Abstract
To investigate the effect of recombinant human aFGF (rhaFGF) on acute wounds in a diabetic mouse model focusing on the transition from acute inflammation to chronic inflammation. Diabetes mellitus (DM) mouse models were induced through intraperitoneal injection of streptozotocin and acute diabetic wounds were created on their hind paws. The mice were divided into four groups: Con, Con + rhaFGF, DM, and DM + rhaFGF. rhaFGF (0.08 µg/cm²) or PBS was daily administered on wound surface for 14 days. The levels of IL-6 and TNF-α in serum and tissues were measured using ELISA, and NLRP3 inflammasome components (NLRP3, ASC and caspase-1) and pro-inflammatory cytokines (IL-1β, IL-18) in tissue were detected by Western blot analysis. CCK8 assay and cell migration were used to assess the proliferation and migration ability of HUVEC, HFF, and HaCaT cells, respectively. Wound healing rates in the DM group decreased significantly, which was effectively alleviated by rhaFGF treatment for 7 days and longer durations. Notably, at day 7 after wound creation, the levels of IL-6 and TNF-α as well as the expressions of NLRP3, ASC, caspase-1, IL-1β, and IL-18 in the DM group were significantly increased, and rhaFGF treatment substantially suppressed these changes. Moreover, when HUVEC, HFF, and HaCaT cells were exposed to high glucose and LPS condition, the proliferation and migration of these cells were significantly inhibited, and rhaFGF treatment effectively reversed this inhibition. rhaFGF could promote the healing of acute DM wounds by preventing chronicity transition of acute inflammation via reducing the release of pro-inflammatory cytokines and inhibiting the activation of NLRP3 in DM wounds.
Similar content being viewed by others
Introduction
Globally, diabetes poses a significant health concern. An estimated 537 million adults live with diabetes, with 6.7 million deaths annually attributed to the disease or its complications, accounting for 12.2% of the total deaths1. China faces a particularly high burden, with the number of diabetics rising from 90 million to 140 million in the past decade (2011–2021). Diabetic foot, one of the most common chronic wounds, is the main cause of hospitalization, amputation, and even death of diabetes patients2. About 30% of diabetes patients will have diabetic foot ulcers, which seriously affect the prognosis of diabetes patients and also bring a huge economic burden to the society.
Wound chronicity refers to the failure of normal, orderly and timely healing process to achieve anatomical and functional integrity of the wound, resulting in transitioning from an acute state to a delayed healing or non-healing chronic wound, such as a diabetic foot3. The delayed healing in diabetes involves complex mechanisms, including impaired blood vessel formation (neo-angiogenesis) local ischemia, reperfusion damage, and aging and cellular dysfunction. However, the contribution of inflammatory response to healing of acute diabetic wounds is largely ignored. Inflammation in wound healing acts as a double-edged sword, with a beneficial initial inflammatory response that clears debris and bacteria and attracts repairing cells to the wound area, as well as a harmful persistent and excessive inflammation that acts as the primary culprit for chronic wounds4. Many studies have implicated inflammatory bodies (inflammasomes) as an important cause of various diseases, while it also participates in wound healing process5. These multi-protein complexes play a critical role in innate immunity which activates by cell injury, tissue damage, and infections6. NLRP3 is a protein 3 containing NACHT, LRR and PYD domains, and its role in inflammation has been extensively studied. It uniquely recognizes the damage-associated molecular patterns (DAMPs)7, and during the acute inflammatory phase, NLRP3 is expressed in pro-inflammatory macrophages4, leading to the production of inflammatory mediators, macrophage recruitment, and polarization into pro-inflammatory mediators. NLRP3 has been shown to play a protective role in normal skin wound healing8, while its over-activation may significantly impede diabetic wound healing9.
Fibroblast growth factor (FGF) is a family of signaling molecules with diverse biological effects. It promotes tissue repair by stimulating the growth of blood vessels (vascular endothelial cells), collagen production, and the differentiation of various cell types essential for wound healing10. Among FGF family members, both acidic FGF (aFGF or FGF1) and basic FGF (FGF2) have been used in clinical practice, and their structures, binding receptors and biological functions are similar. Unlike its counterpart bFGF, aFGF retains well its activity and stability in the acidic environment typical of chronic wounds, and thus exhibits advantages for chronic wound treatment11,12,13. Recombinant human aFGF (rhaFGF) is a human protein obtained through genetic recombination technology. It is a low molecular weight, acidic (pH 5.1) single chain polypeptide with the same efficacy as aFGF. Previous studies have shown that rhaFGF can promote the healing of diabetic wounds14,15. It has also been proven that rhaFGF can accelerate the wound healing process and shorten the healing time in acute wounds including deep partial-thickness burns or skin graft donor sites. However, there is little evidence regarding the effect of rhaFGF on the transition from an acute wound to chronic one in diabetes and the precise mechanism remains to be elucidated16.
Building on the current literature, we hypothesize that rhaFGF may promote DM wound healing by inhibiting excessive inflammation and preventing the occurrence of acute wound chronicity.
Results
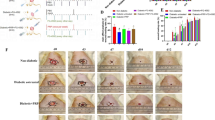
rhaFGF promotes diabetic wound healing
To assess the therapeutic effect of rhaFGF on wound healing, the wound closure rates were compared among the Con, Con + rhaFGF, DM, and DM + rhaFGF groups within 14 days after wound creation. Figure 1 showed the healing process of the wound in the hindlimb paw of the representative mouse in each group. The wound size was quantitated for each mouse at each indicated time point, and the data showed that the initial wound size (day 0) did not differ significantly among the four groups (Fig. 2A). As shown in Fig. 2B, the DM + PBS group exhibited slowest wound healing rate compared to Con, Con + rhaFGF, and DM + rhaFGF groups at all time points (*p < 0.05). No significant differences in wound size were observed among the four groups at day 3 (Fig. 2C), day 5 (Fig. 2D), and day 7 (Fig. 2E) post-wound creation (*p > 0.05). However, starting from day 7, the wound healing rate in the DM + rhaFGF group became significantly higher than the DM + PBS group, and this difference kept increasing over time (day 9, day 11, and day 14; *p < 0.05). Compared to the Con group, the DM + PBS group’s wound sizes were significant larger on day 9 (Fig. 2F), day 11 (Fig. 2G), and day 14 (Fig. 2H) (*p < 0.05). Notably, the Con + rhaFGF group showed no significant difference in wound size compared to Con at any indicated time point (*p > 0.05). These results suggested that prolonged rhaFGF treatment (longer than 7 days) could significantly promote wound healing in diabetic mice, while no significant effect was observed when the treatment duration was less than 7 days.
rhaFGF promotes the proliferation and migration of wound healing-associated cells
We next tested whether rhaFGF improves wound healing through promoting the proliferation in wound healing-associated cells, including vascular endothelial cells and skin cells. Human umbilical vein endothelial cells (HUVEC), human dermal fibroblasts (HFF), and human keratinocytes (HaCat) were cultured and treated with rhaFGF. CCK-8 assays were performed to measure the proliferation of HUVEC (Fig. 3A), HFF (Fig. 3B), and HaCat cells (Fig. 3C) following exposure to rhaFGF at indicated concentrations. rhaFGF treatment increased the proliferation in all three cell types, and HUVEC displayed the strongest response to rhaFGF (Fig. 3A). These findings indicated that rhaFGF promoted the proliferation of all wound healing-associated cells in a dose-dependent manner, and the effects varied among three cell types.
The effects of rhaFGF on the proliferation of HUVEC, HFF and HaCat cells under control and HG + LPS conditions. The cells were incubated with rhaFGF for 48 h and cell proliferation was assessed by CCK-8 assay. A. proliferation following 48 h of incubation with various rhaFGF concentrations in normal culture medium (n = 3/group). rhaFGF treatment increased the proliferation of HUVEC (A), HFF (B) HaCat (C) under control condition in a dose-dependent manner(n = 3/group), The proliferation of HFF (D), HUVEC (E), HaCat (F) were inhibited under diabetic inflammatory conditions mimicked by high glucose and LPS-induced inflammation and rhaFGF treatment (optimal rhaFGF concentration of 20ng/ml,5ng/ml and 50ng/ml respectively) partially reversed this inhibition (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
.
We further investigated the effects of rhaFGF on cell proliferation under simulated diabetic wound conditions (high glucose and lipopolysaccharide-induced inflammation). Compared to the control group, lipopolysaccharide (LPS) + high glucose medium significantly inhibited the proliferation of HaCaT (Fig. 3D), HFF (Fig. 3E), and HUVEC cells (Fig. 3F) (*p < 0.05). Conversely, rhaFGF treatment significantly increased proliferation of all three cell types (*p < 0.05).
To verify the effect of rhaFGF on HFF, HUVEC, and HaCaT cell migration ability, we performed cell scratch tests. The results showed that compared with the LPS + HG group, Hacat cells in the LPS + HG + rhaFGF group with 20ng/ml rhaFGF migrated significantly at 6 h, 12 h, and 24 h after migration (p < 0.05). The migration of HUVEC cells in the LPS + HG + rhaFGF group with 5ng/ml rhaFGF group was significant at 12 h after proliferation, and not significantly at 6–24 h (p < 0.05) compared with the LPS + HG group. And compared with the LPS + HG group, the HFF cells in the 50ng/ml LPS + HG + rhaFGF group migrated significantly at 12 h and 24 h (P < 0.05), but not significantly at 6 h (P > 0.05) (Figs. 4 and 5).
These results suggested that high glucose and inflammatory stimulus impaired HFF, HUVEC, and HaCat cell proliferation and migration, which could be partially reversed by rhaFGF treatment.
rhaFGF reduces Pro-inflammatory factors in diabetic mice
To elucidate the link between impaired healing and inflammation in diabetes, ELISA was used to measure serological and wound tissue levels of pro-inflammatory factors IL-6 and TNF-α in all groups at day 7 after wound creation. Mice in the diabetic group (DM) exhibited significantly elevated serological (Fig. 6A, C) and tissue (Fig. 6B, D) levels of IL-6 and TNF-α compared to controls (*p < 0.05). Conversely, rhaFGF treatment (DM + rhaFGF group) significantly reduced IL-6 and TNF-α levels in both serum and tissues (*p < 0.05).
Serological and wound tissue levels of IL-6 and TNF-α were assessed by ELISA in control and diabetic mice. Diabetic mice (DM) exhibited significantly higher serological (A) and wound tissue (B) IL-6 levels compared to controls. rhaFGF treatment (DM + rhaFGF) significantly reduced IL-6 levels in both serum and wound tissues. Similar to IL-6, serological (C) and wound tissue (D) levels of TNF-α levels were significantly elevated in DM compared to controls. rhaFGF treatment (DM + rhaFGF) significantly decreased TNF-α levels in both serum and wound tissues. *p < 0.05 and ****p < 0.0001.
rhaFGF suppresses NLRP3 inflammasome in diabetic mice
To investigate the mechanism underlying chronic wounds in diabetes, western blot analysis was performed to measure the protein levels of NLRP3, ASC, and other inflammasome-related molecules in all groups at day 7 after wound creation (Fig. 7A). Compared to controls (Con), diabetic mice (DM) exhibited significantly increased NLRP3 expression (Fig. 7B; *p < 0.05). rhaFGF treatment (DM + rhaFGF group) significantly reduced NLRP3 protein levels (*p < 0.05). Similarly, the expression of downstream inflammasome components ASC (Fig. 7C) and Caspase-1 (Fig. 7D) was significantly lower in the DM + rhaFGF group compared to DM (*p < 0.05).
The effects of rhaFGF on the protein levels of NLRP3, ASC, Caspase-1, IL-1β, and IL-18 in wound tissue in control and diabetic mice. (A) Representative immunoblots showed the protein levels of NLRP3, ASC, Caspase-1, IL-1β, and IL-18 in all groups. Band intensities were quantitated for NLRP3 (B), ASC (C), Caspase-1 (D), IL-1β (E), and IL-18 (F) protein after normalization to β-actin. Diabetic mice (DM) exhibited significantly increased expression of all measured proteins compared to controls and rhaFGF treatment (DM + rhaFGF) significantly reduced this increase, while there was no significant difference between Con and Con + rhaFGF groups. *p < 0.05 and ***p < 0.001.
Furthermore, the levels of IL-18 (Fig. 7E) and IL-1β (Fig. 7F), two important markers of NLRP3 inflammasome activity, were significantly elevated in the DM group compared to controls (*p < 0.05). Interestingly, rhaFGF treatment (DM + rhaFGF) significantly reduced IL-18 and IL-1β expression (*p < 0.05), whereas no significant difference in IL-1β was observed between the control and Con + rhaFGF groups (*p > 0.05).
Discussion
There are many factors that lead to the difficulty in healing of diabetic wound. High glucose microenvironment plays an important role in the occurrence and evolution of diabetic wounds. Usually, a high blood sugar environment can lead to pathological and physiological abnormalities throughout the body. When a diabetic patient is injured, a series of changes appear in the local microenvironment of the wound, including prolonged duration of inflammation17, dysangiogenesis18, hypoxia induced oxidative stress19, neuropathy20, accumulation of advanced glycation end products21, and impairment of neuropeptide signal transduction22. Previous studies have confirmed that sustained inflammatory response is an important cause of chronic DM wounds23. The main manifestations are prolonged inflammation and impaired function of wound healing cells during the proliferative phase24.
rhaFGF can inhibit the chronicity of acute wounds in DM
In DM wounds, due to prolonged exposure to high glucose environments, macrophage function changes25 prevents the transition from the inflammatory phase to the proliferative phase and results in persistent inflammation and an excessive response. This leads to an increase in the secretion of pro-inflammatory cytokines, reducing their ability to clear infections and delaying the repair process26. In addition, changes in the function of wound healing cells lead to reduced migration and proliferation of endothelial cells and keratinocytes, resulting in insufficient re-epithelialization of the wound and affecting wound healing27,28,29. Under physiological conditions, fibroblasts can secrete aFGF, promote cell proliferation30 and accelerate wound healing. For the DM wounds, the high glucose environment and sustained inflammatory response affect the function of fibroblast cells31, preventing them from secreting sufficient aFGF to aid wound healing. In addition, inflammatory factors and growth factors are extremely unbalanced in the environment of diabetes, unable to reach the threshold value of growth factors required for normal wound healing, which also makes the wound heal slowly32.
Previous studies have shown that the secretion of endogenous aFGF increases 12 h after human skin injury, reaches its peak at 48 h, and declines in 4 days after injury. Compared with acute wounds, the expression of aFGF is significantly reduced in chronic wounds33. This indicates that aFGF is involved in the wound healing process and its expression is insufficient in chronic wounds. It has been shown that aFGF is beneficial for tissue repair and epidermal regeneration. It can induce fibroblast proliferation and generates collagen, promote the proliferation and differentiation of endothelial cells, facilitate the formation of new capillaries, increase blood supply to wounds and accelerate granulation tissue growth34. Other study has shown that aFGF has a promoting effect on the healing of skin and mucosal injuries35. aFGF can promote mitosis of cells derived from the mesoderm and ectoderm, promote proliferation of epithelial cells in the epidermis, and contribute to epithelialization of wounds36. This is consistent with our study findings. During the wound healing process, there was no significant difference in wound healing rate between the DM group and the Control group in 7 days after wound creation. However, as time went on, the wound healing in the DM group was significantly slower than that in the control group on the 9th, 11th, and 14th day. After 14 days of wound creation, the wound healing speed in the DM group significantly decreased. With the treatment of rhaFGF, the wound healing rate of DM group significantly increased. The single cell culture results also confirmed that under high glucose and inflammatory stimulation, the proliferation and migration of fibroblasts, endothelial cells, and keratinocytes were significantly inhibited. But the proliferation and migration inhibition of these three cell types was significantly reduced after adding rhaFGF. These results indicate that there is a significant trend of chronicity in DM wounds, and the application of rhaFGF can promote DM wound healing and avoid the occurrence of chronicity.
rhaFGF reduces acute wound chronicity in DM mice by inhibiting sustained inflammatory response
The wound healing process includes four stages: hemostasis, inflammation, proliferation, and remodeling37. Acute inflammation plays a crucial role in wound healing process25. After skin injury, immune cells and cytokines are activated and initiate the inflammatory process. The release of pro-inflammatory cytokines constitutes an effective chemical attraction signal, which recruits immune cells to the wound area and is responsible for clearing pathogens and cell debris, thereby promoting wound healing38. During acute inflammation, pro-inflammatory factors such as IL-6 and TNF-α promote the homing of inflammatory cells, triggering pro-apoptotic genes and epithelial repair39. However, continuous stimulation of pro-inflammatory factors can exacerbate the inflammatory response of DM wounds, delaying wound healing and leading to the transition from acute wounds to chronic wounds5. Furthermore, the sustained inflammation induced by a high blood glucose environment leads to immune system dysfunction, resulting in an imbalance between inflammatory and growth factors, which is also an important reason for delayed wound healing23,32,40. Due to the long-term exposure of the body to a high glucose environment, hyperglycemia and oxidative stress can lead to abnormal secretion of pro-inflammatory cytokines, growth factors, and adhesion molecules. The microenvironment of inflammatory cells and wound healing cells around the wound changes, causing acute wounds in DM to be unable to enter the proliferative phase from the inflammatory phase, resulting in persistent inflammation. This is also the reason for the chronicity of acute wounds in DM26. Therefore, preventing the transition from acute inflammation to chronic inflammation is a key step in preventing chronic wounds.
Previous studies have shown that aFGF prevents the development of various inflammatory diseases41. In obese and insulin resistant mice, FGF-1 has a positive effect on glucose intolerance, liver lipid accumulation, and insulin resistance. At the same time, it significantly inhibits the secretion of cytokines (TNF-α and IL-6) in serum, thereby reducing diet induced liver inflammation in obese mice[50]. Other studies have shown that aFGF plays an excellent role in inhibiting inflammation of weak acidic wounds12. In the process of wound healing in diabetes, the nanoenzyme hydrogel system loaded with aFGF can promote the polarization of macrophage phenotype from M1 to M2 and promote angiogenesis, thus improving the potential of diabetes wound from chronic inflammation to wound repair and proliferation42. aFGF also plays a protective role in the central nervous system. It can reduce chronic inflammation and neuronal dysfunction caused by excessive activation of astrocytes or the persistent presence of reactive astrocytes by inhibiting their activation43. In our study, there was no significant difference in the healing rate between normal wounds and DM wounds at 7 days, but with the prolongation of time, the DM group showed a significant delay in wound healing. The expressions of inflammatory factors in the wound tissues and serum of each group showed that there were increased levels of pro-inflammatory factors (TNF-α and IL-6) in the wound tissues and serum of the DM group. After using rhaFGF, the levels of inflammatory factors in the wound tissue and serum of DM group significantly decreased. It can be seen that aFGF has a certain inhibitory effect on sustained inflammatory responses.
rhaFGF May promote wound healing by inhibiting the NLRP3 inflammasome signaling pathway
NLRP3 has recently become a key regulatory factor in chronic inflammatory response44. Previous studies have shown that there is a link between NLRP3 and the development of chronic wounds in diabetes45. The findings of Zhang et al.46 suggest that high expression of NLRP3 may be responsible for the excessive inflammation seen in human diabetic wounds and in high glucose-induced macrophages. The activation of NLRP3 inflammatory vesicles is induced by endogenous and exogenous danger signals such as lipopolysaccharide (LPS) and high glucose (HG)47. Diabetic wounds are accompanied by excessive production of a large number of reactive oxygen species (ROS), and the excessive accumulation of ROS will destroy the cell tissues and trigger inflammatory reactions. ROS promote the expression and activity of metalloproteinases, leading to a state of excessive protein hydrolysis in the wound and significant destruction of the extracellular matrix, which results in the cessation of the repair process ROS48. Overproduction of ROS in cells also leads to loss of cellular homeostasis, oxidative stress and eventual cellular destruction of organs49. In addition, ROS accumulation increases NLRP3 inflammatory vesicle activation, leading to caspase-1 activation and subsequent activation of cytokines IL-1β/IL-18. Activated caspase-1 can cleave inactive storage forms of IL-1β and IL-18 into active cytokines, thus exacerbating the inflammatory response in diabetes, which further leads to insulin resistance and organ dysfunction, making wound healing delayed50,51,5. In the acute inflammatory phase of healthy wounds, NLRP3 is expressed by pro-inflammatory macrophages and has a protective role in wound healing[8]. However, in DM wounds, the inflammatory cascade response caused by a large number of activated NLRP3 inflammatory vesicles becomes an important risk factor for non-healing wounds52.
The use of exogenous rhaFGF can supplement the insufficient growth factors in DM wounds, inhibit the activation of NLRP3 in DM wounds, reduce the secretion of pro-inflammatory factors, weaken inflammatory reactions, prevent acute inflammation from turning into chronic inflammation, and promote wound healing. Sun et al. reported that aFGF can alleviate oxidative stress and inflammation in mitochondria by activating Wnt/β-catenin signaling pathway, thus preventing endothelial damage caused by diabetes53. The findings of Wang D et al.54 indicates that aFGF and its variants can improve chronic kidney disease through PI3K/AKT mediated oxidative stress and inflammation inhibition. Another study has shown that intraventricular injection of aFGF can activate the hypothalamic MAPK/ERK signaling pathway, thereby inhibiting oxidative stress damage caused by hyperglycemia, effectively correcting abnormal increases in blood glucose levels, and reducing the impact of the high glucose microenvironment on cells55. Fan et al.41 reported that aFGF can weaken the c-Jun N-terminal kinase signaling pathway, thereby improving insulin resistance and inhibiting inflammatory responses. In this study, 7 days after wound creation, compared with the Con group, the DM group had higher levels of NLRP3 related molecules and IL-18 expression in the wound tissue of mice. The application of rhaFGF can effectively reduce the expression of NLRP3 related molecules and downstream molecules in the wound tissue of DM group, and significantly accelerate wound healing. This indicates that the excessive production of NLRP3 is an important cause of delayed wound healing. rhaFGF can promote wound healing by reducing the expression of NLRP3 and downstream molecules.
Based on the results of this study, it can be concluded that there is a sustained inflammatory response in DM wounds after the end of the acute inflammatory phase, which is an important reason for the chronic difficult-to-heal DM wounds. The use of rhaFGF can effectively inhibit the excessive inflammatory response of DM wounds, prevent acute inflammation from turning into chronic inflammation, and promote DM wound healing. Its mechanism may be related to the inhibition of excessive activation of NLRP3.
Our study has some limitations. Firstly, we only utilized an animal model, and further investigations in human clinical wounds are warranted in the future. Secondly, the single-cell culture model does not fully capture the complex interactions between human umbilical vein endothelial cells (HUVEC), human keratinocytes (HaCat), and human dermal fibroblasts (HFF) within the in vivo human granulation tissue environment. Thirdly, the 14-day study duration limits our ability to assess rhaFGF’s long-term effects. Fourthly, in exploring the mechanism of NLRP3 inflammasome in chronic rhaFGF induced diabetic acute wounds, we did not use NLRP3 gene knockout or blockade assays, which need to be fulfilled in future study. Finally, the study focused solely on diabetic wounds, excluding other chronic wound types. Therefore, in future study, we hope to broaden the scope of the study, extend the study time, further explore the relevant mechanisms.
rhaFGF promotes the healing of DM wounds by reducing the secretion of pro-inflammatory cytokines, which can prevent acute inflammation from turning into chronic inflammation. The mechanism may be related to its inhibition of NLRP3 activation in DM wounds.
In summary, our study focused on specific stage of the transition from acute to chronic diabetic wound which was seldom mentioned in previous literatures. It has been proven in this study that exogenous rhaFGF could effectively prevent the persistence of inflammation and thus halt the transition of acute diabetic wounds to chronic wounds, which may be related to the inhibition of NLRP3 inflammasome pathway activation. In addition, rhaFGF was proved to suppress the decline in cell proliferation and migration ability under HG + LPS (simulating diabetic wound in vitro) environment. This study provided preliminary theoretical support for rhaFGF application in the prevention of the development of non-healing diabetic wounds targeting suppression of inflammatory chronicity.
Methods
Experimental instruments
Cell culture incubator (Keinda: KT95-115-340), fluorescence microscope (BM/Piaim: A0007606), water bath (SIMPLAB: DN-22 S), portable blood glucose meter (yuwell: LC2023126), centrifuge (Sigma: Z654728CH-1EA), ultrasonic grinder (Sonice, USA: vcx-130pm), developer (Shanghai Qinxiang: 100230179-ICQ).
Experimental reagents
DMEM (Gibco: 12491015), fetal bovine serum (Sigma-Aldrich: F8318), rhaFGF (PEPROTECH: 100–17 A), high glucose medium (Thermo Fisher: C11995500BT), lipopolysaccharide (ACMEC: AC12037), CCK-8 assay kit (DOJINDO: CK04), NLRP3 (abcam: ab263899), ASC (SANTA CRUZ BIOTECHNOLOGY: sc-514414), Caspase-1 (abcam: ab138483), IL-1β (CST: 12242 S), IL-18 antibody (proteintech: 10663-1-AP), mouse IL-6 ELISA assay kit (Multisciences: 0-EK206/ 3–96), mouse TNF-α ELISA detection kit (Multisciences: 70-EK282HS/3–96), lipopolysaccharide (ACMEC: AC12037), streptozotocin (Macklin: S6089-1 g).
Experimental animals and model establishment
A total of 44 male eight-week-old C57BL/6 mice were acclimatized for one week. Body weight and fasting blood glucose were measured. Mice were then randomly divided into four groups (n = 11 / group): (1) control mice with acute wound (Con), (2) control mice with acute wound plus rhaFGF treatment (Con + rhaFGF), (3) diabetic mice with acute wound plus PBS (DM + PBS), (4) and diabetic mice with acute wound plus rhaFGF treatment (DM + rhaFGF). Within each group, the mice were further allocated for specific experimental purposes: 6 mice for wound healing observation and 5 mice for cytokine detection. The mice in the Con group received a regular diet, while the DM group received a high-fat diet for 4 weeks with their blood glucose monitored weekly. After four weeks, the diabetic group received a daily intraperitoneal injection of streptozotocin (STZ) in citrate buffer (40 mg/kg for 5 days) to induce diabetes. The Con group received an equal volume of citrate buffer injection. Three days later, fasting blood glucose was measured again. Mice with blood glucose levels ≥ 11.1 mmol/L were considered as diabetic mice56. A circular full-thickness wound of approximately 4 mm in diameter was created on the dorsal surface of the hindlimb paw to establish the acute wound model57. All mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Peking, China) and housed in an asepsis condition with a controlled temperature of 23 °C and a standardized light - dark cycle.
RhaFGF treatment and wound healing recording
On the day after successful wound creation (day 0), rhaFGF was diluted in fucoidan buffer to 10 µg/mL and topically applied daily to the wound with a dose of 0.08 µg/cm² for consecutive 14 days. The control mice received the same dose of fucoidan buffer. Wound closure was documented photographically on days 0, 3, 5, 7, 9, 11, and 14 (n = 6 per group). Images were analyzed using ImageJ software to calculate the wound healing rate and expressed as [(initial wound area - wound area at a specific timepoint) / initial wound area] x 100%.
Quantitative protein analysis (BCA Assay)
After indicated treatment, wound tissue protein was extracted using RIPA buffer with a tissue and RIPA volume ration of 2 : 30 and the protein contents was assessed using a commercial BCA assay kit. In brief, 15 µL of diluted protein lysate and BSA standards (prepared according to the kit instructions) were mixed with BCA reagents in a 96-well microplate, and after 30-min incubation, the absorbance of each well was measured at 562 nm wavelength using a microplate reader in triplicate.
Western blotting analysis
Total proteins were extracted and separated by SDS-PAGE using 10% polyacrylamide gels. Proteins were then transferred to nitrocellulose membranes, blocked with 5% non-fat milk for 2 h, and incubated overnight at 4℃ with primary antibodies at 1:1000 dilution against NLRP3, ASC, Caspase-1, IL-1β IL-18, or β-actin. The membranes were washed with tris-buffered saline (TBS) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5000 dilution) at room temperature. Protein bands were visualized using a gel imaging system, and band intensity was quantified using β-actin as a loading control.
ELISA analysis of inflammatory markers
Serum and tissue levels of inflammatory cytokines IL-6 and TNF-α were measured using commercially available ELISA kits according to the manufacturer’s instructions (n = 5/group).
Cell culture and treatments
The Human fibroblasts (HFF), Human umbilical vascular endothelial cells (HUVEC), and Human immortalized keratinocytes (HaCat) cells were both purchased from ScienCell Research Laboratories (San Diego, CA, USA). The cell lines were cultured using DMEM medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a humidified incubators gassed with 5% CO2 and 95% room air. Both cells in four- to- eight passages were used for experiments.
To mimic the diabetic inflammatory environment58 of diabetic wounds, the cells were exposed to a combination of high glucose (HG) and lipopolysaccharide (LPS). Three groups were established: control, LPS + HG, and LPS + HG + rhaFGF. The cells were treated as follows: the LPS + HG group was exposed to LPS (10 ng/mL) in high-glucose DMEM for 48 h. rhaFGF was additionally administered to the LPS + HG treatment in the LPS + HG + rhaFGF group. And the cells in control group received an equivalent volume of DMEM medium. Following the 48-hour incubation period, cellular proliferation activity was assessed using the CCK-8 assay. The ranges of aFGF concentrations for the three cells were determined separately based on previous studies59,60,61, manufacturer’s guidelines of rhaFGF and our pilot study, and then the optimal concentration of rhaFGF ( 20ng/ml,5ng/ml and 50ng/ml for HUVEC, HFF and HaCat respectively ) was determined according to the results of our pilot study.
Cell viability assay (CCK-8)
Cell proliferation was assessed using a CCK-8 assay after 48 h of rhaFGF treatment in HFF, HUVEC, and HaCaT cells. Cell viability was determined in triplicate by measuring the absorbance at 450 nm using a microplate reader (n = 6). The following formula was used to calculate cell viability: cell viability (%) = (OD[treated] - OD[blank])/(OD[control] - OD[blank]) x 100%.
Migration assays
Cell Migration assay was used to verify the effect of rhaFGF on the migration ability of HaCat, HUVEC, HFF cells. Cells were taken in good growth condition, digested and resuspended into a single cell suspension, and the cell density (5 × 105cells/mL) was adjusted. Add the cell suspension evenly to a 6-well plate, 2 mL per well, and gently shake to evenly distribute the cells. Place in a 37 °C, 5% CO2 incubator overnight to allow the cells to adhere to the wall and reach 80% and 90% confluence. Use a 200 U sterile pipette tip perpendicular to the bottom of the plate and make scratches in a straight line at one time, and after scratching, gently rinse with PBS 23 times to remove the exfoliated cells. The LPS + HG group was treated with 2 ml of serum-free medium, the LPS + HG + rhaFGF group was diluted with serum-free medium. Hacat, HUVEC and HFF in the LPS + HG + rhaFGF group was treated with serum-free medium containing 20ng/ml, 5ng/ml and 50ng/ml rhaFGF respectively. All the cells was cultured in a 37 °C, 5% CO2 incubator, and the cell migration was recorded at 6 h, 12 h and 24 h, respectively. All images were processed with ImageJ. Migration distance (µm) was calculated by subtracting the distance at 6 h, 12–24 h from that at 0 h.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 9.3.0 for Windows. Continuous data are presented as mean ± standard deviation. Two-group comparisons were performed using unpaired t-tests, and one-way ANOVA was used for comparisons between multiple groups. Statistical significance was set at p < 0.05.
Conclusion
rhaFGF promotes the healing of DM wounds by reducing the expression of pro-inflammatory cytokines, which can prevent acute inflammation from turning into chronic inflammation. The mechanism may be related to its inhibition of NLRP3 activation in DM wounds.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sun, H. et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Armstrong, D. G. et al. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 13, 16 (2020).
Tan, Q. & Xu, Y. [theories and strategies of chronic wound treatment]. Zhonghua Shao Shang Za Zhi. 36, 798–802 (2020).
Raziyeva, K. et al. Immunology of acute and chronic wound healing. Biomolecules 11, 700 (2021).
Wan, L. et al. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 18, 809–825 (2022).
Swanson, K. V., Deng, M. & Ting, J. P. Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Barnett, K. C., Li, S., Liang, K. & Ting, J. P.-Y. A 360° view of the inflammasome: mechanisms of activation, cell death, and diseases. Cell 186, 2288–2312 (2023).
Vinaik, R., Abdullahi, A., Barayan, D. & Jeschke, M. G. NLRP3 inflammasome activity is required for wound healing after burns. Transl Res. 217, 47–60 (2020).
Yang, S. et al. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/caspase-1/GSDMD pathway. Transl Res. 254, 115–127 (2023).
Ornitz, D. M. & Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev. Dev. Biol. 4, 215–266 (2015).
Shi, G. J. et al. Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed. Pharmacother. 101, 510–527 (2018).
Pan, Q. et al. Weakly acidic microenvironment of the wound bed boosting the efficacy of acidic fibroblast growth factor to promote skin regeneration. Front. Bioeng. Biotechnol. 11, 1150819 (2023).
Tan, F., Rui, X., Xiang, X., Yu, Z. & Al-Rubeai, M. Multimodal treatment combining cold atmospheric plasma and acidic fibroblast growth factor for multi-tissue regeneration. FASEB J. 35, e21442 (2021).
Blaber, S. I., Diaz, J. & Blaber, M. Accelerated healing in NONcNZO10/LtJ type 2 diabetic mice by FGF-1. Wound Repair. Regen. 23, 538–549 (2015).
Wang, S. et al. Fibroblast growth factor 1 levels are elevated in newly diagnosed type 2 diabetes compared to normal glucose tolerance controls. Endocr. J. 63, 359–365 (2016).
Ma, B. et al. Randomized, multicenter, double-blind, and placebo-controlled trial using topical Recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site. Wound Repair. Regen. 15, 795–799 (2007).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
Yang, J., Chen, Z., Pan, D., Li, H. & Shen, J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int. J. Nanomed. 15, 5911–5926 (2020).
Dworzański, J. et al. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with epstein-barr virus. PLoS One. 15, e0230374 (2020).
Köhler, G. et al. [diabetic neuropathy and diabetic foot syndrome (update 2023)]. Wien Klin. Wochenschr. 135, 164–181 (2023).
Park, H. Y. et al. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 20, 969–974 (2011).
Zhang, Y. et al. Role of VIP and Sonic Hedgehog signaling pathways in mediating epithelial wound healing, sensory nerve regeneration, and their defects in diabetic Corneas. Diabetes 69, 1549–1561 (2020).
Xiao, J., Li, J., Cai, L., Chakrabarti, S. & Li, X. Cytokines and diabetes research. J. Diabetes. Res. 2014, 920613 (2014). (2014).
Komi, D. E. A. & Khomtchouk, K. Santa Maria, P. L. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 58, 298–312 (2020).
Aitcheson, S. M., Frentiu, F. D., Hurn, S. E. & Edwards, K. Murray, R. Z. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules 26, 4917 (2021).
Rai, V., Moellmer, R. & Agrawal, D. K. Role of fibroblast plasticity and heterogeneity in modulating angiogenesis and healing in the diabetic foot ulcer. Mol. Biol. Rep. 50, 1913–1929 (2023).
Koliaraki, V., Prados, A., Armaka, M. & Kollias, G. The mesenchymal context in inflammation, immunity and cancer. Nat. Immunol. 21, 974–982 (2020).
Uchiyama, A. et al. SOX2 epidermal overexpression promotes cutaneous wound healing via activation of EGFR/MEK/ERK signaling mediated by EGFR ligands. J. Invest. Dermatol. 139, 1809–1820e8 (2019).
Wilkinson, H. N. & Hardman, M. J. Wound healing: cellular mechanisms and pathological outcomes. Open. Biol. 10, 200223 (2020).
Elseoudi, A. et al. Bipartite regulation of cellular communication network factor 2 and fibroblast growth factor 1 genes by fibroblast growth factor 1 through histone deacetylase 1 and fork head box protein A1. J. Cell. Commun. Signal. 15, 81–91 (2021).
Wan, R. et al. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair. Regen. 29, 573–581 (2021).
Liu, Y. et al. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 13, 918223 (2022).
Yamakawa, S. & Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 7, 10 (2019).
Xie, L. et al. Improved refractory wound healing with administration of acidic fibroblast growth factor in diabetic rats. Diabetes Res. Clin. Pract. 93, 396–403 (2011).
Jiang, C. et al. Application of collagen-chondroitin sulfate scaffolds with different pore sizes combined with acidic fibroblast growth factor in repairing full thickness skin defects in nude mice. Biomed Mater 17, (2022).
Huang, H. W., Yang, C. M. & Yang, C. H. Fibroblast growth factor type 1 ameliorates high-glucose-induced oxidative stress and neuroinflammation in retinal pigment epithelial cells and a streptozotocin-induced diabetic rat model. Int. J. Mol. Sci. 22, 7233 (2021).
Eming, S. A., Martin, P. & Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl Med. 6, 265sr6 (2014).
Almogbel, E. & Rasheed, N. Protein mediated oxidative stress in patients with diabetes and its associated neuropathy: correlation with protein carbonylation and disease activity markers. J. Clin. Diagn. Res. 11, BC21–BC25 (2017).
Nanda, R., Patel, S., Ghosh, A., Asha, K. S. & Mohapatra, E. A study of Apolipoprotein A1(ApoA1) and interleukin-10(IL-10) in diabetes with foot ulcers. Biomed. (Taipei). 12, 30–38 (2022).
Li, D. et al. miR-19a/b and miR-20a promote wound healing by regulating the inflammatory response of keratinocytes. J. Invest. Dermatol. 141, 659–671 (2021).
Fan, L. et al. Fibroblast growth factor-1 improves insulin resistance via repression of JNK-mediated inflammation. Front. Pharmacol. 10, 1478 (2019).
Gao, S. et al. Multifunctional bioactive nanozyme systems for enhanced diabetic wound healing. Adv. Healthc. Mater. e2401580 https://doi.org/10.1002/adhm.202401580 (2024).
Peng, D. et al. Extracellular vesicles derived from astrocyte-treated with haFGF14-154 attenuate alzheimer phenotype in AD mice. Theranostics 12, 3862–3881 (2022).
Accipe, L., Abadie, A., Neviere, R. & Bercion, S. Antioxidant activities of natural compounds from Caribbean plants to enhance diabetic wound healing. Antioxid. (Basel). 12, 1079 (2023).
Bracey, N. A. et al. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. J. Biol. Chem. 289, 19571–19584 (2014).
Zhang, X., Dai, J., Li, L., Chen, H. & Chai, Y. NLRP3 inflammasome expression and signaling in human diabetic wounds and in high glucose induced macrophages. J. Diabetes Res. 2017, 5281358 (2017).
Dai, J., Chen, H. & Chai, Y. Advanced glycation end products (AGEs) induce apoptosis of fibroblasts by activation of NLRP3 inflammasome via reactive oxygen species (ROS) signaling pathway. Med. Sci. Monit. 25, 7499–7508 (2019).
Qi, X. et al. An Immunomodulatory hydrogel by hyperthermia-assisted self-cascade glucose depletion and ROS scavenging for diabetic foot ulcer wound therapeutics. Adv. Mater. 35, e2306632 (2023).
Jacobson, M. D. Reactive oxygen species and programmed cell death. Trends Biochem. Sci. 21, 83–86 (1996).
Feng, H. et al. High glucose and lipopolysaccharide prime NLRP3 inflammasome via ROS/TXNIP pathway in mesangial cells. J. Diabetes Res. 2016, 6973175 (2016).
Ding, Y. et al. Relevance of NLRP3 inflammasome-related pathways in the pathology of diabetic wound healing and possible therapeutic targets. Oxid. Med. Cell Longev. 2022, 9687925 (2022).
Zhao, Y. et al. Bletilla striata polysaccharide promotes diabetic wound healing through Inhibition of the NLRP3 inflammasome. Front. Pharmacol. 12, 659215 (2021).
Sun, J. et al. aFGF alleviates diabetic endothelial dysfunction by decreasing oxidative stress via wnt/β-catenin-mediated upregulation of HXK2. Redox Biol. 39, 101811 (2021).
Wang, D. et al. FGF1∆HBS ameliorates chronic kidney disease via PI3K/AKT mediated suppression of oxidative stress and inflammation. Cell. Death Dis. 10, 464 (2019).
Brown, J. M. et al. Role of hypothalamic MAPK/ERK signaling and central action of FGF1 in diabetes remission. iScience 24, 102944 (2021).
Zhang, J. et al. Establishment of a diabetic myocardial hypertrophy model in mus musculus castaneus mouse. Int. J. Exp. Pathol. 99, 295–303 (2018).
Carrejo, N. C. et al. Multidomain peptide hydrogel accelerates healing of full-thickness wounds in diabetic mice. ACS Biomater. Sci. Eng. 4, 1386–1396 (2018).
Ayala, T. S. et al. High glucose environments interfere with bone marrow-derived macrophage inflammatory mediator release, the TLR4 pathway and glucose metabolism. Sci. Rep. 9, 11447 (2019).
Sancar, G. et al. FGF1 and insulin control lipolysis by convergent pathways. Cell. Metab. 34, 171–183e6 (2022).
Perry, R. J. et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Commun. 6, 6980 (2015).
Lin, Q. et al. FGF1∆HBS delays the progression of diabetic nephropathy in late-stage type 2 diabetes mouse model by alleviating renal inflammation, fibrosis, and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166414 (2022).
Funding
We acknowledge the financial support provided by the Shenzhen Science and Technology Program (JCYJ20180228163014668 & KCXFZ2023073109410002), Shenzhen Second People’s Hospital Clinical Research Fund of Shenzhen High-level Hospital Construction Project (No. 20253357007), National Natural Science Foundation of China (No. 82471574) and Shenzhen Clinical Research Center for Trauma treatment (LCYSSQ20220823091405012).
Author information
Authors and Affiliations
Contributions
T.P., Y.S. and L.Z. completed the experimental content and wrote the draft paper. T.P. drew pictures. Z.D. planned the study and modified the article. Z.W., P.X., Y.Z. and L.X have substantively revised the manuscript. All authors gave the final approval and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Ethics Committee for Animal Experiments of the Second People’s Hospital of Shenzhen, China (Approval No. 20220090) and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85 − 23, Revised 1996). All procedures were performed in accordance with the ethical standards of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, and ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pang, T., Shao, Y., Zhou, L. et al. rhaFGF promotes acute diabetic wound healing by suppressing chronicity of inflammation. Sci Rep 15, 19085 (2025). https://doi.org/10.1038/s41598-025-03086-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03086-5