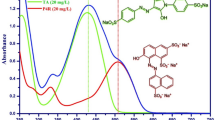
Abstract
Edible dyes have become an essential part of modern life, widely appreciated for their safety and effectiveness in food coloring. Given the fundamental role of hydrogen bonding in chemical and biological systems, this study explores the photophysical properties of three commonly used aromatic azo food dyes–Ponceau 4R, Sunset Yellow, and Tartrazine–across both isotropic solvents and anisotropic environments. To better understand their environmental interactions in ground and excited states, the influence of media polarity on these dyes was also explored. The study further evaluated the applicability of the Kamlet–Abboud–Taft polarity scale in capturing these effects. The results highlight the crucial role of hydrogen bonding in facilitating azo-hydrazone tautomerization and enhancing structural resonance. Additionally, the toxicity of these dyes on kidney cells was assessed using the MTT assay, revealing that Ponceau 4R exhibits notable toxicity, primarily due to solute–solvent interactions. Notably, the hydrazone tautomeric form was identified as the predominant species in this dye.
Similar content being viewed by others
Introduction
Dyes have played a significant role in human culture since ancient times, with natural food colorants dating back to 1500 B.C. Today, synthetic dyes are widely used in the food industry to enhance appearance and maintain product consistency. Among them, azo dyes, aromatic compounds characterized by one or more –N = N– groups, stand as some of the oldest synthetic colorants. These dyes account for approximately 50% of all commercial dyes due to their availability, low cost, stability, and ability to enhance food appearance and customer appetite1,2,3,4,5,6,7. Despite their benefits, the chemical properties and interactions of azo dyes require careful consideration. They adopt various spatial and isomeric forms, which can significantly influence their isomeric transitions and structural resonance. Once introduced into biological environments, azo food dyes may undergo chemical transformations that influence their stability and toxicity, potentially triggering undesirable reactions due to the complexity of these environments. Regulatory agencies conduct preliminary studies on food color additives to ensure consumer safety. However, some color additives exhibit different interactional resonance forms in different environments, which can influence their bioavailability and toxicity8,9,10,11. A 2010 report highlighted the potential toxicity associated with tautomerism, raising concerns about the safety of tautomeric food dyes and need for further tautomeric investigation into their molecular behavior12.
Synthetic aromatic azo dyes like Ponceau 4R, Sunset Yellow, and Tartrazine are extensively used to enhance food appearance. These dyes exhibit tautomerism, existing in a dynamic equilibrium between azo and hydrazone forms, depending on environmental factors like solvent polarity, pH, and temperature. The tautomeric balance significantly influences their stability, electronic properties and biological interactions13,14,15. Notably, Ponceau 4R mostly exists in the hydrazone form in the condensed phase, influencing its thermodynamic stability and spectroscopic properties. Once introduced into biological environments, these dyes may undergo chemical transformations, potentially influencing their toxicity and metabolic pathways. Research on their cytotoxicity suggests potential adverse effects, with Ponceau 4R linked to oxidative stress, genotoxicity, and mitochondrial dysfunction, ultimately leading to apoptosis16,17,18,19,20. Sunset Yellow has been linked to histamine release, which can trigger allergic reactions, asthma symptoms, and inflammatory responses. Some reports indicate that Sunset Yellow can cause DNA instability in cell cultures, raising concerns about potential long-term effects. Additionally, evidence suggests that Sunset Yellow interferes with cellular signaling pathways, potentially disrupting key enzymes involved in detoxification and metabolism21,22,23,24. Tartrazine, undergoing tautomeric shifts, has been linked to neurobiochemical alterations affecting brain function and inducing apoptosis in special cell types. Additionally, evidence suggests that Tartrazine can cross the blood–brain barrier, interfering with neurotransmitter balance and raising concern about neurotoxicity25,26,27. Although these findings highlight a connection between azo dye tautomerism and cytotoxcity, the direct relationship between tautomeric stability and biological impact remains largely unexplored. (Fig. S1).
The International Union of Pure and Applied Chemistry (IUPAC) defines tautomerism as the facile interconversion of isomers via hydrogen atom transfer between two hydrogen-bonding-capable atoms. Nowadays, the physical organic chemistry of tautomers is an active research topic that has attracted significant attention from researchers28. Azo-hydrazone tautomerism is a crucial process governing the physicochemical properties of azo compounds, leading to two distinct tautomeric forms with contrasting technical and dye behaviors. The hydroxyl functional group plays a key role in this equilibrium, with its spatial arrangement directly affecting molecular interactions9,10. Specifically, the hydroxy group donates a hydrogen bond to the nitrogen, transforming into its keto form– a process known as azo-hydrazone tautomeric conversion– while simultaneously undergoing the enol-keto tautomerism29. Both transformations occur when the hydroxy group is suitably positioned relative to the azo moiety. Polar solvents elevated temperatures, neutral pH, and electron-withdrawing substituents favor the keto form through intramolecular hydrogen bonding, while non-polar solvents, lower temperatures, high pH, and electron-donating groups promote the enol form28.
Food additives, including azo dyes, interact with biological systems and undergo multiple environmental transformations before reaching their molecular targets, like proteins and nucleic acids30. The surrounding media and functional groups within a molecule can significantly influence tautomeric equilibria, occasionally enabling the coexistence of multiple tautomeric states. The solvation environment plays a decisive role in dictating the stability and prevalence of tautomeric forms. The intricate interplay between solvent properties and solute molecules results in varied behavior and physiochemical attributes9,10. Consequently, researchers have developed various models to analyze solvent effects on isomeric equilibria. Given the complexity of these interactions, multi-parameter solvent models offer the most effective qualitative and quantitative insights. The Kamlet–Abboud–Taft multi-parametric solvent polarity scale based on the Linear Solvation Energy Relationship (LSER) model, provide robust predictions of inter- and intramolecular interactions. The stability of tautomers depends on the general solvent interactions, such as dielectric constant, alongside specific stabilizing forces like hydrogen bonding31.
Intramolecular hydrogen bonding also plays a key role in stabilizing specific tautomeric forms of o-hydroxy Schiff bases, influencing their physicochemical properties. Studies by Filarowski et al.32 highlight strong hydrogen bonds between the hydroxyl group and the imine nitrogen, forming stable quasi-aromatic six-membered rings. Hansen33 characterized these bonds using isotope effects on chemical shifts, while Dziembowska et al.34 studied proton transfer and electronic interactions in N-oxide Schiff bases via FT-IR and NMR spectroscopy, further elucidating the influence of intramolecular hydrogen bonding on molecular behavior. These studies underscore the profound impact of hydrogen bonding on tautomeric equilibrium, molecular stability, and key biological functions including DNA integrity and enzyme activity35.
In many studies, the behavior of azo food dyes has been examined in conventional solvents to simulate biological environments, as toxicity tests are also conducted in aqueous and cellular conditions. However, one of the major challenges in this field is the orientation and anisotropy of the cell membrane, which significantly influences the interaction of these substances. To address this limitation, in addition to using conventional solvents, we utilized liquid crystals (LCs). Like the cell membrane, LCs exhibit both orientational properties and anisotropy, providing a more accurate simulation of the cellular environment36,37,38. The novelty of our study lies in employing LCs as a unique medium to investigate the tautomeric properties and solvatochromic behavior of Ponceau 4R, Sunset Yellow, and Tartrazine across both isotropic solvents and anisotropic environments. Furthermore, our study evaluates the effectiveness of the Kamlet–Abboud–Taft model in describing solvent effects on azo-hydrazone tautomerism and correlates tautomer stability with cytotoxicity via the MTT assay. The results showed that the behavior of these dyes in LCs closely resembles their toxicity patterns in cellular environments. For instance Sunset Yellow, due to its complete azo-hydrazone transition pathway and its compatibility with the polar nature of the solvent, did not exhibit toxicity in kidney cells. In contrast, in Ponceau 4R, this transition pathway is incomplete, leading to the accumulation of the hydrazo form in high-alpha environments, which ultimately results in toxicity. A similar trend was observed in Tartrazine, as its azo-hydrazone transition pathway is also incomplete, leading to comparable toxic effects. These findings suggest that using LCs as a simulation medium can enhance our understanding of the interactions of these dyes in biological systems. Additionally, dipole moment calculations in both ground and excited states provide deeper insights into the electronic structures of each tautomeric form and the polarity-dependent behavior of these dyes. By advancing our knowledge of solvent interactions, hydrogen bonding, and molecular stability, this research contributes to a more assessment of azo dyes safety in food products.
Experimental
Materials
Azo family food color additives including Ponceau 4R, Sunset Yellow, and Tartrazine were purchased from Merck and used without further purification (Table 1). The isotropic solvents used in this study were of the highest purity available from Merck, with their spectroscopic solvent polarity parameters detailed in Table 2 ref.31,39. Additionally, six Nematic liquid crystal mixtures (Merck Ltd.) were selected as anisotropic solvents based on their varying dielectric constants. The solvent polarity parameters for these mixtures at 25 °C are also presented in Table 2 ref.40,41,42.
Absorption and emission spectroscopy
Absorption spectra in the range of 300–800 nm were recorded using a double-beam Shimadzu UV-2450 Scan UV–visible spectrophotometer. Solution evaluations were performed using standard quartz cuvettes (5 × 1 × 1 cm), while for the liquid crystal medium, standard quartz cells (5 × 1 × 0.2 cm) were used. Fluorescence measurements were conducted using a JASCO FP-750 with sample concentrations set at 1 × 10–5 M. The fluorescence quantum yield (Ф) was determined using Coumarin 2 (C 450) in acetonitrile as the reference standard (ΦF = 0.8) and calculated according to the well-established equation:
where n represents the refractive indices of the experimental (unk) and standard (std) solvents, I is the integrated fluorescence intensity over the emission range of 400–600 nm. A denotes the absorbance at the excitation wavelength43.
Estimation of the dipole moment
The dipole moment was estimated using quantum mechanical second order perturbation theory for a polarizable dipole moment. This approach provides equations for the difference and sum of va and vf (the maxima of absorption and emission bands in various surrounding media, expressed in cm−1). The solvatochromic spectral shift is a commonly used method for determining dipole moment.
Straight line slopes can be used to determine the parameters m1and m2 for sum and difference of wave numbers, which are linear relationships with solvent polarity \(f\left(\varepsilon , n\right)\) and \(\mathsf{g}\left(n\right)\).
The dipole moments of the solute in the ground and excited states are denoted as \(\mu_{g}\) and \(\mu_{e}\) respectively. The Planck constant is donated as h and the light velocity in vacuum is c. Radii of Onsager cavities (a)44. \(f\left(\varepsilon , n\right)\) and \(\mathsf{g}\left(n\right)\) can alternatively be expressed as:
In these correlations, \(\varepsilon\), n, \(a\) and \(\alpha\) represent the dielectric permittivity, refractive index, spherical cavity radius of the solute, and average polarizability, respectively. The criteria \(\frac{2\alpha }{{a^{3} }} = 1\) is usually met for isotropic polarizability of the solute, and Bakhshiev45 established the following relationships:
The dipole moments in ground and excited states, when expressed in parallel form, can be expressed as follows:
Evaluating cytotoxicity by MTT assay
The maximum solubility of the dyes in water
Maximum solubilities of Ponceau 4R, Sunset Yellow, and Tartrazine in water were measured by adding 0.2 g of each dye in 1000 ml of deionized water in a 2-L glass container. The containers were shaken for three hours at 37 °C. The mixtures were filtered through Watman paper 40 (Cat Number: 1440) and then through a 0.2 µm filter to remove any particles larger than 200 nm. The clear filtrates (800 ml) were poured into pre-weighted plastic containers and dried under vacuum (0.6 bar) for a day at room temperature to reach a constant weight. The solubility of each dye in water was measured as the dry net weight of the corresponding dye divided by the applied volume. For MTT assay, the dyes were dissolved in RPMI medium and then filtered through Watman paper 40 and then 0.2 µm filter. The concentrations of dissolved dyed in RPMI were assumed to be similar to that of water.
MTT assay
Previous research46,47,48 indicates that food colorants such as Tartrazine, Sunset Yellow, and Ponceau 4R are primarily excreted via the kidneys. Their small molecular size and high water solubility allow them to pass easily through the glomerular filtration process, leading to their rapid elimination in the urine. Since their absorption in the gastrointestinal tract is minimal, and the small fraction that does get absorbed remains unchanged and is efficiently excreted via the kidneys. Given these properties, this study investigates the cytotoxicity effects of Ponceau 4R, Sunset Yellow, and Tartrazine using MTT assay. Since the kidneys contain a mechanism for excreting these dyes, the human embryonic kidney (HEK293) cell line was chosen as the test system. The cells were seeded in 48-well culture plate at a density of 10,000 per well, and allowed to adhere overnight in a typical CO2 incubator. Following the adhesion, the medium was removed and the cells were treated with the mixtures of RPMI alone and dye-containing RPMI with different volume ratios with negative controls in triplicates. Then, the cells were incubated for 16 h. Then MTT reagent was added to each well and incubated for four hours at 37 °C to allow the formation of formazan crystals. The crystals were dissolved in DMSO after removing the medium, and the absorbances were recorded at 570 nm using a microplate reader (BioTekELx 800, USA).
Results and discussion
The media effects on the absorption and emission spectral behaviors
This study examines the spectral behavior of three widely used food dyes–Ponceau 4R, Sunset Yellow, and Tartrazine. Despite their structural differences, all three dyes contain azo and hydroxyl groups. These similarities lead to coexistence of both azo and hydrazone tautomeric forms, while the structural differences cause different interactions in diverse solvent environments. The presence of these tautomeric forms adds complexity to their spectral behavior.
To investigate this issue, the absorbance and fluorescence spectra of these dyes were recorded in solvents with different polarities and specific interactions capabilities such as hydrogen bonds donor and acceptors. The absorption spectra of these dyes are broad, typically featuring a sharp peak alongside a shoulder. Since they can tautomerize between azo and hydrazo forms, both forms may be visible in the absorption spectra, provided there is no solvent interference or spectral overlap. As shown in Fig. 1, the absorbance spectra of the azo and hydrazone forms overlap. In alcoholic and hydrogen bond donor solvents, the hydrazone peak appears sharper while the azo peak appears as a shoulder. However, in solvents like DMF and DMSO, which have high hydrogen bond acceptor abilities, the hydrazone peak disappears, leaving a sharp azo form peak for all three dyes. As we know, two key phenomena—bathochromic and hypsochromic shifts—play a fundamental role in the optical properties of dyes. A bathochromic shift refers to the spectral shift toward longer wavelengths (red shift), typically resulting from increased conjugation or environmental changes in the molecular structure. In contrast, a hypsochromic shift describes the spectral transition toward shorter wavelengths (blue shift), which may be caused by reduced conjugation or alterations in the electronic structure27. Fluorescence quantum yield analysis of dyes clearly confirms the hypsochromic behavior of the azo-hydrazo form and its associated fluorescence variations. These findings underscore the significant influence of structural and environmental factors in determining the optical characteristics of dyes, highlighting their crucial role in optical behavior. To determine the precise absorption wavelengths of the azo and hydrazone forms, spectral deconvolution was performed, with the results presented in Table 3.
Fluorescence spectra were also recorded in various solvents of varying polarity to assess the fluorescence behavior of these dyes. Since fluorescence excitation corresponds to the maximum absorption wavelength of each tautomeric form, the recorded fluorescence spectra reflect the contributions of both azo and hydrazone forms. Figures 2 and 3 illustrate the fluorescence spectra of the azo and hydrazone forms, respectively. In the study of the hydrazone form of dyes, several key points are noteworthy. One of the most significant observations is the appearance of a shoulder peak in the fluorescence spectrum, which can indicate the presence of different forms in the excited state. This phenomenon is generally attributed to solvent–solute interactions and possible vibrational structures in the excited state. Solvents such as alcohols can influence electronic transitions by forming specific interactions, thereby altering the vibrational structures of these dyes. The prominence of the shoulder peak may be affected by the degree of freedom in the excited-state structure, leading to variations in vibrational modes and peak intensity. While other factors may also contribute to this effect, they might not be less relevant to these specific dyes. Overall, various factors can play a role in this behavior including different conformers in the excited state, solvent–solute interactions, vibrational structures, tautomerism in the excited state, and molecular aggregation. In alcoholic solvents, excited-state interactions can cause rearrangements in molecular structure, potentially leading to the emergence of new peaks. The shoulder peak may arise from vibronic coupling, where electronic transitions are accompanied by vibrational modes. This effect is particularly noticeable in Sunset Yellow and Tartrazine dyes. The excited-state structures of these dyes are strongly influenced by hydrogen bonding with alcohols, which enhances vibronic coupling. As a result, the shoulder peak becomes more intense in Tartrazine. A similar trend is observed in Sunset Yellow, especially in solvents such as 2-Propanol, Ethanol, and Ethylene glycol, where these peaks appear more prominently. Ponceau 4R also exhibits a comparable pattern.
A similar approach was applied to study these dyes in a liquid crystal (LC) environment. Figures 4, 5, 6 present the absorption and fluorescence spectra in various LCs, while Table 4 provide the corresponding spectral data. In LCs environment, absorption spectra reveal a dominant hydrazone peak of Ponceau 4R, with the azo form appearing as a weak shoulder. Conversely, in Tartrazine, the azo form exhibits a sharp peak, while the hydrazone form appears as a shoulder. Sunset Yellow behaves as an intermediate case, showing both azo and hydrazone peaks with comparable sharpness. The relative intensities of these peaks indicate the predominant tautomeric form in each environment. In the LC environment, the hydrazone form dominates for Ponceau 4R, whereas the azo form is more prevalent in Tartrazine. Meanwhile, Sunset Yellow exhibits nearly equal populations of both forms.
A key distinction between solvent and LC environments lies in the factors influencing tautomeric stability. In solvent environments, molecular interactions–particularly hydrogen bond donation and acceptance–determine the dominant form. However, in the structural LC matrix, stereoselectivity plays a more significant role. The reduced Tartrazine within the LC environment suggests that stereoselectivity incompatibility prevents its stabilization. Based on the obtained quantum yield for the fluorescence of these dyes, it is evidence that the azo and hydrazone forms exhibit difference in overall emission efficiency. The azo form, due to its greater stability in the ground state and lower energy level, demonstrates a higher quantum yield compared to the hydrazone form. This trend is fully consistent with the dipole moments result presented in Section “Results and discussion”.
To gain a more precise understanding of the solvent environment effect on the photophysical behavior of these dyes, we will analyze their solvent-dependent spectral shifts using multi-parameter solvent polarity model, such as the Kamlet–Abboud–Taft49,50,51 model, in the following section.
Solvent environment contribution to interaction and tautomerism
As stated in the previous section, the structural nature of the solvent and the type of interactions between the solvent and the studied dyes significantly influence the photophysical behavior of Ponceau 4R, Sunset Yellow, and Tartrazine. To quantitatively assess these effects, the Kamlet–Abboud–Taft multi-parameter solvent polarity model (Eq. 13) provides valuable insights. This equation quantitatively describes how various solvent polarity parameters affect the photophysical behavior of dyes through a linear combination of these parameters.
This model evaluates solute–solvent interactions by accounting for specific and non-specific interactions. Specific interactions include hydrogen bond donor ability (acidity) and hydrogen bond acceptor ability (basicity), while non-specific interactions involve polarity/polarizability effects. The corresponding solvent parameters in this model is as follows: acidity (α)49, basicity (β)50, polarity/polarizability (π*)51. The coefficients \(a\), b, and s represent the contributions of these parameters to the wavenumber associated with the solute, while the regression value of the solute property,\(v_{0}\), corresponds to the intrinsic property in the reference solvent. The α, β and π* values for conventional solvents, as reported in previous studies are listed in Table 2. Similarly, the corresponding values for liquid crystals, as determined by Zakerhamidi et al.32,33,34 are also provided in Table 2.
According to Tables 5, 6, 7, 8, the negative sign of all three evaluated solvent polarity parameters suggests that the ground state of the azo tautomers of Ponceau 4R, Sunset Yellow and Tartrazine dyes is the destabilized, as predicted by the Kamlet–Abboud–Taft equation. For fluorescence, the Kamlet–Abboud–Taft model highlights the solvent’s hydrogen bond acceptor and donor abilities as the most significant factors. The negative signs of solvent polarity parameters in fluorescence wavenumbers indicate that these interactions stabilize the excited state of the dyes.
The Stokes shift, which reflects solvent reorientation around solute molecules, is primarily influenced by the solvent’s hydrogen bond acceptor ability and dipolarity/polarizability in these dyes. The negative signs of these parameters further support this conclusion, aligning with the presence of hydrogen bonding and polar functional groups in these dyes.
For the hydrazo-tautomeric form, hydrogen bond donor ability and dipolarity/polarizability are the most influential factors affecting absorption, fluorescence wavenumber, and Stokes shift. The negative sign of hydrogen bond donor ability in the absorption process suggests instability in the ground state, while its negative sign in the fluorescence indicates excited-state stabilization in the solvent. Conversely, the positive sign of the dipolarity/polarizability parameter suggests opposite effects in the ground and excited states. Regarding the Stokes shift, the negative coefficient of hydrogen bond donor ability indicates reduced solvent molecule rearrangement, while the positive coefficient of dipolarity/polarizability suggests increased molecular rearrangement.
Figure 7 summarizes these observations. Except for hydrogen bond donor ability, which behaves similarity in both azo and hydrazone form, the relationship between these tautomeric forms is evident. The hydrogen bond accepting capacity and their dipolarity/polarizability show opposite trends, consistent with excited-state intramolecular proton transfer (ESIPT) between azo and hydrazo tautomers.
In liquid crystal (LC) environments, investigating the influence of polarity on spectral behavior is more complex than in conventional solvents. As shown in Fig. 7, the dominant solvation interactions in both the azo and hydrazone forms are hydrogen bond acceptor ability and dipolarity / polarizability, respectively, while hydrogen bond donor ability has the least impact. This can be attributed to the directional nature of hydrogen bonding between the liquid crystal environment and the solutes containing hydrogen bond acceptor groups.
The study further reveals that the hydrogen bond donor ability of LC environments in solvent–solute interactions does not significantly affect the azo-hydrazone conversion in the excited state. Similarity, the hydrogen bond acceptor ability of LC environments does not contribute to the stabilizing the hydrazone ground state during its conversion to the azo form. As a result, the probability of ESIPT is low, and the dominant tautomeric form is determined by thermodynamic stability rather than interactional stability. In Tartrazine, the azo form is predominant; in Ponceau 4R, the hydrazone form is dominant; while Sunset Yellow exhibits an intermediate behavior between the two. A detailed analysis of the ground and excited dipole moments of Ponceau 4R, Sunset Yellow, and Tartrazine in solvent and liquid crystal environments will be provided for further discussion.
Dipole moment and possible resonance structures
The dipole moment is a key parameter representing charge distribution in molecules, offering significant insights into the electrical and geometrical structures in both ground and excited states. This property is directly linked to molecular activity, making it crucial for understanding the behavior of Ponceau 4R, Sunset Yellow, and Tartrazine dyes. To estimate their dipole moments, the solvent polarity functions,\(f(\varepsilon ,n)\) and, \(f(\varepsilon ,n) + 2g(n)\),were calculated and reported in Tables 2. The ground-state (µg) and excited-state (µe) dipole moments were determined from the slopes of these lines using Eqs. (10) and (11), with the final results summarized in Table 9. In the liquid crystal environment, the dipole moments were calculated using the average dielectric permittivity and refractive index (see Fig. 8).
According to Table 9, the excited-state dipole moment of Ponceau 4R, Sunset Yellow, and Tartrazine is consistently higher than their ground-state dipole moment in both azo and hydrazo tautomeric forms. The azo for, in particular, exhibits a significantly larger excited-state dipole moment compared to the hydrazone form in both solvent and liquid crystal media. However, in the ground-state, the azo form displays a lower dipole moment than the hydrazone form in solvents, confirming the presence of excited-state intramolecular proton transfer (ESIPT) between the two tautomers, as discussed earlier. Interestingly, this trend is not seen in the liquid crystal environment, especially for Ponceau 4R and Tartrazine, where the ground-state dipole moment of the azo form is higher than that of the hydrazone form. This suggests a low probability of ground-state intramolecular proton transfer (GSIPT) in the liquid crystal environments.
It is important to note that the occurrence of ESIPT does not imply a complete conversion between azo and hydrazo tautomeric forms. Various factors, including molecular structure and thermodynamic stability, influence the extent to which ESIPT take place. While ESIPT provides pathway or the azo forms to return to the ground state from the excited state in a solvent environments, its efficiency may vary.
In solvent environments, ESIPT facilitates the conversion of the excited azo form into the hydrazone form. To reverse this process-converting the hydrazo form back into the azo form- in the solvent medium, GSIPT takes place between the ground states in solvent media. To reverse this process–returning the hydrazo form to the azo form–GSIPT takes place in the ground state. However, based on the findings from dipole moment interactions and dye interactions in solvent and the LC environments, GSIPT appears to be unlikely in LC environments. Figure 8 shows the resonance structure of Ponceau 4R, Sunset Yellow, and Tartrazine in solvent media, summarizing the key charge movement pathways and interactions discussed above.
Evaluating cytotoxicity by MTT assay
The toxicity of Ponceau 4R, Sunset Yellow and Tartrazine was evaluated using the MTT assay. The maximum solubility of these dyes was determined as follows: Ponceau 4R (94 mg/L), Sunset Yellow (70 mg/L), and Tartrazine (73 mg/L). Among them, Ponceau 4R exhibited the highest solubility and proved to be the most toxic with the IC-50 of 2.23 ng/µl. Sunset yellow did not show increasing toxicity by increasing the concentration from 10 to 47 ng/µl, however the viability has dropped from 100% to between 50 and 80% by applying 10 to 47 ng/µl. In contrast, Tartrazine exhibited an unusual cytotoxicity effect. Applying from 13 to 27 ng/µl, higher viabilities were observed respect to the control showing a promoting effect in cell propagation. By increasing the concentration above this, viability has decreased to 82% with the IC-50 of 68.17 ng/µl.
As illustrated in Fig. 9, Tartrazine, at low concentrations, does not pose a hazard to kidney cells and, may even promote the proliferation. Conversely, Ponceau 4R is highly toxic to kidney cells, causing 80% cell death even at a concentration of 5 ng/ml. Sunset Yellow demonstrated a steady toxicity trend across the tested concentrations, leading to approximately 25% cell death in kidney cells.
The observed toxicity of these dyes towards kidney cells appears to be linked to the prevalence of their hydrazo-tautomeric forms in the liquid crystal environment. Notably, cell walls share structural similarities with liquid crystal environment due to their organized and dynamic nature.
Tartrazine exhibited the lowest percentage of hydrazo forms, while Ponceau 4R had the highest, mirroring their behavior in liquid crystal environment. The toxicity patterns of these dyes align with previous studies, which suggest that the hydrazo form is less stable and more toxic.
Conclusions
The study explored the absorbance and fluorescence spectra of Ponceau 4R, Sunset Yellow, and Tartrazine in solvents of varying polarity and specific interactions. These dyes undergo tautomerization between azo and hydrazo forms, allowing both tautomers to be observed. The dominant tautomeric form is primarily determined by molecular interactions, particularly the hydrogen bond donor and acceptor properties of the solvents.
The spectral behaviors of these food dyes in their azo form are primarily dictated by the hydrogen bond acceptor capacity of the solvent, which shapes their absorption and emission properties. In contrast, in the hydrazo form, is predominantly influenced by the hydrogen bond donor ability of the solvent. In liquid crystal environments, the spectroscopic behavior of both tautomeric forms is governed by the hydrogen bond acceptance ability of the media.
Dipole moment, a key parameter that reflects charge distribution within molecules, provides valuable insights into their electronic and geometric structures. Across various solvents and liquid crystal environments, the studied dyes exhibited significantly larger excited-state dipole moments in both azo and hydrazone tautomeric forms. These findings confirm the existence of excited-state intramolecular proton transfer (ESIPT) between tautomeric forms in solvent environments, alongside notable charge transfer within excited molecules. However, due to structural hindrance, the ESIPT process is unlikely to occur in liquid crystal media.
The MTT assay was used to evaluate the toxicity of the food dyes under investigation. Ponceau 4R predominantly present in its hydrazo-tautomeric form emerged as both the most soluble and toxic. In contrast, Sunset Yellow did not exhibit outright toxicity; it did lead to reduced cell viability. Tartrazine displayed an unusual cytotoxic profile, with low concentration even promoting kidney cells proliferation.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author (P.T.) upon reasonable request.
References
Barciela, P., Perez-Vazquez, A. & Prieto, M. A. Azo dyes in the food industry: Features, classification, toxicity, alternatives, and regulation. Food Chem Toxicol. 178, 113935 (2023).
Benkhaya, S., M’rabet, S. & El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6, e03271 (2020).
Burrows, J. D. Palette of our palates: a brief history of food coloring and its regulation. Compr. Rev. Food Sci. Food Saf. 8, 394–408 (2009).
Dey, S. & Nagababu, B. H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Adv. 1, 100019 (2022).
Luzardo-Ocampo, I., Ramírez-Jiménez, A. K., Yañez, J., Mojica, L. & Luna-Vital, D. A. Technological applications of natural colorants in food systems: A review. Foods 10, 634 (2021).
Mpountoukas, P. et al. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 48, 2934–2944 (2010).
Singh, R. P., Singh, P. K. & Singh, R. L. Bacterial decolorization of textile azo dye acid orange by Staphylococcus hominis RMLRT03. Toxicol. Int. 21, 160 (2014).
Chen, X. C. et al. Azo-hydrazone tautomerism observed from UV-vis spectra by pH control and metal-ion complexation for two heterocyclic disperse yellow dyes. Dalton Trans. 41, 11107–11115 (2012).
Zakerhamidi, M. S., Ahmadian, S. S. & Kian, R. The specific and nonspecific solvatochromic behavior of Sudan dyes in different solvents. Can. J. Chem. 93, 639–647 (2015).
Zakerhamidi, M. S., Sorkhabi, S. G. & Shamkhali, A. N. Polar and low polar solvents media effect on dipole moments of some diazo Sudan dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 127, 340–348 (2014).
Zheng, D. et al. Hydrogen bonding promoted tautomerism between Azo and Hydrazone forms in Calcon with multistimuli responsiveness and biocompatibility. J. Chem. Inf. Model. 59, 2110–2122 (2019).
Katritzky, A. R., Hall, C. D., El-Gendy, B. E. D. M. & Draghici, B. Tautomerism in drug discovery. J. Comput. Aided Mol. Des. 24, 475–484 (2010).
Amchova, P., Siska, F. & Ruda-Kucerova, J. Food safety and health concerns of synthetic food colors: an update. Toxics 12, 466 (2024).
Sinha, R., Mukherjee, A. & Chatterjee, S. Influence of solvent polarity on the azo-hydrazone tautomerism of sunset yellow. J. Mol. Liq. 208, 286–291 (2015).
Zhao, H., Wang, X. & Song, X. Spectroscopic and theoretical studies on the azo-hydrazone tautomerism of Tartrazine in aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 189, 342–349 (2018).
Almeida, M. R., Stephani, R., Dos Santos, H. F. & Oliveira, L. F. C. D. Spectroscopic and theoretical study of the “Azo”-Dye E124 in condensate phase: Evidence of a dominant hydrazo form. J. Phys. Chem. A 114, 526–534 (2009).
Saleh, T. S. & El-Shishtawy, R. M. Spectroscopic and computational studies of tautomerism in azo dyes: The case of Ponceau 4R. Dyes Pigm. 124, 276–284 (2016).
EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific opinion on the re-evaluation of Ponceau 4R (E124) as a food additive. EFSA J. 7(11), 1328 (2009).
Moutinho, I. L. D., Bertges, L. C. & Assis, R. V. Prolonged use of the food dye Ponceau 4R (E124) provokes oxidative stress in the rat kidney. Food Chem. Toxicol. 45, 876–880 (2007).
Mpountoukas, P. et al. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 48, 2934–2944 (2010).
Berktay, E., Çelik, İ & Çelik, S. In ovo given sunset yellow adversely affects embryonic development of chick thymus and bursa Fabricii as evidenced by histological and enzyme histochemical findings. Beni Suef Univ. J. Basic Appl. Sci. 12, 62 (2023).
Şensoy, E. Comparison of the effect of Sunset yellow on the stomach and small intestine of developmental period of mice. Heliyon. 10, e31998 (2024).
Poul, M., Jarry, G., Elhkim, M. O. & Poul, J. M. Lack of genotoxic effect of food dyes amaranth, sunset yellow and tartrazine in the gut micronucleus assay in mice. Food Chem. Toxicol. 47, 443–448 (2009).
Akin, F. E., Akin, M. & Koruk, M. Food additives and hyperactivity in children: Sunset Yellow and behavioral alterations. Int. J. Pediatr. Res. 41, 127–135 (2014).
Bhatt, D., Vyas, K., Singh, S., John, P. J. & Soni, I. Tartrazine induced neurobiochemical alterations in rat brain sub-regions. Food Chem. Toxicol. 113, 322–327 (2018).
Sharma, A. & Sharma, S. Neurobehavioral effects of Tartrazine in mice: Role of oxidative stress. Toxicol. Mech. Methods 22, 520–525 (2012).
Lau, K., McLean, W. G., Williams, D. P. & Howard, C. V. Synergistic interactions between commonly used food additives in a neurotoxicity model. Toxicol. Sci. 90, 178–187 (2006).
Bharatam, P. V., Valanju, O. R., Wani, A. A. & Dhaked, D. K. Importance of tautomerism in drugs. Drug Dis. Today 28, 103494 (2023).
Moayedniya, A., Gilani, A. G., Zakerhamidi, M. S., Dezhampanah, H., & Kian, R. Investigation of the effective interactions of two bioactive compounds in different media. J. Mol. Struct. 1274, 134534 (2023).
Sahrai, H., Kian, R., Shamkhali, A. N., Kheradmand, R. & Zakerhamidi, M. S. Evaluation of solvent effect on the effective interactions of Isotretinoin and Tretinoin: Isomeric forms of vitamin A. Heliyon 10, e25174 (2024).
Reichardt, C. & Welton, T. Solvents and Solvent Effects in Organic Chemistry (John Wiley & Sons, 2011).
Filarowski, A., Koll, A. & Sobczyk, L. Intramolecular hydrogen bonding in o-hydroxy aryl Schiff bases. Curr. Org. Chem. 13, 172–193 (2009).
Hansen, P. E. Isotope effects on chemical shifts in the study of intramolecular hydrogen bonds. Molecules 20, 1205–2424 (2015).
Dziembowska, T., Majewski, E., Rozwadowski, Z. & Brzezinski, B. Intramolecular hydrogen bonds in N-oxides of Schiff bases. J. Mol. Struct. 403, 183–187 (1997).
Jeffrey, G. A. & Saenger, W. Hydrogen Bonding in Biological Structures (Springer Science & Business Media, 2012).
Landrein, B. & Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: history, experiments and revisited theories. Plant J. 75, 324–338 (2013).
Jarvis, M. C. & McCann, M. C. Macromolecular biophysics of the plant cell wall: concepts and methodology. Plant Physiol. Biochem. 38, 1–13 (2000).
Voxeur, A. & Höfte, H. Cell wall integrity signaling in plants: “To grow or not to grow that’s the question. Glycobiol. 26, 950–960 (2016).
Kian, R., Zakerhamidi, M. S., Kandjani, S. A. & Dadashzadeh, M. The effective interactions of Fuchsine and Pararosaniline as two bioactive compounds in different solvent media: a comparative study. J. Mol. Struct. 1264, 133285 (2022).
Ranjkesh, A. et al. Determination of polarity parameters for liquid crystals using solvatochromic method in anisotropic and isotropic phases. Liq. Cryst. 44, 695–704 (2017).
Ranjkesh, A., Kiani, S., Parast, M. H., Zakerhamidi, M. S. & Yoon, T. H. Characterization of short-range molecular interaction by empirical solvent polarity scale to analyze the Kerr effect of nematic liquid crystals in the isotropic phase. J. Mol. Liq. 295, 111653 (2019).
Ranjkesh, A., Parast, M. H., Strzeżysz, O., Zakerhamidi, M. S. & Yoon, T. H. New linear solvation energy relationships for empirical solvent scales using the Kamlet–Abboud–Taft parameter sets in nematic liquid crystals. RSC Adv. 8, 22835–22845 (2018).
Alzard, R. H. et al. Solubilization of pyridone-based fluorescent tag by complexation in cucurbit [7] uril. ACS Omega 4, 953–960 (2019).
Kabatc, J., Ośmiałowski, B. & Pączkowski, J. The experimental studies on the determination of the ground and excited state dipole moments of some hemicyanine dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 63, 524–531 (2006).
Bakhshiev, N. G. Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in 2-component solutions. 7. Theory (general case for isotopic solution). Opt. Spektrosk. 16, 821–832 (1964).
Pizzorno, J. The kidney dys mic, part 1: causes. Integr. Med. Clin. J. 14.6, 8 (2015).
EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific opinion on the re-evaluation Tartrazine (E 102). EFSA J. 7(11), 1331 (2009).
EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific opinion on the re-evaluation of sunset yellow FCF (E 110) as a food additive. EFSA J. 7(11), 1330 (2009).
Abboud, J. L., Kamlet, M. J. & Taft, R. W. Regarding a generalized scale of solvent polarities. J. Am. Chem. Soc. 99, 8325–8327 (1977).
Kamlet, M. J. & Taft, R. W. The solvatochromic comparison method. I. The beta-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 98, 377–383 (1976).
Kamlet, M. J. & Taft, R. W. Linear solvation energy relationships Part 3 Some reinterpretations of solvent effects based on correlations with solvent π* and α values. J. Chem. Soc. Perkin Trans. 2, 349–356 (1979).
Author information
Authors and Affiliations
Contributions
Bita Taheri studied, analyzed and evaluated the data, and wrote the manuscript. Mohammad Sadegh Zakerhamidi studied, analyzed and evaluated the data, and wrote and edited the manuscript. Amir Nasser Shamkhali analyzed and evaluated theoretical section Roshanak Kian studied, analyzed and evaluated the data, and wrote and edited the manuscript. Amir Amiri-Sadeghan analyzed and evaluated MTT data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Taheri, B., Zakerhamidi, M.S., Shamkhali, A.N. et al. Solvent polarity effect on photophysical properties of some aromatic azo dyes with focus on tautomeric and toxicity competition. Sci Rep 15, 15716 (2025). https://doi.org/10.1038/s41598-025-00001-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00001-w