Abstract
Melatonin supplementation during in vitro maturation (IVM) improves porcine oocyte maturation and embryonic development by exerting antioxidative effects. Nevertheless, the mechanism by which melatonin prevents polyspermy after in vitro fertilization (IVF) remains unclear. Here, we examined the effects of melatonin on cytoplasmic maturation and the incidence of polyspermic penetration in porcine oocytes. No statistically significant difference was observed in the rate of first polar body formation between the groups (Control, Melatonin, Melatonin + Luzindole, and Melatonin + 4-P-PDOT). Interestingly, melatonin supplementation significantly improved the cytoplasmic maturation of porcine oocytes by enhancing the normal distribution of organelles (Golgi apparatus, endoplasmic reticulum and mitochondria) and upregulating organelle-related gene expressions (P < 0.05). However, these promotional effects were counteracted by melatonin antagonists, suggesting that melatonin enhances cytoplasmic maturation through its receptors in porcine oocytes. Melatonin supplementation also significantly improved the rate of diploid and blastocyst formation after IVF by promoting the normal distribution of cortical granules (P < 0.05). In conclusion, melatonin supplementation during in vitro maturation of porcine oocyte improves fertilization efficiency and embryonic developmental competence by enhancing cytoplasmic maturation.
Similar content being viewed by others
Introduction
Embryo in vitro production (IVP) in pigs offers several critical advantages that contribute to advancements in swine reproduction and genetics. These benefits include shortening generational intervals, allowing for increased selection intensity, facilitating the transport of genetics across international borders, ensuring bio secure farm restocking, and preserving rare breeds. Understanding and enhancing the efficiency of in vitro fertilization (IVF) techniques, particularly in addressing challenges such as polyspermy, can significantly improve breeding programs and contribute to the sustainability of pig farming.
Fertilization involves the fusion of a sperm and an oocyte within ampulla of the fallopian tube, resulting in the formation of a zygote. However, during IVF, multiple spermatozoa typically penetrate the oocytes, leading to polyspermy. The high frequency of polyspermic fertilization presents a significant scientific challenge in the in vitro maturation (IVM) of oocytes and continues to be a persistent concern in IVF1. Polyspermic fertilization may result in abnormal embryonic development and even failure to reach full-term due to chromosomal abnormalities2. In humans, the incidence of polyspermy during IVF varies from a few percent to more than 30%3. The high frequency of polyspermy hinders the successful IVP of normal porcine embryos, with incidence rates ranging from 50 to 90%4. The use of porcine oocytes as a natural and optimal model for studying polyspermic penetration after IVF is justified by the higher incidence of polyspermic fertilization in pigs compared to other species5.
Melatonin, a potent antioxidant, has the remarkable ability to effectively counteract oxidative stress and scavenge reactive oxygen species in oocytes6. Our previous studies demonstrated that supplementation with melatonin or its agonist ramelteon can enhance the in vitro maturation (IVM) of oocytes by promoting lipid metabolism, which serves as a crucial energy source for oocyte and embryo development through the activation of melatonin receptor 2 (MT2)7,8,9. Both melatonin receptor 1 (MT1) and MT2 are abundantly expressed in mammalian oocytes10,11,12,13. However, in pigs, only MT2 is expressed in their oocytes, with no expression of MT114,15. Additionally, the inclusion of melatonin receptor antagonists (Luzindole, which antagonizes both MT1 and MT2, and 4P-PDOT, which specifically targets MT2) facilitates a comprehensive understanding of the intricate molecular regulatory mechanisms underlying porcine oocyte maturation mediated by MT28,16. These findings highlight the indispensable role of MT2 in the maturation of porcine oocytes.
Cytoplasmic maturation in mammalian oocytes is a complex biological process that involves the accumulation of mRNA, proteins, and nutrients necessary for achieving oogenesis competence17. It has been demonstrated that cytoplasmic maturation is crucial for the fertilization capacity and developmental potential of oocytes18. Indeed, during IVF, the high incidence of polyspermy leads to lower developmental competence of embryos compared to those developed in vivo, especially in pigs2. This phenomenon is closely associated with the cytoplasmic maturation of oocytes and suggests that high-quality oocytes effectively prevent polyspermy. Thus, understanding the mechanism of melatonin-mediated cytoplasmic maturation could help explain how melatonin enhances the fertilization capacity and developmental potential of oocytes. In our previous research, we found that melatonin could enhance the cytoplasmic maturation of porcine oocytes by modulating lipid metabolism7,8. Therefore, we speculate that the improved developmental capacity of porcine oocytes after melatonin treatment will effectively prevent polyspermy during IVF. Additionally, melatonin has been shown to enhance the fertilization capacity and developmental potential of bovine oocytes by increasing the rate of monospermic fertilization in IVF systems for cattle19,20. To the best of our knowledge, there is a lack of research investigating the impact of melatonin on porcine polyspermy in IVF procedures, and the precise mechanisms by which melatonin hinders polyspermic fertilization remain elusive. The aim of this study is to test the hypothesis that melatonin increases IVF efficiency, specifically by reducing polyspermy in porcine oocytes. The findings of this study will contribute to understanding the mechanisms through which melatonin enhances the fertilization capacity and developmental potential of oocytes.
Results
Effects of melatonin on porcine oocyte nuclear maturation
During porcine IVM, we enriched the oocyte maturation medium with 10−9 M melatonin and its antagonist (10−9 M Luzindole or 4-P-PDOT). Our findings revealed no statistically significant difference in oocyte nuclear maturation among groups (Control: 89.7 ± 0.6%; Melatonin: 91.5 ± 1.5%; Melatonin + Luzindole: 88.9 ± 0.5%; and Melatonin + 4-P-PDOT: 89.2 ± 0.8%, as shown in Table 1).
Effects of melatonin on distribution of Golgi and CGs
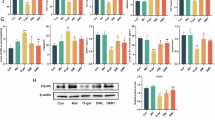
With melatonin supplementation, the normal distribution rate of Golgi was significantly increased compared to the control group (62.9 ± 2.2% vs. 49.4 ± 2.3%, P < 0.05, Fig. 1A). Additionally, melatonin supplementation increased the mRNA expression of Golgi-related genes PAQR3 and RAB8A (P < 0.05, Fig. 1C). Conversely, treatment with melatonin antagonists (Luzindole and 4P-PDOT) reduced normal distribution of the Golgi apparatus.
Effects of melatonin on the distribution of Golgi apparatus (Golgi) and cortical granule (CG) in porcine oocytes. (A) Golgi distribution: a - homogeneous distribution (normal); b–d - unequal distribution (abnormal). (B) CG distribution: a - peripheral CG distribution (normal); b and c - homogeneous distribution (abnormal); d - cortical distribution (abnormal). (C) Gene expression related to Golgi and CG was analyzed. Bars with different letters within the same indicator denote significant differences at P < 0.05. The results are presented as the mean ± standard error of the mean (SEM) from at least three independent experimental replicates. Each replicate involved the analysis of 30 oocytes per group. Melatonin: 10⁻⁹ M Melatonin; Mtn + Lu: 10⁻⁹ M Melatonin + 10⁻⁹ M Luzindole; Mtn + 4P: 10⁻⁹ M Melatonin + 10⁻⁹ M 4-P-PDOT.
As depicted in Fig. 1B, the melatonin group exhibited a significantly higher rate of cortical granules (CGs) following a normal distribution compared to the control group (64.5 ± 2.2% vs. 47.0 ± 1.6%, P < 0.05). However, this increase was inhibited by melatonin antagonists (Melatonin + Luzindole: 49.7 ± 1.4% and Melatonin + 4-P-PDOT: 51.7 ± 2.0%). Consistent with the CG distribution results, there was a significant increase in the expression of CG-related genes (ASTL and RAB3A) in the melatonin-treated group (P < 0.05).
Effects of melatonin on distribution of ER and mitochondria
As shown in Fig. 2A, the normal distribution rate of the endoplasmic reticulum (ER) was significantly improved with melatonin supplementation compared to the other groups (57.1 ± 2.9% vs. 45.6 ± 2.6%, 44.2 ± 1.6%, and 46.3 ± 1.9%, P < 0.05). In line with the ER distribution results, the ER-related gene CHOP was significantly decreased, and RNC1 was significantly increased with melatonin supplementation compared to the other groups (P < 0.05, Fig. 2C).
Effects of melatonin on the distribution of endoplasmic reticulum (ER) and mitochondria in porcine oocytes. (A) ER distribution: a - homogeneous distribution (normal); b–d - unequal distribution (abnormal). (B) Mitochondrial distribution: a - peripheral (normal); b–d - cortical or diffused (abnormal). (C) Expression of genes related to ER and mitochondrial biogenesis. Bars with different letters within the same indicator denote significant differences at P < 0.05. The results are presented as the mean ± standard error of the mean (SEM) from at least three independent experimental replicates. Each replicate involved the analysis of 30 oocytes per group. Melatonin: 10⁻⁹ M Melatonin; Mtn + Lu: 10⁻⁹ M Melatonin + 10⁻⁹ M Luzindole; Mtn + 4P: 10⁻⁹ M Melatonin + 10⁻⁹ M 4-P-PDOT.
The abnormal distribution rate of mitochondria was significantly higher in the control, Melatonin + Luzindole, and Melatonin + 4-P-PDOT groups compared to the melatonin treatment group (46.4 ± 0.77%, 46.2 ± 2.6%, and 46.36 ± 0.6% vs. 25.5 ± 1.5%, P < 0.05, Fig. 2B). Mitochondrial biogenesis was significantly improved with melatonin supplementation, as indicated by the increased expression of POLG, PRDX2, TFAM, TFB1M, NRF1, and PGC1α (P < 0.05, Fig. 2C). However, these effects were inhibited by melatonin antagonists (Luzindole and 4P-PDOT).
Polyspermic penetration incidence and embryonic development after IVF
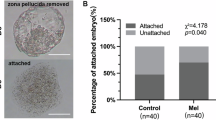
To determine the timing of pronucleus formation, we examined nuclear morphology 0 to 14 h post-fertilization. We found that pronucleus formation peaked at 12 h compared to other time points (Fig. 3A and B). Therefore, the polyspermy rate was assessed at 12 h after fertilization. As shown in Table 2, the normal fertilization rate (2 pronuclei) was significantly improved with melatonin supplementation. In contrast, the polyspermy rate was significantly reduced with melatonin supplementation compared to the other groups (P < 0.05). Additionally, the expression of anti-polyspermic genes such as JUNO, CD9, ZP2, and ZP4 was significantly increased with melatonin treatment compared to the other groups (P < 0.05, Fig. 3C). However, these effects were attenuated by the administration of a melatonin antagonist. Notably, although there was no significant difference in cleavage rate and total cell number, the rate of blastocyst formation was significantly improved by melatonin treatment compared to the other groups (P < 0.05, Table 3).
(A) Determination of pronucleus formation time. (B) Number of pronuclei: a - unfertilized (white arrow: first polar body; yellow arrow: oocyte nucleus); b - diploid; c and d - polyploid. (C) Polyspermy-related gene expression. Bars with different letters within the same indicator denote significant differences at P < 0.05. The results are presented as the mean ± standard error of the mean (SEM) from at least three independent experimental replicates. PN: pronucleus; Melatonin: 10⁻⁹ M Melatonin; Mtn + Lu: 10⁻⁹ M Melatonin + 10⁻⁹ M Luzindole; Mtn + 4P: 10⁻⁹ M Melatonin + 10⁻⁹ M 4-P-PDOT.
Discussion
The efficiency of IVP of pig embryos is lower compared to other species, primarily due to the high occurrence of polyspermy during IVF, making it challenging to avoid polyspermic fertilization. This study investigated the effects of melatonin on cytoplasmic maturation and the incidence of polyspermic penetration in porcine oocytes. Our findings revealed a significant increase in monospermic fertilization rates, indicating that melatonin supplementation had a positive effect on early embryo development up to the blastocyst stage following IVF. Supplementation with melatonin also resulted in an increased percentage of normal organelle distribution, suggesting that embryos derived from IVF with melatonin-supplemented oocytes underwent molecular changes indicative of enhanced embryonic developmental competence and improved fertilization ability.
Cytoplasmic maturation in mammalian oocytes is a complex biological process that involves the accumulation of mRNA, proteins, and nutrients essential for achieving oogenesis competence17. Additionally, the high developmental potential of oocytes is attributed to the precise spatial distribution and coordinated temporal dynamics of organelles21. Melatonin supplementation did not significantly increase the nuclear maturation rate during IVM compared to the control group. However, melatonin notably enhanced the normal distribution of organelles, suggesting a positive effect on oocyte cytoplasmic maturation. In a previous study, we demonstrated that melatonin did not enhance oocyte nuclear maturation but significantly improved cytoplasmic maturation indicators such as GDF9 and BMP15 through MT2 activation8. Furthermore, we detected MT2 mRNA in porcine oocytes, but not MT114. Our collective findings suggest that melatonin supplementation during IVM enhances the cytoplasmic maturation of oocytes by promoting the normal distribution of organelles through MT2 signaling. Melatonin has also been shown to enhance the fertilization capacity and developmental competence of bovine oocytes by upregulating cytoplasmic maturation19.
The robust developmental potential of oocytes is ensured by the maintenance of homeostasis and the reorganization of organelles22. The Golgi apparatus plays a crucial role in intracellular trafficking processes, including protein transport and modifications21. Additionally, the Golgi apparatus releases cortical granules (CGs) stored in the oocyte cortex to prevent polyspermy23. The present study demonstrated that melatonin supplementation significantly enhanced the proper localization of the Golgi apparatus and upregulated the expression of the Golgi-associated genes PAQR3 and RAB8A. Furthermore, melatonin effectively reduced the aberrant distribution of CGs by upregulating the expression of CG-associated genes ASTL and RAB3A compared to the control group. However, these effects were inhibited by melatonin antagonists. Therefore, our findings suggest that melatonin supplementation reduces the abnormal distribution of cortical granules, effectively decreasing the incidence of polyspermic fertilization by promoting Golgi function via MT2 signaling. This inhibits polyspermy formation and improves blastocyst development after IVF.
The role of mitochondria in ATP production, which is crucial for regulating cellular metabolism during oocyte maturation and embryonic development, is widely acknowledged24. Mitochondria are closely associated with a specialized domain of the endoplasmic reticulum, leading to the formation of mitochondria-associated ER membranes22. The ER functions as the primary intracellular reservoir of calcium ions and plays a pivotal role in maintaining calcium homeostasis21. Additionally, the cortical reaction is a calcium-dependent exocytotic process that is crucial for reducing the occurrence of polyspermic fertilization25. Therefore, maintaining functional homeostasis in the ER is essential for preventing polyspermy during cortical granule exocytosis. Our findings demonstrate that melatonin enhances ER and mitochondrial organization by upregulating genes (RCN1, POLG, PRDX2, TFAM, TFB1M, NRF1, and PGC1α) associated with these organelles, while simultaneously suppressing the expression of CHOP, a gene involved in ER stress. With melatonin supplementation during IVM, there was significant upregulation in the expression of the polyspermy-related genes JUNO, CD9, ZP2, and ZP4, whereas their downregulation was observed in the presence of melatonin antagonists. In a previous study, we found that melatonin could enhance lipid metabolism in porcine oocytes7,8. This improved metabolic environment may indirectly protect mRNA from degradation, either by reducing the mRNA degradation rate and prolonging its half-life or by regulating the activity of specific mRNA-degrading enzymes, thereby enhancing the cytoplasmic maturation of oocytes and embryonic development competence. Indeed, melatonin treatment of porcine oocytes results in an elevated rate of monospermic fertilization and blastocyst formation following IVF. The administration of melatonin to bovines has been demonstrated to enhance the rate of monospermic fertilization and promote early embryo development following IVF20. Additionally, melatonin upregulates CD9 and JUNO protein levels in bovine oocytes19. Collectively, promoting ER and mitochondrial redistribution involved in oocyte cytoplasmic maturation enhances oocyte quality and embryo developmental competence through melatonin supplementation.
Our findings suggest that melatonin supplementation enhances oocyte cytoplasmic maturation, promotes monospermic fertilization, and increases the blastocyst formation rate by augmenting normal distribution of organelles during IVM. This study’s findings not only shed light on the mechanisms by which melatonin enhances the cytoplasmic maturation of porcine oocytes but also have broader implications for the future of pig IVP. By reducing the incidence of polyspermic fertilization, our research supports the notion that improved oocyte quality can lead to more successful embryo development and higher pregnancy rates. This advancement will facilitate the rapid propagation of desirable genetic traits, support the international exchange of genetics, and promote the conservation of rare pig breeds, ultimately enhancing the overall efficiency and viability of swine production systems.
Methods
The protocol for animal use was approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (NEAUEC202316) in accordance with the Guide for the Care and Use of Laboratory Animals of Northeast Agricultural University.
Chemicals
Unless otherwise specified, all chemicals and reagents used in this study were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Porcine oocyte in vitro maturation
Porcine ovaries were obtained from at a local slaughterhouse and transported to the laboratory in 0.9% NaCl solution at 25–32 °C. The contents of follicles (3–6 mm in diameter) were recovered by aspiration with an 18-gauge needle using a 10 mL syringe. The cumulus-oocyte complexes (COCs) were washed with 10 mM HEPES-buffered TCM199 (Invitrogen, Carlsbad, CA, USA) containing 2 mM of sodium bicarbonate, 5 mM sodium hydroxide, 1% Pen-Strep (Invitrogen) and 0.3% polyvinyl alcohol (PVA). The COCs were then cultured in a porcine oocyte in vitro maturation solution (TCM199 with 10% porcine follicular fluid, 10 IU/mL FSH, 0.57 mM cysteine, 10 IU/mL LH and 0.91 mM sodium pyruvate) with 10⁻⁹ M melatonin and 10⁻⁹ M Luzindole or 10⁻⁹ M M 4-P-PDOT (A stock solution of melatonin and its antagonist was dissolved in DMSO). The COCs were incubated for 21 h at 38.5 °C. After 21 h of maturation with hormone, the COCs were cultured for an additional 21 h in the hormone-free (without FSH and LH) maturation medium with 10⁻⁹ M melatonin and 10⁻⁹ M Luzindole or 10⁻⁹ M 4-P-PDOT. After 42 h of IVM, the nuclei were categorized into three distinct groups based on microscopic observations: immature, mature (oocytes with the first polar body), and degenerated.
Detection of embryo development and polyspermy after IVF
Sample of oocytes were denuded by gently pipetting with 0.1% hyaluronidase. Thirty fully matured oocytes were then transferred to a modified Tris-buffer medium (mTBM) containing 113.1 mM NaCl, 3 mM KCl, 20 mM Tris, 11 mM glucose, 5 mM sodium pyruvate, 7.5 mM CaCl₂·2 H₂O, 0.67 mg/mL caffeine, and 2 mg/mL BSA, with a volume of 50 µL per well, after 42 h of IVM, while fresh semen was prepared for IVF. The semen sample was washed twice with DPBS supplemented with 0.1% BSA by centrifuging at 2,000 rpm for 2 min. The spermatozoa pellet was resuspended in mTBM medium at a concentration of 1.0 × 106 spermatozoa/mL and subsequently added to a 50 µL mTBM droplet. Immediately before insemination, sperm motility was assessed, and > 80% motile sperm was used in each experiment. After 6 h of IVF, the presumptive zygotes were washed thrice in mTBM and subsequently transferred to porcine zygote medium 3 (PZM3) for in vitro culture at 38.5 °C under a humidified atmosphere of 5% O2, 5% CO2, and 90% N2. The zygotic pronucleus was visualized through nuclear staining in the absence of the zona pellucida after 2 mg/mL pronase treatment for 5 min, using a final concentration of 10 µg/mL Hoechst 33342 for 4 min. Additionally, the embryos were assessed for cleavage and blastocyst formation using a stereomicroscope 48 and 168 h post-IVF, respectively. Blastocysts were harvested on day 7 and stained with Hoechst33342 at a concentration of 10 µg/mL for 4 min to determine the total cell count per blastocyst.
Staining of Golgi, CG, ER, and mitochondria in oocytes
Following IVM, the mature oocytes were fixed in 4% paraformaldehyde for 30 min. Subsequently, they were washed thrice with 0.2% PVA-PBS before staining for ER and Golgi apparatus. The samples were then incubated in a solution containing either 1 µM ER-Tracker Red (BODIPYTR Glibenclamide, E34250; Invitrogen) or 5 mM NBD C6-Ceramide (C049-E; GeneCopoeia, Heidelberg, Germany) at 4 °C in the dark for 30 min. The labelled samples were mounted and imaged using a Nikon epifluorescence microscope (TE2000-S; Nikon, Tokyo, Japan). The ER and Golgi distribution was categorized into two groups: organelles uniformly distributed throughout the cytoplasm (normal distribution) and those with incomplete cytoplasmic localization, clustered organelle granulations, irregular cavity formations, or uneven distribution along the plasma membrane.
Fully mature porcine oocytes were fixed with 4% PFA for 30 min, blocked with a solution of 0.3% BSA and 1 M glycine, and permeabilized in 0.1% Triton X-100 for 5 min. After washing three times, the oocytes were incubated with 100 µg/mL fluorescein isothiocyanate conjugated to peanut agglutinin (FITC-PNA, L7381; Sigma, St. Louis, MO, USA) for 30 min. The labeled oocytes were mounted, and the cortical granule (CG) distribution was observed under a laser-scanning confocal microscope (TE2000-U). The distribution of CGs was classified as follows: peripheral distribution, where CGs are adjacent to the plasma membrane; homogeneous distribution, where CGs are distributed across half of the plasma membrane; and cortical distribution, where CGs are spread throughout the cytoplasm and plasma membrane.
To label the mitochondria of the oocytes, MitoTracker GreenFM (M7514; Invitrogen) was used for staining. To block the samples, 2% BSA-PBS was added and incubated for 2 h. The samples were then stained with 200 nM MitoTracker Green for 30 min. The distribution of mitochondria was classified as follows: uniformly delicate, with small granules dispersed throughout the cytoplasm; and non-uniform, with either large granules scattered throughout the cytoplasm or clusters of mitochondria found in the periphery or center.
Real-time polymerase chain reaction
Briefly, the TRIZOL reagent (Invitrogen) was used to extract total mRNA from 200 oocytes in each group, following the manufacturer’s protocol. For cDNA synthesis, the amfiRivert cDNA Synthesis Platinum Master Mix from GenDEPOT (random primers) was used. Quantitative real-time PCR utilized the following parameters: an initial denaturation phase at 95 °C for 10 min, followed by 40 cycles of a two-step process involving 5 s at 95 °C and annealing/extension at 60 °C for 1 min. The dissociation stage involved a denaturation step at 95 °C for 15 s, followed by annealing at 60 °C for 1 min, and concluded with a final denaturation at 95 °C. For each sample, the CT values were obtained by averaging the results of three replicates, using the appropriate equation R = 2-△△Ct. Oligonucleotide primer sequences are listed in Table 4.
Statistical analyses
Percentage data were subjected to arcsine transformation before statistical analysis. The data were analyzed using ANOVA followed by Tukey’s test, with the aid of SPSS statistical software (version 19.0; SPSS Inc., Chicago, IL, USA), and presented as the mean ± SEM. Each experiment was repeated at least three times. Statistical significance was set at P < 0.05.
Data availability
All data generated or analysed in this study are included in this article.
References
Li, Y. H. et al. Reduced polyspermic penetration in porcine oocytes inseminated in a new in vitro fertilization (IVF) system: straw IVF. Biol. Reprod. 69, 1580–1585. https://doi.org/10.1095/biolreprod.103.018937 (2003).
Nguyen, H. T. et al. Selection based on morphological features of porcine embryos produced by in vitro fertilization: timing of early cleavages and the effect of polyspermy. Anim. Sci. J. 91, e13401. https://doi.org/10.1111/asj.13401 (2020).
Balakier, H. Tripronuclear human zygotes: the first cell cycle and subsequent development. Hum. Reprod. 8, 1892–1897. https://doi.org/10.1093/oxfordjournals.humrep.a137955 (1993).
Kitaji, H., Ookutsu, S., Sato, M. & Miyoshi, K. A new rolling culture-based in vitro fertilization system capable of reducing polyspermy in porcine oocytes. Anim. Sci. J. 86, 494–498. https://doi.org/10.1111/asj.12327 (2015).
Sun, J. T. et al. Tannin reduces the incidence of polyspermic penetration in porcine oocytes. Antioxidants (Basel) 11, https://doi.org/10.3390/antiox11102027 (2022).
Tamura, H. et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 44, 280–287. https://doi.org/10.1111/j.1600-079X.2007.00524.x (2008).
Jin, J. X. et al. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 62, https://doi.org/10.1111/jpi.12388 (2017).
Jin, J. X. et al. Melatonin regulates lipid metabolism in porcine cumulus-oocyte complexes via the melatonin receptor 2. Antioxid. (Basel) 11, https://doi.org/10.3390/antiox11040687 (2022).
Sun, J. T. et al. Ramelteon reduces oxidative stress by Maintenance of lipid homeostasis in porcine oocytes. Antioxid. (Basel) 11, https://doi.org/10.3390/antiox11091640 (2022).
Wang, J. et al. Melatonin alleviates the suppressive effect of hypoxanthine on oocyte nuclear maturation and restores meiosis via the melatonin receptor 1 (MT1)-Mediated pathway. Front. Cell. Dev. Biol. 9, 648148. https://doi.org/10.3389/fcell.2021.648148 (2021).
Sampaio, R. V. et al. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod. Biol. Endocrinol. 10, 1–7 (2012).
Guo, S. et al. Melatonin promotes in vitro maturation of vitrified-warmed mouse germinal vesicle oocytes, potentially by reducing oxidative stress through the Nrf2 pathway. Animals 11, 2324 (2021).
Tian, X. et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 18, https://doi.org/10.3390/ijms18040834 (2017).
Lee, S. et al. Melatonin influences the sonic hedgehog signaling pathway in porcine cumulus oocyte complexes. J. Pineal Res. 63, https://doi.org/10.1111/jpi.12424 (2017).
Kang, J. T. et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 46, 22–28. https://doi.org/10.1111/j.1600-079X.2008.00602.x (2009).
Zhu, T. et al. Melatonin modulates lipid metabolism in porcine cumulus-oocyte complex via its receptors. Front. Cell. Dev. Biol. 9, 648209. https://doi.org/10.3389/fcell.2021.648209 (2021).
Watson, A. J. Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. J. Anim. Sci. 85, E1-3. https://doi.org/10.2527/jas.2006-432 (2007).
Paczkowski, M. & Krisher, R. Aberrant protein expression is associated with decreased developmental potential in porcine cumulus–oocyte complexes. Mol. Reprod. Dev. 77, 51–58 (2010).
Zhao, X. M. et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 64, https://doi.org/10.1111/jpi.12445 (2018).
Gutierrez-Anez, J. C. et al. Melatonin improves rate of monospermic fertilization and early embryo development in a bovine IVF system. PLoS ONE 16, e0256701. https://doi.org/10.1371/journal.pone.0256701 (2021).
Mao, L., Lou, H., Lou, Y., Wang, N. & Jin, F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod. Biomed. Online 28, 284–299. https://doi.org/10.1016/j.rbmo.2013.10.016 (2014).
Guzel, E. et al. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int. J. Mol. Sci. 18, https://doi.org/10.3390/ijms18040792 (2017).
Bello, O. D. et al. Rab3A, a possible marker of cortical granules, participates in cortical granule exocytosis in mouse eggs. Exp. Cell. Res. 347, 42–51. https://doi.org/10.1016/j.yexcr.2016.07.005 (2016).
Roth, Z. Symposium review: reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J. Dairy Sci. 101, 3642–3654 (2018).
Tsai, P. S., van Haeften, T. & Gadella, B. M. Preparation of the cortical reaction: maturation-dependent migration of SNARE proteins, clathrin, and complexin to the porcine oocyte’s surface blocks membrane traffic until fertilization. Biol. Reprod. 84, 327–335. https://doi.org/10.1095/biolreprod.110.085647 (2011).
Acknowledgements
We would like to thank Editage (https://www.editage.co.kr) for their assistance with English language editing.
Funding
This study was supported by the National Key R&D Program of China (2021YFA0805902, 2022YFD1302203), the National Natural Science Foundation of China (No. 32372884), and the Self-financing Project of Harbin Science and Technology Plan (2023ZCZJCG013).
Author information
Authors and Affiliations
Contributions
Conceptualization: Jing-Tao Sun, Jia-Hui Liu and Bao-Xiu Zhang; Data curation: Jing-Tao Sun, Jia-Hui Liu and Hang-Yu Chen; Formal analysis: Jing-Tao Sun; Funding acquisition: Xiao-Gang Weng, Jun-Xue Jin and Jing-Tao Sun; Investigation: Jing-Tao Sun, Jia-Hui Liu and Lu Zhao; Methodology: Jing-Tao Sun, Jia-Hui Liu and Jun-Xue Jin; Project administration: Jun-Xue Jin and Zhong-Hua Liu; Resources: Qian Shen, Bao-Xiu Zhang and Jun-Xue Jin; Software: Jing-Tao Sun, Lu Zhao, Hang-Yu Chen and Ren-Fei Wang; Supervision: Jun-Xue Jin; Validation: Jing-Tao Sun and Yong-Jia Li; Visualization: Jing-Tao Sun and Jia-Hui Liu; Writing—original draft: Jun-Xue Jin, Bao-Xiu Zhang and Jing-Tao Sun; Writing—review & editing: Xiao-Gang Weng, Bao-Xiu Zhang and Zhong-Hua Liu; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, JT., Liu, JH., Zhao, L. et al. Melatonin decreases excessive polyspermy for single oocyte in pigs through the MT2 receptor. Sci Rep 14, 23153 (2024). https://doi.org/10.1038/s41598-024-74969-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74969-2