Abstract
In recent decades, the impact of air pollution on eye health has been emphasized, but the effect of air pollution on changes in refractive error is still unknown. A comprehensive retrospective cohort study was conducted to address this, utilizing the China Medical University Hospital Clinical Research Data Repository (CMUH-CRDR). It included 4,399 participants aged 6–12 years, with 2,166 females and 2,223 males, who visited the ophthalmology department between 2003 and 2019, ensuring a robust and representative sample. The mean age was 7.04 years (± 2.20), and the change in refractive error was highest in subjects aged between 7 and 9. Cumulative exposure to delicate particulate matter (PM2.5), carbon monoxide (CO), nitrogen oxides (NOx), ozone (O3), and sulfur dioxide (SO2) from the index date to the end of the follow-up was calculated to examine the association between air pollutants and myopia progression. The study found a clear dose-effect relationship, as the change in refractive error progressively increased with higher levels of PM2.5, CO, and O3, with exposure to the highest levels resulting in a considerably significant change (p < 0.05). For every quantile increase in PM2.5, CO, NOx, O3, and SO2, the average change in refractive error decreased by approximately 0.3 D. Among the five air pollutants analyzed, CO had the most significant effect with just one unit increase. PM2.5 had the most significant impact on refractive error in patients among different age groups (age 5–6, β: − 0.40; age 7–9, β: − 0.47; age 10–12, β: − 0.35). In conclusion, exposure to air pollutants, including PM2.5, CO, SO2, NOx, and O3, increases the risk of myopic progression in children aged between 6 and 12 years old, emphasizing the need for better air quality control measures to protect children’s eye health and prevent myopia progression.
Similar content being viewed by others
Introduction
Myopia is widely considered a worldwide public health issue due to its association with untreatable visual impairments and is increasing in prevalence. These complications, including cataracts, myopic maculopathy, and retinal detachment, significantly burden healthcare systems1,2. By 2050, 50% of the people are expected to suffer from myopia. This will correspond with a two-fold increase in the prevalence of myopia (from 22% in 2000) and will affect nearly 5 billion people worldwide3.
Notably, the prevalenceis especially high in Asian countries. Taiwan is particularly concerned because it has a significantly higher prevalence and incidence than the global average (Tsai et al., 2021; Wan et al., 2018; Wu et al., 2020). One nationwide survey demonstrated that the prevalence of myopia in 6-year-old students increased fivefold from 1983 to 2017. In students around 12, the prevalence more than doubled from 30.66–76.67%4.
However, the causes underlying the widespread occurrence of myopia remain unclear. Current evidence suggests that myopia is a complex trait in which environmental risk factors, lifestyle, and genetic variation participate in its development5,6. Identifying independent modifiable risk factors is crucial for effective myopic prevention, especially environmental factors amenable to policy changes.
The impact of air pollution on public health has been emphasized in recent decades7. Air pollution comprises a complex mixture including pollutant gaseous components such as carbon monoxide (CO), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and particulate matter (PM) with different aerodynamic diameters. PM2.5refers to fine PM with an aerodynamic diameter of 2.5 μm or less and is widely recognized as a significant contributor to health problems associated with PM exposure8.
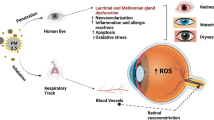
Since eyes make direct contact with the atmosphere, they are particularly vulnerable to the effects of air pollution. Through direct contact, air pollution particles can irritate foreign body sensations and cause allergic reactions, eventually resulting in dry and inflamed eyes9. Furthermore, air pollution may impose oxidative stress, endothelial dysfunction, and immune-related inflammatory reactions on human organs10,11. Epidemiological evidence links these pollutions to myopia development12,13,14,15. However, current evidence linking air pollution with refractive error is scarce, either from databases, interviews, or self-reported questionnaires of spectacle users12,13,14.
To further evaluate the potential causal role of air pollutants (PM2.5, CO, SO2, O3, and NOx) in myopia, we conducted a longitudinal cohort study in children aged 6–12 in the China Medical University Hospital (CMUH). We aimed to stratify the risk of each air pollutant and quantify the impact on the progression of myopic refraction.
Methods
Health data
The CMUH’s Big Data Centre established the CMUH Clinical Research Data Repository (CRDR) in 2017. This repository integrates and validates electronic health records, providing consolidated patient information such as demographics, diagnosis, prescriptions, medical and surgical procedures, hospitalization, physiological monitoring, and a registry for catastrophic illness patients. Additionally, the CMUH-CRDR contains the national death registry for 2,988,912 patients as of the end of 201916. All individuals enrolled in the CMUH-CRDR were followed up until December 31, 2019, or until their death if it occurred before this date. Approval for this study was obtained from the Big Data Center and the Research Ethics Committee/Institutional Review Board of CMUH (CMUH109-REC2-185). All methods were carried out according to relevant guidelines and regulations, and informed consent was obtained from all subjects.
Study population
Our study population comprised individuals aged 6–12 years who had visited the ophthalmology department at CMUH between 2003 and 2019. Individuals with a refractive error between + 1 D and − 2D were included. We excluded individuals who had been diagnosed with retinopathy, prematurity, stickler syndrome, or Marfan syndrome. Individuals without records at 18 years of age were also eliminated. Diagnoses were recorded using the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM; ICD-10-CM). Supplementary Fig. 1 demonstrates the cohort selection process in detail.
Air quality data
The Environmental Protection Administration Executive Yuen’s website provided air pollution statistics (Taiwan). A geographic information system (GIS) was utilized to identify and manage the locations of monitoring stations and air pollution sources (ArcGIS version 10.8.2; ESRI, Redlands, CA, USA). The monitoring data were combined with annual point data and extrapolated to pollutant surfaces using the inverse distance weighting technique (IDW)17. For the IDW method, a suitable spatial resolution (100 m) and an inverse squared distance (1/five squared distance) weighted average of the five closest surveillance stations were utilized to calculate pollutant concentration from ambient air quality surveillance stations across Taiwan. From 2003 to 2019, data from 73 surveillance stations were utilized to estimate the levels of air pollutants in the residences of study participants. The cumulative exposure was calculated using standard methods and the data they generated and obtain estimated time-series data. The process described (using IDW interpolation based on monitoring station data) would result in estimated pollutant concentrations for each participant’s residence, which represents the sophisticated step of calculating the pollution level at specific locations where no direct monitor exists, using data from nearby monitors (via IDW). To get the cumulative exposure, the researchers would take these estimated values, representing concentrations over specific time units like days or years, and add them together over each participant’s total period of interest. Our models demonstrated good performance, with cross-validated R2 values of 0.91 for PM2.5, 0.51 for CO, 0.76 for NOx, 0.45 for O3, and 0.91 for SO2.
Covariables and comorbidities
The primary focus of the study was the relationship between the change in refractive error and cumulative exposure to air pollutants. Comorbidities were diseases linked to myopia progression, such as systemic lupus erythematosus, asthma, atopic dermatitis, allergic conjunctivitis, allergic rhinitis, and Kawasaki disease. In our study, using atropine and orthokeratology served as dependent variables. According to Liu et al. (2006), urbanization level can be categorized based on several factors, including population density (people/km2), the educational level of the population, the proportion of older people aged 65 years and older, and the ratio of agriculture workers to physicians per 100,000 people18. To guarantee an adequate sample size for each subgroup, we combined levels 3 and 4 for our study. As a result, areas classified as level 1 are considered urban, level 2 as suburban, and levels 3 and 4 form the rural group collectively.
Statistical analysis
The mean and standard deviation of the left and right refractive errors on the index date and at the end of the follow-up period were compiled. The outdoor air pollutant data were collected from the seventy-four ambient air quality surveillance stations in the Ministry of Environment of Taiwan. The mean of the change in refractive error, representing the trend of refractive error changes among various quantile levels of air pollutants, was also generated from computer-aid estimation. Pearson’s correlation between air pollutants and refractive error was plotted. A linear regression model was used to examine the relationship between air pollutants, which were represented as the annual concentration of air pollutants and the evolution of refractive error to obtain the trend’s p and β coefficients. Age, sex, systemic lupus erythematosus, asthma, atopic dermatitis, allergic conjunctivitis, allergic rhinitis, Kawasaki disease, atropine, Orthokeratology Contact Lens (Ortho-K lens), and urbanization were considered. Refractive error decline is defined as progressive myopia using the estimation of computer-aided instruments. The odds ratio of progressive myopia was estimated using the logistic regression model. The subgroup analysis included stratification by age and sex. All analyses were conducted using version 4.2 of R. The p-value was deemed statistically significant when it was less than 0.05.
Results
There is limited evidence linking air pollution to the progression of myopia, with most studies focusing on the risk of myopia. In a previous retrospective cohort study of 6- to 12-year-old children, we found that exposure to PM2.5 increased the incidence rate of myopia from 15.8 to 24.5 per 1,000 person-years, and exposure to NOx increased it from 13.7 to 34.4. The adjusted hazard ratios for elevated PM2.5and NOx exposure concentrations in quartile 4 were 1.57 and 2.60, respectively12. A cross-sectional analysis by Dadvand et al. in school children showed that exposure to NO2 and PM2.5was associated with odds ratios for spectacle use of 1.16 (95% CI:1.03, 1.29) and 1.13 (95% CI:0.99, 1.28), respectively13. In a cross-sectional survey of adults aged over 50 years by Ruan et al., a J-shaped association was found between PM2.5 and O3with myopia, with adjusted prevalence ratios of 1.12 (95% CI:1.05, 1.21) and 1.26 (95% CI:1.14, 1.38) for each standard deviation increase in concentrations, respectively14. A recent cross-sectional study of 6 to 18-year-old children by Yang et al. reported that interquartile range increases in PM2.5 and NO2were associated with 1.267- and 1.276-fold increased odds of visual impairment, respectively. However, no specific etiologies were further analyzed15.
We conducted a longitudinal cohort study to investigate the impact of air pollution on myopia progression. All the patients were from a single hospital in Taichung, Taiwan. As shown in Fig. 1, most patients originated from central Taiwan. Table 1 displays the baseline clinical characteristics of 4,399 participants. Of these, 2,116 (48.77%) were female and 2,223 (51.23%) were male. Most study patients were between 5 and 6 years old, with a mean age of 7.04 years (± 2.20). Allergic rhinitis (17.52%), atopic dermatitis (14.96%), and asthma (11.75%) were the three most prevalent comorbidities. Atropine was used by 1,818 (41.90%) patients, with an average dosage of 16.4 ± 18.9 bottles per year. Only 204 (4.07%) patients used orthokeratology; most users were 7–9 years old. The age group of 7–9 years showed the most significant change in refractive error. Atropine use was associated with the most significant change in refractive error (right: −1.66 ± 1.83 D; left: −1.55 ± 2.03 D) from the index date to the end of the follow-up period. Most patients resided in suburban (48.19%) and urban (43.91%) areas.
Table 2 displays the daily mean concentration of air pollutants and the total amount of air pollutants exposed throughout the study. The daily average concentrations of PM2.5, CO, NOx, O3, and SO2 were 24.47 ± 3.08 µg/m3, 0.26 ± 0.14 ppm, 10.78 ± 5.73 ppb, 29.82 ± 17.74 ppb, and 1.93 ± 1.04 ppb, respectively.
The association between cumulative air pollutant exposure and changes in refractive error was analyzed, and the results are presented in Table 3. The change in refractive index increased proportionally with increasing PM2.5, CO, and O3 concentrations. The most significant change in refractive error was associated with cumulative exposure to the highest levels of PM2.5 (P < 0.001), CO (P < 0.05), and O3 (P < 0.005). Although the P for trend was not statistically significant for NOx and SO2, there is a general trend of decreasing refractive error with increasing levels of air pollutants. Air pollutant exposure had the most significant impact on changes in refractive error in children aged between 5 and 6 years old. Pearson’s correlation was used to assess further the relationship between cumulative air pollutant exposure and changes in refractive error, as shown in Fig. 2. The plots demonstrated that higher cumulative air pollutant exposure was associated with more significant changes in refractive error.
Scatter plots depict the relationship between cumulative exposure to each air pollutant and the change in refractive error of either the left or right eye. The blue line in each plot represents the linear trend fit (Pearson’s correlation coefficient, R), the x-axis shows each air pollutant’s cumulative exposure, and the y-axis represents the change in refractive error for the left or right eye. (a) PM2.5, (b) CO, (c) NOx, (d) O3, and (e) SO2.
Table 4 displays the results of the linear regression analysis, which revealed that all air pollutants had a negative coefficient for the change in refractive error. After adjusting for age, sex, comorbidities, and the use of atropine, orthokeratology and urbanization, every quantile increase in PM2.5, CO, NOx, O3, and SO2 was associated with an average decrease in refractive error of around 0.30 D. When analyzing the change in refractive error separately for the right and left eyes, the coefficient for the suitable eye regression model was approximately − 2.45 to − 3.55, while that for the left eye regression model was around − 2.38 to − 3.23.
Table 5. demonstrates that a one-unit increase in CO had the most significant impact on refractive error among the five air pollutants in all sub-age group analyses. On the other hand, O3 had the most minor effect on refractive error among the air pollutants. In terms of quantile changes in air pollutants, PM2.5 had the most significant impact on refractive error in patients among different age groups (age 5–6, β: − 0.40; age 7–9, β: − 0.47; age 10–12, β: − 0.35). CO was the most influential air pollutant (with a one-unit change) on refractive error across all age groups, regardless of sex (Table 6). One quantile change in PM2.5 significantly reduced refractive error in all age groups (Table 6). Table seven revealed that regardless of the level of urbanization, CO was the most impactful air pollutant (with one-unit change) on refractive error. One quantile of change of PM2.5 significantly reduced the refractive error across all urbanization levels (Table 7).
Discussion
Based on the findings above, there was a significant negative correlation between cumulative exposure to air pollutants and changes in refractive errors. The five air pollutants (PM2.5, CO, SO2, NOx, and O3) had variable toxicity levels. In addition to PM2.5 and NOx, which have received more public attention, we suggest that CO, SO2, and O3should be given equal consideration for protecting children’s health. However, it’s worth noting that exposure levels to air pollutants vary across different studies. Moreover, the incidence and prevalence of myopia in Taiwan are significantly higher than in Western countries, as reported in previous studies4,19. Therefore, our conclusions should be interpreted cautiously when extrapolating to regions with different pollution levels or populations with varying degrees of susceptibility.
Patients whose refractive error did not change or increase were assigned to the control group to determine further the effect of air pollutant exposure on myopia progression. In contrast, those whose refractive error decreased were assigned to the disease group. We used these two groups to determine the progression risk of myopia related to exposure to air pollution. As shown in Table 8, a one-unit increase in air pollutants increases the risk of myopia by 1.01 to 1.15 times, whereas a one-quantile change in air pollutants increases the risk by 1.22 to 2.80 times. The results further demonstrate the association between air pollution and myopia progression.
The current study has multiple strengths. Firstly, all participants were recruited retrospectively from our outpatient clinic, ensuring precise quantification of refraction changes. Secondly, we verified patients’ residential addresses through outpatient registration and CMUH-CRDR follow-up visits, and most patients did not change residential locations during the study period, thus ensuring accurate air pollution exposure levels for each participant. Thirdly, in contrast to previous studies that focused on the general population, we included children aged 6–12 years old with refraction between + 1 D and − 2 D. Lastly, as the degree of urbanization is known to be associated with the incidence of myopia, our findings suggest that urbanization influences the progression of myopia independently of exposure to air pollutants.
The current study is not without limitations. Firstly, the registered addresses of the children in the database may not entirely represent where they spent most of their time. Some children may have gone to school in another area or had different outdoor activity levels, which could result in heterogeneous exposure levels between individuals. Despite adjusting for various potential confounders at the individual level, other pollutants or regional characteristics may still play a role. Indoor air quality, such as tobacco exposure or the use of air purifiers, was not considered in this study. Secondly, the absence of socioeconomic factors such as near-work activities and the education and income levels of the patients and their parents are known to play significant roles in the incidence of myopia. These socioeconomic factors may mitigate the impact of air pollution on the progression of myopia. As myopia is a multifaceted condition influenced by environmental risk factors, lifestyle, and genetic variations, it is challenging to account for all confounding factors. Lastly, the retrospective design of this study makes it difficult to establish a causal relationship between air pollution and myopia. A large-scale prospective cohort study must confirm the association between air pollutants and myopia progression.
The biological mechanisms responsible for the association between air pollution and myopia progression are not fully understood. However, several studies have proposed potential pathophysiological mechanisms, such as the dopamine (DA) pathway, allergic inflammation, reactive oxygen species (ROS), free radical-related tissue hypoxia, and direct tissue remodeling. In addition, William et al. suggested that cumulative ultraviolet (UV) exposure could influence the presence of myopia by affecting the DA pathway20. There is similar evidence from studies conducted on guinea pigs with form-deprived or lens-induced myopia, where scleral cross-linking with riboflavin/ultraviolet A (UVA) slowed the progression of myopia21,22. The physical properties of PM2.5have been associated with the obstruction of UV exposure from reaching the Earth’s surface23. Thus, we hypothesize that air pollution may obstruct the presence of UVA, leading to a downregulation of the DA pathway and ultimately causing myopia progression. This conjecture could be substantiated by studying the correlation between outdoor activity and a slower rate of myopia progression19.
Clinical studies have shown a significant association between air pollution and allergic inflammation. The frequency of allergic conjunctivitis (AC) increased with the concentration of PM2.5, O3, NOx, and CO9,12,24. Our previous study found a correlation between allergic inflammation and the risk of developing myopia in rats with allergic conjunctivitis. The expression levels of c-Fos, nuclear factor κB (NF-κB), interleukin (IL)−6, and tumor necrosis factor α (TNF-α) were found to be the potential causative factors25. Furthermore, in the microenvironment characterized by high levels of inflammatory mediators in AC, there was an increase in axial length elongation and corneal curvature25,26. A cohort study indicated that children with AC had a higher incidence and subsequent risk of myopia (hazard ratio 2.35, 95% CI 2.29–2.40) than those without AC5. This hypothesis proposes a link between the increasing incidence of AC in the presence of air pollution and provides further evidence at the molecular level. AC causes mast cell recruitment and degranulation, releasing TNF-α and IL-6, impairing corneal epithelial cells’ tight junctions. As a result, inflammatory cytokines migrate across the cornea, stimulating retinal pigment epithelial cells to secrete TNF-α, IL-6, and NF-κB. The activation of NF-κB increases the levels of matrix metalloproteinase (MMP)−2 and transforming growth factor β (TGF-β), both of which are associated with the progression of myopia12,25,26. The downregulation of the DA pathway may also promote myopia by influencing the inflammatory response in the eye. It has been demonstrated that the dopamine-D1R signaling pathway modulates myopia development by decreasing the activation of the nucleotide-binding oligomerization domain (NOD), leucine-rich repeat (LRR), and pyrin domain-containing protein 3 (NLR) family pyrin domain-containing protein 3 (NLRP3) inflammasome27.
Another possible mechanism is tissue hypoxia. Scleral ischemia and hypoxia may be due to decreased choroidal blood flow, resulting in the elongation of the sclera, which then contributes to the presence and progression of myopia28. In addition, the hypoxic microenvironment itself might upregulate tissue hypoxia markers and reduce the cell density of the retinal pigment epithelium in the retro-equatorial region, causing axial elongation28,29. Air pollutants, including PM2.5, NOx, and O3, have been negatively correlated with the diameter of the central retinal artery in both adults and school-aged children in multiple studies30,31,32,33,34. MicroRNA regulation due to air pollution-induced oxidative stress and inflammatory processes may contribute to arteriolar narrowing33,35. ROS and free radicals produced under the stimulation of PM2.5and NOx may also play a role in the hypoxic environment12,35,36. These ROS and free radicals can uncouple endothelial nitric oxide synthase and downregulate nitric oxide (NO), a known vasodilator. The decrease in NO results in endothelial dysfunction and vasoconstriction, which subsequently diminishes retinal blood flow and impairs retinal neuroactivity31,34,37. Finally, direct scleral tissue remodeling, including loss of collagen fiber I and activation of MMP-2, a scleral extracellular matrix degradation enzyme, can cause scleral thinning38. The thinning and weakening sclera are prone to elongation, resulting in myopia progression. This study found a significant association between cumulative exposure to all five air pollutants (PM2.5, CO, SO2, NOx, and O3) and myopia progression. This association showed a positive dose-effect relationship and remained significant even after adjusting for confounding factors. Myopic control methods, such as atropine and orthokeratology, did not appear to mitigate the effect. Males were found to be more susceptible than females, and the age group of 7–9 years old showed the most significant progression of myopia (0.2 D to 0.4 D) compared to the age groups of 5–6 or 10–12 years old. Although PM2.5 appeared to cause slightly more myopia than the other pollutants, this difference was not statistically significant. These findings suggest an independent association between air pollutants and myopia progression and highlight the need for myopia prevention efforts. We recommend that the public be made aware of the harmful effects of these five pollutants on children’s health and that policies should be implemented to regulate and minimize these modifiable exposures for effective myopic prevention and control.
Conclusion
The present study demonstrates a significant and independent association between cumulative exposure to five common air pollutants—PM2.5, CO, SO2, NOx, and O3—and myopia progression in children aged 6–12. The observed positive dose-effect relationship, consistent even after adjusting for confounders and not mitigated by standard myopic control methods, underscores the concerning impact of air pollution on children’s visual health. While PM2.5 has received considerable attention, our findings emphasize the equal importance of CO, SO2, and O3 as contributors to myopia progression. Furthermore, the study highlights a heightened susceptibility in males and children aged 7–9. Although limited by its retrospective design, which precludes definitive causal conclusions, this research strongly suggests that air pollution is a modifiable environmental risk factor for myopia progression. These findings call for increased public awareness regarding the detrimental effects of these pollutants on children’s health and advocate for implementing policies aimed at regulating and minimizing air pollution exposure. The present study mentions that future approaches using Land Use Regression (LUR) or atmospheric dispersion models would be accompanied to further validate these findings by providing a more detailed exposure assessment. Future prospective cohort studies are essential to confirm these associations and further elucidate the underlying biological mechanisms. These studies will ultimately inform effective strategies for myopia prevention and control in the face of growing urbanization and air pollution. For example, the internal and external exposure dosage should be clearly explained, and the causal relationship between exposure and disease risk should be clarified.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to the data exploration and administrative support of the iHi Clinical Research Platform, which belongs to the Big Data Center of China Medical University Hospital, Taiwan, but they are available from the corresponding author upon reasonable request.
References
Morgan, I. G. et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 62, 134–149 (2018).
Verkicharla, P. K., Ohno-Matsui, K. & Saw, S. M. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol. Opt. 35 (5), 465–475 (2015).
Holden, B. A. et al. Global prevalence of myopia and high myopia and Temporal trends from 2000 through 2050. Ophthalmology 123 (5), 1036–1042 (2016).
Tsai, T. H. et al. Evolution of the prevalence of myopia among Taiwanese schoolchildren: A review of survey data from 1983 through 2017. Ophthalmology 128 (2), 290–301 (2021).
He, M. et al. Effect of time spent outdoors at school on the development of myopia among children in China: A randomized clinical trial. Jama 314 (11), 1142–1148 (2015).
Tedja, M. S. et al. IMI - Myopia genetics report. Invest. Ophthalmol. Vis. Sci. 60 (3), M89–m105 (2019).
Cohen, A. J. et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet 389 (10082), 1907–1918 (2017).
Rajagopalan, S., Al-Kindi, S. G. & Brook, R. D. Air pollution and cardiovascular disease: JACC State-of-the-Art review. J. Am. Coll. Cardiol. 72 (17), 2054–2070 (2018).
Miyazaki, D. et al. Epidemiological aspects of allergic conjunctivitis. Allergol. Int. 69 (4), 487–495 (2020).
Hahad, O. et al. Ambient air pollution increases the risk of cerebrovascular and neuropsychiatric disorders through induction of inflammation and oxidative stress. Int. J. Mol. Sci., 21(12). (2020).
Lin, Y. T. et al. Particulate air pollution and progression to kidney failure with replacement therapy: an advanced CKD Registry-Based cohort study in Taiwan. Am. J. Kidney Dis. 76 (5), 645–657e1 (2020).
Wei, C. C. et al. PM2.5 and NOx exposure promote myopia: clinical evidence and experimental proof. Environ. Pollut. 254(Pt B), p113031 (2019).
Dadvand, P. et al. Traffic-related air pollution and spectacles use in schoolchildren. PLoS One. 12 (4), e0167046 (2017).
Ruan, Z. et al. Ambient fine particulate matter and Ozone higher than certain thresholds associated with myopia in the elderly aged 50 years and above. Environ. Res. 177, 108581 (2019).
Yang, B. Y. et al. Exposure to ambient air pollution and visual impairment in children: A nationwide cross-sectional study in China. J. Hazard. Mater. 407, 124750 (2021).
Chiang, H. Y. et al. Electronic medical record-based deep data cleaning and phenotyping improve the diagnostic validity and mortality assessment of infective endocarditis: medical big data initiative of CMUH. Biomed. (Taipei). 11 (3), 59–67 (2021).
Jung, C. R., Lin, Y. T. & Hwang, B. F. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 8 (9), e75510 (2013).
Liu, C. Y. et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 4 (1), 1–22 (2006).
Wu, P. C. et al. Increased time outdoors is followed by reversal of the Long-Term trend to reduced visual acuity in Taiwan primary school students. Ophthalmology 127 (11), 1462–1469 (2020).
Williams, K. M. et al. Association between myopia, ultraviolet B radiation exposure, serum vitamin D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a multicountry European study. JAMA Ophthalmol. 135 (1), 47–53 (2017).
Han, D. et al. Protective effects of riboflavin-UVA-mediated posterior sclera collagen cross-linking in a guinea pig model of form-deprived myopia. Int. J. Ophthalmol. 14 (3), 333–340 (2021).
Lai, L. et al. Comparing the differences in slowing myopia progression by riboflavin/ultraviolet A scleral Cross-linking before and after Lens-induced myopia in Guinea pigs. Curr. Eye Res. 47 (4), 531–539 (2022).
Riva, M. et al. High pressure inside Nanometer-Sized particles influences the rate and products of chemical reactions. Environ. Sci. Technol. 55 (12), 7786–7793 (2021).
Hong, J. et al. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 6, 23858 (2016).
Lin, H. J. et al. Role of chronic inflammation in myopia progression: clinical evidence and experimental validation. EBioMedicine 10, 269–281 (2016).
Wei, C. C. et al. Allergic Conjunctivitis-induced retinal inflammation promotes myopia progression. EBioMedicine 28, 274–286 (2018).
Chen, C. S. et al. Acupuncture modulates development of myopia by reducing NLRP3 inflammasome activation via the dopamine-D1R signaling pathway. Acupunct Med, : p. 9645284231170886. (2023).
Liu, Y. et al. The influence of the choroid on the onset and development of myopia: from perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 99 (7), 730–738 (2021).
Wu, P. C. et al. Update in myopia and treatment strategy of Atropine use in myopia control. Eye (Lond). 33 (1), 3–13 (2019).
Adar, S. D. et al. Air pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA). PLoS Med. 7 (11), e1000372 (2010).
Louwies, T. et al. Retinal microvascular responses to short-term changes in particulate air pollution in healthy adults. Environ. Health Perspect. 121 (9), 1011–1016 (2013).
Louwies, T. et al. Blood pressure changes in association with black carbon exposure in a panel of healthy adults are independent of retinal microcirculation. Environ. Int. 75, 81–86 (2015).
Louwies, T. et al. MiRNA expression profiles and retinal blood vessel calibers are associated with short-term particulate matter air pollution exposure. Environ. Res. 147, 24–31 (2016).
Korsiak, J. et al. Air pollution and retinal vessel diameter and blood pressure in school-aged children in a region impacted by residential biomass burning. Sci. Rep. 11 (1), 12790 (2021).
Yuan, T. & Zou, H. Effects of air pollution on myopia: an update on clinical evidence and biological mechanisms. Environ. Sci. Pollut Res. Int. 29 (47), 70674–70685 (2022).
Lasagni Vitar, R. M. et al. Urban air pollution induces redox imbalance and epithelium hyperplasia in mice cornea. Toxicol. Appl. Pharmacol. 384, 114770 (2019).
Ikeda, S. I. et al. Oral bovine milk lactoferrin administration suppressed myopia development through matrix metalloproteinase 2 in a mouse model. Nutrients, 12(12). (2020).
Wan, L. et al. The synergistic effects of orthokeratology and Atropine in slowing the progression of myopia. J. Clin. Med., 7(9). (2018).
Acknowledgements
We thank the Big Data Center of China Medical University Hospital, Taiwan, for the data exploration and administrative support of the iHi Clinical Research Platform. This study was supported in part by Ministry of Science and Technology, Taiwan, R.O.C. (MOST107-2320-B-039-049-MY3; 111-2314-B-039 -054; 111-2314-B-039 -053 -MY3), China Medical University Hospital, Taichung, Taiwan (DMR-113-121; DMR-114-183) and China Medical University, Taichung, Taiwan (CMU112-MF-49). The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
Y-T Huang: Formal analysis, Writing – Original Draft; P-T Tien: Investigation; J J-Y Chen: Formal analysis; M-Y Wu: Formal analysis; P-P Meng: Conceptualization; H-T Yip: Formal analysis Data Curation; H-J Lin: Conceptualization, Resources, Funding acquisition, Writing – Original Draft; T-Y Yang: Supervision; Writing – original draft; Writing—review & editing; L Wan: Conceptualization, Funding acquisition, Writing—original Draft, Project administration, Writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, YT., Tien, PT., Chen, J.JY. et al. Association between outdoor air pollutant exposure and refractive error changes: A retrospective clinical practice study. Sci Rep 15, 16716 (2025). https://doi.org/10.1038/s41598-025-01006-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01006-1