Abstract
Patients with traumatic brain injury (TBI) frequently exhibit concomitant immunosuppression. In this study, we evaluated the predictive values of soluble programmed death-1 (sPD-1) and soluble programmed death ligand-1 (sPD-L1) in patients with severe TBI. Peripheral blood sPD-1 and sPD-L1 levels were measured within 48 h of patient admission. A total of 20 healthy volunteers and 82 patients were enrolled in this study. The levels of sPD-1 and sPD-L1 were upregulated in patients with severe TBI (P < 0.001). They were significantly increased in the post-TBI severe pneumonia group and among non-survivors (P < 0.001). The area under the curves (AUCs) for sPD-1 and sPD-L1 levels to predict severe pneumonia were 0.714 and 0.696, respectively, and the AUCs to predict mortality were 0.758 and 0.735. The levels of sPD-1 and sPD-L1 are correlated with the GCS scores at admission, APACHE II scores, length of MV, and time elapsed to mortality. The levels of sPD-1 and sPD-L1 emerged as independent predictive factors for severe pneumonia and mortality. This study demonstrates that upregulation of sPD-1 and sPD-L1 in severe TBI patients is significantly associated with severe pneumonia and mortality, suggesting their potential as predictive biomarkers for these outcomes.
Similar content being viewed by others
Introduction
Traumatic Brain Injury (TBI) is a major global health concern, and severe TBIs are particularly associated with high rates of mortality and disability1. Following severe TBI, a cascade of detrimental reactions can lead to secondary brain damage and systemic inflammatory responses, exacerbating the clinical condition and yielding unfavorable outcomes2. The suppression of the cellular immune system at this stage is a profound consequence of TBI, often resulting in infections likely stemming from bacterial translocation3,4. Negative co-stimulatory molecules play an important role in regulating the immune response by negatively regulating immune cell activation and function5.
As important constituents of the negative co-stimulatory molecules, Programmed Death-1 (PD-1) and Programmed Death Ligand-1 (PD-L1) are pivotal in moderating immune responses, significantly impacting T and B cell functionalities, including cytokine production and cytotoxic activity6,7. Soluble PD-1 (sPD-1) and soluble PD-L1 (sPD-L1) are recognized as vital natural inhibitors that regulate the balance within the PD-1/PD-L1 signaling pathway and have been found in patients and experimental animals with autoimmune diseases, sepsis, and cancer8,9,10. Although many studies have explored the roles of the sPD-1 and sPD-L1 pathway across different diseases, few have explored the relationship between these markers and the risk stratification of severe TBI patients. Thus, we hypothesize that sPD-1 and sPD-L1 may also be associated with severe pneumonia and prognostic evaluation in severe TBI patients. In this study, we measured the peripheral blood levels of sPD-1 and sPD-L1 to evaluate their predictive value for pneumonia severity and mortality in patients with severe TBI.
Methods
This observational clinical study was conducted in the 15-bed neurointensive care unit (NCU) of Shengli Oilfield Central Hospital, a tertiary care teaching hospital. Consecutive patients with a primary diagnosis of severe TBI, defined by Glasgow Coma Scale (GCS) scores of 3-811, were enrolled between January 2022 and January 2024. Healthy volunteers were also recruited as controls. The exclusion criteria included age under 18 years, hematologic disease, liver disease, end-stage renal disease, cancer, hormone therapy, and death within 48 h. Clinical characteristics, including demographic data and past medical history, along with laboratory examinations (white blood cell count (WBC), Lymphocytes, C-reactive protein (CRP), blood gas analysis, procalcitonin (PCT), blood biochemistry, imaging examination, etc.), were collected upon enrollment. The Marshall CT, Revised trauma score (RTS), duration of mechanical ventilation (MV), duration of intensive care unit (ICU), and Acute Physiology and Chronic Health Evaluation system II (APACHE II) score was calculated using relevant clinical and demographic data. All patients were treated according to current guidelines for the management of severe TBI12. Patients were categorized into mild pneumonia and severe pneumonia groups based on the severity of pneumonia during hospitalization, diagnosed according to the Infectious Diseases Society of America/American Thoracic Society consensus guidelines13. According to 90-day survival, severe TBI patients were classified as survivors or non-survivors. During the follow-up period, enrolled patients who died from any cause were classified as non-survivors. This study was approved by the Shengli Oilfield Central Hospital Ethics Committee (approval no. Q/ZXYY-ZY-YWB-LL202119) and conducted in accordance with the Declaration of Helsinki of 1975, revised in 2013. Written informed consent was obtained from all patients or, when patients were unable to consent, by first-degree relatives.
Samples of peripheral blood were collected between 48 and 72 h after the onset of TBI. Serum samples were collected and stored at − 80 °C for a uniform assay. Quantitative enzyme-linked immunosorbent assay (ELISA) kits were used to determine the levels of sPD-1 (DuoSet Human PD-1, R&D systems, Minneapolis, MN) and sPD-L1 (Multiskan MK3; Thermo, West Palm Beach, FL).
Descriptive statistics included the mean and standard deviation for normally distributed data or the median and interquartile range for non-normally distributed data. For data that were normally distributed, we utilized the Analysis of Variance (ANOVA) test to compare means across groups. For data that exhibited non-normal distribution, we applied the Kruskal-Wallis one-way analysis, which is a non-parametric alternative to ANOVA. Receiver operating characteristic (ROC) curves were used to determine the area under the curve (AUC) for predicting severe pneumonia and mortality. Prognostic parameters, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), were calculated based on optimal thresholds identified through ROC curve analysis. AUC comparisons were conducted using the Z-test formula. Binary logistic regression analysis was used to identify independent predictors of severe pneumonia and 90-day mortality. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant. All data were analyzed using IBM SPSS Statistics 26.0 and GraphPad Prism 9.0.
Results
Clinical data characteristics and baseline data
During the investigation period, a total of 82 TBI patients were enrolled after screening 133 individuals (Fig. 1). Among the enrolled, 4 were under 18 years old, 3 had hematologic disorders, 7 had liver disease, 6 had end-stage renal disease, 9 had cancer, and 6 were undergoing hormone therapy. Notably, 16 died within 48 h of admission. The study ultimately included these 82 TBI patients and 20 healthy volunteers. Among the 82 patients with TBI, the primary injury mechanisms were cerebral contusions, traumatic subdural hematomas, and traumatic subarachnoid hemorrhages; 64 of whom received surgical procedures. During their hospital stay, 30 (36.6%) developed severe pneumonia, and the time to progression averaged 6.0 ± 2.5 days. Characteristics of patients with mild and severe pneumonia are detailed in Table 1. No significant differences in age, gender, or medical history were found among the three groups. The median values of WBC, lymphocytes, lactate, CRP, PCT, surgical procedures and APACHE II scores significantly differed between TBI patients and healthy controls, as well as between the mild and severe pneumonia groups. There were no significant differences in surgical procedures and Marshall CT scores across the groups. The RTS was significantly lower in the severe pneumonia group compared to the mild pneumonia group. Interestingly, the mild pneumonia group had a significantly longer ICU stay than the severe pneumonia group.
Based on the 90-day follow-up, there were ultimately 24 deaths (23.5%), with an average time from hospital admission to death of 13.6 ± 3.7 days. The characteristics between survivors and non-survivors among TBI patients are shown in Table 2. The median values of WBC, lymphocytes, lactate, CRP, PCT, and APACHE II scores significantly differed, with higher values in non-survivors than in survivors and controls (all P < 0.001). There was no significant difference in the distribution of Marshall CT scores and surgical procedures between survivors and non-survivors. The RTS was significantly lower in non-survivors (P = 0.002). The duration of MV and ICU stay were also reported, showing a significant difference in ICU stay duration between survivors and non-survivors (P < 0.001).
sPD-1 and sPD-L1 expression in severe TBI patients
The median levels of sPD-1 and sPD-L1 for each group are presented in Table 1. In healthy volunteers, the median values were 0.95 ng/mL for sPD-1 and 0.58 ng/mL for sPD-L1. There was a significant increase in both sPD-1 and sPD-L1 levels in TBI patients with pneumonia compared to healthy controls (P < 0.001). Furthermore, levels of sPD-1 and sPD-L1 were markedly higher in patients with severe pneumonia than in those with mild pneumonia (all P < 0.01, as shown in Fig. 2A and B). Similarly, levels of sPD-1 and sPD-L1 were significantly elevated in non-survivors compared to survivors (P < 0.001, Fig. 2C and D). Detailed data are provided in Table 2.
sPD-1 and sPD-L1 as predictors of severe pneumonia and mortality in TBI patients
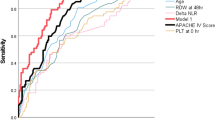
The AUC for predicting severe pneumonia was 0.714 for sPD-1 and 0.696 for sPD-L1, not significantly different from PCT (0.699) or APACHE II (0.702) according to the Z test (all P > 0.05; see Table 3; Fig. 3A). The optimal threshold for sPD-1 was 1.97 ng/mL, with a sensitivity of 80.0%, specificity of 50.0%, PPV of 48.0%, and NPV of 81.3%. For sPD-L1, the optimal threshold was 0.83 ng/mL, with a sensitivity of 63.3%, specificity of 69.2%, PPV of 54.3%, and NPV of 76.6%. The AUCs for predicting severe pneumonia when combining PCT or APACHE II score with sPD-1 or sPD-L1 were 0.733, 0.742, 0.721, and 0.730, respectively, slightly higher than those of PCT or APACHE II alone (P > 0.05). The results are detailed in Table 3; Fig. 3A.
Similarly, the AUC for predicting mortality was 0.758 for sPD-1 and 0.735 for sPD-L1, slightly higher than that of PCT (0.718) or APACHE II (0.723) (all P > 0.05; see Table 3; Fig. 3B). The best cutoff for sPD-1 was 2.07 ng/mL, with a sensitivity of 46.6%, specificity of 91.7%, PPV of 41.5%, and NPV of 90.3%. The optimal threshold for sPD-L1 was 1.14 ng/mL, with a sensitivity of 48.3%, specificity of 87.5%, PPV of 41.2%, and NPV of 90.3%. The AUCs for predicting mortality when combining PCT or APACHE II score with sPD-1 or sPD-L1 were 0.782, 0.792, 0.767, and 0.780, respectively, higher than those of PCT or APACHE II scores alone, though not statistically significant (P > 0.05). The detailed data are shown in Table 3; Fig. 3B.
Correlation of sPD-1 and sPD-L1 levels with other variables
Correlation of sPD-1 and of sPD-L1 levels with GCS scores at admission, APACHE II scores, length of MV, and time elapsed to mortality were performed. Correlation analysis revealed a strong correlation between sPD-1 levels in TBI patients and the GCS scores (r=-0.758, P < 0.001, Fig. 4A). There was a moderate correlation with the APACHE II score (r = 0.580, P < 0.001, Fig. 4B). A weak correlation was observed with the duration of MV (r = 0.274, P = 0.013). Among TBI patients who died, a strong correlation was found between sPD-1 levels and time elapsed to mortality (r=-0.721, P < 0.001, Fig. 4C).
Similarly, sPD-L1 levels in TBI patients also showed a strong correlation with GCS scores (r=-0.721, P < 0.001, Fig. 4D), a moderate correlation with the APACHE II score (r = 0.550, P < 0.001, Fig. 4E), and a weak correlation with the duration of MV (r = 0.331, P = 0.004). In deceased TBI patients, a strong correlation was identified between sPD-L1 levels and the time interval until death (r=-0.715, P < 0.001, Fig. 4F).
sPD-1 and sPD-L1 as independent predictors of severe pneumonia and mortality in TBI patients
Univariate and multivariate logistic regression was used to identify independent predictors associated with severe pneumonia in patients with TBI. In the multivariate analysis, WBC, lymphocytes, lactate, CRP, PCT, GCS score, RTS score, APACHE II score, and duration of ICU stay were included in the logistic regression model. Binary logistic regression analysis revealed that sPD-1 (odds ratio (OR) = 2.461, 95%CI: 1.179 to 5.139, P = 0.016) and increased sPD-L1 (OR = 5.553, 95%CI: 1.647–18.720, P = 0.006) were independent predictors of severe pneumonia in TBI patients. The detailed results are shown in Table 4.
WBC, lymphocytes, lactate, CRP, PCT, GCS score, RTS score, APACHE II score, and duration of ICU stay were also included in the multivariate logistic regression model to determine independent predictors of mortality in patients with TBI. Multivariate logistic regression analysis revealed that sPD-1 (OR = 2.746, 95%CI: 1.055–7.144, P = 0.038) and elevated sPD-L1 levels (OR = 5.290, 95%CI: 1.010–27.693, P = 0.049) were associated with mortality. The detailed results are demonstrated in Table 4.
Discussion
In this study, we found that sPD-1 and sPD-L1 were upregulated in patients with severe TBI. The peripheral blood levels of these biomarkers were significantly correlated with post-TBI severe pneumonia and mortality. sPD-1 and sPD-L1 emerged as independent predictive factors for both conditions. Our findings suggest the potential utility of sPD-1 and sPD-L1 as immunological biomarkers for risk stratification and prognosis prediction in patients with severe TBI.
Several studies have explored the relationship between TBI and immune suppression, particularly in the context of pneumonia. TBI has been shown to induce lung injury and systemic immune suppression, leading to an increased vulnerability to pneumonia14,15. The mechanisms underlying this immune suppression and its impact on the incidence of pneumonia following TBI are areas of ongoing research. sPD-1 and sPD-L1 function as negative regulatory factors within the immune system16. High levels of sPD-1 and sPD-L1 can lead to a suppression of immune function, which may have both beneficial and detrimental effects17,18. On one hand, this suppression helps prevent excessive inflammation that can cause further damage to the brain and other tissues. On the other hand, it can impair the body’s ability to fight infections, as evidenced by the association of elevated sPD-1 and sPD-L1 with severe pneumonia and higher mortality rates in TBI patients.
Previous studies have demonstrated a positive correlation between the peripheral blood levels of sPD-1 and sPD-L1 with the expression levels of their membrane-bound counterparts in patients with sepsis19. Additionally, overexpression of membrane-bound PD-1 on CD4 + and CD8 + T cells, along with elevated serum levels of sPD-1, has been observed in patients with immune disease20,21. These findings indicate that a marked increase in sPD-1 levels may signify more severe immune impairment in patients with severe TBI. Research has also identified elevated sPD-1 levels in cases of critical illness, suggesting its potential as a biomarker for acute respiratory distress syndrome (ARDS)22. In the context of TBI, our study indicates that high levels of both sPD-1 and sPD-L1 could serve as reliable biomarkers for predicting the onset of severe pneumonia and mortality post-injury.
Our study showed that sPD-1 and sPD-L1, as immunological markers, offered predictive validity for severe pneumonia and mortality in TBI patients comparable to established markers like PCT and the APACHE II score23. While the APACHE II score and PCT are comprehensive for critical illness assessment, they do not encompass an evaluation of immune function24,25. Integrating sPD-1 or sPD-L1 could enhance the predictive accuracy of these tools regarding TBI prognosis. sPD-1 and sPD-L1 are reliable prognostic indicators for severe pneumonia and mortality in TBI patients and are valuable for risk stratification, as determined by the optimal cutoff values from ROC curve analysis. They also aid in patient stratification in clinical trials focused on immunomodulation in TBI. Interestingly, our study found that patients with mild pneumonia had longer ICU stays than those with severe pneumonia. The primary reason may be the higher incidence of in-hospital mortality among patients with severe pneumonia. Consequently, our results also indicate that the length of stay for survivors was longer than that for patients who died.
Our study has several limitations. Firstly, our study population primarily consisted of patients with a principal diagnosis of severe cranial injury, excluding patients with multisystem trauma or severe shock, in whom sPD-1 and sPD-L1 may exhibit varying expressions. Secondly, we mainly recruited TBI patients who survived beyond 48 h after admission, yet many patients with severe TBI may die within the first 48 h, and those who die early might exhibit different immune profiles. Thirdly, our study population had an average age of 58 years, largely because younger patients’ families often declined participation; thus, it is important to consider the variations in immune function across different age groups26. Fourthly, the immune function of TBI patients may change over time; therefore, the dynamic monitoring of sPD-1 and sPD-L1 levels could provide more information.
Conclusions
In conclusion, the present study demonstrated that sPD-1 and sPD-L1 levels were upregulated in patients with severe TBI and were significantly associated with severe pneumonia and mortality after TBI. These findings suggest that sPD-1 and sPD-L1 could potentially serve as predictors of severe pneumonia and death in patients with severe TBI.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Change history
11 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-78428-w
References
Guan, B., Anderson, D. B., Chen, L., Feng, S. & Zhou, H. Global, regional and national burden of traumatic brain injury and spinal cord injury, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. BMJ Open. 13, e075049 (2023).
Lassarén, P. et al. Systemic inflammation alters the neuroinflammatory response: a prospective clinical trial in traumatic brain injury. J. Neuroinflammation. 18, 221 (2021).
Sharma, R. et al. Infections after a traumatic brain injury: the complex interplay between the immune and neurological systems. Brain Behav. Immun. 79, 63–74 (2019).
Bao, W., Lin, Y. & Chen, Z. The Peripheral Immune System and Traumatic Brain Injury: insight into the role of T-helper cells. Int. J. Med. Sci. 18, 3644–3651 (2021).
Chen, Y. et al. The implication of targeting PD-1:PD-L1 pathway in treating sepsis through immunostimulatory and anti-inflammatory pathways. Front. Immunol. 14, 1323797 (2023).
Peng, Q. et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun.11, 4835 (2020).
Gianchecchi, E., Delfino, D. V. & Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 12, 1091–1100 (2013).
Du, Y. et al. Serum levels of soluble programmed death-1 (sPD-1) and soluble programmed death ligand 1(sPD-L1) in systemic lupus erythematosus: Association with activity and severity. Scand. J. Immunol. 92, e12884 (2020).
Niu, M., Liu, Y., Yi, M., Jiao, D. & Wu, K. Biological characteristics and clinical significance of Soluble PD-1/PD-L1 and exosomal PD-L1 in Cancer. Front. Immunol. 13, 827921 (2022).
Sari, M. I. & Ilyas, S. The expression levels and concentrations of PD-1 and PD-L1 proteins in septic patients: a systematic review. Diagnostics (Basel) 12, (2022).
Mehta, R. & Chinthapalli, K. Glasgow coma scale explained. Bmj. 365, l1296 (2019).
Carney, N. et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 80, 6–15 (2017).
Mandell, L. A. et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44 (Suppl 2), S27–72 (2007).
Vermeij, J. D. et al. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J. Neurotrauma. 30, 2073–2079 (2013).
Geng, X. et al. Construction and validation of a predictive model of pneumonia for ICU patients with traumatic brain injury (TBI). Neurosurg. Rev. 46, 308 (2023).
Liu, M. et al. Serum sPD-L1, upregulated in Sepsis, May reflect Disease Severity and clinical outcomes in septic patients. Scand. J. Immunol. 85, 66–72 (2017).
Sun, S. et al. Serum-soluble PD-L1 may be a potential diagnostic biomarker in sepsis. Scand. J. Immunol. 94, e13049 (2021).
Chen, R. & Zhou, L. PD-1 signaling pathway in sepsis: does it have a future? Clin. Immunol. 229, 108742 (2021).
Zhao, Y., Jia, Y., Li, C., Shao, R. & Fang, Y. Predictive value of Soluble programmed Death-1 for severe Sepsis and septic shock during the First Week in an Intensive Care Unit. Shock. 51, 289–297 (2019).
Wan, B. et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J. Immunol. 177, 8844–8850 (2006).
Wu, H. et al. Soluble PD-1 is associated with aberrant regulation of T cells activation in aplastic anemia. Immunol. Invest. 38, 408–421 (2009).
Monaghan, S. F. et al. Soluble programmed cell death receptor-1 (sPD-1): a potential biomarker with anti-inflammatory properties in human and experimental acute respiratory distress syndrome (ARDS). J. Transl Med. 14, 312, (2016).
Wang, R., Hua, Y., He, M. & Xu, J. Prognostic value of serum procalcitonin based model in moderate to severe traumatic brain Injury patients. J. Inflamm. Res. 15, 4981–4993 (2022).
Vincent, J. L. & Moreno, R. Clinical review: scoring systems in the critically ill. Crit. Care. 14, 207 (2010).
Raj, R. et al. Predicting six-month mortality of patients with traumatic brain injury: usefulness of common intensive care severity scores. Crit. Care. 18, R60 (2014).
Magatti, M. et al. Systemic immune response in young and elderly patients after traumatic brain injury. Immun. Ageing 20, 41, (2023).
Funding
This work was supported by the Foundation for the School Health Association of Shandong (SDWS2022186) and Dongying Natural Science Foundation (2023ZR036).
Author information
Authors and Affiliations
Contributions
Lei Liu and Pengpeng Lan performed the experiments, collected data, analyzed and interpreted the data, and wrote the paper. Guiping Wu, Xiaojie Zhu, Hongfeng Shi, Yan Li, Ruili Li and Ling Zhao conducted the trial and collected data. Juan Xu and Min Xu designed this study and obtained research funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, Min Xu was incorrectly affiliated. Full information regarding the correction made can be found in the correction for this Article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L., Lan, P., Wu, G. et al. Prognostic value of soluble programmed death-1 and soluble programmed death ligand-1 in severe traumatic brain injury patients. Sci Rep 14, 23791 (2024). https://doi.org/10.1038/s41598-024-74520-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74520-3