Abstract
Antibodies to Ro52/tripartite motif-containing 21 (TRIM21), referred to as anti-Ro52, are found in patients diagnosed with diverse systemic autoimmune rheumatic disease and associated with interstitial lung diseases. However, little is known about the clinical characteristics of anti-Ro52 in patients with idiopathic interstitial pneumonias (IIPs). We aimed to analyze the prevalence, co-existent autoantibodies, and clinical characteristics of anti-Ro52 in patients with IIP. The study enrolled 288 patients diagnosed with IIP. Clinical, laboratory and radiographic findings of IIP patients were compared between anti-Ro52 positives and negatives. Anti-Ro52 (20/288; 6.9%), anti-ARS (18/288; 6.3%), and anti-Ro60/SS-A (16/288; 5.6%) were the most common autoantibodies detected in IIP patients. Among 20 IIP patients who had anti-Ro52, anti-ARS was present in 8 (40%) patients. The criteria for interstitial pneumonia with autoimmune features (IPAF) were significantly better fulfilled by patients with anti-Ro52 than those without (P = 0.001). Meeting serological domain (P < 0.001) and Raynaud’s phenomenon (P = 0.009) were significantly more common in the anti-Ro52-positive patients. Anti-Ro52-positive IIP patients have clinical features consistent with IPAF. Anti-Ro52 may have an important role in detecting the autoimmune phenotype in IIP patients.
Similar content being viewed by others
Introduction
Idiopathic interstitial pneumonias (IIPs) are diffuse fibrotic lung disorders that exclude known causes of interstitial lung diseases (ILDs) such as systemic autoimmune rheumatic disease (SARD), environmental exposure, and medication toxicity1,2. Patients with IIP who have autoimmune features, but do not meet established diagnostic/classification criteria of SARD3,4 are categorized as “interstitial pneumonia with autoimmune features (IPAF)”5. Autoantibodies such as anti-aminoacyl-tRNA synthetases (anti-ARS) have proved clinically significant in diagnosis, treatment, and prediction of prognosis of IIP patients fulfilling the IPAF criteria5,6,7,8.
Ro52/tripartite motif-containing 21 (TRIM21), an E3 ubiquitin ligase involved in ubiquitination, plays a prominent regulatory role in inflammation, apoptosis, and oxidative stress9,10,11. Anti-Ro52/TRIM21 antibodies (anti-Ro52) are commonly detected in the sera of patients diagnosed with different types of SARD, including Sjögren's syndrome12, polymyositis/dermatomyositis (PM/DM)13,14, systemic sclerosis (SSc)15,16, and systemic lupus erythematosus (SLE)17. Co-existence of anti-Ro52 and anti-ARS (particularly anti-Jo-1 and anti-PL-7) is common in patients with PM/DM14,18,19. Anti-Ro52 is associated with the presence of ILD in SSc, PM/DM, and mixed connective tissue disease (MCTD)13,15,16,20. One study in PM/DM reported higher prevalence of ILD in patients with anti-Ro52 than without, however, it might be due to an association of anti-Ro52 and anti-ARS14. Another study on patients with anti-Ro52-positive ILD reported the absence of an established diagnosis of SARD in the majority (78%) of patients while nearly half (49.3%) fulfilled the IPAF criteria21. Further studies are warranted to explore the prevalence, co-existent autoantibodies, and clinical characteristics associated with the presence of anti-Ro52 in IIP patients.
This study aims to analyze the clinical significance of anti-Ro52 in patients with IIPs and the associated clinical and immunological characteristics. The findings from this study may contribute to more accurate classification of IIP.
Results
Autoantibodies in sera of patients with IIP
Of the 288 IIP patients enrolled in the study, ELISA revealed the presence of anti-Ro52 in the sera of 20 patients (6.9%) (Table 1). ANA and RF positivity in the serological domain of the IPAF were as follows; ANA titer ≥ 1:320, diffuse, speckled, homogeneous patterns (n = 20; 7.1%), ANA any titer, nucleolar or centromere patterns (n = 14; 5.0%), RF ≥ 2 × upper limit of normal (n = 24; 9.4%). Anti-ARS was detected in 18 patients (6.3%), including autoantibodies to Jo-1 (n = 5; 1.7%), PL-7 (n = 2; 0.7%), PL-12 (n = 1; 0.3%), EJ (n = 4; 1.4%), OJ (n = 1; 0.3%), and KS (n = 5; 1.7%) (Table 1). In addition, autoantibodies to the following antigens were detected; Ro60/SS-A (n = 16; 5.6%), La/SS-B (n = 1; 0.3%), CCP (n = 9; 5.0%), double stranded DNA (n = 6; 3.3%), U1RNP (n = 2; 0.7%), topoisomerase I (Scl-70) (n = 1; 0.3%), MDA5 (n = 1; 0.3%), TIF1β (n = 2; 0.7%), CENP-A (n = 3; 1.0%), CENP-B (n = 2; 0.7%), and Anti-RNA Polymerase III (n = 1; 0.3%) (Table 1).
Autoantibodies co-existing with anti-Ro52
Co-existence of anti-Ro52 with other autoantibodies was analyzed (Table 2). The presence of ANA (ANA titer ≥ 1:320, diffuse, speckled, or homogeneous patterns, 20% vs. 6.2%, P = 0.04), anti-ARS (40% vs. 3.7%, P < 0.001), and anti-Ro60/SS-A (30% vs. 3.7%, P < 0.001) was significantly more common in anti-Ro52-positive than in anti-Ro52-negative patients (Table 2). Among eight patients positive for both anti-ARS and anti-Ro52, three were positive for anti-Jo-1, two for anti-KS, and one each for anti-PL-7, anti-PL-12, and anti-EJ.
Anti-Ro52: patient characteristics and clinical course
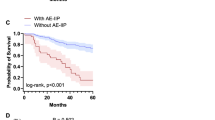
Clinical characteristics of anti-Ro52-positive vs. -negative patients are summarized in Table 3. Anti-Ro52-positive patients frequently met the IPAF criteria (50% vs. 17%, P = 0.001), had the clinical domain of IPAF criteria (20% vs. 8%, P = 0.09), and showed a greater percentage of fulfillment of serological domain (75% vs. 26%, P < 0.001), but had similar prevalence of morphological domain (40% vs. 44%) compared to anti-Ro52-negative patients. Raynaud's phenomenon was significantly more common in anti-Ro52-positive than in anti-Ro52-negative patients (15% vs. 2%, P = 0.009). Laboratory findings indicated significantly higher levels of serum Krebs von den Lungen-6 (KL-6) in anti-Ro52-positive than in anti-Ro52-negative patients (1258 U/mL vs. 858 U/mL, P = 0.01) (Table 4). HRCT analyses revealed more frequent lower distribution (90% vs. 69%, P = 0.03) and less frequent ground-glass attenuations (45% vs. 72%, P = 0.02) in anti-Ro52-positive than in anti-Ro52-negative patients (Table 5). Significant differences were not detected in HRCT patterns in the presence or absence of serum anti-Ro52 in IIP patients. However, OP and DAD were seen exclusively in anti-Ro52-negative patients. Patient characteristics and details of each domain are shown in Supplementary Tables S1 and S2. There were no patients who developed and fulfilled the classification/diagnostic criteria of SARD during a median observation period of 771 days in this cohort study. Kaplan–Meier curves showed no significant difference in the overall survival rate between patients with and without serum anti-Ro52 (log-rank P = 0.51) (Fig. 1).
Kaplan–Meier survival curves of idiopathic interstitial pneumonia (IIP) patients with/without anti-Ro52/tripartite motif-containing 21 antibodies (anti-Ro52). Kaplan–Meier curves representing the survival rate of IIP patients in the presence (dotted line; n = 20) and absence (dashed line; n = 268) of serum anti-Ro52. Statistically relevant difference was not observed between the analyzed groups (log-rank P = 0.51).
Anti-Ro52 in anti-ARS-positive cases: patient characteristics and survival
The clinical characteristics and survival of anti-Ro52-positive vs. -negative patients who were also positive for anti-ARS are summarized in Supplementary Tables S3–5 and Supplementary Fig. S1. Clinical characteristics and Kaplan–Meier curves showed no significant difference in anti-ARS-positive patients with and without serum anti-Ro52.
Discussion
This is the first study investigating the frequency of serum anti-Ro52 antibodies in unselected patients with IIP. Similar to the prevalence of anti-ARS (6.3%), anti-Ro52 was detected in 6.9% of patients with IIP. Presence of serum anti-Ro52 was significantly associated with fulfillment of IPAF criteria, particularly with respect to the serological domain and Raynaud’s phenomenon, in IIP patients.
Anti-Ro52 is mostly present in patients with different types of SARD22, as seen in nearly half of the patients with Sjögren's syndrome12, SSc15,16, and SLE17 and 20–30% of patients with PM/DM13,14, In this study, the prevalence of anti-Ro52 (6.9%) in IIP was lower than in SARD but higher than in healthy individuals (< 0.2–1%)23. In addition, as in patients with PM/DM14,18,19, anti-Ro52 frequently co-existed with anti-ARS. Among the anti-ARS that co-existed with anti-Ro52, anti-Jo-1 found in three cases was the most common, in addition to anti-PL-7, anti-PL-12, anti-EJ, and anti-KS.
IPAF criteria (P = 0.001) related to the serological domain (P < 0.001) were more frequently fulfilled by anti-Ro52-positive (50%) than anti-Ro52-negative patients (17%) in our IIP cohort. A previous retrospective study showed that 49.3% of the ILD patients who had anti-Ro52 met the IPAF criteria, similar to our result21. Anti-Ro52-positive patients could be negative in immunofluorescence ANA tests, however, anti-Ro52 was associated with IPAF serological domain, indicating that it frequently coexists with the other autoantibodies included in the IPAF serological domain (Table 2). Co-existence of anti-Ro52 with other specific autoantibodies in various SARD have been reported14,15,18. Although anti-Ro52 is not specific for a particular type of SARD diagnosis, a 14-fold increased risk of developing SARD was reported in IIP patients who met the IPAF criteria24. Thus, presence of anti-Ro52 might be considered as a useful clinical diagnostic tool for the early detection of SARDs in patients with IIP who pose a higher risk of developing in the future.
IPAF criteria are used for the identification of a subset of IIP patients exhibiting autoimmune features but lacking a definitive diagnosis of SARD5 The ATS/ERS task force has suggested the need for further validation and revision of IPAF criteria5. Accordingly, there has been a proposal for the inclusion of several myositis-specific antibodies (anti-NXP-2, anti-TIF1γ) in the IPAF criteria25. In contrast, anti-double stranded DNA, anti-Sm, anti-topoisomerase I (Scl-70), and anti-MDA5 are disease-specific diagnostic antibodies that have a proven link to the diagnosis of SLE26, SSc27, and clinically amyopathic DM (CADM)28. These disease-specific marker antibodies are produced prior to the clinical manifestation of the associated SARD and the association of IPAF with these antibodies might be an indication of early stage SARD. The appropriateness of the inclusion of these antibodies in the IPAF criteria is controversial.
Some commercial assays separately measure antibodies to Ro60 and Ro52, while other anti-Ro/SS-A immunoassays use a mixture of Ro60 and Ro52 as antigen. However, recent literature indicates that Ro60 and Ro52/TRM21 are unrelated molecule and Ro52/TRIM21 is not a part of Ro60/SS-A-hYRNAs complex29,30,31. Thus, separate measurement of anti-Ro52 and anti-Ro60/SS-A is recommended because of their biochemical and immunological differences32. In our study, 70% (14 of 20) of anti-Ro52 positive were negative for anti-Ro60/SS-A. It remains unclear whether the definition of “anti-Ro (SS-A)” in the IPAF criteria meant a mixture of anti-Ro52 and anti-Ro60/SS-A or anti-Ro60/SS-A alone; therefore, our findings suggested that the definition of “anti-Ro (SS-A)” in the IPAF criteria should be clarified and testing anti-Ro52/TRIM21 and anti-Ro60/SS-A separately to identify the autoimmune phenotype in IIP patients.
The frequency of Raynaud's phenomenon was significantly higher in anti-Ro52-positive patients than in anti-Ro52-negative patients in our IIP cohort (P = 0.009) (Table 3). Nearly half of the IPAF patients exhibit at least one clinical domain with Raynaud's phenomenon as the most common symptom33,34. In this study, all three patients with anti-Ro52 who had Raynaud's phenomenon were classified as IPAF (Supplementary Tables S1 and S2). Thus, testing for serum anti-Ro52 might be helpful in classifying IIP patients with Raynaud’s phenomenon as those meeting the IPAF criteria. Raynaud's phenomenon is associated with underlying or future development of SARD35 but is not considered a predictor for its prognosis or development in IPAF patients33,34 probably due to the low prevalence and short follow-up periods. Thus, the clinical significance of Raynaud's phenomenon in IPAF patients remains controversial.
Patients with anti-Ro52 have a higher frequency of rapidly progressive ILD and a higher rate of mortality than those without anti-Ro52 in SARD13,15,16,20. Herein, presence of anti-Ro52 was not significantly associated with overall mortality, possibly due to the heterogeneity of IIPs and the limited number of patients.
Patients with anti-ARS are associated with a unique subset characterized by clinical features, including ILDs, called anti-synthetase syndrome (AS), and several criteria of AS have been proposed36,37. However, AS is a “syndrome” developed for research settings, and its concept is still controversial. Recent research has reported the heterogeneity related to the prognosis and response to treatment of IIP patients with anti-ARS, wherein, certain patients were refractory to treatment with poor prognosis, while others responded well7,38. Patients with PM/DM positive for both anti-Ro52 and anti-ARS had severe myositis and joint impairment with a higher prevalence of ILD14,16. In this study, among 18 anti-ARS-positive patients, significant differences were not seen in symptoms, characteristics (Supplementary Tables S3–5), and prognosis (Supplementary Fig. S1) related to SARD, between anti-Ro52-positive and -negative patients. However, these findings might considerably be affected by the small number of IIP patients with anti-ARS and further research is thus required.
Several limitations of this study are acknowledged. First, this study was a retrospective study with variable follow-up intervals and periods. Second, the sample size was relatively small and comprised only of Japanese individuals from two university hospitals. Third, possible missed signs and symptoms in the clinical domain of IPAF criteria may have resulted in inaccurate IPAF diagnoses because our cohort included patients enrolled before IPAF criteria was proposed. However, we routinely consulted ILD patients with rheumatologists and requested appropriate evaluations to exclude the presence of collagen vascular diseases. Fourth, although none of the patients with IIP developed any autoimmune diseases during the follow-up period, the observation period was short. It is possible that some might develop SARD in the future because ILD could precede the development of SARD in certain patients24.
In conclusion, the fulfillment of IPAF criteria and presence of Raynaud's phenomenon were more frequently seen in the presence than in the absence of anti-Ro52 in patients with IIP. Our findings may suggest that testing for anti-Ro52 help to identify the autoimmune phenotype and predict the development of SARD in IIP patients. Further prospective studies on a large cohort are needed to elucidate the clinical significance of anti-Ro52 in patients with IIP.
Methods
Study participants
A two-center retrospective cohort study was conducted by the Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan, and the Department of Respiratory Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan. Patients with IIP were enrolled in the study between March 2007 and October 2016 (n = 288). At the first visit, IIP was diagnosed based on clinical, laboratory, and radiological findings as per the definition of the American Thoracic Society/European Respiratory Society (ATS/ERS) international multidisciplinary consensus classification1,2. The study was conducted in accordance with the amended Declaration of Helsinki. The Institutional Review Board of the Nagasaki University Hospital, Nagasaki, Japan (Approval No: 16042517), and the University of Occupational and Environmental Health, (Approval No: H27-238) approved the protocol. Informed consent was obtained from all subjects. Observation and follow-up of each patient was conducted on an annual basis and was censored on April 30, 2020. Patients who were lost to follow-up were censored at the date of last contact/follow-up and those alive as on April 30, 2020, were censored for overall survival analyses.
Detection of serum autoantibodies
Serum samples of patients were obtained during their first visit and stored at − 20 °C until further use. For the analyses of autoantibodies, 35S-methionine radiolabeled K562 cell extract was immunoprecipitated with IgG purified from 8 µL of human serum samples. The immunoprecipitated proteins were electrophoresed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as described previously39. Briefly, cells were labeled with 35S-methionine and cysteine, lysed in 0.5 M NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 50 mM Tris (pH 7.5), 0.3% octylphenyl polyethylene glycol (IGEPAL CA-630) buffer containing 0.5 mM phenylmethylsulfonyl fluoride and 0.3 trypsin inhibitory units (TIU)/mL aprotinin, and immunoprecipitated using protein-A-Sepharose beads coated with IgG. Immunoprecipitates were then washed with 0.5 M NaCl-NET/IGEPAL CA-630 and analyzed by SDS-PAGE and autoradiography. The specificity of autoantibodies was confirmed by the use of human reference sera39. Antibodies to Ro52/TRIM21, histidyl-tRNA synthetase (Jo-1), and melanoma differentiation-associated protein 5 (MDA5) were tested by enzyme-linked immunosorbent assay (ELISA) as described previously39. All recombinant proteins were purchased from Diarect (Freiburg, Germany). Briefly, 96-well microtiter plates (Immobilizer Amino; Nunc Naperville, IL, USA) were coated with 0.5 μg/ml of recombinant protein and blocked with 0.5% bovine serum albumin (BSA)-NET/ IGEPAL CA-630 for 1 h at room temperature. Patients’ sera (1:250) and alkaline phosphatase-conjugated goat anti-human IgG (1:1000; γ-chain specific; Jackson Immunoresearch, Hershey, PA, USA) diluted in 0.5% BSA-NET/ IGEPAL CA-630 were used as the sample and secondary antibodies, respectively. A standard curve was generated using serial 1:5 dilutions of a high-titer prototype serum. Optical density of samples measured at 405 nm was converted into units based on the standard curve.
Anti-nuclear antibodies (ANA) and rheumatoid factor (RF) positivity was defined in accordance with the classification criteria of IPAF5: ANA titer ≥ 1:320, diffuse, speckled, homogeneous patterns; ANA any titer, nucleolar or centromere patterns; RF ≥ 2 × upper limit of normal.
Clinical data collection and analyses
Demographic data, clinical information, results of laboratory and pulmonary function tests, and analyses of bronchoalveolar lavage fluid were obtained from medical records. Physical findings were confirmed by rheumatologists when appropriate. Classification criteria of IPAF were based on the 2015 ERS/ATS Task Force research statement5. Although patient data (n = 281) used in this study partially overlapped with a retrospective study published earlier6, our research data related to anti-Ro52 are unique.
Radiographic evaluation
At the first visit, patients were examined by high-resolution chest computed tomography (HRCT), and evaluated independently by two pulmonologists (N. S. and H. I.) for volume loss, distribution and presence of reticular shadows, honeycombing, traction bronchiectasis, ground-glass attenuation, consolidations, thickening of bronchovascular bundles, small nodules (φ < 5 mm), nodules (φ > 5 mm), and pleural effusion. According to the international IIPs classification2, HRCT patterns were classified into usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), and diffuse alveolar damage (DAD).
Statistical analyses
Data are presented as the median [interquartile range] or frequency (%). Fisher’s exact test was used to compare categorical variables. Comparisons between groups were made using the Mann–Whitney U test. Survival analyses were performed using the Kaplan–Meier method and the log-rank test. All analyses were conducted at a significance level of α = 0.05. All statistical analyses were performed using the STATA 16.1 software (StataCorp, College Station, TX, USA).
Ethics declarations
The Institutional Review Board of the Nagasaki University Hospital, Nagasaki, Japan (Approval No: 16042517), and the University of Occupational and Environmental Health, (Approval No: H27-238) approved the protocol. Informed consent was obtained from all subjects.
Data availability
The datasets used for the current study are available from the corresponding author on reasonable request.
References
American Thoracic, S., European Respiratory, S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 165, 277–304. https://doi.org/10.1164/ajrccm.165.2.ats01 (2002).
Travis, W. D. et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 188, 733–748. https://doi.org/10.1164/rccm.201308-1483ST (2013).
Kinder, B. W. et al. Idiopathic nonspecific interstitial pneumonia: Lung manifestation of undifferentiated connective tissue disease?. Am. J. Respir. Crit. Care Med. 176, 691–697. https://doi.org/10.1164/rccm.200702-220OC (2007).
Vij, R., Noth, I. & Strek, M. E. Autoimmune-featured interstitial lung disease: A distinct entity. Chest 140, 1292–1299. https://doi.org/10.1378/chest.10-2662 (2011).
Fischer, A. et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 46, 976–987. https://doi.org/10.1183/13993003.00150-2015 (2015).
Yura, H. et al. Clinical characteristics of patients with anti-aminoacyl-tRNA synthetase antibody positive idiopathic interstitial pneumonia. Respir. Med. 132, 189–194. https://doi.org/10.1016/j.rmed.2017.10.020 (2017).
Tanizawa, K. et al. The long-term outcome of interstitial lung disease with anti-aminoacyl-tRNA synthetase antibodies. Respir. Med. 127, 57–64. https://doi.org/10.1016/j.rmed.2017.04.007 (2017).
Tomonaga, M. et al. Comparison of pulmonary involvement between patients expressing anti-PL-7 and anti-Jo-1 antibodies. Lung 193, 79–83. https://doi.org/10.1007/s00408-014-9665-7 (2015).
Espinosa, A. et al. Anti-Ro52 autoantibodies from patients with Sjogren’s syndrome inhibit the Ro52 E3 ligase activity by blocking the E3/E2 interface. J. Biol. Chem. 286, 36478–36491. https://doi.org/10.1074/jbc.M111.241786 (2011).
Oke, V. & Wahren-Herlenius, M. The immunobiology of Ro52 (TRIM21) in autoimmunity: A critical review. J. Autoimmun. 39, 77–82. https://doi.org/10.1016/j.jaut.2012.01.014 (2012).
Arai, T. et al. Heterogeneity of incidence and outcome of acute exacerbation in idiopathic interstitial pneumonia. Respirology 21, 1431–1437. https://doi.org/10.1111/resp.12862 (2016).
Espinosa, A. et al. The Sjogren’s syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J. Immunol. 176, 6277–6285. https://doi.org/10.4049/jimmunol.176.10.6277 (2006).
Cruellas, M. G., Viana Vdos, S., Levy-Neto, M., Souza, F. H. & Shinjo, S. K. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics 68, 909–914. https://doi.org/10.6061/clinics/2013(07)04 (2013).
Yamasaki, Y. et al. Clinical subsets associated with different anti-aminoacyl transfer RNA synthetase antibodies and their association with coexisting anti-Ro52. Mod. Rheumatol. 26, 403–409. https://doi.org/10.3109/14397595.2015.1091155 (2016).
Wodkowski, M. et al. Monospecific anti-Ro52/TRIM21 antibodies in a tri-nation cohort of 1574 systemic sclerosis subjects: Evidence of an association with interstitial lung disease and worse survival. Clin. Exp. Rheumatol. 33, S131-135 (2015).
Hudson, M. et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res. Ther. 14, R50. https://doi.org/10.1186/ar3763 (2012).
Smith, S. et al. Estrogen receptor alpha regulates tripartite motif-containing protein 21 expression, contributing to dysregulated cytokine production in systemic lupus erythematosus. Arthritis Rheumatol. 66, 163–172. https://doi.org/10.1002/art.38187 (2014).
Ghillani, P. et al. Clinical significance of anti-Ro52 (TRIM21) antibodies non-associated with anti-SSA 60kDa antibodies: Results of a multicentric study. Autoimmun. Rev. 10, 509–513. https://doi.org/10.1016/j.autrev.2011.03.004 (2011).
Mahler, M., Miller, F. W. & Fritzler, M. J. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: A comprehensive review. Autoimmun. Rev. 13, 367–371. https://doi.org/10.1016/j.autrev.2014.01.022 (2014).
Gunnarsson, R. et al. Associations between anti-Ro52 antibodies and lung fibrosis in mixed connective tissue disease. Rheumatology 55, 103–108. https://doi.org/10.1093/rheumatology/kev300 (2016).
Sclafani, A. et al. Presentations and outcomes of interstitial lung disease and the anti-Ro52 autoantibody. Respir. Res. 20, 256. https://doi.org/10.1186/s12931-019-1231-7 (2019).
De Soyza, A. et al. Bronchiectasis rheumatoid overlap syndrome is an independent risk factor for mortality in patients with bronchiectasis: A multicenter cohort study. Chest 151, 1247–1254. https://doi.org/10.1016/j.chest.2016.12.024 (2017).
Satoh, M. et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 64, 2319–2327. https://doi.org/10.1002/art.34380 (2012).
Alevizos, M. K., Giles, J. T., Patel, N. M. & Bernstein, E. J. Risk of progression of interstitial pneumonia with autoimmune features to a systemic autoimmune rheumatic disease. Rheumatology 59, 1233–1240. https://doi.org/10.1093/rheumatology/kez404 (2020).
De Sadeleer, L. J. et al. Prevalence of myositis-specific antibodies in idiopathic interstitial pneumonias. Lung 196, 329–333. https://doi.org/10.1007/s00408-018-0108-8 (2018).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686. https://doi.org/10.1002/art.34473 (2012).
van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 72, 1747–1755. https://doi.org/10.1136/annrheumdis-2013-204424 (2013).
Bailey, E. E. & Fiorentino, D. F. Amyopathic dermatomyositis: Definitions, diagnosis, and management. Curr. Rheumatol. Rep. 16, 465. https://doi.org/10.1007/s11926-014-0465-0 (2014).
Kelekar, A., Saitta, M. R. & Keene, J. D. Molecular composition of Ro small ribonucleoprotein complexes in human cells. Intracellular localization of the 60- and 52-kD proteins. J. Clin. Investig. 93, 1637–1644. https://doi.org/10.1172/JCI117145 (1994).
Sim, S. & Wolin, S. L. Emerging roles for the Ro 60-kDa autoantigen in noncoding RNA metabolism. Wiley Interdiscip. Rev. RNA 2, 686–699. https://doi.org/10.1002/wrna.85 (2011).
Boccitto, M. & Wolin, S. L. Ro60 and Y RNAs: Structure, functions, and roles in autoimmunity. Crit. Rev. Biochem. Mol. Biol. 54, 133–152. https://doi.org/10.1080/10409238.2019.1608902 (2019).
Schulte-Pelkum, J., Fritzler, M. & Mahler, M. Latest update on the Ro/SS-A autoantibody system. Autoimmun. Rev. 8, 632–637. https://doi.org/10.1016/j.autrev.2009.02.010 (2009).
Oldham, J. M. et al. Characterisation of patients with interstitial pneumonia with autoimmune features. Eur. Respir. J. 47, 1767–1775. https://doi.org/10.1183/13993003.01565-2015 (2016).
Sambataro, G. et al. Clinical, serological and radiological features of a prospective cohort of Interstitial Pneumonia with Autoimmune Features (IPAF) patients. Respir. Med. 150, 154–160. https://doi.org/10.1016/j.rmed.2019.03.011 (2019).
Belch, J. et al. ESVM guidelines—the diagnosis and management of Raynaud’s phenomenon. Vasa 46, 413–423. https://doi.org/10.1024/0301-1526/a000661 (2017).
Connors, G. R., Christopher-Stine, L., Oddis, C. V. & Danoff, S. K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years?. Chest 138, 1464–1474. https://doi.org/10.1378/chest.10-0180 (2010).
Solomon, J., Swigris, J. J. & Brown, K. K. Myositis-related interstitial lung disease and antisynthetase syndrome. J. Bras. Pneumol. 37, 100–109. https://doi.org/10.1590/s1806-37132011000100015 (2011).
Watanabe, K. et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir. Med. 105, 1238–1247. https://doi.org/10.1016/j.rmed.2011.03.022 (2011).
Satoh, M., Ceribelli, A., Hirakata, M. & Chan, E. K. L. Immunodiagnosis of autoimmune myopathies. (eds Detrick, B. et al.) 878–887 (Manual of Molecular and Clinical Laboratory Immunology, American Society of Microbiology Press, 2016).
Funding
This work was supported by Japan society for the promotion of science (JSPS) KAKENHI (Grant-in-Aid for Scientific Research) [grant number 17K09617 to HM, 15K08790 and 19K08617 to MS].
Author information
Authors and Affiliations
Contributions
M.T. designed the study, had full access to all the data in this study, performed statistical analyses, and wrote the initial draft. M.S., T.H., and S.T. tested autoantibodies in the sera by immunoprecipitation and ELISA. N.S., M.S., H.I., K.Yatera, and H.M. made substantial contribution to the conception and design of the study. M.T., N.S., H.I., H.Y., and T.K. acquired the data. N.S., M.S., K.Yamasaki, K.Yatera, and M.H. participated in drafting and critically revising the article for important intellectual content. Y.F. gave advice on the statistical analyses. K.Yatera was attributable for the final responsibility for the decision to submit the article for publication. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahara, M., Sakamoto, N., Satoh, M. et al. Clinical characteristics of idiopathic interstitial pneumonias with anti-Ro52/tripartite motif-containing 21 antibodies. Sci Rep 12, 11122 (2022). https://doi.org/10.1038/s41598-022-15321-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-15321-4
This article is cited by
-
Revisiting the INSPIRE trial: antibody profiling reveals high prevalence of occult autoimmunity
Respiratory Research (2026)