Chiyo, M. et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: Analyses of short-term and long-term outcomes. J. Thorac. Cardiovasc. Surg. 126, 1141–1146. https://doi.org/10.1016/s0022-5223(03)00791-8 (2003).
Venereau, E. et al. HMGB1 as biomarker and drug target. Pharmacol. Res. 111, 534–544. https://doi.org/10.1016/j.phrs.2016.06.031 (2016).
Haruna, M. et al. Docetaxel upregulates HMGB1 levels in non-small cell lung cancer. Biol. Pharm. Bull. 43, 399–403. https://doi.org/10.1248/bpb.b19-00702 (2020).
Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 11, 39–51. https://doi.org/10.1016/j.jtho.2015.09.009 (2016).
Raghu, G. et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, e44–e68. https://doi.org/10.1164/rccm.201807-1255ST (2018).
Tsutani, Y. et al. Outcomes after lobar versus sublobar resection for clinical stage I non-small cell lung cancer in patients with interstitial lung disease. J. Thorac. Cardiovasc. Surg. 154, 1089-1096.e1081. https://doi.org/10.1016/j.jtcvs.2017.03.116 (2017).
Sato, T. et al. A simple risk scoring system for predicting acute exacerbation of interstitial pneumonia after pulmonary resection in lung cancer patients. Gen. Thorac. Cardiovasc. Surg 63, 164–172. https://doi.org/10.1007/s11748-014-0487-6 (2015).
Collard, H. R. et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am. J. Respir. Crit. Care Med. 194, 265–275. https://doi.org/10.1164/rccm.201604-0801CI (2016).
Acknowledgements
This work was supported by JSPS KAKENHI (grant number 18K15951).
Author information
Authors and Affiliations
Contributions
K.Y. and S.N. performed data analysis and interpretation; K.Y. drafted the manuscript; and K.Y. designed the study; K.Y., S.N., H.I., Y.T., and Y.M. interpreted the data. All authors edited the manuscript and were involved in patient enrollment and data collection. Additionally, all authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamaguchi, K., Nakao, S., Iwamoto, H. et al. Predictive role of circulatory HMGB1 in postoperative acute exacerbation of interstitial lung disease in lung cancer patients. Sci Rep 11, 10105 (2021). https://doi.org/10.1038/s41598-021-89663-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-89663-w
This article is cited by
-
Predictive role of circulatory levels of high-mobility group box 1 for radiation pneumonitis in patients with non-small cell lung cancer treated with definitive thoracic radiotherapy
International Journal of Clinical Oncology (2022)