Abstract
The lung is one of the most frequently metastasized organs from various cancer entities, especially colorectal cancer (CRC). The occurrence of lung metastasis correlates with worse prognosis in CRC patients. Here, we aimed to investigate the role of IL-10 in lung metastasis development and identify the cellular source and target cells of IL-10 during lung metastatic establishment. To induce lung metastasis in mice, we injected MC38 murine colon cancer cells intravenously. Mice with Il10-deficiency were used to test the role of IL-10. The lung metastatic burden was assessed both macroscopically and histologically. IL-10- and Foxp3-reporter mice were employed to identify the cellular source and target cells of IL-10 in lung metastasis using flow cytometry. These findings were further confirmed using mice with cell-specific deletion of Il10- and IL-10 receptor (Il10ra). Interestingly, Il10 ablation led to reduced lung metastasis formation, suggesting a pathogenic role of IL-10 in lung metastasis. Moreover, using reporter mice, we identified Foxp3 + regulatory T cells (Tregs) as the predominant cellular source of IL-10 in lung metastasis. Accordingly, Foxp3 + Treg-specific deletion of Il10 resulted in decreased lung metastasis formation. In terms of target cells, myeloid cells and Foxp3 + Tregs expressed high IL-10Ra levels. Indeed, IL-10 signaling blockade in these two immune cell populations resulted in reduced lung metastatic burden. In conclusion, Foxp3 + Treg-derived IL-10 was found to act on Foxp3 + Tregs and myeloid cells, thereby promoting lung metastasis formation. These findings provide insights into lung metastasis-related immunity and establish the groundwork for optimizing metastasis-targeting immunotherapies through targeting of IL-10 as a novel therapeutic strategy.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) remains one of the most prevalent and deadly forms of cancer worldwide, with high incidence of distant metastasis, which leads to worse prognosis1,2. Lung metastasis in CRC represents a complex process that involves the migration of cancer cells from the primary tumor site to the lungs. This migration occurs through a series of steps known as the metastatic cascade, comprising local invasion, intravasation, survival in the circulatory system, extravasation, and colonization in the pulmonary environment3. Since the lung is one of the most frequently targeted organs, lung metastasis significantly contributes to the mortality rate associated with CRC4. Therefore, understanding how colorectal cancer cells establish secondary colonization in the lung is crucial for developing targeted therapies that improve patient outcomes.
Recent studies have highlighted the importance of immune system interactions in various cancer entities5,6,7,8. Previously, we have identified Interleukin-10 (IL-10) as a critical cytokine in the process of CRC-derived liver metastasis formation9. Specifically, Foxp3 + regulatory T cell (Treg)-derived IL-10 acted on monocytes and promoted liver metastasis formation via the upregulation of programmed death-ligand 1 (PD-L1)9. However, the role of IL-10 in lung metastasis has not yet been fully understood. IL-10 is well known for its anti-inflammatory and immunosuppressive capabilities, affecting a wide range of diseases10,11,12. IL-10 binds to the receptor IL-10R, majorly IL-10Ra, to initiate a cascade of signaling activation that broadly modulates the immune response13,14. The expression of IL-10Ra is relatively narrowly distributed, primarily found on cells of hematopoietic origin, including certain subsets, such as T cells, B cells, macrophages, and dendritic cells (DCs)10,15. This selective expression allows for a better definition of cells responding directly to IL-10.
IL-10 is a pleiotropic cytokine and has divergent roles in different diseases13,16. The effects of IL-10 vary according to the experimental setting and the cell types of interest. In metastasis, the traits of disseminated cancer cells show organ-specific patterns17,18. It warrants clarification whether IL-10 shares the same role in different metastatic sites, and more interestingly, whether the cellular source(s) and target cell(s) of IL-10 in different metastatic sites are the same. This is of high interest in clinical practice, since side effects upon systemic treatment are ought to be considered beforehand. For example, if the role of IL-10 is paradoxical in lung and liver metastasis, IL-10 inhibition can reduce liver metastasis, while adversely, facilitating lung metastasis. Therefore, more caution should be taken when employing systemic IL-10 treatment. Addressing this could ameliorate the management of colorectal cancer patients with distant metastasis by inhibiting IL-10 signaling systemically, or in an organ-specific way.
In this study, we aimed to investigate the role of IL-10 in lung metastasis using mouse models. In addition, we aimed to identify the major cellular source and target cells of IL-10 during lung metastasis. Taken together, our findings seek to provide novel insights into lung metastasis formation and uncover novel immunotherapeutic targets, such as IL-10, thereby optimizing treatment approaches for CRC patients with distant metastasis.
Materials and methods
Mice
C57BL/6 J, Il10-/-, Il10flox/flox;Foxp3cre+ , Il10eGFP;Foxp3RFP, Il10raflox/flox;Lysmcre+ , Il10raflox/flox;Foxp3cre+ mice were housed in the animal facility of the University Medical Center Hamburg-Eppendorf under specific pathogen-free conditions. Il10raflox/flox mice were kindly provided by Prof. Richard Flavell. The Il10flox/flox and Il10raflox/flox mice have previously been validated and characterized12,19. Age- (8–14 weeks) and sex-matched littermates were used for experiments. All animal experiments were approved by the Institutional Review Board “Behörde für Justiz und Verbraucherschutz, Lebensmittelsicherheit und Veterinärwesen” (Hamburg, Germany). All methods were performed in accordance with the relevant guidelines and regulations. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Cancer cell lines
Colon adenocarcinoma (MC38) cancer cells were cultured in 10% FBS DMEM medium with penicillin–streptomycin. Cells were cultured in 37 °C incubator under 5% CO2. The cells were split using trypsin–EDTA (0.25%) once they had reached around 80% confluency. Similarly, cells with around 80% confluency were harvested for lung metastasis induction.
Mouse models for lung metastasis induction
Forced lung metastasis was induced by injecting cancer cells intravenously (i.v.)20. As described previously, 100 μL of the resuspended cancer cells were administered into the tail vein of mice under anesthesia. The lungs were harvested 3 weeks post injection for lung metastatic burden assessment.
Immune cell isolation
Murine lungs were harvested after PBS perfusion through the vena cava. Subsequently, the lungs were cut into small pieces and digested in a shaking incubator at 37 °C for 25 min. The media used for digestion of the organs consisted of HBSS (with Ca2+ and Mg2+) containing 10 U/ml DNase and 1 mg/ml Collagenase. After digestion, the lungs were smashed using metal cell strainers and washed using 1% FBS PBS into a 50 ml falcon. After centrifugation at 400 g for 8 min, the pellet was collected. Immune cells were then enriched from the pellet by Percoll gradient centrifugation (GE Healthcare, Chicago, IL).
Flow cytometry
Lung immune cells were isolated as mentioned above. To block the Fc-γ receptors, a mAb (clone 2.4G2) was used. The cells were then washed and stained with fluorochrome-conjugated antibodies (Supplementary Table 1) for 15 min at 4 °C. The BD LSRFortessa (BD Biosciences, San Jose, CA) was used for flow cytometry. Data analysis was performed using FlowJo v.10 (TreeStar, Ashland, OR).
Hematoxylin and Eosin (H&E) staining
Lung specimens were immediately fixed after harvesting in 4% buffered formalin. Subsequently, lungs were embedded in paraffin or OCT embedding matrix (Sakura, Tokyo, Japan) and stored at room temperature or -80 ̊C, respectively, for future staining. Before staining, lung tissue sections (4 mm) were prepared and stained with H&E. Pictures were taken using the Axio Vert.A1 (Zeiss, Jena, Germany) microscope.
Statistical analysis
All data were analyzed using the GraphPad Prism statistical software (GraphPad software, San Diego, CA, USA). Mouse data are presented as mean ± SEM. Comparison of means was performed using the Mann–Whitney U test for paired group comparisons or the one-way ANOVA (Bonferroni) for multiple group comparisons, as appropriate. P-values below 0.05 were considered significant.
Results
Il10-deficiency protects mice against CRC-derived lung metastasis
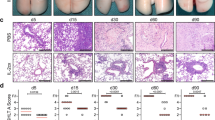
To pinpoint the role of IL-10 in CRC-derived lung metastasis formation, we injected MC38 colon cancer cells i.v. in Il10-/- and wild type (Wt) mice, and assessed the lung metastatic burden 21 days later (Fig. 1A). Interestingly, Il10-/- mice developed significantly fewer lung metastatic sites compared to the Wt mice (Fig. 1B), thereby suggesting a pathogenic role of IL-10 during CRC-derived lung metastasis formation.
Il10-deficiency protects mice against CRC-derived lung metastasis. (A) Schematic overview of forced lung metastasis induction following intravenous MC38 cancer cell injection. (B) Representative macroscopic and histological pictures of lung metastasis following H&E staining, as well as number of macroscopic lung metastases in Wt and Il10-/- mice (n ≥ 18 mice per group). Scale bar: 2 mm. Data are presented as mean ± SEM. **p ≤ 0.01calculated by Mann–Whitney U test.
Foxp3+ Tregs are the major source of IL-10 during lung metastasis formation
To better understand how IL-10 signaling affects lung metastasis formation, we next aimed to identify the cellular source of IL-10 during lung metastasis formation. We have previously identified Foxp3 + Tregs as the major producer of IL-10 during liver metastasis formation9. To investigate whether Foxp3 + Tregs are the predominant cellular source of IL-10 in lung metastasis as well, a Il10GFP; Foxp3RFP reporter mouse was used for lung metastasis induction (Fig. 2A)21. Compared to steady state, IL-10 production in lung metastasis increased significantly (Fig. 2B). Of note, increased IL-10 production was not only seen in CD3- immune cells, but also in T cells, especially in CD4 + T cells (Fig. 2C,D). Specifically, increased IL-10 production was found both in Foxp3 + Tregs and in Foxp3-CD4 + T cells (Fig. 2E–G). To pinpoint the population that contributed the most to IL-10 production in lung metastasis, we focused on IL-10-producing cells and analyzed the proportion of each immune cell population of interest (Fig. 2H). Even though the frequency of IL-10-producing CD3- cells increased in lung metastasis compared to steady state, the proportion of CD3- cells in IL-10-producing cells reduced. This could be explained by a profound expansion of IL-10-producing Foxp3 + Tregs, from 5.7% in steady state, to 31% in lung metastasis, among all IL-10-producing cells (Fig. 2H). Moreover, Foxp3 + Tregs were indeed the main IL-10-producing cells among all immune cell populations.
Foxp3 + Tregs are the major source of IL-10 in lung metastasis formation. (A) Schematic overview of the forced lung metastasis induction using intravenous injection of MC38 cancer cells in Foxp3RFP; Il10GFP reporter mice (n ≥ 12 mice per group). (B–D) Frequency of IL-10 + cells in (B) all CD45 + cells, (C) CD3- cells and T cells, and in (D) CD8 + T cells as well as in CD4 + T cells. (E–G) IL-10 expression in (F) Foxp3- IL-10 + cells and (G) Foxp3 + Tregs. (H) General distribution of all IL-10 producing CD45 + cells in healthy lung and lung with metastasis. Data are presented as mean ± SEM. Non-significant (ns): p > 0.05; *p < 0.05; **p ≤ 0.01; ***p < 0.001, as calculated by Mann–Whitney U test.
Taken together, our results demonstrate that Foxp3 + Tregs are the major cellular source of IL-10 in CRC-derived lung metastasis.
Foxp3 + Treg-derived IL-10 promotes CRC-derived lung metastasis
Next, we aimed to investigate whether IL-10 expressed by the major producer, Foxp3 + Tregs, could affect lung metastasis development. To this end, we employed another mouse model to delete IL-10 from Foxp3 + Tregs, namely Il10flox/flox; Foxp3cre+ mice. Lung metastasis was induced as described before (Fig. 3A), and the lung metastatic load was assessed three weeks later. Indeed, IL-10 deletion in Foxp3 + Tregs resulted in decreased lung metastases (Fig. 3B), a finding suggesting that Foxp3 + Treg-derived IL-10 promotes CRC-derived lung metastasis.
Foxp3 + Tregs-derived IL-10 promotes CRC-derived lung metastasis. (A) Schematic overview of lung metastasis induction following MC38 colon cancer cell intravenous injection in mice harboring Foxp3 + Treg cell-specific Il10 deletion and respective controls (n ≥ 6 mice per group). (B) Representative images and number of macroscopic lung metastases. Scale bar: 2 mm. Data are presented as mean ± SEM. **p ≤ 0.01, as calculated by Mann–Whitney U test.
Foxp3+ Tregs and myeloid cells express high IL-10Ra levels
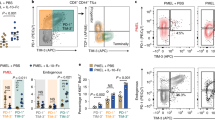
As a next step, we aimed to identify the target cells of IL-10 during CRC-derived lung metastasis formation. To this end, we first characterized the IL-10Ra expression in various immune cell populations in the lung. In the healthy lung, CD3- cells exhibited the highest IL-10Ra expression, significantly higher than T cells (Fig. 4A,B). Among T cells, the highest IL-10Ra expression was observed in Foxp3 + Tregs (Fig. 4A,B). Additionally, we determined the IL-10Ra expression in different immune cell populations in lungs with metastasis (Fig. 4C,D). Similar to our findings in steady state conditions, the highest IL-10Ra expression was also identified in CD3- cells in the metastatic lung. Foxp3 + Tregs still exhibited higher IL-10Ra expression compared to other T cell subtypes. Within CD3- cells, dendritic cells (DCs) and macrophages showed higher IL-10Ra levels compared to other immune cell populations (Fig. 4E,F). In conclusion, myeloid cells, especially DCs and macrophages, as well as Foxp3 + Tregs, were found to express high IL-10Ra levels during lung metastasis formation.
Foxp3 + Tregs and myeloid cells exhibit high IL-10Ra expression. (A–D) Representative FACS plots and ΔMFI quantification of IL-10Ra expression in immune cells isolated from (A,B) healthy lungs (n ≥ 4 mice per group) or (C,D) lungs with metastasis 21 days post i.v. MC38 cancer cell injection (n ≥ 5 mice per group). (E) Representative FACS plots and (F) ΔMFI quantification of IL-10Ra expression in lung CD3- immune cells isolated from lungs with metastasis. Data are presented as mean ± SEM. Non-significant (ns): p > 0.05; *p < 0.05; ***p < 0.001; ****p < 0.0001, as calculated by one-way ANOVA (Bonferroni) with Bonferroni post hoc tests.
IL-10 signaling in Foxp3+ Tregs and myeloid cells promotes lung metastasis
Finally, we aimed to examine whether Foxp3 + Tregs and myeloid cells are directly targeted by IL-10 during lung metastasis development. To this end, lung metastasis was induced in transgenic mice allowing a respective cell-specific IL-10Ra deletion in Foxp3 + Tregs (Il10raflox/flox; Foxp3cre+ mice) or in myeloid cells (Il10raflox/flox; Lysmcre+ mice).The lungs of these mice were harvested after three weeks (Fig. 5A). Of note, impaired IL-10 signaling in either Foxp3 + Tregs or myeloid cells resulted in lung metastasis reduction (Fig. 5B,C). These findings suggest that Foxp3 + Tregs and myeloid cells not only express high IL-10Ra levels, but also directly mediate the role of IL-10 in lung metastasis formation.
IL-10 signaling in Foxp3 + Tregs and myeloid cells promotes lung metastasis. (A) Schematic overview of forced lung metastasis induction in mice with cell-specific Il10ra deletion (n ≥ 6 mice per group). (B,C) Representative images and number of lung metastases in mice with (B) Treg−, or (C) myeloid cell-specific Il10ra deletion. Scale bar: 2 mm. Data are presented as mean ± SEM. Non-significant (ns): p > 0.05; *p < 0.05; **p ≤ 0.01; as calculated by Mann–Whitney U test.
Discussion
In this study, we investigated the role of IL-10 in lung metastasis. By using the Il10-deficient mouse model, we found that, similar to liver metastasis9, IL-10 promotes lung metastasis formation. These results highlight the important role of Foxp3 + Treg-derived IL-10 in promoting CRC-derived distant metastasis, through its IL-10Ra-mediated action on myeloid cells and Foxp3 + Tregs. These observations are consistent with previous studies identifying IL-10 as an immunosuppressive agent that can promote tumor growth by inhibiting effective anti-tumor immune responses22. Our findings provide a new dimension in understanding the immunological metastatic niche, where IL-10 acts as a facilitator of metastatic progression in both the lung and the liver environment.
Using reporter mice and forced lung metastasis induction, we found that, among all examined immune cell populations, Foxp3 + Tregs are the predominant cellular source of IL-10 in lung metastasis. This is an interesting finding, since reports have often highlighted diverse sources of IL-10 in various diseases, including myeloid-derived suppressor cells (MDSCs) and macrophages13,23,24,25. Here, we demonstrate that Foxp3 + Tregs present as a major IL-10 producing cell source in lung metastasis, similar to liver metastasis, but also indeed functionally contribute to lung metastasis formation. The significant involvement of Foxp3 + Treg-derived IL-10 in metastasis formation suggests that these cells are consistent central drivers for CRC cancer cell colonization and growth in the lung and liver metastatic microenvironment. Of note, lung and liver are the most frequently metastasized organs in CRC patients. This suggests that targeting Foxp3 + Tregs or their IL-10 production may provide a strategic approach for therapeutic interventions aimed at reducing distant metastasis of CRC patients. Higher IL-10 production by CD3- immune cells and CD4 + Foxp3 + Tregs was observed in lungs with metastasis compared to healthy lungs. However, the proportion of CD3- cells in all IL-10-producing cells decreased in lung metastasis, which could be explained by a drastic increase in Foxp3 + Tregs. The fraction of CD4 + Foxp3- cells in IL-10 producing cells was slightly elevated. Although CD4 + Foxp3- cells were not identified as the most predominant IL-10-producing cells, further studies focusing on this particular cell population are warranted.
Furthermore, the higher expression of IL-10Ra on myeloid cells and Foxp3 + Tregs in the metastatic lung was consistent with that in liver metastasis. We have observed that in metastatic lungs, IL-10RA expression was not elevated in T cells, which is similar to our observation in the liver9. Nevertheless, we found that IL-10 signaling in T cells does play a role. Thus, it seems that an upregulation of IL-10RA is not essential in order to allow IL-10 to exert its function in metastasis. The fact that deletion of IL-10Ra on myeloid cells and Foxp3 + Tregs resulted in reduced lung metastatic burden, strongly supports a direct effect of IL-10 on these cells during lung metastasis formation. These findings align with our recent reports suggesting that IL-10 signaling in Foxp3 + Tregs and monocytes can facilitate a pro-metastatic microenvironment in the liver9.
However, our study has a few limitations. The models used may not fully recapitulate the complexity of human CRC or the heterogeneity of the tumor microenvironment. For example, the microenvironment in lung metastasis may differ upon synchronous presence of liver metastasis, as compared to the sole occurrence of lung metastasis, since the presence of liver metastasis induces systemic immune alterations26. However, inducing both lung and liver metastasis in a mouse at the same time will highly burden it, and is therefore not recommended. Future studies could explore the implications of IL-10 signaling in more clinically relevant models, using for example, direct clinical sample-derived organotypic slides. Additionally, therapeutical targeting of IL-10 or its pathway could carry risks given its broad role in controlling inflammation and maintaining immune homeostasis10,11,13. A thorough investigation of the side effects of such targeting in clinical settings is warranted27.
We recently deciphered the molecular mechanism by which IL-10 promotes metastasis in the liver. IL-10 does not affect cancer cell extravasation, but rather influences later stages of the metastatic cascade. Specifically, Foxp3 + Treg-derived IL-10 acts on Foxp3 + Tregs themselves, thereby amplifying IL-10 production. Furthermore, IL-10 promotes PD-L1 upregulation on monocytes, which subsequently suppresses CD8 + T-cell-mediated immune surveillance and this causing metastasis9. Previous studies have shown that deletion of IL-10 or its receptor IL-10Ra can significantly affect the cytotoxic response, particularly involving CD8 + T cells and other cytotoxic effector mechanisms. IL-10 is known for its immunosuppressive role, and in its absence, CD8 + T cell activation typically increases. This is because IL-10 suppresses the production of pro-inflammatory cytokines and costimulatory molecules that are essential for CD8 + T cell activation. CD8 + T cells are more likely to produce higher levels of interferon-gamma (IFN-γ) in the absence of IL-10 or IL-10Ra. IFN-γ is a key cytokine for cytotoxic function and the immune response against tumors. In the absence of IL-10 signaling, CD8 + T cells tend to have higher expression of these molecules, allowing them to more effectively induce apoptosis in target cells28,29. Further studies are warranted to test if this mechanism would also apply in lung metastasis.
In conclusion, our data provide evidence that Foxp3 + Tregs are the major cellular source of IL-10 in lung metastasis. Additionally, we demonstrate that Foxp3 + Treg-produced IL-10 promotes CRC-derived lung metastasis formation. Lastly, we report that Foxp3 + Tregs and myeloid cells are direct target cells of IL-10 during lung metastasis formation. These findings highlight the potential therapeutic benefit of IL-10Ra inhibition against CRC-derived lung metastasis. Of note, this study further suggests that anti-IL-10Ra administration could serve as a promising treatment to CRC patients with distant metastasis, without triggering metastatic progression in other organs.
Limitation of the study
Due to mouse background only one mouse CRC cell line could be used, a point that could be considered as a limitation of the study.
Data availability
All data from this study are provided. Primary data from flow cytometry are available upon reasonable request. Further information and requests for resources and reagents should be directed to Anastasios Giannou (a.giannou@uke.de).
Change history
23 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-17854-w
Abbreviations
- MC38:
-
Murine colon cancer cells
- Tregs:
-
Foxp3 + regulatory T cells
- CRC:
-
Colorectal cancer
- Pen/Strep:
-
Penicillin–streptomycin
- DC:
-
Dendritic cell
- i.v.:
-
Intravenous
References
Cervantes, A. et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34(1), 10–32 (2023).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263 (2024).
Gomez-Cuadrado, L., Tracey, N., Ma, R., Qian, B. & Brunton, V. G. Mouse models of metastasis: Progress and prospects. Dis. Model. Mech. 10(9), 1061–1074 (2017).
Jordens, M. S. et al. Prevalence of lung metastases among 19,321 metastatic colorectal cancer patients in eight countries of Europe and Asia. Curr. Oncol. 28(6), 5035–5040 (2021).
Mukherjee, A. G. et al. Role of immune cells and receptors in cancer treatment: An immunotherapeutic approach. Vaccines 10(9), 1493 (2022).
Hiam-Galvez, K. J., Allen, B. M. & Spitzer, M. H. Systemic immunity in cancer. Nat. Rev. Cancer 21(6), 345–359 (2021).
Leone, R. D. & Powell, J. D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 20(9), 516–531 (2020).
Huppert, L. A. et al. Tissue-specific Tregs in cancer metastasis: Opportunities for precision immunotherapy. Cell Mol. Immunol. 19(1), 33–45 (2022).
Shiri, A. M. et al. IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction. J. Hepatol. 78, S525 (2023).
Bedke, T., Muscate, F., Soukou, S., Gagliani, N. & Huber, S. IL-10-producing T cells and their dual functions. Semin. Immunol. 44, 101335 (2019).
Rallis, K. S. et al. IL-10 in cancer: An essential thermostatic regulator between homeostatic immunity and inflammation: A comprehensive review. Future Oncol. 18(29), 3349–3365 (2022).
Yogev, N. et al. CD4(+) T-cell-derived IL-10 promotes CNS inflammation in mice by sustaining effector T cell survival. Cell Rep. 38(13), 110565 (2022).
Mannino, M. H. et al. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 367(2), 103–107 (2015).
Saraiva, M., Vieira, P. & O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217(1), 418 (2020).
Shouval, D. S. et al. Interleukin 10 receptor signaling: Master regulator of intestinal mucosal homeostasis in mice and humans. Adv. Immunol. 122, 177–210 (2014).
Silva, F. S. et al. A dual-role for IL-10: From leukemogenesis to the tumor progression in acute lymphoblastic leukemia. Cytokine 171, 156371 (2023).
Obenauf, A. C. & Massague, J. Surviving at a distance: Organ-specific metastasis. Trends Cancer 1(1), 76–91 (2015).
Izraely, S. & Witz, I. P. Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment. Int. J. Cancer 148(6), 1308–1322 (2021).
Rubtsov, Y. P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28(4), 546–558 (2008).
Lucke, J. et al. Protocol for generating lung and liver metastasis in mice using models that bypass intravasation. STAR Protoc. 5(1), 102696 (2024).
Kamanaka, M. et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25(6), 941–952 (2006).
Sullivan, K. M. et al. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut 72, 325–337 (2022).
Yaseen, M. M., Abuharfeil, N. M., Darmani, H. & Daoud, A. Mechanisms of immune suppression by myeloid-derived suppressor cells: The role of interleukin-10 as a key immunoregulatory cytokine. Open Biol. 10(9), 200111 (2020).
Shouval, D. S. et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40(5), 706–719 (2014).
Mocellin, S., Marincola, F. M. & Young, H. A. Interleukin-10 and the immune response against cancer: A counterpoint. J. Leukoc. Biol. 78(5), 1043–1051 (2005).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27(1), 152–64 (2021).
Ganesh, K. & Massague, J. Targeting metastatic cancer. Nat. Med. 27(1), 34–44 (2021).
Jaime-Sanchez, P. et al. Cell death induced by cytotoxic CD8+ T cells is immunogenic and primes caspase-3-dependent spread immunity against endogenous tumor antigens. J. Immunother. Cancer 8(1), e000528 (2020).
Groux, H., Bigler, M., de Vries, J. E. & Roncarolo, M. G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J. Immunol. 160(7), 3188–3193 (1998).
Acknowledgements
The authors thank Cathleen Haueis, Sandra Wende, and Tom Blankenburg for technical assistance, the in vivo Optical Imaging Core Facility and the Cytometry und Cell Sorting Core Unit at the University Medical Center Hamburg-Eppendorf for their technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
A.M.S., M.F.A, and A.D.G. conceived, designed, and carried out most experiments, analyzed data, and wrote the manuscript; T.B., E.D.P., M.S., D.E.Z., J.L., S.Z., P.S. carried out in vivo experiments; M.E., T.H., K.J.O, G.E.G. provided critical intellectual input; S.H. and A.D.G. conceived the idea and supervised the study, designed experiments, and wrote the manuscript. All authors reviewed and concurred with the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: Figures 2 and 4 were corrected, the author by-line was updated.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shiri, A.M., Fard-Aghaie, M., Bedke, T. et al. Foxp3 + Treg-derived IL-10 promotes colorectal cancer-derived lung metastasis. Sci Rep 14, 30483 (2024). https://doi.org/10.1038/s41598-024-80437-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80437-8