Abstract
The serotonergic system modulates the neural circuits involved in jaw movement; however, the role of serotonin (5-HT) neurons in masticatory movement remains unclear. Here, we investigated the effect of selective activation of 5-HT neurons in the dorsal raphe nucleus (DRN), or the raphe obscurus nucleus (ROb), on voluntary masticatory movement using transgenic mice expressing the channelrhodopsin-2 (ChR2) mutant (C128S) in central 5-HT neurons. During voluntary mastication, DRN blue light illumination increased masticatory frequency and decreased the root mean square peak amplitude of electromyography (EMG) in the masseter muscles. DRN blue light illumination also decreased EMG burst duration in the masseter and digastric muscles. These changes were blocked by a 5-HT2A receptor antagonist. Conversely, ROb blue light illumination during voluntary mastication did not affect masticatory frequency and EMG bursts in the masseter and digastric muscles. DRN or ROb blue light illumination during the resting state did not induce rhythmic jaw movement such as mastication but induced an increase in EMG activity in masseter and digastric muscles. These results suggest that both DRN and ROb 5-HT neurons may facilitate jaw movement. Furthermore, DRN 5-HT neuron may contribute to changes in masticatory patterns during the masticatory sequence.
Similar content being viewed by others
Introduction
Masticatory jaw movements—characterized by rhythmic alternating contractions of jaw-closing and -opening muscles—are known to be modulated by a number of neuromodulators (e.g., serotonin (5-HT), orexin, histamine, and neuropeptide Y)1,2,3,4. 5-HT neurons, originating in the raphe nuclei, innervate almost the entire brain region and play a critical role in various behaviors such as social5, sleep-awake6, and feeding behaviors7. In addition, 5-HT is involved in oral motor functions. 5-HT neuron activity in the dorsal raphe nucleus (DRN)8,9 and caudal raphe—including the raphe obscurus nucleus (ROb)10—is increased during rhythmic oral motor behavior (e.g., chewing, licking, and grooming). Systemic injection of the 5-HT agonist quipazine decreases the electromyography (EMG) activity burst amplitude in the masseter and digastric muscles. Quipazine also raises the electrical threshold for inducing rhythmic jaw movements elicited by repetitive stimulation of the masticatory area of the cerebral cortex in anesthetized guinea pigs11. These results indicate that 5-HT affects masticatory jaw movements. Additionally, 5-HT has an influence on the neural circuits containing trigeminal motor neurons which transmit coordinated motor outputs to the masticatory muscles and receive 5-HT immunoreactive boutons from the ROb and raphe pallidus nucleus (RPa)12. Our previous study using in vitro brainstem slice preparations showed that 5-HT increases the excitability of jaw-closing motoneurons by inducing membrane depolarization and enhancing glutamate receptor-mediated postsynaptic potentials13,14,15. Therefore, we hypothesized that the serotonergic system, which is involved in controlling jaw movement, plays an important role in mastication.
Optogenetic techniques using a combination of genetic and optic approaches can control the activity of specific neuronal types at any time in freely moving animals and are used in various motor behavior experiments16,17,18. Here, we analyzed EMG activity in the masseter and digastric muscles, a well-established method in the analysis of masticatory patterns3,19,20,21, using double transgenic mice expressing the channelrhodopsin-2 (ChR2) mutant (C128S) only in central serotonergic neurons22,23 to ascertain the role of 5-HT neurons in voluntary mastication.
Results
Effect of optogenetic activation of ChR2-expressing 5-HT neurons in DRN on masticatory movements
To determine whether DRN 5-HT neuron activation affects masticatory movements, we investigated the effects of DRN blue light illumination on EMG activity during voluntary mastication. To activate DRN 5-HT neurons, we used transgenic mice expressing specifically the ChR2 (C128S) in central 5-HT neurons22,23 and implanted an optic fiber near the DRN (Fig. 1a,b). To quantify masticatory muscle activity, we recorded EMG signals from both the masseter and digastric muscles. Optogenetic activation of DRN 5-HT neurons by blue light (470 nm) illumination during voluntary mastication changed rhythmic masticatory activity to a higher frequency and decreased masseter activity (Fig. 1c).
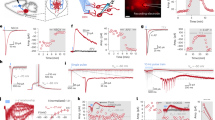
Optogenetic activation of DRN 5-HT neurons during voluntary mastication alters muscle activity patterns. (a) Schematic illustration of the position of the optical fiber stereotaxically inserted into the DRN of double transgenic mice expressing ChR2 in central 5-HT neurons for DRN 5-HT neuron activation. (b) Representative images showing Tph2-IR neurons (Alexa Fluor 594, red), YFP-IR neurons (Alexa Fluor 488, green) indicating ChR2 (C128S) expression, and the merged image in DRN. Scale bars = 300 μm. The dashed white lines indicate optic fiber placement. (c) and (d) Representative EMG recordings of digastric and masseter muscles during voluntary mastication before and after blue light application (470 nm; blue bar) for optogenetic activation of DRN 5-HT neurons as activation (c, top), or yellow light application (590 nm; yellow bar) as control (d, top). Expanding EMG recordings picked from top EMG recordings (dotted box outlines); before and after light application are indicated at the bottom in c and d. DRN dorsal raphe nucleus, EMG electromyography, IR immunoreactive.
To verify that this masticatory movement change was indeed due to ChR2 blue light activation and no other non-specific light stimulation, we substituted yellow light (590 nm) in the DRN as a control (Fig. 1d). Subsequently, we analyzed and compared 10 consecutive masticatory cycles after applying blue light (activation) and yellow light (control) (Fig. 2a). The DRN blue light illumination during voluntary mastication increased the masticatory rhythm frequency (control: 4.7 ± 0.9 Hz; activation: 8.7 ± 0.7 Hz, n = 8; P < 0.001; Fig. 2b). Furthermore, DRN blue light illumination inhibited pellet biting, and a high masticatory cycle frequency period (> 7 Hz) persisted for 12.8 ± 4.1 s (n = 8; Fig. S2a).
Optogenetic activation of DRN 5-HT neurons during voluntary mastication increases masticatory frequency and decreases EMG bursts. (a) Representative of raw and RMS EMG of masseter muscles during mastication and measured parameters. The masticatory frequency was calculated from the interval between RMS peaks. (b) Pooled data for masticatory frequency after yellow (control) or blue light (activation) application. (c) and (d) Pooled data for RMS peak amplitude (c) and duration (d) in masseter EMG bursts after yellow or blue light application. (e) and (f) Pooled data for RMS peak amplitude (e) and duration (f) in digastric EMG bursts after yellow or blue light application. **P < 0.01, ***P < 0.001 (paired Student’s t test). Comparisons with n.s. have P > 0.05 and are considered not significant. Data are expressed as mean ± SEM. RMS root mean square.
Regarding the EMG bursts, DRN blue light illumination during voluntary mastication significantly decreased the root mean square (RMS) peak amplitude of the masseter muscles (control: 0.60 ± 0.26 mV; activation: 0.22 ± 0.10 mV, n = 8, P < 0.01; Fig. 2c) and duration of the masseter EMG bursts (control: 102.8 ± 16.8 ms; activation: 65.5 ± 14.0 ms, n = 8, P < 0.01; Fig. 2d). Conversely, DRN blue light illumination during voluntary mastication did not significantly change the RMS peak amplitude of the digastric muscles (control: 0.29 ± 0.13 mV; activation: 0.22 ± 0.10 mV, n = 7, P = 0.45; Fig. 2e), but significantly decreased the duration of the digastric EMG bursts (control: 112.5 ± 20.1 ms; activation: 85.7 ± 27.2 ms, n = 7, P < 0.01; Fig. 2f). The phase relationships indicated by circular statistics did not differ between yellow and blue light illumination of the DRN for masseter/digastric muscle coordination (Fig. 3; Table 1).
Optogenetic activation of DRN 5-HT neurons does not involve coordination between masseter and digastric muscle activity during voluntary mastication. (a) Representative circular plots showing the relative burst phases recorded from the masseter muscles relative to the digastric muscles. The black dots on each circle indicate the phase shift between the corresponding RMS peak of the masseter-digastric muscle activity alternation after yellow (left) or blue (right) light application. Black straight lines indicate mean coordination r-vectors (b) Circular plot summary showing the phasing of all seven animals. Gray and straight red lines indicate the mean coordination r-vectors for individual animals and animal groups, respectively. Comparisons with n.s. have P > 0.05 and are considered not significant. The Watson–Williams test for phase comparisons did not show any statistically significant differences between the application of yellow and blue light for individual animals.
Optogenetic activation of DRN 5-HT neurons does not induce masticatory movement initiation but modulates ongoing mastication movement. (a) Representative EMG recordings of digastric and masseter muscles before and after blue light application (blue bar) during resting state. (b) Pooled data for the percentage of EMG activity evoked after yellow (control) or blue light (activation) illumination to that during the resting state before yellow or blue light illumination in the digastric and masseter muscles. (c) Representative EMG recording of digastric and masseter muscles before and after blue light application (blue bar) during incision (left). The dashed line indicates the start of feeding. Expanding EMG recordings in dotted box outlines of left EMG recordings (right). (d) Representative EMG recording of digastric and masseter muscles before and after blue light application (blue bar) during chewing stage (left). Expanding EMG recordings in dotted box outlines of left EMG recordings (right). (e) Pooled data for masticatory frequency after blue light application during incision and chewing stages. (f) Pooled data for periods showing high frequency (> 7 Hz) masticatory muscle activity after blue light application during incision and chewing. (g) and (h) Pooled data for RMS peak amplitude (f) and duration (g) in masseter EMG bursts after blue light application during incision and chewing. (i) and (j) Pooled data for RMS peak amplitude (h) and duration (i) in digastric EMG bursts after blue light application during incision and chewing. **P < 0.01, ***P < 0.001 (unpaired Student’s t test). Comparisons with n.s. have P > 0.05 and are considered not significant. Data are expressed as mean ± SEM.
These results indicate that activation of DRN 5-HT neurons during voluntary mastication drastically alters the characteristics of masticatory muscle activity without alternation of masseter/digastric muscle coordination.
Effects of optogenetic activation of DRN 5-HT neurons on masticatory movements at different masticatory stages
To determine whether DRN 5-HT neuron activation induced the initiation of rhythmic EMG activity, we examined the effects of optogenetic activation of DRN 5-HT neurons while the mice were at rest. We defined a state in which masseter and digastric muscle activity did not exceed 2 S.D. for 30 s as “resting”. Unlike during voluntary mastication, DRN blue light illumination during the resting state did not induce mastication-like rhythmic EMG activity in the masseter or digastric muscles (Fig. 5a). However, DRN blue light illumination during the resting state significantly increased EMG activity in masseter (control: 90.8 ± 27.8%; activation: 214.0 ± 48.9%, n = 5, P < 0.01; Fig. 5b) and digastric muscles (control: 113.4 ± 40.1%; activation: 296.0 ± 61.1%, n = 5, P < 0.05; Fig. 5b).
5-HT2Areceptors are involved in induced fast masticatory movement by optogenetic activation of DRN 5-HT neurons. (a) Representative EMG recording of digastric and masseter muscles during voluntary mastication at 30 min after the administration of 0.3 mg/kg MDL100907 (s.c.) (left). Expanding EMG recordings in dotted box outlines of left EMG recordings (right). (b) Pooled data for masticatory frequency after yellow (control) or blue light (activation) application with the administration of MDL 100,907. (c) and (d) Pooled data for RMS peak amplitude (c) and duration (d) in masseter EMG bursts after yellow or blue light application with the administration of MDL 10,090. (e) and (f) Pooled data for RMS peak amplitude (e) and duration (f) in digastric EMG bursts after yellow or blue light application with the administration of MDL 100,907. Comparisons with n.s. have P > 0.05 and are considered not significant. Data are expressed as mean ± SEM.
The masticatory sequence consists of two stages—incision and chewing stages2,19. The transgenic mice used here also had an incision stage, with unstable but partially fast (> 7 Hz), small bursts of the masseter muscles, and a chewing stage with slow, large bursts of these muscles (Fig. S1). To verify whether DRN blue light illumination induced a masticatory pattern change at any masticatory stage, we applied light at two points of the masticatory sequence—the incision (immediately after the start of feeding) and chewing (incision stage followed by large masseter muscle activity) stages. These two stages were confirmed by visual observation and EMG recordings. DRN blue light illumination during both stages induced rapid and small rhythmic EMG activity (Fig. 5c,d).
After DRN blue light illumination during the incision and chewing stages, there was no difference in the masticatory frequency (unpaired Student’s t test, incision: 8.6 ± 0.7 Hz, n = 7; chewing: 8.8 ± 1.6 Hz, n = 6, P = 0.82; Fig. 5e) and high frequency period of the masticatory cycle (unpaired Student’s t test, incision: 13.0 ± 5.0 s, n = 7; chewing: 10.2 ± 4.4 s, n = 6, P = 0.76; Fig. 5f). Similarly, in the incision and chewing stages, there was no significant difference in the properties of the induced small EMG bursts in the masseter (unpaired Student’s t test, incision: n = 7; chewing: n = 6; RMS peak amplitude: P = 0.40; duration: P = 0.67; Fig. 5g,h) and digastric (unpaired Student’s t test, incision: n = 7; chewing: n = 5; RMS peak amplitude: P = 0.86; duration: P = 0.97; Fig. 5i,j) muscles. These results suggest that DRN 5-HT neuron activation may not be responsible for masticatory movement initiation, but may modulate ongoing masticatory muscle activity, irrespective of the masticatory stage.
5-HT2Areceptor involvement in the modulation of masticatory movements by optogenetic activation of DRN 5-HT neurons
Because 5-HT2A/2C and 5-HT1A receptors contribute to the generation of trigeminal rhythmic activity24, we examined whether these receptors were involved in increased masticatory frequency and decreased EMG bursts by the DRN blue light illumination. After systemic administration of the selective 5-HT2A receptor antagonist MDL100907 (0.3 mg/kg, s.c.), DRN blue light illumination did not induce rapid masticatory frequency (control: 4.0 ± 0.2 Hz, activation: 4.4 ± 0.7 Hz, n = 5; P = 0.29; Fig. 6a,b). Furthermore, DRN blue light illumination did not change masseter EMG bursts (RMS peak amplitude: control: 0.47 ± 0.12 mV; activation: 0.44 ± 0.16 mV, n = 5, P = 0.62; duration: control: 111.8 ± 25.8 ms; activation: 102.2 ± 19.2 ms, n = 5, P = 0.46; Fig. 6a,c,d) and digastric EMG busts (RMS peak amplitude: control: 0.18 ± 0.04 mV; activation: 0.20 ± 0.05 mV, n = 4, P = 0.38; duration: control: 121.5 ± 32.7 ms; activation: 125.3 ± 23.7 ms, n = 4, P = 0.38, Fig. 6a,e,f) after MDL100907 application.
Optogenetic activation of ROb 5-HT neurons during voluntary mastication does not affect masticatory movement patterns. (a) Schematic illustration of the position of the optical fiber stereotaxically inserted into the ROb of double transgenic mice expressing ChR2 in central 5-HT neurons. (b) Representative EMG recordings of digastric and masseter muscles during the resting state before and after optogenetic activation of ROb 5-HT neurons by blue light application (blue bar). (c) Pooled data for the percentage of EMG activity evoked after yellow (control) or blue light (activation) illumination to that during the resting state before illumination in the digastric and masseter muscles. (d) Representative EMG recordings of digastric and masseter muscles during voluntary mastication before and after blue light application (blue bar) for optogenetic activation of ROb 5-HT neurons (left). Expanding EMG recordings in dotted box outlines of left EMG recordings (right). (e) Pooled data for masticatory frequency after yellow or blue light application. (f) and (g) Pooled data for RMS peak amplitude (f) and duration (g) in masseter EMG bursts after yellow or blue light application. (h) and (i) Pooled data for RMS peak amplitude (h) and duration (i) in digastric EMG bursts after yellow or blue light application. *P < 0.05 (paired Student’s t-test). Comparisons with n.s. have P > 0.05 and are considered not significant. Data are expressed as mean ± SEM. ROb raphe obscurus nucleus.
DRN blue light illumination induced rapid, small masticatory movements with systemic administration of the 5-HT2c receptor antagonist SB242084 (1 mg/kg, i.p.) or the selective 5-HT1A receptor antagonist WAY100635 (1 mg/kg, i.p.), similar to the results recorded in the absence of any antagonists (Fig. S2). These results suggest that 5-HT2A receptors contribute to altered masticatory patterns evoked by DRN blue light illumination.
Effects of optogenetic activation of ROb 5-HT neurons on masticatory movements
5-HT neurons in the caudal raphe, including the ROb, innervate many brainstem regions including the trigeminal motor nucleus and central pattern generator (CPG) for mastication25. We therefore investigated the effects of optogenetic activation of ROb 5-HT neurons using transgenic mice with implanted optical fibers above the ROb (Fig. 7a). To determine whether activation of ROb 5-HT neurons induced the initiation of rhythmic EMG activity, we examined the effect of optogenetic activation of ROb 5-HT neurons during the resting state. This activation did not induce rhythmic EMG activity such as mastication (Fig. 7b), but significantly increased EMG activity in masseter (control: 90.8 ± 27.8%; activation: 214.0 ± 48.9%, n = 5, P < 0.01; Fig. 7c) and digastric (control: 113.4 ± 40.1%; activation: 296.0 ± 61.1%, n = 5, P < 0.05; Fig. 7c) muscles. Subsequently, we examined the effects of optogenetic activation of ROb 5-HT neurons on EMG activity during voluntary mastication. ROb blue light illumination during voluntary mastication did not affect masticatory frequency (control: 4.1 ± 0.5 Hz; activation: 4.4 ± 0.6 Hz, n = 5, P = 0.61; Fig. 7d,e), masseter EMG bursts (RMS peak amplitude: control: 0.56 ± 0.21 mV; activation: 0.55 ± 0.17 mV, n = 5, P = 0.98, duration: control: 92.6 ± 9.1 ms; activation: 96.3 ± 12.7 ms, n = 5, P = 0.66; Fig. 7f,g) and digastric EMG bursts (RMS peak amplitude: control: 0.4 ± 0.1 mV; activation: 0.4 ± 0.1 mV, n = 5, P = 0.95; duration: control: 75.0 ± 12.9 ms; activation: 86.7 ± 14.4 ms, n = 5, P = 0.09; Fig. 7h,i). The phase relationships indicated by circular statics did not differ between ROb yellow and blue light illumination for masseter/digastric muscle coordination (n = 5; Fig. 4; Table 1). These results indicate that the activation of ROb 5-HT neurons increased EMG activity in masseter and digastric muscles during the resting state; however, no significant change in masticatory movement was observed after ROb blue light illumination during voluntary mastication.
Optogenetic activation of ROb 5-HT neurons does not involve coordination between masseter and digastric muscle activity during voluntary mastication. (a) Representative circular plots showing the relative burst phases recorded from the masseter muscles relative to the digastric muscles. The black dots on each circle indicate the phase shift between the corresponding RMS peak of the masseter-digastric muscle activity alternation after yellow (left) or blue (right) light application. Black straight lines indicate mean coordination r-vectors. (b) Circular plot summary showing the phasing of all five animals. Gray and straight red lines indicate the mean coordination r-vectors for individual animals and animal groups, respectively. Comparisons with n.s. have P > 0.05 and are considered not significant. The Watson–Williams test for phase comparisons did not show any statistically significant differences between the application of yellow and blue light for individual animals.
Discussion
Here, we provide evidence for the involvement of the serotonergic system in voluntary masticatory movements. We found that DRN blue light illumination during voluntary mastication increased masticatory frequency (4.7 ± 0.9 to 8.7 ± 0.7 Hz) and decreased the RMS peak amplitude in masseter muscles and duration of EMG bursts in masseter and digastric muscles. The masticatory sequence is classified into two stages based on the EMG activity of the constituent masticatory cycles: incision and chewing in mice19,20. In these studies, EMG recordings revealed that, during the incision stage, rapid (7 Hz), small bursts of masseter muscles were evident, reflecting pellet biting. In the chewing stage, slow (5 Hz), large bursts of masseter muscles were evident, reflecting crushing and grinding.
Fast, small masticatory movements induced by DRN blue light illumination in the present study showed similar characteristics, in which the frequency of masticatory muscle activity during the incision stage of the masticatory cycle was > 7 Hz. DRN 5-HT neuron activation may be involved in the transition from the chewing to the incision stage during mastication. However, single application of the blue light induced rapid masticatory movements for 12.8 ± 4.1 s without pellet biting, while the incision stage duration was < 3 s in normal mastication in mice19,20. This prolonged rapid masticatory movement may be due to ChR2 (C128S) characteristics, with a closing time constant of 100 s and causing vigorous, sustained spiking after short blue light application26,27.
We found that the activation of 5-HT2A receptors was involved in masticatory pattern modulation by DRN blue light illumination during voluntary mastication. 5-HT2A receptors are widely expressed in the brain regions such as the cortex, striatum, amygdala, and hypothalamus to which DRN 5-HT neurons project28,29. Furthermore, these regions are involved in the central control of masticatory movements3,30,31,32. These pathways may be candidates for modulating masticatory patterns via the serotonergic system. However, future studies must clearly determine the projection sites that mediate the observed effects of DRN 5-HT neurons on masticatory movements.
Masticatory patterns—the bite force and duration of the masticatory cycle—are affected by sensory information from periodontal mechanoreceptors and muscle spindles based on the physical properties33,34. The mesencephalic trigeminal nucleus (MesV) neurons, which innervate the periodontal ligament and muscle spindles, While electrophysiological evidence indicates that activation of 5-HT1A receptors reduces hyperpolarization-activated inward current in MesV35, and activation of 5-HT1B receptors suppresses excitatory inputs from MesV to motoneurons36, the role of 5-HT2A receptors in MesV is not comprehensively understood. However, the MesV receives inputs from both DRN and ROb37, and expresses 5-HT2 receptors4, suggesting potential involvement in the alterations of voluntary masticatory patterns induced by activation of DRN 5-HT neurons.
Under conditions in which EMG activity does not exceed 2 S.D. for 30 s (resting state), ROb blue light illumination increased EMG activity in the masseter and digastric muscles. ROb 5-HT neurons project primarily to the brainstem, including the trigeminal motor nucleus and regions in which jaw movement-related neurons are located25. Trigeminal motoneurons receive 5-HT terminals, and electrical stimulus application to the RPa and ROb complex induces a monosynaptic excitatory postsynaptic potential in jaw-closing and -opening motoneurons in cats12. Furthermore, 5-HT directly increases the excitability of jaw-closing motoneurons13,14,15. ROb 5-HT neurons may be related to EMG activity regulation in these muscles by directly activating jaw-closing and -opening motoneurons in the brainstem. ROb 5-HT neurons also project to the spinal cord38 and spinal motoneuron activity is modulated by serotonin39. ROb 5-HT neurons may affect masticatory muscles as well as systemic activation of skeletal muscles. However, in the present study, ROb blue light illumination did not reveal any initiation of locomotion during the resting state. Our data also showed that DRN blue light illumination during the resting state increased EMG activity in the masseter and digastric muscles. DRN 5-HT neurons project mainly to the forebrain40, and optogenetic activation of DRN 5-HT neurons during the sleep state increased the EMG power in trapezius muscles due to induced wakefulness16. Therefore, DRN 5-HT neurons may indirectly increase masticatory muscle activity via neural circuits involving the forebrain rather than directly activating trigeminal motoneurons as in ROb 5-HT neurons. In contrast, DRN blue light illumination during mastication decreased EMG bursts in the masseter and digastric muscles. The neural circuits that control rhythmic jaw movements, such as mastication, include inhibitory inputs from GABA and glycine neurons in the brainstem to MoV, which regulate masticatory muscle activity41,42,43. The small pattern of masticatory movements induced by activation of DRN 5-HT neurons may be attributed to an increase in these inhibitory inputs.
Our present data show that ROb blue light illumination did not induce rhythmic jaw movements such as mastication during the resting state and did not affect rhythmic mastication activities during voluntary mastication. CPG activity may not be critically dependent upon ROb 5-HT neurons. Conversely, DRN blue light illumination altered masticatory movements but did not induce rhythmic jaw movements during the resting state. Optogenetic activation of DRN 5-HT neurons during free movement does not induce stereotypical behavior in mice—freezing, grooming, and rearing27—and reduces spontaneous locomotion, as found in an open field test in mice44. Therefore, DRN 5-HT neuron activation may not be involved in rhythmic movement initiation. DRN 5-HT neurons activation alone cannot activate the CPG for mastication, but it may be able to alter CPG activity patterns activated by other inputs.
Conclusion
Here, we demonstrated that both DRN and ROb blue light illumination increased the EMG activity in the masseter and digastric muscles during the resting state. These results suggest that DRN and ROb may facilitate jaw movement, possibly via different pathways. We showed also that the optogenetic activation of DRN 5-HT neurons, but not ROb 5-HT neurons, induces a fast, small pattern of masticatory movements similar to the incision stage characteristics during voluntary mastication. We also revealed that this modulation was mediated via 5-HT2A receptor activation. Since masticatory movements are accomplished by alternating between the incision and chewing stages in mice, DRN 5-HT neurons may enable sustained masticatory movements by switching from the chewing to the incision stage. Changes in masticatory patterns aid in proper food processing and efficient nutrient absorption. Our experimental results suggest that drug therapies or behavioral therapies that modulate 5-HT levels may be useful in the treatment of masticatory dysfunction. Although further studies examining the effects of 5-HT neuron inhibition on masticatory movements are needed to more precisely determine the functional role of DRN 5-HT neurons on masticatory jaw movements, our study provides a better understanding of the neuro-moludatory mechanisms controlling voluntary mastication.
Methods
Animals
All experiments were conducted with the approval of the Institutional Animal Research Committee of Showa University (approval reference 14028), operating in accordance with Japanese Government Law No. 105 and the Animal Research: Reporting of In Vivo Experiments (ARRIVE 2.0) guidelines. All animals had ad libitum access to food and water and were housed in an air-conditioned room with 12–12 h dark-light cycles. Double-transgenic mice (tph2-tTA::tetO-ChR2(C128S)-EYFP, n = 29) were obtained by crossing Tph2-tTA and tetO-ChR2(C128S)-EYFP mice. All lines were obtained from the RIKEN BioResource Center (Ibaraki, Japan). Genotyping was performed using polymerase chain reaction (PCR), as previously described22,23. The following PCR primer sets were used in the mouse genotyping: ChR2 (5’-ACCGACCCTTTGGCACAGTATG-3’) for tetO-ChR2(C128S) and tTA (5’-CCAGGGTCTCGTACTGCTTC3’) for Tph2-tTA mice. Mice with the transgene expression confirmed were randomly assigned to different experiments, and the same individuals were used across different experimental conditions for direct comparisons. Male mice aged 10–28 weeks were used for the experiments. Each experiment included 4–8 mice.
Surgical procedures
All surgical procedures were performed using a stereotaxic system (Model 940; David Kopf Instruments, Tujunga, CA, USA). All mice were anesthetized with intraperitoneal injection of a mixture of medetomidine hydrochloride (0.75 mg/kg, Domitor; Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan), midazolam hydrochloride (4.0 mg/kg, Dormicum; Sandoz K.K., Tokyo, Japan), and butorphanol tartrate (5.0 mg/kg, Vetorphale; Meiji Seika Pharma Co., Ltd., Tokyo, Japan).
For optogenetic stimulation at DRN, a craniotomy (− 8.75 mm anteroposterior, 0 mm mediolateral, 3.0 mm dorsoventral from bregma) was prepared, and an optical fiber (400 μm diameter, 0.39 NA, Core Multimode Optical Fiber, Thorlabs, Inc. Newton, NJ, USA) inserted 3.0 mm horizontally from the posterior to the anterior45. For optogenetic stimulation at ROb, an optical fiber was inserted vertically above ROb (− 4.2 mm anteroposterior, 0 mm mediolateral, − 4.5 mm dorsoventral from bregma).
For the EMG recordings of the masseter and digastric muscles, optical fiber-implanted mice were used. The detailed protocol has been previously described46. A pin connector to which a stainless-steel screw (M1-2, Unique Medical, Tokyo, Japan) for grounding and pairs of Teflon-coated stainless-steel wires (#AS631, Cooner Wire Co., Chatsworth, CA, USA) were soldered were attached to the skull using dental resin cement (56849; 3 M Dental Products, St Paul, MN, USA). A pair of wires was located in the left masseter and digastric muscles. Atipamezole (0.75 mg/kg, Antisedan, Nippon Zenyaku Kogyo Co., Ltd., Fukushima, Japan) as an antagonist of medetomidine hydrochloride was administered postoperatively.
Rerecording procedures and EMG analysis
Mice were allowed a recovery period of at least 1 week after implant surgery. Before EMG recording, the mice were deprived of food for 24 h with free access to water. Following the food-deprivation period, EMG recordings were made, while the mice ate the uniform size of food (diameter: approx. 10 mm, weight: 0.2 ~ 0.3 g) to eliminate the effects of differences in size on masticatory movement.
The EMG signals were filtered (100–1000 Hz) and amplified (AB-611 J device, Nihon Kohden Co., Tokyo, Japan). The EMG data were digitized at 4 kHz using a PowerLab 8/35 analog-to-digital converter (PL3508, AD Instruments Inc., Dunedin, New Zealand) and stored on a computer using the Lab Chart 7 software (AD Instruments).
Individual bursts were then detected when the rectified EMG exceeded the mean level by 2 SD. To evaluate masticatory frequency and EMG masticatory movement bursts, values were obtained from consecutive gnaw 10 bursts of rectified EMG activity exceeding the mean level by 2 S.D after light application. Masseter muscles activity was measured to determine masticatory frequency because digastric muscles activity often occurs twice in a single cycle2,19. EMG amplitude was estimated using the RMS EMG calculated over a 40-ms sliding window. EMG activities of the masseter and digastric muscles were rectified and integrated using Lab Chart 7 (AD Instruments). For EMG activity, the change in the mean integrated values for masseter and digastric EMG activities during 30 s after light illumination was measured using the mean integrated values for masseter and digastric EMG activities during 30 s before light illumination as 100%.
Optogenetic stimulation
Optogenetic stimulation was carried out using blue (470 nm) or yellow (590 nm) light with an optical fiber-coupled 470 nm light-emitting diode (LED) light source (M470F3, Thorlabs) or a 590 nm LED light source (M590F3, Thorlabs) controlled by an LED driver (DC2200, Thorlabs) and an electronic stimulator (SEN-8203MG, Nihon Kohden). The light power intensity of both blue and yellow at the fiber optic tip was 1–2 mW. For optogenetic stimulation, blue light (1 s duration) was used with yellow light (1 s duration) used as the control.
Drug application
The drugs used were the selective 5-HT2A receptor antagonist (R)-(+)-α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperinemethanol (MDL100907; Tocris Bioscience, Ellisville, MO, USA), the selective 5-HT2C receptor antagonist 6-chloro-5methyl-1[2-(2-methylpyridyl-3-oxy)-pyri-5-yl carbomyl] indoline (SB 242084; Tocris Bioscience), and the selective 5-HT1A receptor antagonist N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (Way-100635; Merck KGaA, Darmstadt, Germany). MDL100907 was dissolved in water containing 5% Tween 80. SB242084 and Way-100,635 was dissolved in isotonic saline. Respective drugs were administered 30 min before the recording.
Histology
At the experiment conclusion, the mice perfused with a 4% paraformaldehyde phosphate buffer solution (Wako, Osaka, Japan). The brain was removed and postfixed in the same fixative at 4 ℃ overnight. The specimens were then immersed in 0.1 M phosphate buffer containing 15% and 30% sucrose at 4 °C until submerged. Sagittal 40-µm-thick serial sections were cut using a cryostat (Leica CM3050S; Leica Biosystems, Nussloch, Germany). The sections were incubated with Alexa 488-labeled anti-GFP antibody (1:500; Santa Cruz biotechnology, Dallas, TX, USA) overnight at 4 °C. The sections were then incubated with rabbit anti-tryptophan hydroxylase antibody (1:400; Merck KGaA) overnight at 4 °C and incubated with Alexa 594 donkey anti-rabbit IgG (1:500; Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature (24–26 ℃). Sections were mounted and examined under a light microscope (BZ-X710; Keyence, Osaka, Japan).
Circular statistics
Phase relationships between the masseter and digastric muscle activities were analyzed using circular plots47. MATLAB (R2020b, MathWorks Inc., Natick, MA, USA) and Excel (Microsoft Co., Redmond, WA, USA) were used for data analysis. The 25 RMS peak phases of the masseter muscles from each animal were calculated in accordance with the RMS peak of the digastric muscles. The direction of an individual vector indicates the phase (0 or 360, synchrony; 180, alteration). The r-value (vector length) indicates the concentration of the phase-shift values around the mean, indicating the strength of coordination between the analyzed RMS peaks (dots at the circle). Rayleigh’s test was used to determine whether r-values associated with coupling were significant. Coupling was regarded as significant when the Rayleigh test result was P < 0.05. Multiple sample testing of the angles was performed using the Watson–Williams test to compare the resulting mean phase values.
Statistics
Statistical analyses were performed using SPSS software (version 22.0; IBM Co., Armonk, NY, USA). Pooled data are expressed as the mean ± standard error of the mean (SEM). To compare EMG activity after blue light illumination with that after yellow light illumination, we used Student’s paired t-tests. Differences between groups were analyzed using unpaired Student’s t-tests or one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc multiple comparison test, when appropriate. Statistical significance was set at P < 0.05. The sample size was not calculated beforehand; instead we determined the sample size based on previous studies with similar experimental designs2,3,19,20,48.
Data availability
All data and material are available from the corresponding author on reasonable request.
Change history
07 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-96547-w
References
Sakata, T., Yoshimatsu, H. & Kurokawa, M. Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism. Nutrition 13, 403–411. https://doi.org/10.1016/s0899-9007(97)91277-6 (1997).
Tsuji, T., Yamamoto, T., Tanaka, S., Bakhshishayan, S. & Kogo, M. Analyses of the facilitatory effect of orexin on eating and masticatory muscle activity in rats. J. Neurophysiol. 106, 3129–3135. https://doi.org/10.1152/jn.01108.2010 (2011).
Ushimura, A., Tsuji, T., Tanaka, S., Kogo, M. & Yamamoto, T. Neuropeptide-Y modulates eating patterns and masticatory muscle activity in rats. Behav. Brain Res. 278, 520–526. https://doi.org/10.1016/j.bbr.2014.10.031 (2015).
Kolta, A., Dubuc, R. & Lund, J. P. An immunocytochemical and autoradiographic investigation of the serotoninergic innervation of trigeminal mesencephalic and motor nuclei in the rabbit. Neuroscience 53, 1113–1126. https://doi.org/10.1016/0306-4522(93)90494-z (1993).
Walsh, J. J. et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594. https://doi.org/10.1038/s41586-018-0416-4 (2018).
Monti, J. M. Serotonin control of sleep-wake behavior. Sleep. Med. Rev. 15, 269–281. https://doi.org/10.1016/j.smrv.2010.11.003 (2011).
He, Y. et al. 5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding. Mol. Psychiatry 26, 7211–7224. https://doi.org/10.1038/s41380-021-01220-z (2021).
Fornal, C. A., Metzler, C. W., Marrosu, F., Ribiero-do-Valle, L. E. & Jacobs, B. L. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 716, 123–133. https://doi.org/10.1016/0006-8993(96)00006-6 (1996).
Veasey, S. C., Fornal, C. A., Metzler, C. W. & Jacobs, B. L. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79, 161–169. https://doi.org/10.1016/s0306-4522(96)00673-2 (1997).
Ribeiro-do-Valle, L. E. Serotonergic neurons in the caudal raphe nuclei discharge in association with activity of masticatory muscles. Braz. J. Med. Biol. Res. 30, 79–83. https://doi.org/10.1590/s0100-879x1997000100013 (1997).
Chandler, S. H., Goldberg, L. J. & Alba, B. Effects of a serotonin agonist and antagonist on cortically induced rhythmical jaw movements in the anesthetized guinea pig. Brain Res. 334, 201–206. https://doi.org/10.1016/0006-8993(85)90211-2 (1985).
Nagase, Y. et al. Serotonergic axonal contacts on identified cat trigeminal motoneurons and their correlation with medullary raphe nucleus stimulation. J. Comp. Neurol. 384, 443–455. (1997).
Inoue, T. et al. Serotonergic modulation of the hyperpolarizing spike afterpotential in rat jaw-closing motoneurons by PKA and PKC. J. Neurophysiol. 82, 626–637. https://doi.org/10.1152/jn.1999.82.2.626 (1999).
Inoue, T. et al. Involvement of 5-HT7 receptors in serotonergic effects on spike afterpotentials in presumed jaw-closing motoneurons of rats. Brain Res. 954, 202–211. https://doi.org/10.1016/s0006-8993(02)03286-9 (2002).
Dantsuji, M. et al. 5-HT2A receptor activation enhances NMDA receptor-mediated glutamate responses through src kinase in the dendrites of rat jaw-closing motoneurons. J. Physiol. 597, 2565–2589. https://doi.org/10.1113/JP275440 (2019).
Kato, T. et al. Oscillatory population-level activity of dorsal raphe serotonergic neurons is inscribed in sleep structure. J. Neurosci. 42, 7244–7255. https://doi.org/10.1523/jneurosci.2288-21.2022 (2022).
Lemieux, M. & Bretzner, F. Glutamatergic neurons of the gigantocellular reticular nucleus shape locomotor pattern and rhythm in the freely behaving mouse. PLoS Biol. 17, e2003880. https://doi.org/10.1371/journal.pbio.2003880 (2019).
Singer, M. L. et al. Optogenetic activation of the tongue in spontaneously breathing mice. Respir. Physiol. Neurobiol. 309, 103998. https://doi.org/10.1016/j.resp.2022.103998 (2023).
Kobayashi, M. et al. Electrophysiological analysis of rhythmic jaw movements in the freely moving mouse. Physiol. Behav. 75, 377–385. https://doi.org/10.1016/s0031-9384(01)00662-x (2002).
Kobayashi, M. et al. Characteristics of mastication in the anodontic mouse. J. Dent. Res. 81, 594–597. https://doi.org/10.1177/154405910208100903 (2002).
Yamada, M. et al. Longitudinal electromyographic analysis of jaw-closing muscle activities during ingestive behaviors from pre-weaning to juvenile periods in rats. Physiol. Behav. 265, 114173. https://doi.org/10.1016/j.physbeh.2023.114173 (2023).
Tanaka, K. F. et al. Expanding the repertoire of optogenetically targeted cells with an enhanced gene expression system. Cell. Rep. 2, 397–406. https://doi.org/10.1016/j.celrep.2012.06.011 (2012).
Ohmura, Y., Tanaka, K. F., Tsunematsu, T., Yamanaka, A. & Yoshioka, M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int. J. Neuropsychopharmacol. 17, 1777–1783. https://doi.org/10.1017/S1461145714000637 (2014).
Mori, A. et al. Effect of serotonin (5-HT) on trigeminal rhythmic activities generated in in vitro brainstem block preparations. J. Dent. Res. 81, 598–602. https://doi.org/10.1177/154405910208100904 (2002).
Jacobs, B. L. & Azmitia, E. C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229. https://doi.org/10.1152/physrev.1992.72.1.165 (1992).
Berndt, A., Yizhar, O., Gunaydin, L. A., Hegemann, P. & Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 12, 229–234. https://doi.org/10.1038/nn.2247 (2009).
Miyazaki, K. W. et al. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr. Biol. 24, 2033–2040. https://doi.org/10.1016/j.cub.2014.07.041 (2014).
Pompeiano, M., Palacios, J. M. & Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Mol. Brain Res. 23, 163–178. https://doi.org/10.1016/0169-328x(94)90223-2 (1994).
Cornea-Hébert, V., Riad, M., Wu, C., Singh, S. K. & Descarries, L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 409, 187–209. (1999).
Iwata, K., Muramatsu, H., Tsuboi, Y. & Sumino, R. Input-output relationships in the jaw and orofacial motor zones of the cat cerebral cortex. Brain Res. 507, 337–340. https://doi.org/10.1016/0006-8993(90)90293-k (1990).
Nakamura, S., Muramatsu, S. & Yoshida, M. Role of the basal ganglia in manifestation of rhythmical jaw movement in rats. Brain Res. 535, 335–338. https://doi.org/10.1016/0006-8993(90)91620-v (1990).
Ueno, Y. et al. Motor representation of rhythmic jaw movements in the amygdala of guinea pigs. Arch. Oral Biol. 135, 105362. https://doi.org/10.1016/j.archoralbio.2022.105362 (2022).
Inoue, T. et al. Modifications of masticatory behavior after trigeminal deafferentation in the rabbit. Exp. Brain Res. 74, 579–591. https://doi.org/10.1007/bf00247360 (1989).
Morquette, P. et al. Generation of the masticatory central pattern and its modulation by sensory feedback. Prog. Neurobiol. 96, 340–355. https://doi.org/10.1016/j.pneurobio.2012.01.011 (2012).
Tanaka, S. et al. Serotonergic modulation of slow inward rectification in mesencephalic trigeminal neurons. Brain Res. 1718, 126–136. https://doi.org/10.1016/j.brainres.2019.05.013 (2019).
Nagata, A. et al. Serotonin(1B) receptor-mediated presynaptic inhibition of proprioceptive sensory inputs to jaw-closing motoneurons. Brain Res. Bull. 149, 260–267. https://doi.org/10.1016/j.brainresbull.2019.05.001 (2019).
Li, J., Xiong, K. H., Li, Y. Q., Kaneko, T. & Mizuno, N. Serotonergic innervation of mesencephalic trigeminal nucleus neurons: a light and electron microscopic study in the rat. Neurosci. Res. 37, 127–140. https://doi.org/10.1016/s0168-0102(00)00108-5 (2000).
Martin, R. F., Jordan, L. M. & Willis, W. D. Differential projections of cat medullary raphe neurons demonstrated by retrograde labelling following spinal cord lesions. J. Comp. Neurol. 182, 77–88. https://doi.org/10.1002/cne.901820106 (1978).
Perrier, J. F. & Cotel, F. Serotonergic modulation of spinal motor control. Curr. Opin. Neurobiol. 33, 1–7. https://doi.org/10.1016/j.conb.2014.12.008 (2015).
Hensler, J. G. Serotonergic modulation of the limbic system. Neurosci. Biobehav. Rev. 30, 203–214. https://doi.org/10.1016/j.neubiorev.2005.06.007 (2006).
Nozaki, S., Iriki, A. & Nakamura, Y. Role of corticobulbar projection neurons in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J. Neurophysiol. 55, 826–845. https://doi.org/10.1152/jn.1986.55.4.826 (1986).
Inoue, T., Chandler, S. H. & Goldberg, L. J. Neuropharmacological mechanisms underlying rhythmical discharge in trigeminal interneurons during fictive mastication. J. Neurophysiol. 71, 2061–2073. https://doi.org/10.1152/jn.1994.71.6.2061 (1994).
Noguchi, T. et al. Developmental changes in GABAergic and glycinergic synaptic transmission to rat motoneurons innervating jaw-closing and jaw-opening muscles. Brain Res. 1777, 147753. https://doi.org/10.1016/j.brainres.2021.147753 (2022).
Correia, P. A. et al. Transient inhibition and long-term facilitation of locomotion by phasic optogenetic activation of serotonin neurons. eLife 6, e20975 (2017). https://doi.org/10.7554/eLife.20975
Yoshida, K., Drew, M. R., Mimura, M. & Tanaka, K. F. Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat. Neurosci. 22, 770–777. https://doi.org/10.1038/s41593-019-0376-5 (2019).
Ikawa, Y. et al. Effects of citalopram on jaw-closing muscle activity during sleep and wakefulness in mice. Neurosci. Res. 113, 48–55. https://doi.org/10.1016/j.neures.2016.07.004 (2016).
Batschelet, E. Circular Statistics in Biology (Academic, 1981).
Stanek, E., Rodriguez, E., Zhao, S., Han, B. X. & Wang, F. Supratrigeminal bilaterally projecting neurons maintain basal tone and enable bilateral phasic activation of jaw-closing muscles. J. Neurosci. 36, 7663–7675. https://doi.org/10.1523/jneurosci.0839-16.2016 (2016).
Acknowledgements
This work was supported by research grants from JSPS KAKENHI (grant numbers: JP19K18951 [M. D.], JP23K15966 [M. D.], JP21K19614 [T. I.], and JP22K09918 [S. N.]).
Author information
Authors and Affiliations
Contributions
M.D. contributed to the conception, design, data acquisition, analysis, and interpretation, and drafted and critically revised the manuscript; A.M., K.N., M.K., M.I., and K.T. contributed to data interpretation and critically revised the manuscript; T.I. contributed to the conception and design and critically revised the manuscript; S.N. contributed to the conception, design, data acquisition, analysis, and interpretation, and drafted and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, the legends of Figures 4, 5, 6, and 7 were switched. Full information regarding the correction made can be found in the correction for this Article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dantsuji, M., Mochizuki, A., Nakayama, K. et al. Optogenetic activation of serotonergic neurons changes masticatory movement in freely moving mice. Sci Rep 14, 27703 (2024). https://doi.org/10.1038/s41598-024-79429-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79429-5