Abstract
Expanded hemodialysis (HDx) with medium cutoff (MCO) membranes, which remove middle-to-large molecules well, may be a good option to replace online hemodiafiltration (online-HDF). To provide more evidence, this randomized controlled trial compared several cardiovascular parameters between patients undergoing HDx and online-HDF. Eighty patients undergoing thrice-weekly hemodialysis were randomly assigned to receive either HDx with a Theranova membrane (n = 43) or online-HDF (n = 37). The primary endpoints were changes in brachial-ankle pulse wave velocity (baPWV), echocardiographic parameters, and coronary artery calcium (CAC) scores over 1 year, and the secondary endpoints included blood cardiovascular biomarkers, mortality, and patient-reported outcomes. A linear mixed model and log-rank test were used to estimate the group differences. 65 patients had completed the trial. The changes in baPWV and echocardiographic parameters did not differ between the two groups. The CAC scores remained stable in the online-HDF group, whereas an increasing trend was shown in the HDx group (P = 0.012). Other endpoints, including cardiovascular and all-cause mortalities, were similar between the two groups. The changes in cardiovascular parameters did not differ between HDx with an MCO membrane and online-HDF. However, attention may be needed in patients with high CAC scores or scores with an increasing tendency when online-HDF is replaced with HDx with an MCO membrane.
Similar content being viewed by others
Introduction
Cardiovascular disease is a leading cause of morbidity and mortality in patients with end-stage renal disease (ESRD)1. In addition to traditional cardiovascular risk factors, nontraditional factors, including uremic toxins, electrolyte and fluid imbalance, anemia, and inflammation, contribute to worsening cardiovascular outcomes in ESRD2,3,4,5. In particular, uremic toxins induce oxidative stress and vascular inflammation and remodeling6,7,8,9. Furthermore, uremic conditions induce platelet activation and aggregation, which leads to thrombus formation7,8. All of these factors can explain the high risk of cardiovascular diseases in patients with ESRD.
Uremic toxins are removed by hemodialysis (HD), but diffusion-based approaches in the conventional mode are limited in their ability to completely remove these toxins10. Given the potential association of middle-to-large uremic molecules but not small molecules with a high risk of cardiovascular disease11,12, the goal of treatment has been to enhance removal of these uremic toxins via HD membranes with increased permeability13,14. The technique used to increase the removal rate of middle-to-large molecules includes the use of high-flux membranes plus high dialysis intensity and alternative hemofiltration15. Although high-flux membranes improve the clearance of smaller middle molecules (e.g., β2-microglobulin), larger middle solutes (e.g., free light chains, most cytokines) may not be the target10. An increase in dialysis intensity has less effect on the plasma levels of potential cardiovascular toxins, which ultimately cannot improve patient survivals16,17. Online hemodialfiltration (online-HDF) is a great option for removing middle-to-large molecules with the help of ultrafiltration and subsequent convection. The online-HDF seems to represent the gold standard in removing uremic toxins among hemodialysis types18. This dialysis technique was related to improved cardiovascular outcomes in some studies19,20, although others did not show cardiovascular benefits related to online-HDF21,22,23,24,25,26. Despite the enhanced removal of middle-to-large molecules, it is difficult to make wide use of online-HDF due to the high cost, technical burden, and simultaneous risk of albumin loss15.
Expanded HD (HDx) is a treatment in which diffusion and convection are integrated inside a dialyzer equipped with a medium cutoff (MCO) membrane27. This novel HD method has shown greater removal of middle-to-large molecules than conventional HD28,29,30,31. However, it is unknown whether the use of MCO membranes confers cardiovascular benefits compared with other dialysis techniques. Because online-HDF was associated with improved cardiovascular survival compared with a high-flux dialyzer in some pooled analyses19,20, this randomized, controlled study tried to address the noninferiority of HDx with an MCO membrane to online-HDF in terms of cardiovascular outcomes. For this purpose, several cardiovascular parameters, such as brachial-ankle pulse wave velocity (baPWV), echocardiography, and blood markers, were traced over a one-year study period.
Methods
Study design
The study was a multicenter, prospective, open-label, randomized trial with prevalent HD patients from the dialysis units of four tertiary referral hospitals in South Korea. The study was approved by the review boards of all hospitals (Samsung Medical Center, 2018-04-047; Seoul St. Mary’s Hospital, KC18DEDV0209; Seoul National University Bundang Hospital, B-1804-463-401; and Seoul National University Hospital, D-1711-065-899) and was conducted in accordance with the principles of the Declaration of Helsinki. The study was registered at Clinical Research Information Service (https://cris.nih.go.kr/cris; ID, KCT0003188; date of registration, September 19th, 2018). The protocol was registered at Clinicaltrials.gov (ID: NCT03448887). All patients were recruited between April 2018 and May 2019 and signed an informed consent form to participate in the study. No changes were made to the procedures or study outcomes after trial commencement.
Patients
Patients who underwent thrice-weekly in-center maintenance HD for > 3 months were eligible for the study if they met the following criteria: age 18 years old or older, receiving HD with a high-flux dialyzer for at least 1 month before enrollment, and providing informed consent. The exclusion criteria were as follows: receiving online-HDF or using MCO membranes before study enrollment, undergoing HD more or less than three times per week, having concurrent peritoneal dialysis, planning kidney transplantation within 1 year, having advanced or active cancer, having monoclonal and polyclonal gammopathies, being pregnant or planning to be pregnant, and being enrolled in other clinical trials.
Treatment procedures
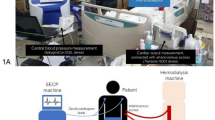
Patients were randomly assigned in a 1:1 ratio to receive HDx with a Theranova membrane (Theranova 400; Baxter International Inc., Hechingen, Germany) in the study group or online-HDF (Artis Physio system; Baxter International Inc.) with a high-flux dialyzer (Polyflux 170H or 210H dialyzer; Baxter International Inc.) in the control group using a web-based randomization system. Randomization was stratified by the participating center and patient age (≥ 65 or < 65 years old). HD was conducted in three 4-h sessions per week, and the postdilution volume-controlled mode with a target convective ultrafiltration volume of ≥ 19 L and a dialysate flow rate of ≥ 500 mL/min was used in online-HDF. Adverse events during the study period were noted whether or not they were related to the treatments.
Data collection
Baseline clinical information was collected, such as age, sex, dialysis vintage, type of vascular access, comorbidities including diabetes mellitus, and previous history of myocardial infarction, cerebrovascular disease, congestive heart failure, peripheral vascular disease, and kidney transplantation. Kt/V was calculated by the second-generation formula for single-pool values to determine the appropriate level of HD.
All cardiovascular parameters were recorded at baseline and 6 and 12 months after enrollment. The primary endpoints were the changes in cardiovascular parameters, such as baPWV, echocardiographic parameters, and coronary artery calcium (CAC) scores, from baseline to 12 months after enrollment. baPWV was measured using a noninvasive vascular testing device (VP-1000 plus; Colin Co. Ltd., Japan). The values were obtained from the arm contralateral to the patient’s vascular access. Transthoracic two-dimensional echocardiography was conducted to estimate the left ventricular ejection fraction (LVEF) and left ventricular mass index (LVMI)32. Using pulsed-wave spectral Doppler tissue images, the early transmitral inflow (E) and early diastolic mitral annular peak (e′) velocities were calculated to display E/e′ as an indicator of LV diastolic function. Noncontrast cardiac computed tomography was performed to obtain the CAC score. The Agatston score was used to calculate the CAC scores, which ranged from 0 to several thousand, indicating extensive coronary atherosclerosis33. The CAC scores were available in 41 patients in the HDx group and 35 patients in the online-HDF group, because 4 patients had stents in coronary arteries. Blood cardiovascular biomarkers, such as troponin I and T, brain natriuretic peptide (BNP), and N-terminal prohormone of BNP (NT-proBNP), were measured using electrochemiluminescence immunoassays. Plasma interleukin (IL)-6 was measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Inc., Minneapolis, MN, USA). Other blood markers, such as albumin, calcium, phosphate, and high-sensitivity C-reactive protein, were also recorded by immunoturbidimetry and colorimetric assays.
Patient-reported outcomes, such as the Dialysis Symptom Index (DSI)34, the degree of fatigue35, and the recovery time after dialysis36, were collected as secondary endpoints. The DSI contained 30 items targeting specific and common physical and emotional symptoms of HD patients. The degree of fatigue after dialysis ranged from 0 to 10, with high degrees indicating worse fatigue. The patients were requested to respond to a single open-ended question, “How long does it take you to recover from a dialysis session?” The recovery time was scored at five levels as follows: 1, within minutes; 2, upon arriving home; 3, by bedtime; 4, by the next morning; and 5, by the next dialysis session. Mortality and its causes were also evaluated based on prospective monitoring.
Statistical analysis
The sample size was driven by the primary endpoint, baPWV. When standard deviations of 0.5 m/s and 0.4 m/s in the online-HDF and conventional HD, respectively, from the best estimates were assumed37, a total of 74 patients were needed to provide 80% power at a two-sided alpha level of 0.05, to detect a mean difference of 0.3 m/s between the effect of the two modalities. We had added approximately 10% more participants to account for potential dropouts, resulting in a final enrollment goal of 80 participants (40 per group).
All analyses were conducted based on the intention-to-treat principle. Categorical and continuous variables are expressed as proportions and the means ± standard deviations for normally distributed variables and as the median with interquartile range for nonnormally distributed variables. A comparison of baseline characteristics was performed with the unpaired t-test or Mann–Whitney U test for continuous variables and with the chi-square test for categorical variables. Linear mixed-effects models were used to assess changes from baseline and differences between groups in primary and secondary endpoints with treatment assignment and time as fixed effects and patients as random effects. The estimated changes at 6 and 12 months in each group and the differences between groups (i.e., the HDx group relative to the online-HDF group) are presented with means and 95% confidence intervals. Because there was no prespecified plan to adjust for multiple comparisons, P values and their corresponding 95% confidence intervals were not adjusted for multiple tests, and a Bonferroni-adjusted significance of 0.025 (i.e., 0.05 divided by two) was applied to account for two tests for between-group differences at 6 and 12 months. Kaplan–Meier survival curves were drawn to evaluate the all-cause and cardiovascular mortalities. The log-rank test was used to compare survival curves. Cox proportional hazard ratio models were conducted to calculate hazard ratios of the mortality risk. A P value less than 0.05 was considered significant in other analyses. All of the analyses were performed using STATA software (version 16.1; StataCorp LP, College Station, TX, USA) and GraphPad Prism (version 9.0.2; GraphPad Software, Inc., La Jolla, CA, USA).
Results
Baseline characteristics of patients
Eighty-six patients were initially assessed for eligibility for the study between April 2018 and May 2019. Six patients were excluded because they refused to participate in the study. Finally, a total of 80 patients were randomized, and 65 patients were followed up until the end of the study (Fig. 1). Among 5 patients who withdrew consents in the HDx group after starting trial, 2 patients refused to receive tests, 2 patients switched to other hemodialysis modes due to patient need, and 1 patient was transferred to another center. There was no withdrawal of consent in the online-HDF group after starting trial. The mean age of all patients was 62 ± 14 years old, and 58.8% were men. The mean Kt/V value was 1.71 ± 0.33. The mean value of achieved convective volume adjusted by weight was 0.3 ± 0.1 L/kg/session in the online-HDF group. Other baseline characteristics are shown in Table 1. These baseline characteristics did not differ between the two groups.
Cardiovascular parameters
At baseline, the cardiovascular parameters did not differ between the two groups (Table 2). The changes in baPWV and echocardiographic parameters such as LVEF, LVMI, and E/e′ were not different between the two groups (Table 2). Figure S1A,B, which show the trends of the parameters over the study period, support these results. The CAC scores remained stable in the online-HDF group, whereas an increasing trend was shown in the HDx group (Table 2, Fig. S1C). When some of the baseline characteristics that had a P value of < 0.1 in the comparison analysis (e.g., sex, diabetes mellitus, previous history of cardiovascular disease, and dialysis vintage) were further adjusted, the overall results remained unaltered (Table S1). As a reference, the baseline values of and changes in calcium and phosphate did not differ between the two groups (Table S2).
Blood cardiovascular biomarkers
The changes in blood biomarkers, including troponins I and T, BNP, NT-proBNP, high-sensitivity C-reactive protein, and IL-6, did not differ between the two groups (Table 3). When additional factors were adjusted, the overall results remained unaltered (Table S1). The change in albumin levels over the study period did not differ between the two groups, with between-group differences of − 0.06 (− 0.20 to 0.09) at 6 months and − 0.10 (− 0.25 to 0.05) at 12 months (Ps > 0.05).
All-cause and cardiovascular mortality
There were 6 deaths (7.5%; 3 in the HDx group and 3 in the online-HDF group) during the study period (Table S3). Of them, 2 patients died due to cardiovascular events. As shown in Fig. 2, the survival curves for cardiovascular and all-cause mortality did not differ between the two groups. When the Cox models were applied, the HDx and online-HDF groups had similar risks of cardiovascular and all-cause mortality (Table S4).
Patient-reported outcome
The baseline DSI scores, degree of fatigue, and recovery time after dialysis were not different between the two groups (Table 4). The change trends in these endpoints did not differ between the two groups.
Discussion
Uremic toxin, a result of ESRD, is associated with high cardiovascular risk. However, conventional HD may not be enough to remove all uremic toxins, particularly middle-to-large molecules. This prospective, open-label, multicenter, randomized, controlled trial indicated that HDx with an MCO membrane was not inferior to online-HDF in terms of cardiovascular risk according to the change trends in the values of baPWV, LVEF, LVMI, E/e′, and other blood biomarkers. Additionally, the changes in patient-reported outcomes did not differ between the two groups. However, compared with the online-HDF group, the HDx group had an increasing trend in CAC scores, although this endpoint was not followed in some patients.
Previous studies have shown the superiority of HDx with a Theranova membrane to conventional HD in removing middle-to-large molecules while ensuring retention of albumin14,28,29,30,31,38. A representative randomized, controlled trial wherein 86 patients received HDx with a Theranova membrane and 86 other patients received conventional high-flux dialyzer identified a great reduction in middle-to-large molecules such as free light chains, complements, and cytokines when using HDx, but serum albumin levels were maintained similarly between the two groups30. However, there are no studies that address the effects of HDx on cardiovascular endpoints, such as surrogate markers and mortality, and this clinical trial was the first to compare cardiovascular parameters between HDx and online-HDF.
Five randomized, controlled trials have been conducted in different European countries to determine the benefits for survival and other outcomes (e.g., cardiovascular events, blood biomarkers, and quality of life) in online-HDF22,23,24,25,26. Of them, the Estudio de Supervivencia de Hemodiafiltración On-Line trial was the only one to show a reduction in all-cause and cardiovascular mortalities for the online-HDF arm compared with the high-flux HD arm. None of the others showed elevated cardiovascular survival, although the clearance of middle-to-large molecules improved in online-HDF. Corresponding meta-analyses reported survival and cardiovascular benefits for online-HDF compared with conventional HD20,21,39,40,41. Post hoc and pooled analyses suggest that a high convection volume may be needed to show better outcomes in online-HDF than in conventional HD22,23,25. European regulatory authorities have allowed online-HDF to be used in patients with uncontrolled hyperphosphatemia and those suffering from complications such as amyloidosis21,42, but this issue may not be similarly accepted in other countries because of limitations in setting up online-HDF. Before conducting online-HDF, new machines, quality control in substituting fluids, and trained staff are needed, all of which limit its utilization, particularly in primary clinics or developing countries43. The present results may support the interchangeability of HDx with an MCO membrane when online-HDF is recommended in terms of cardiovascular benefit.
In terms of uremic status, vascular remodeling, characterized by a high degree of both intimal and medial calcifications, is promoted via bone-related proteins and transformation of vascular smooth muscle cells into osteoblastic-like cells7,9,44. The CAC, a marker of vascular remodeling in ESRD patients45,46, seemed to be worsening in the HDx group compared with the online-HDF group. However, not only were these results based on subgroup analysis because of missing data in some patients, but the normal aging process might mask the changes caused by uremia-related calcification, particularly in elderly patients9. Nevertheless, the choice of HD modality between HDx with an MCO membrane and online-HDF may be affected, particularly when CAC scores are aggravated along with HD.
Previous studies have shown that patients on online-HDF had better quality of life than patients on conventional HD47,48, but one randomized, controlled trial (n = 334 in high-flux HD; n = 347 in online-HDF) did not demonstrate a benefit of online-HDF using the Kidney Disease Quality of Life-Short form survey49. In the case of HDx with an MCO membrane, a randomized, controlled trial (n = 24 in HDx with a Theranova membrane; n = 25 in high-flux dialyzer) demonstrated the benefit of HDx in quality of life, although another randomized, controlled trial (n = 86 in HDx with a Theranova membrane; n = 86 in high-flux dialyzer) showed no difference between groups50. The present trial measured three different patient-reported outcomes, because these are more predictive of outcomes than the Kidney Disease Quality of Life-Short form35,36,51,52, and found no difference between HDx and online-HDF. Based on these results, HDx with an MCO membrane may be a good alternative to online-HDF in patients receiving conventional HD who want to use the latter in order to improve their quality of life.
Although the trial results are informative, there are some issues that need to be discussed. The trial was conducted only in Korean patients, and thus, the dialysis settings or the risk of cardiovascular disease may be different in other populations. The study duration and sample size were relatively short and low, respectively. Most of the measurements were conducted in the individual hospitals, not in a central laboratory, which might increase the measurement variability, although the same protocol was used. Other important parameters were not examined, such as residual kidney function, intradialytic hemodynamic stability, and nutritional status.
HDx with an MCO membrane was not inferior to online-HDF in terms of cardiovascular parameters. Accordingly, the former can be a good alternative to the latter. This issue may be more important in clinics where online-HDF is not available. However, when online-HDF is replaced with HDx with an MCO membrane, attention may be needed in patients with a high CAC score or a CAC score with an increasing trend. Large clinical trials with other populations and long-term follow-up durations are needed to confirm the present results.
References
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32, S112–S119. https://doi.org/10.1053/ajkd.1998.v32.pm9820470 (1998).
Zoccali, C., Mallamaci, F. & Tripepi, G. Novel cardiovascular risk factors in end-stage renal disease. J. Am. Soc. Nephrol. 15(Suppl 1), S77–S80. https://doi.org/10.1097/01.asn.0000093240.84097.fe (2004).
Zoccali, C., Mallamaci, F. & Tripepi, G. Traditional and emerging cardiovascular risk factors in end-stage renal disease. Kidney Int. Suppl. https://doi.org/10.1046/j.1523-1755.63.s85.25.x (2003).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538. https://doi.org/10.1046/j.1523-1755.2002.00600.x (2002).
Cheung, A. K. et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 58, 353–362. https://doi.org/10.1046/j.1523-1755.2000.00173.x (2000).
Ravid, J. D. & Chitalia, V. C. Molecular mechanisms underlying the cardiovascular toxicity of specific uremic solutes. Cells. https://doi.org/10.3390/cells9092024 (2020).
Fujii, H., Goto, S. & Fukagawa, M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins (Basel). https://doi.org/10.3390/toxins10050202 (2018).
Moradi, H., Sica, D. A. & Kalantar-Zadeh, K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am. J. Nephrol. 38, 136–148. https://doi.org/10.1159/000351758 (2013).
London, G. M. Cardiovascular calcifications in uremic patients: Clinical impact on cardiovascular function. J. Am. Soc. Nephrol. 14, S305–S309. https://doi.org/10.1097/01.asn.0000081664.65772.eb (2003).
Ronco, C. & Clark, W. R. Haemodialysis membranes. Nat. Rev. Nephrol. 14, 394–410. https://doi.org/10.1038/s41581-018-0002-x (2018).
Sun, J. et al. Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin. J. Am. Soc. Nephrol. 11, 1163–1172. https://doi.org/10.2215/CJN.10441015 (2016).
Spoto, B. et al. Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin. J. Am. Soc. Nephrol. 10, 232–240. https://doi.org/10.2215/CJN.07000714 (2015).
Humes, H. D., Fissell, W. H. & Tiranathanagul, K. The future of hemodialysis membranes. Kidney Int. 69, 1115–1119. https://doi.org/10.1038/sj.ki.5000204 (2006).
Boschetti-de-Fierro, A., Voigt, M., Storr, M. & Krause, B. MCO membranes: Enhanced selectivity in high-flux class. Sci. Rep. 5, 18448. https://doi.org/10.1038/srep18448 (2015).
Schiffl, H. Online hemodiafiltration and mortality risk in end-stage renal disease patients: A critical appraisal of current evidence. Kidney Res. Clin. Pract. 38, 159–168. https://doi.org/10.23876/j.krcp.18.0160 (2019).
Eknoyan, G. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 347, 2010–2019. https://doi.org/10.1056/NEJMoa021583 (2002).
FHN Trial Group et al. In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 363, 2287–2300. https://doi.org/10.1056/NEJMoa1001593 (2010).
Ronco, C. Hemodiafiltration: Technical and clinical issues. Blood Purif. 40(Suppl 1), 2–11. https://doi.org/10.1159/000437403 (2015).
Mercadal, L. et al. Hemodiafiltration versus hemodialysis and survival in patients with ESRD: The French renal epidemiology and information network (REIN) registry. Am. J. Kidney Dis. 68, 247–255. https://doi.org/10.1053/j.ajkd.2015.11.016 (2016).
Mostovaya, I. M. et al. Clinical evidence on hemodiafiltration: A systematic review and a meta-analysis. Semin. Dial. 27, 119–127. https://doi.org/10.1111/sdi.12200 (2014).
Peters, S. A. et al. Haemodiafiltration and mortality in end-stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transplant. 31, 978–984. https://doi.org/10.1093/ndt/gfv349 (2016).
Ok, E. et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transplant. 28, 192–202. https://doi.org/10.1093/ndt/gfs407 (2013).
Maduell, F. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 24, 487–497. https://doi.org/10.1681/ASN.2012080875 (2013).
Locatelli, F. et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J. Am. Soc. Nephrol. 21, 1798–1807. https://doi.org/10.1681/ASN.2010030280 (2010).
Grooteman, M. P. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J. Am. Soc. Nephrol. 23, 1087–1096. https://doi.org/10.1681/ASN.2011121140 (2012).
Morena, M. et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 91, 1495–1509. https://doi.org/10.1016/j.kint.2017.01.013 (2017).
Zweigart, C. et al. Medium cut-off membranes—Closer to the natural kidney removal function. Int. J. Artif. Organs 40, 328–334. https://doi.org/10.5301/ijao.5000603 (2017).
Allawati, H. et al. A pharmacokinetic study comparing the clearance of vancomycin during haemodialysis using medium cut-off membrane (theranova) and high-flux membranes (revaclear). Toxins (Basel). https://doi.org/10.3390/toxins12050317 (2020).
Sevinc, M. et al. Comparison of circulating levels of uremic toxins in hemodialysis patients treated with medium cut-off membranes and high-flux membranes: Theranova in Sisli Hamidiye Etfal (THE SHE) randomized control study. Blood Purif. 49, 1–10. https://doi.org/10.1159/000508061 (2020).
Weiner, D. E. et al. Efficacy and safety of expanded hemodialysis with the theranova 400 dialyzer: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 15, 1310–1319. https://doi.org/10.2215/CJN.01210120 (2020).
Reque, J. et al. Is expanded hemodialysis an option to online hemodiafiltration for small- and middle-sized molecules clearance?. Blood Purif. 47, 126–131. https://doi.org/10.1159/000493910 (2019).
Devereux, R. B. et al. Echocardiographic detection of pressure-overload left ventricular hypertrophy: Effect of criteria and patient population. J. Clin. Hypertens. 3, 66–78 (1987).
Hecht, H. S. et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J. Thorac. Imaging 32, W54–W66. https://doi.org/10.1097/RTI.0000000000000287 (2017).
Weisbord, S. D. et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J. Am. Soc. Nephrol. 16, 2487–2494. https://doi.org/10.1681/ASN.2005020157 (2005).
Rayner, H. C. et al. Recovery time, quality of life, and mortality in hemodialysis patients: The dialysis outcomes and practice patterns study (DOPPS). Am. J. Kidney Dis. 64, 86–94. https://doi.org/10.1053/j.ajkd.2014.01.014 (2014).
Lindsay, R. M. et al. Minutes to recovery after a hemodialysis session: A simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin. J. Am. Soc. Nephrol. 1, 952–959. https://doi.org/10.2215/CJN.00040106 (2006).
Georgianos, P. I. et al. Hemodiafiltration does not have additional benefits over hemodialysis on arterial stiffness, wave reflections and central aortic pressures. Blood Purif. 37, 18–26. https://doi.org/10.1159/000355945 (2014).
Hutchison, C. A. & Wolley, M. The rationale for expanded hemodialysis therapy (HDx). Contrib. Nephrol. 191, 142–152. https://doi.org/10.1159/000479262 (2017).
Wang, A. Y. et al. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: A systematic review and meta-analysis of randomized trials. Am. J. Kidney. Dis. 63, 968–978. https://doi.org/10.1053/j.ajkd.2014.01.435 (2014).
Nistor, I. et al. Convective versus diffusive dialysis therapies for chronic kidney failure: An updated systematic review of randomized controlled trials. Am. J. Kidney Dis. 63, 954–967. https://doi.org/10.1053/j.ajkd.2013.12.004 (2014).
Susantitaphong, P., Siribamrungwong, M. & Jaber, B. L. Convective therapies versus low-flux hemodialysis for chronic kidney failure: A meta-analysis of randomized controlled trials. Nephrol. Dial. Transplant 28, 2859–2874. https://doi.org/10.1093/ndt/gft396 (2013).
Lee, Y. H. et al. Effects of online hemodiafiltration on anemia and nutritional status in chronic hemodialysis patients. Kidney Res. Clin. Pract. 39, 103–111. https://doi.org/10.23876/j.krcp.19.082 (2020).
Kim, Y. W. & Park, S. Confronting practical problems for initiation of on-line hemodiafiltration therapy. Electrol. Blood Press. 14, 1–4. https://doi.org/10.5049/EBP.2016.14.1.1 (2016).
Giachelli, C. M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 15, 2959–2964. https://doi.org/10.1097/01.ASN.0000145894.57533.C4 (2004).
Alexopoulos, N. & Raggi, P. Calcification in atherosclerosis. Nat. Rev. Cardiol. 6, 681–688. https://doi.org/10.1038/nrcardio.2009.165 (2009).
Blaha, M. J., Silverman, M. G. & Budoff, M. J. Is there a role for coronary artery calcium scoring for management of asymptomatic patients at risk for coronary artery disease?: Clinical risk scores are not sufficient to define primary prevention treatment strategies among asymptomatic patients. Circ. Cardiovasc. Imaging 7, 398–408. https://doi.org/10.1161/CIRCIMAGING.113.000341 (2014).
Lin, C. L. et al. Clinical improvement by increased frequency of on-line hemodialfiltration. Ren. Fail. 23, 193–206. https://doi.org/10.1081/jdi-100103491 (2001).
Knezevic, M. Z. et al. Influence of dialysis modality and membrane flux on quality of life in hemodialysis patients. Ren. Fail. 34, 849–855. https://doi.org/10.3109/0886022X.2012.684555 (2012).
Mazairac, A. H. et al. Effect of hemodiafiltration on quality of life over time. Clin. J. Am. Soc. Nephrol. 8, 82–89. https://doi.org/10.2215/CJN.00010112 (2013).
Lim, J. H. et al. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci. Rep. 10, 7780. https://doi.org/10.1038/s41598-020-64622-z (2020).
Breckenridge, K. et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: An expert consensus meeting. Nephrol. Dial. Transplant. 30, 1605–1614. https://doi.org/10.1093/ndt/gfv209 (2015).
Jhamb, M. et al. Design and rationale of health-related quality of life and patient-reported outcomes assessment in the frequent hemodialysis network trials. Blood Purif. 31, 151–158. https://doi.org/10.1159/000321855 (2011).
Acknowledgements
This work was an investigator-initiated research grant from Baxter International, Inc. (Deerfield, IL, USA).
Author information
Authors and Affiliations
Contributions
S.S.H. and K.W.J. designed the study. Y.L., M.J. and J.J. collected the data, analyzed and interpreted the results. Y.L. and M.J. made the figures. J.E.L., W.H., B.S.C., C.W.P., H.J.C., C.L.K. and D.K.K. conceived the study and assisted in the analyses. Y.L., S.S.H. and K.W.J. drafted and revised the paper. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, Y., Jang, Mj., Jeon, J. et al. Cardiovascular Risk Comparison between Expanded Hemodialysis Using Theranova and Online Hemodiafiltration (CARTOON): A Multicenter Randomized Controlled Trial. Sci Rep 11, 10807 (2021). https://doi.org/10.1038/s41598-021-90311-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-90311-6
This article is cited by
-
Comparative efficacy of expanded hemodialysis and online hemodiafiltration: a systematic review and meta-analysis
International Urology and Nephrology (2025)
-
Effect of hemodiafiltration and hemodialysis on mortality of patients with end-stage kidney disease: a meta-analysis
BMC Nephrology (2024)