Abstract
This retrospective study aimed to assess different macular neovascular network characteristics in relation to changes in best corrected visual acuity (BCVA) over 3 and 12 months following treatment. Using optical coherence tomography angiography, we reviewed the medical records of 46 treatment-naïve patients with neovascular age-related macular degeneration (nAMD) who received intravitreal aflibercept injections. The change in BCVA from baseline to 3 months and 12 months after treatment was recorded. The mean vessels percentage area, junctions density, lacunarity, and fractal dimension were significantly correlated with the change of BCVA from baseline to month 3 (P = 0.003, 0.046, 0.007, and 0.005 respectively). Fractal dimension and vessels percentage area were correlated with the change of BCVA from baseline to month 12 (P = 0.023 and 0.023 respectively). The findings suggest that baseline characteristics of macular neovascular complexes may serve as predictors for BCVA changes following treatment with aflibercept in nAMD patients.
Similar content being viewed by others
Introduction
Macular neovascularization (MNV) in the context of neovascular age-related macular degeneration (nAMD) is among the leading causes of blindness in the world resulting in significant disabilities despite treatment1. The cornerstone of treatment of the disease is intravitreal anti-vascular endothelial growth factor (Anti-VEGF) injection. There are several types of Anti-VEGFs and various kinds of treatment regimens, however, every person could have a different treatment response that may dependent on patient demographics, basic characteristics, genetics background, etc2. Generally, the activity of the disease is defined by optical coherence tomography (OCT) as the presence of subretinal fluid (SRF), intraretinal fluid (IRF), and subretinal hyperreflective material(SHRM)2. This imaging modality has a crucial role in planning treatment and modifying regimens. In addition, several studies have attempted to develop some OCT biomarkers to estimate the treatment response1,3. With the advent of optical coherence tomography angiography (OCTA) as a fast-none invasive imaging modality, it has garnered a lot of attention from investigators as a useful diagnostic tool for retinal diseases4. Today, advanced machines with robust imaging algorithms are able to provide a depth-resolved high-quality image of MNVs architecture in detail. Some previous studies have shown that OCTA outperforms fluorescein angiography (FA) and indocyanine green angiography (ICGA) in demonstrating a MNV network5,6. Several studies tried to describe MNV appearance based on OCTA images to develop criteria for disease activity and treatment response estimation7,8. some of these studies are qualitative and others just used a limited number of measures for quantitative analysis9,10. Moreover, the presence of contradictory findings could be attributed to varying inclusion criteria, such as distinguishing between previously treated eyes and treatment-naïve eyes, or it might be related to differences in interobserver variability when describing the shape of MNV or assessing qualitative indices9,11,12,13,14,15. In this study, our objective was to quantitatively evaluate the OCTA characteristics of treatment-naïve eyes with nAMD. We aimed to investigate any potential correlation between these OCTA features at baseline and the visual recovery observed after administering anti-VEGF treatments.
Methods
This study is a retrospective case series conducted at the Retina Clinic of Farabi Eye Hospital in Tehran, Iran. It involved reviewing the medical records of patients diagnosed with nAMD who received intravitreal aflibercept injections and were followed for 12 months. Informed consent was obtained from all subjects or their legal guardians. The study was approved by the institutional review board of Tehran University of Medical Sciences (IR.TUMS.FARABIH.REC.1401.014) and complies with tenets of the Declaration of Helsinki.
Inclusion criteria were treatment-naïve patients with active nAMD confirmed by two retina specialists (H.R. and E.K.) based on funduscopy, subretinal and/or intraretinal fluid on macular OCT (Spectralis SDOCT, Heidelberg Engineering, Germany), and dye leakage on FA (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany) or presence of MNV complex in OCTA (RTVue-XR; Optovue, Inc., Freemont, CA). Patients with type 1 or mixed type neovascularization were included in the study. Pachychoroid spectrum diseases and other causes of MNV were excluded based on a fundus examination, OCT, OCTA, and FA. When the diagnosis was uncertain, Enhanced depth imaging optical coherence tomography (EDI-OCT) and Indocyanine green angiography (ICGA) were performed. Best corrected visual acuity (BCVA) was measured based on early treatment diabetic retinopathy study (ETDRS) charts.
Patients with diabetes mellitus, occlusive vascular disorders, refractive error exceeding ± 3 diopters of spherical equivalent, a history of intraocular surgery (except cataract surgery), MNV originating from causes other than AMD, image quality score below 6/10, and MNV complexes located outside the 3 × 3 mm scanning area were excluded from the study.
Patients received monthly intravitreal injections of either aflibercept (Eylea, Regeneron, US) or its biosimilar, Tyalia (CinnaGen Company, Iran), for three doses. The previously published investigation demonstrated the non-inferiority of this biosimilar to the reference drug16 Subsequently, injections were continued every two months for up to 12 months.
Patient evaluations were conducted at three time points: baseline, month 3, and the end of 12 months. These evaluations included dilated fundus examination, macular OCT, and measurement of BCVA.
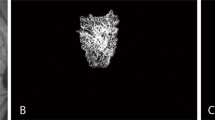
All patients underwent macular OCTA at baseline (RTVue-XR; Optovue, Inc., Freemont, CA) with 3 × 3 mm and 6 × 6 mm scan sizes at the central macular area. Removal of the projection artifacts is done using the device’s built-in software. The presence of MNV was evaluated on the outer retina and choriocapillaris slabs. Segmentation error was manually corrected to include the entire lesion then images were loaded in the FIJI software (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij). In the FIJI software, the image type was converted to “8-bit,” and subsequently, the “mean threshold” was applied. After manually extracting MNV complexes using the “polygon selection” tool, the images were binarized and skeletonized (Fig. 1). Vascular dispersion (VD) and fractal dimension (FD) were then calculated using the “directionality” and “fractal box count” functions, respectively17.
A patient with Neovascular age-related macular degeneration. (A) and (B): Infrared and corresponding spectral domain optical coherence tomography image demonstrates intraretinal fluid, subretinal hyperreflective material, and disruption of outer retinal hyperreflective bands. (C): En face outer retina slab of optical coherence tomography angiography shows macular neovascular complex. (D): The neovascular complex that is extracted from the rest of the outer retina slab. (E) and (F): Corresponding binarized and skeletonized images of macular neovascular complex.
The explant area, vessels area, vessels percentage area, total number of junctions, junctions density, total vessels length, average vessels length, total number of endpoints, and mean lacunarity were calculated using AngioTool software version 0.6 (National Institutes of Health®, Bethesda, Maryland, United States)17 (Fig. 2). The image analysis was reviewed by two independent retina specialists (H.R. and E.K.). Any disagreements existed between interpretations were adjudicated by a third senior retina specialist (H.F.).
Analysis of the Macular Neovascular Complex. (A): En face outer retina slab of optical coherence tomography angiography displaying the macular neovascular complex. (B): Extraction of the neovascular complex from the outer retina slab after thresholding, performed using ImageJ. (C): Corresponding binarized image of the macular neovascular complex. (D): Macular neovascular complex after processing with AngioTool.
The vessels percentage area represents the ratio of the area occupied by the vessels. Junction density represents the number of vascular junctions per unit of total length of vessels which reflects the density of branching18. Lacunarity accounts for structural nonuniformity of vascular complex and more value represents higher voids and gaps and less homogeneity. Fractal dimension is a parameter that reflects morphological complexity, the higher values indicate a more complex vascular network19.
To present data we used mean, standard deviation, minimum and maximum, frequency and percentage. To assess the relation of different parameter on VA and its changes during the follow up we used Pearson correlation coefficient as well as scatter plot which demonstrate the simple linear regression equation. To adjust for the effect of age and sex, we used partial correlation coefficient and multiple logistic regression analysis. All statistical analysis performed by SPSS (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). P-value less than 0.05 considered statistically significant.
Results
We recruited 46 eyes of 46 patients with nAMD to the study. Thirty-two patients (69.6%) were male and 14 were female (30.4%). The mean age of participants was 68.41 ± 5.8 years (range: 55–80). All patients completed the 12-month follow-up period and received seven intravitreal aflibercept injections (either Eylea or its biosimilar, Tyalia). The mean baseline BCVA was 55.7 ± 11.03 EDTRS letters which increased to 63.04 ± 12.89 and 62.65 ± 16.53 ETDRS letters at the months three and twelve after treatment, respectively. The mean change in BCVA from baseline to months 3 and 12 were 7.35 ± 9.94 and 6.96 ± 14.74 ETDRS letters, respectively. Regarding the anatomical outcomes, all patients had either SRF, IRF, or both at baseline; 34 patients were fluid-free at 3 months, and 33 were fluid-free at 12 months (Table 1).
Among the baseline parameters, The mean vessels percentage area was 56.15 ± 9.98 and its correlation with BCVA changes in month 3 and 12 was statistically significant (P = 0.003, r = 0.442, and P = 0.023, r = 0.345, respectively) (Figs. 3 and 4). The correlation between junctions density and BCVA changes was only statistically significant in month 3 (P = 0.046, r = 0.306) (Fig. 3). The correlation between mean lacunarity (0.157 ± 0.078) and BCVA changes was statistically significant in month 3 (P = 0.007, r = -0.403) (Fig. 3), However, This effect was not seen in month 12 (P = 0.155). The mean of FD was 1.508 ± 0.091 at baseline, and the correlation between baseline FD and BCVA changes in both months 3 and 12 was statistically significant (P = 0.005, r = 0.413, and P = 0.023, r = 0.343, respectively) (Fig. 5). The mean explant area, vessels area, total number of junctions, total vessels length, average vessels length, total number of end points, and vascular dispersion were not significantly correlated with BCVA changes from baseline to both months three and twelve (Table 2).
Comparison of OCTA imaging between nAMD patients with different visual outcomes at 12 months post-treatment with aflibercept. (A-C) Images from a patient with a 23-letter gain in ETDRS letters show the outer retina slab on OCTA (A), with corresponding binarized (B) and skeletonized (C) images, revealing a vessel percentage area of 58.24% and a fractal dimension of 1.485. (D–F) Images from a patient with a 12-letter loss in ETDRS letters show the outer retina slab on OCTA (D), with corresponding binarized (E) and skeletonized (F) images, revealing a vessel percentage area of 50.13% and a fractal dimension of 1.416.
We stratified patients into two groups based on changes in BCVA from baseline to month 3 and 12. Patients exhibiting a visual gain of more than 15 ETDRS letters (8 patients) were compared to those with a change in BCVA equal to or less than 15 ETDRS letters (38 patients) using multiple logistic regression analysis. Among these subgroups, the only OCT-A quantitative parameters that demonstrated significant differences were vessels percentage area and mean lacunarity, both observed at the 3rd month (Table 3). These differences were not observed at month 12 (Table 4).
The mean vessels percentage area in the subgroup of patients with a visual gain of more than 15 ETDRS letters and patients with a change of equal to or less than + 15 letters were 62.49 ± 6.89 and 54.82 ± 10.08, respectively. The vessels percentage area was significantly greater in patients with a visual gain of more than 15 letters from baseline to month 3 (P = 0.049).
The mean lacunarity in the subgroup of patients with a visual gain of more than 15 ETDRS letters and patients with a change of equal to or less than + 15 letters were 0.10 ± 0.02 and 0.17 ± 0.08, respectively. The mean lacunarity was significantly smaller in patients with a visual gain of more than 15 letters from baseline to month 3 (P = 0.037).
Discussion
In this study, we assessed the connections between baseline OCTA quantitative morphological MNV parameters and visual acuity improvement following the treatment. To overcome confounding parameters like “normalization” we included treatment naïve active MNVs and measured the visual acuity at month three and one year of follow-up. The normalization hypothesis is a phenomenon that describes the evolution of an immature vascular network to a truncal and mature network over time due to treatment by anti-VEGFs10,20.
Previously Coscas et al., developed a system to score the MNV activity based on some OCTA qualitative parameters including the shape of MNV (sea fan or lacy wheel shape), the presence of tiny capillaries, anastomoses and vessel loops, peripheral arcade, and hyporeflective halo around the lesion7. However, the qualitative assessment is highly subject to inter-rater and intra-rater variability. The same group presented an additional intriguing predictive model utilizing quantitative OCTA parameters. They identified variables within the lesion area including vascular density and FD, which prove effective in distinguishing between active and in remission nAMD. The study revealed that measurable blood flow characteristics in OCTA, such as lesion area and FD, seem to be more closely linked with exudation in OCT21. Based on these findings, our objective was to ascertain whether specific baseline quantitative morphological parameters of MNV tufts could serve as predictors for the functional response to anti-VEGF treatment in nAMD. Therefore, we tried to implement a quantitative assessment using AngioTool which provides a quantitative measurements.
Choi et al. (2023) explored the predictive role of OCTA in assessing exudation recurrence in type 1 nAMD patients22. They highlighted that, after three loading doses of intravitreal aflibercept injections, higher values of MNV area, MNV length, total number of endpoints, and junctions were crucial parameters correlated with early recurrence of exudation22. In line with their findings, our study also observed that vessel percentage area and junction density at baseline were significantly correlated with early visual improvements. Specifically, our results showed that a higher baseline vessel percentage area and junction density were associated with better visual outcomes at 3 months, indicating a more active and responsive neovascular network. However, while Choi et al. did not observe long-term correlations with these parameters, we found that vessel percentage area and fractal dimension could predict visual improvement even after one year of treatment.
Lee et al. (2020) examined the association of treatment response with quantitative changes in choroidal neovascularization and choroidal vessels23. They reported significant correlations between vessel area, vessel diameter, branch vessel length, fractal dimension, and lacunarity with treatment outcomes23. Our study supports these findings, particularly with the significant correlations we found for vessels percentage area, lacunarity, and FD. Similar to Lee et al., we observed that a higher FD, indicative of a more complex vascular network, correlated positively with visual improvement. Additionally, lower lacunarity, suggesting a more homogeneous and less disrupted vascular structure, was associated with better outcomes at 3 months. However, unlike Lee et al., we did not find a significant long-term correlation for lacunarity.
Jia et al. (2022) focused on the short-term efficacy of anti-VEGF therapy and its prediction using OCTA24. They evaluate different OCTA parameters -MNV area, vessel area, greatest vascular caliber (GVC), and greatest linear dimension (GLD)- and only GVC was identified as predictive parameters24. However, we did not specifically measure GVC; our parameter of total vessel length did not show a significant correlation. This suggests that while vessel area and complexity (as indicated by FD) are important, the overall length of vessels may not be as critical in predicting treatment response.
Our findings suggest that neovascular lesions exhibiting higher baseline vessel percentage area, junction density, and fractal dimension, along with lower lacunarity, may display increased responsiveness to anti-VEGF treatment at 3 months. Additionally, the vessel percentage area and mean lacunarity are key baseline quantitative factors that can distinguish patients who experience a visual gain of more than 15 ETDRS letters after three months of anti-VEGF therapy from those who do not. The observation that the vessel percentage area and mean lacunarity were significant predictors of visual gain at three months but not at twelve months can be interpreted in several ways. The initial response to anti-VEGF treatment might be influenced by the baseline morphology of the neovascular network, including the percentage area of vessels and lacunarity. However, over a longer period, other factors such as the normalization hypothesis, the development of resistance, and vascular maturation due to treatment could alter the correlation with long-term visual outcomes and might play a more dominant role10,20,23. This shift can diminish the predictive power of the initial OCTA biomarkers.
It has been shown that immature MNVs with more tangle-young vessels have more values of FD and vessels percentage area9. Like our study, Costanzo et al. showed that lower perfusion at baseline was associated with poorer treatment response to intravitreal aflibercept injections at the end of the study9. This is consistent with previous studies that have shown anti-VEGFs are more effective on capillary micro-vessels compared to mature truncal vessels11,25,26. In contrast, lacunarity as a heterogeneity index of vascular texture which usually has more value in old and previously treated MNVs was negatively correlated with treatment response. These old mature vessels and heterogenous vascular tufts show more resistance to treatment compared to fresh-tangles’ new capillaries11,25. Out of the measured biomarkers, only vessels percentage area and FD demonstrated the capability to predict visual improvement after one year of treatment. Studies have revealed that MNV lesions displaying heightened arterialization and reduced new vascular sprouting and tiny ramifications, leading to lower FD values, might be associated with a less favorable treatment response26. Among the quantitative parameters investigated by Al-Sheikh et al., higher fractal dimension, along with an elevated rate of small vessel branching were significantly more in actively leaking MNVs in contrast to quiescent ones27.
However, Told et al. didn’t show any correlation between BCVA at month three after treatment with aflibercept and baseline OCTA MNV features measured by AngioTool28. They found that vessel density doesn’t change over time and is not a good predictor for treatment response. Miere et al. found that there are no different qualitative OCTA features including pruned vascular tree, vascular loop, tangled network, dark halo, and large flow void between active and none active MNVs8. Similarly Roberts et al., compared the qualitative and quantitative variables measured by AngioTool between two groups of “good responder” and “poor responder” and they didn’t find any significant parameters correlated with disease activity10. However these studies had recruited previously treated eyes, such that in the latter study the median number of previous injections was 34 and they speculated that their contradictory finding might be due to the normalization hypothesis.
The prediction of treatment response is a complex entity and several other parameters may play a significant role, making it challenging to rely just on morphological features without considering other factors29. Additionally, there are limitations in OCTA image acquisition, as some images have significant artifacts that affect quantitative measurements, making it difficult to use as a routine modality for predicting treatment response in daily practice. Future development of image analysis algorithms incorporating these biomarkers could enable physicians to identify patients at higher risk of visual loss. By closely monitoring these patients and considering earlier switching between various anti-VEGF drugs or incorporating newer, more potent anti-VEGF drugs, physicians may enhance treatment outcomes. However, given that the patients in this study were treated with Aflibercept or its biosimilar, further research is needed to confirm whether these findings are applicable across different treatment regimens.
The strength of this study was to include a homogeneous population of nAMD patients treated with the same therapeutic strategy in order to eliminate the confounding factors as the number of injections or the type of drug used.
The main drawback of our study primarily arises from the limited sample size, which can be attributed to the stringent inclusion criteria applied to our study population and the retrospective nature of this investigation. Further, we didn’t take into account changes in OCTA parameters over time. Also, this study was conducted in a tertiary care center, which could have led to the recruitment of excessive numbers of chronic cases and selection bias. Our study is also limited by a follow-up period of only one year. Early vision improvement has been demonstrated as the most robust clinical predictor of long-term visual outcomes. This likely reflects that, in patients who exhibit a strong initial response, the disease remains primarily VEGF-driven. In contrast, for those who respond less favorably, other irreversible pathological processes—such as inflammation, fibrosis, and macular atrophy—may play a more significant role in determining their outcomes30,31. Further research is needed to explore how these biomarkers might affect visual outcomes over longer follow-up periods. With the advances in machine learning algorithms and computer vision science, future studies to reveal the baseline OCTA predictors will be more facilitated and promising and will also help in interobserver concordance.
In conclusion, our study delved into the connection between baseline quantitative OCTA parameters and visual recovery in treatment-naïve nAMD cases treated with aflibercept. We observed a significant positive correlation between early visual improvement and baseline FD and vessels percentage area values. Conversely, lacunarity displayed a negative correlation with early treatment response. Among the biomarkers analyzed, only vessels percentage area and FD showcased the ability to predict visual improvement after a year of treatment.
Data availability
All data during this study are included in this article and can be directed to the corresponding author.
References
Oliveira, M. A. et al. Macular atrophy development in neovascular age-related macular degeneration during first year of treatment: Incidence and risk factors. Eur. J. Ophthalmol. 31, 521–528 (2021).
Amoaku, W. et al. Initiation and maintenance of a treat-and-extend regimen for ranibizumab therapy in wet age-related macular degeneration: Recommendations from the UK retinal outcomes group. Clin. Ophthalmol. 12, 1731–1740 (2018).
Shijo, T. et al. Incidence and risk of advanced age-related macular degeneration in eyes with drusenoid pigment epithelial detachment. Sci. Rep. 12, 4715 (2022).
Coscas, F. et al. Optical coherence tomography angiography in exudative age-related macular degeneration: A predictive model for treatment decisions. Br. J. Ophthalmol. 103, 1342–1346 (2019).
Bousquet, E. et al. Optical coherence tomography angiography of flat irregular pigment epithelium detachment in chronic central serous chorioretinopathy. Retina 38, 629–638 (2018).
de Carlo, T. E. et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology 122, 1228–1238 (2015).
Coscas, G. J. et al. A new diagnostic challenge. Retina 35, 2219–2228 (2015).
Miere, A. et al. Optical coherence tomography angiography features of subretinal fibrosis in age-related macular degeneration. Retina 35, 2275–2284 (2015).
Costanzo, E. et al. Imaging biomarkers of 1-year activity in type 1 macular neovascularization. Transl Vis. Sci. Technol. 10, 18 (2021).
Roberts, P. K., Nesper, P. L., Gill, M. K. & Fawzi, A. A. Semiautomated quantitative approach to characterize treatment response in neovascular age-related macular degeneration: A real-world study. Retina 37, 1492–1498 (2017).
Phasukkijwatana, N., Tan, A. C. S., Chen, X., Freund, K. B. & Sarraf, D. Optical coherence tomography angiography of type 3 neovascularisation in age-related macular degeneration after antiangiogenic therapy. Br. J. Ophthalmol. 101, 597–602 (2017).
Mastropasqua, L. et al. Optical coherence tomography angiography assessment of vascular effects occurring after aflibercept intravitreal injections in treatment-naive patients with wet age-related Macular Degeneration. Retina 37, 247–256 (2017).
Coscas, G. et al. Optical coherence tomography angiography during follow-up: Qualitative and quantitative analysis of mixed type I and II choroidal neovascularization after vascular endothelial growth factor trap therapy. Ophthalmic Res. 54, 57–63 (2015).
Huang, D., Jia, Y., Rispoli, M., Tan, O. & Lumbroso, B. Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina 35, 2260–2264 (2015).
Mathis, T. et al., Retinal vascularization analysis on optical coherence tomography angiography before and after intraretinal or subretinal fluid resorption in exudative age-related macular degeneration: A pilot study. J. Clin. Med. 10 (2021).
Karkhaneh, R. et al., Evaluating the efficacy and safety of aflibercept biosimilar (P041) compared to originator product in patients with neovascular age-related macular degeneration. Ophthalmol Retina (2024).
Riazi-Esfahani, H. et al. Pachychoroid neovasculopathy versus macular neovascularization in age-related macular degeneration with and without shallow irregular pigment epithelial detachment. Sci. Rep. 13, 19513 (2023).
Sacconi, R. et al. Quantitative changes in the ageing choriocapillaris as measured by swept source optical coherence tomography angiography. Br. J. Ophthalmol. 103, 1320–1326 (2019).
Zudaire, E., Gambardella, L., Kurcz, C. & Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One 6, e27385 (2011).
Jain, R. K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Coscas, F. et al. Quantitative optical coherence tomography angiography biomarkers for neovascular age-related macular degeneration in remission. PLoS One 13, e0205513 (2018).
Choi, M., Kim, S. W., Yun, C., Oh, J. H. & Oh, J. Predictive role of optical coherence tomography angiography for exudation recurrence in patients with type 1 neovascular age-related macular degeneration treated with pro-re-nata protocol. Eye (Lond) 37, 34–41 (2023).
Lee, H., Lee, M., Kim, M. A., Chung, H. & Kim, H. C. Association of treatment response with quantitative changes in choroidal neovascularization and choroidal vessel in neovascular age-related macular degeneration. Retina 40, 1704–1718 (2020).
Jia, H. et al. Prediction of the short-term efficacy of anti-VEGF therapy for neovascular age-related macular degeneration using optical coherence tomography angiography. Eye Vis. (Lond) 9, 16 (2022).
Kuehlewein, L. et al. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am. J. Ophthalmol. 160, 739–748 (2015).
Miere, A. et al. Vascular remodeling of choroidal neovascularization after anti-vascular endothelial growth factor therapy visualized on optical coherence tomography angiography. Retina 39, 548–557 (2019).
Al-Sheikh, M., Iafe, N. A., Phasukkijwatana, N., Sadda, S. R. & Sarraf, D. Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography. Retina. 38, 220–230 (2018).
Told, R. et al. Profiling neovascular age-related macular degeneration choroidal neovascularization lesion response to anti-vascular endothelial growth factor therapy using SSOCTA. Acta Ophthalmol. 99, e240–e246 (2021).
Mettu, P. S., Allingham, M. J. & Cousins, S. W. Incomplete response to anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog Retin Eye Res. 82, 100906 (2021).
Nguyen, V. et al. Projection of long-term visual acuity outcomes based on initial treatment response in neovascular age-related macular degeneration. Ophthalmology 126, 64–74 (2019).
Ying, G. S. et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT). Ophthalmology 122, 2523–2531 (2015).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: S.F, H.F, F.B, H.R, E.K, and E.A. Data collection, interpretation of results, draft manuscript preparation, and reviewed the manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Faghihi, S., Faghihi, H., Bazvand, F. et al. Baseline optical coherence tomography angiography biomarkers predict visual outcomes in treatment-naïve neovascular age-related macular degeneration patients. Sci Rep 14, 24528 (2024). https://doi.org/10.1038/s41598-024-75530-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75530-x