Abstract
The estimated glucose disposal rate (eGDR), as a novel metric of insulin resistance (IR), has been demonstrated to correlate with hepatic steatosis in patients with diabetes. Although IR is considered as a factor affecting lipid metabolism, the correlation between eGDR and metabolic associated fatty liver disease (MAFLD) is still unclear. Data from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) were employed in this cross-sectional study designed to examine the association between eGDR and MAFLD through the application of weighted multivariate logistic regression model. The nonlinear relationship was described utilizing restricted cubic spline (RCS). Subgroup analysis was performed to assess the consistency of the results and causal mediation analysis was employed to explore potential mediators. The study included 3,585 participants, 2,002 of whom diagnosed MAFLD, with a prevalence rate of 55.84%. After adjusting for all confounding factors, the risk of MAFLD decreased by 19% with each unit increase in the eGDR (odds ratio [OR] 0.81, 95% confidence interval [CI] 0.76, 0.88, P < 0.0001). Analysis of eGDR in quartiles further supported this finding. The restricted cubic spline analysis revealed a significant nonlinear negative association between eGDR and MAFLD. Causal mediation analysis indicated that the relationship between eGDR and MAFLD was partially mediated by the atherogenic index of plasma (AIP), with a mediation ratio of 8.75%. Our results demonstrate that eGDR has a significant nonlinear negative association with the prevalence of MAFLD, in which AIP plays a mediating role, offering new insights into the prevention and management of MAFLD.
Similar content being viewed by others
Introduction
Metabolic-associated fatty liver disease, formerly referred to as non-alcoholic fatty liver disease (NAFLD), has emerged as a major public health issue due to the rising prevalence of obesity and associated insulin resistance1. It is widely recognized that metabolic dysregulation of glucose and lipids in the liver is a defining characteristic of MAFLD. Recent studies indicate that excessive lipid accumulation in the liver can lead to endoplasmic reticulum (ER) stress, while ER stress can also contribute to hepatic steatosis and insulin resistance2. Moreover, observational studies have elucidated a correlation between hepatic fibrosis, steatosis, hepatocellular carcinoma, and the escalating risk of mortality from all causes3. Consequently, effective prevention, diagnosis, and assessment strategies for MAFLD are crucial to improve clinical prognosis in high-risk populations.
Currently, liver biopsy remains the gold standard for the assessment of hepatic steatosis and fibrosis. However, the practice is invasive, expensive, and has complications, making it difficult for the public to accept. Vibration-controlled transient elastography (VCTE) is an ultrasound-based examination technique that employs liver stiffness measurement (LSM) and controlled attenuation parameters (CAP) to quantitatively assess the degree of liver fibrosis, and recent observational studies have demonstrated its strong accuracy4.
MAFLD is currently recognized as a metabolic disease closely associated with metabolic syndrome and its components, including hypertension, diabetes, dyslipidemia and obesity5. Evidence suggests that IR is a key driver in the development of MAFLD. It not only facilitates lipid accumulation within the liver but also intensifies hepatocellular injury and inflammatory responses triggered by lipid buildup, which progressively leads to hepatic fibrosis6. The gold standard for measuring insulin resistance is the hyperinsulinemic euglycemic clamp (HEC)7. Although it is highly accurate, its complex procedure, high cost, and time-consuming nature make it difficult to implement widely in clinical practice. The eGDR index, as a novel parameter incorporating changes in various indicators such as hemoglobinA1c (HbA1c), hypertension, and waist circumference (WC), can exhibit a strong association with IR, providing a more comprehensive view and effectively capturing trends in metabolic disorders and associated disease risks compared to conventional biochemical markers8. Previous studies have indicated its correlation with lipid metabolism, cardiovascular mortality and all-cause mortality9. However, there has been little research on the correlation between eGDR and MAFLD. Studies examining the interrelationship among these variables may offer new insights for monitoring MAFLD risk by using only HbA1c, hypertension, and WC. eGDR has the potential to serve as a convenient and cost-effective tool for disease evaluation. Therefore, we conduct this study.
Materials and methods
Study population
The data for this study were obtained from the NHANES database, covering a diverse and representative sample of the non-institutionalized population in the United States. As a result of COVID-19 pandemic, data collection for NHANES was halted in March 2020. Therefore, the study utilized data exclusively from the NHANES 2017 to March 2020 period to investigate the correlation between eGDR and MAFLD. The NHANES survey has been granted approval by the National Health Statistics Institutional Review Board, and all participants provided written informed consent.
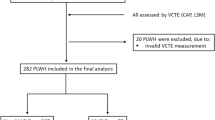
Initially, 15,560 individuals were included in the study. Among 15,560 subjects, those under 18 years old (n = 5,867), those with hepatitis B or C infection (n = 231), those missing VCTE data or lacking sufficient information to diagnose MAFLD (n = 1365), those with incomplete data preventing calculation of eGDR (n = 626), and those with other incomplete information (n = 2553) were excluded. Additionally, considering that alcohol can directly interfere with insulin sensitivity and glucose metabolism, which may confound the association analysis between eGDR and MAFLD. Subjects with excessive alcohol consumption (defined as consuming 4 or more drinks daily for males, 3 or more drinks daily for females, or binge drinking on 5 or more days per month) (n = 1333) were also excluded. Finally, 3585 subjects were included in this study, as shown in Fig. 1.
Definition of eGDR, AIP and MAFLD
eGDR = 21.158 − (0.09*WC) − (3.407*HT) − (0.551*HbA1c) [WC = waist circumference (cm), HT = hypertension (yes = 1/no = 0), and HbA1c = HbA1c (% DCCT)]10.
AIP = Log [TG (mg/dL)/HDL-C (mg/dL)] (TG = triglycerides and HDL-C = high-density lipoprotein cholesterol)11.
MAFLD was diagnosed based on the evidence of hepatic steatosis and any of the following three conditions: over-weight/obesity, diabetes mellitus or metabolic dysfunction. According to the definition of MAFLD, the metabolic dysfunction was defined as the presence of at least two of the following conditions: (1) WC greater than 102 cm in male and 88 cm in female, (2) hypertension(arterial blood pressure > 130/85 mmHg or under antihypertension therapy), (3) hyperlipidemia (TG > 1.70 mmol/L or with specific drug treatment), (4) HDL-C level (< 1.0 mmol/for male and < 1.3 mmol/L for female), (5) prediabetes, (6) hypersensitive C-reactive protein level > 2 mg/L12.
Vibration controlled transient elastography (VCTE)
The VCTE of liver data collected from NHANES subjects between 2017 and 2020 using the FibroScan machine was deemed an appropriate and suitable source for the purposes of this study. In order to assess the fatness and cirrhosis in the liver, validated parameters such as LSM and CAP were employed. It is used to determine that patients with CAP ≥ 248 dB/m had liver steatosis13.
Covariates
Through a comprehensive examination on existing literature, potential confounding covariates were identified in the multivariable-adjusted model for the relationship between eGDR and MAFLD. Demographic covariates in the study encompassed race, age, gender, martial, education and poverty income ratio (PIR), while anthropometric and laboratory covariates included aminotransferase (AST), alanine aminotransferase (ALT), body mass index (BMI), total cholesterol (TC), WC, albumin, gamma-glutamyl transferase (GGT), creatinine, alkaline phosphatase (ALP), HDL-C, total bilirubin, uric acid, and TG. Serum samples are collected at the Mobile Examination Centers and stored in an appropriate refrigerated environment. They are then transported to the central laboratory, where analysis is conducted using the Roche Cobas 6000 analyzer. Hepatitis testing is conducted using standardized immunoassay products, and fasting samples are collected after an 8-h fasting period. More detailed testing procedures can be accessed publicly in the NHANES database. Medical history covariates included the presence or absence of hypertension, diabetes and hyperlipidemia, smoking, alcohol consumption, use of sex hormones, use of steroid medications and use of antidepressants.
Patients are diagnosed with hypertension if they meet any of the following criteria: (1) SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, (2) use of antihypertensive medication, (3) being informed by a doctor that they have hypertension14. Diabetes was defined as HbA1c > 6.5%, fasting glucose ≥ 7.0 mmol/L, random blood glucose or 2-h Oral Glucose Tolerance Test (OGTT) blood glucose ≥ 11.1 mmol/L, use of diabetes medications or a previous diagnosis of diabetes15. Impaired fasting glucose (IFG) is defined as a fasting blood glucose level between 6.1 and 7.0 mmol/L. Hyperlipidemia was defined as TG ≥ 150 mg/dL, TC ≥ 200 mg/dL, HDL < 40 mg/dL for males and < 50 mg/dL for females, or the use of lipid-lowering medications.
Statistical analysis
All the analyses in this study were performed using the sampling weights to account for the complex sampling design. To identify differences between eGDR quartiles, weighted one-way analysis of variance (ANOVA) was used for continuous variables and weighted chi-squared test was employed for categorical variables. The correlation between eGDR and MAFLD were examined with three weighted multivariable logistic regression models. In Crude model, any of covariates was not adjusted, while in Model 1, adjustments were made for race, age and gender. In Model 2, adjustments were made for age, sex, race, education, PIR, marital status, BMI, AST, GGT, ALT, TG, TC, HDL-C, TBIL, ALP, albumin, creatinine, uric acid, drinking, smoking, diabetes, hyperlipidemia, use of sex hormones, use of steroid medications and use of antidepressants.
RCS was employed to assess the nonlinear correlation between eGDR and MAFLD. The optimal number of knots was based on the minimum Akaike Information Criterion (AIC). Additionally, to investigate the presence of heterogeneity and interactions within specific subpopulations, subgroup analysis was conducted. In this analysis, stratified multivariable logistic regression models with covariates were utilized, including age, gender, race, PIR, BMI, drinking, smoking, hyperlipidemia, diabetes and hypertension. In addition, we also used multiple logistic regression models to explore the relationship between AIP and MAFLD. Further causal mediation analysis was conducted to investigate whether AIP mediates the link between eGDR and MAFLD, and the bootstrap method was employed to assess the proportion of the mediation effect. All analysis were performed with R software (version 4.3.1). Statistical significance was determined as a two-sided p ≤ 0.05.
Results
Characteristics of subjects
In this study, a total of 3,585 subjects aged 18 years old or more, with the mean age of 50.11 years old, were included, among which, 47.06% of the subjects were males and 52.94% were females. The eGDR index was divided into quartiles ranging as Q1: < 5.271, Q2: 5.271–7.692, Q3: 7.692–9.837, Q4: ≥ 9.837, respectively. The subjects were stratified into four groups according to eGDR quartiles, revealing notable variations in age, race, gender, education level, marital status, BMI, HbA1c, hypertension, diabetes, hyperlipidemia, smoking, albumin, ALT, AST, GGT, ALP, HDL-C, TG, TC, WC, creatinine, uric acid, AIP, use of antidepressants and MAFLD (all P < 0.05). Among the quartiles, the subjects in Quartile 1 had elevated BMI, WC, HbA1c, GGT, ALP, ALT, AST, uric acid, creatinine, as well as a higher incidence of hyperlipidemia, hypertension and diabetes mellitus compared to those in other groups (P < 0.05). Notably, the prevalence of MAFLD in the Quartile 1 group was significantly higher than that of other groups (P < 0.0001). Details are shown in Table 1.
Correlation between eGDR and MAFLD
Initially, the relationship between eGDR and the risk of MAFLD was assessed without controlling for covariates. A negative correlation was observed between higher eGDR and the risk of MAFLD. Table 2 illustrates that after adjusting for other risk factors, there is a significant correlation between eGDR and MFALD (OR = 0.81, 95% CI 0.76–0.88, p < 0.0001). Further dividing eGDR index into quartiles, the negative association between eGDR and MAFLD remained.
Compared with individuals with lower eGDR in Q1 group (< 5.271), the adjusted eGDR and the risk of MAFLD in the Q2 group (5.271–7.692), the Q3 group (7.692–9.837), and the Q4 group (≥ 9.837) were 0.66 (95% CI 0.0.45–0.98, P = 0.04), 0.55 (95% CI 0.35–0.89, P = 0.02), and 0.20 (95% CI 0.13–0.30, P < 0.0001) (Table 2), indicating that people with higher eGDR have a lower risk of MAFLD than others.
Subgroup analysis
Weighted subgroup analysis was conducted to explore the consistency of the relationship between eGDR and MAFLD in different population subgroups, stratified by age, gender, race, PIR, BMI, drinking, smoking, hyperlipidemia, diabetes and hypertension. As presented in Fig. 2, the findings revealed that age, BMI, hypertension, and diabetes have significant interactions in the relationship between eGDR and MAFLD (P for interaction < 0.05). The association between eGDR and MAFLD is more pronounced in individuals aged 60 and above, as well as in those who are overweight or have diabetes and hypertension, highlighting the importance of prioritizing attention to these high-risk groups.
Subgroup analysis of the association between eGDR and MAFLD in adults. Each stratification was adjusted for age, sex, race, education, PIR, marital status, BMI, AST, GGT, ALT, TG, TC, HDL-C, TBIL, ALP, albumin, creatinine, uric acid, drinking, smoking, diabetes, hyperlipidemia, use of sex hormones, use of steroid medications and use of antidepressants except for the stratification variable.
Non-linear relationship between eGDR and MAFLD
According to Fig. 3, a non-linear association was observed between eGDR and the risk of MAFLD (P for nonlinear < 0.001). In Fig. 3a, prior to adjusting for any covariates, a nonlinear negative relationship between eGDR and the incidence of MAFLD is evident. Following full adjustment for covariates, this non-linear relationship persists in Fig. 3b, with the recursive algorithm identifying an inflection point at 7.6919 mg/kg/min.
The non-linear relationship between eGDR and the risk of MAFLD in the population. The upper and lower limits of the 95% CI are shaded. (a) adjusted for none. (b) adjusted for age, sex, race, education, PIR, marital status, BMI, AST, GGT, ALT, TG, TC, HDL-C, TBIL, ALP, albumin, creatinine, uric acid, drinking, smoking, diabetes, hyperlipidemia, use of sex hormones, use of steroid medications and use of antidepressants.
The intermediary role of AIP
AIP is a comprehensive biomarker that effectively reflects lipid metabolism disorders. It plays a crucial role in fat metabolism and insulin resistance. We hypothesize that AIP may influence the relationship between eGDR and MAFLD. First, we explored the relationship between AIP and the risk of MAFLD. As shown in Table 3, AIP was positively associated with the risk of MAFLD (OR = 2.16, 95% CI 1.77–2.62, P < 0.0001). After categorizing AIP into quartiles, its positive relationship with MAFLD remained unchanged. Subsequently, we conducted a causal mediation analysis to explore the potential mediating role of AIP in the relationship between eGDR and MAFLD. Figure 4 depicts the mediation model and pathway, where eGDR is treated as the independent variable, AIP as the mediator, and MAFLD as the dependent variable. The findings indicated that eGDR had a notable indirect impact on the prevalence of MAFLD via AIP, exhibiting an indirect effect size of − 0.0021 (95% CI − 0.0031, − 0.0010, P < 0.001), indicating that AIP served as a partial mediator in the association between eGDR and MAFLD. Furthermore, even after adjusting for AIP, the relationship between eGDR and MAFLD remained statistically significant (P < 0.001), demonstrating a significant direct effect. This suggests that there are both direct and indirect effects of AIP on eGDR and MAFLD occurrence, with approximately 8.75% being mediated by AIP. The results of the mediation analyses, including direct effects, indirect effects, total effects, and mediation ratios, are presented in Table 4.
Mediated analysis model path diagram. Notes: eGDR was defined as the independent variable; MAFLD as the dependent variable; and AIP as the mediating variable. Path a represents the regression coefficient of the association between eGDR and AIP. Path b represents the regression coefficient of the association between AIP and MAFLD. Path c represents the simple total effect of eGDR on MAFLD. Path c’ represents the direct effect of eGDR on MAFLD when controlling for AIP.
Discussion
In this cross-sectional study, we identified the mediating role of AIP in the relationship between eGDR and MAFLD, which helps to better understand the link between insulin resistance and lipid metabolism disorders. By analyzing clinical data from 3585 adults, we found that lower eGDR was associated with a higher prevalence of MAFLD. In the weighted characteristic table of the study population, individuals with lower eGDR exhibited higher levels of hepatic steatosis and fibrosis (P < 0.0001). This may be attributed to higher levels of insulin resistance, which promote lipid accumulation and exacerbate inflammation. After adjusting for confounding factors, the negative correlation between eGDR and MAFLD remained stable. Subgroup analysis revealed that the association between eGDR and MAFLD was more pronounced in individuals aged ≥ 60 years, overweight individuals, and those with diabetes and hypertension. Regardless of whether covariates were adjusted for, the nonlinear relationship between eGDR and MAFLD remained consistent on the RCS curve, with an inflection point at 7.6919. Further exploration of the relationship between AIP and MAFLD showed that individuals with higher AIP levels had an increased risk of MAFLD (OR = 2.16, 95% CI 1.77–2.62, P < 0.0001). Mediation analysis indicated that AIP plays a significant mediating role in the association between eGDR and MAFLD. In summary, higher eGDR levels are linked to a lower incidence of MAFLD, and this relationship is partially mediated by AIP.
Currently, it is believed that MAFLD is closely associated with insulin resistance, obesity, dyslipidemia, gut microbiome dysbiosis, and genetic risk factors16. A systematic review showed that NAFLD is often comorbid with type 2 diabetes, and the prevalence of NAFLD in diabetic patients is twice that of normal individuals, which is mediated by insulin resistance17. In recent years, an increasing number of studies have explored the biological and lipid biomarkers associated with MAFLD, highlighting the role of insulin resistance and lipid metabolism in the development of MAFLD18. A cross-sectional study estimated IR using the triglyceride-glucose index and found that the estimated IR partially mediated the association between inflammation scores and MAFLD, with a mediation percentage of 33%19. In a cross-sectional study involving 151 patients with type 1 diabetes mellitus, researchers calculated the indirect IR markers including eGDR index, visceral adiposity index (VAI), and the triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C) for each participant. The results indicated that a lower eGDR was associated with a higher likelihood of having NAFLD (OR = 0.86, 95% CI 0.77–0.97, P = 0.01), and participants with NAFLD exhibited lower insulin sensitivity20. Another study recruiting 198 adults with type 1 diabetes found that MAFLD was significantly associated with a lower eGDR (β = − 0.367, 95% CI − 0.472 to − 0.261, p < 0.001)21. A retrospective cohort study including 16,689 participants explored the association between eGDR and MAFLD. The odds ratio for MAFLD events in the highest eGDR quartile was 0.147 (95% CI 0.110–0.194), and eGDR demonstrated superior predictive ability for MAFLD compared to the TG/HDL-C ratio and triglyceride glucose index (AUC = 0.8, 95% CI 0.79–0.81)22. As a new type of insulin resistance indicator, eGDR has great potential in predicting metabolic diseases10, and some studies have shown that there is a clear association between MAFLD and elevated levels of IR23. Systemic metabolic dysregulation is the principal proximate cause for the development and progression of MAFLD. For example, IR increases serum free fatty acid levels and promotes de novo lipogenesis of glucose and protein, leading to excess lipid storage in the liver24. This process enhances lipid deposition and increases oxidative stress, which further damages liver cells and results in cirrhosis. In individuals with IR, reduced insulin receptor responsiveness can enhance hepatic stellate cell growth and selectively mediate TGFβ-induced hepatic stellate cell activation, leading to liver fibrosis25. Inflammation is a key mediator in the connection between IR and MAFLD. Elevated levels of free fatty acids (FFAs) and adipokines, commonly observed in IR patients, activate inflammatory pathways in the liver26. This inflammation further impairs insulin signaling in the liver, creating a vicious cycle that accelerates the progression of MAFLD. The negative correlation between eGDR and MAFLD observed in this study can be attributed to IR’s role in promoting hepatic lipid accumulation. IR impairs the liver’s ability to process glucose and lipids effectively, leading to excess fat storage in hepatocytes. This process is exacerbated by increased circulating free fatty acids and impaired fat oxidation, all of which contribute to the progression of MAFLD.
Apart from being associated with atherosclerosis, AIP, as a novel lipid marker, has also been related to a variety of systemic diseases. Research has shown that there is a linear dose–response relationship between AIP and MAFLD, indicating that as AIP increases, the risk of MAFLD also rises (OR = 3.85, 95% CI 1.55–9.52)27. This is consistent with our research. A study specifically evaluated the relationship between AIP and MAFLD using data from the NHANES 2007-2016 dataset. The results demonstrated that AIP has predictive ability for MAFLD with an AUC of 0.74928. Lin et al.29 demonstrated that AIP has a stronger correlation with the risk of NAFLD and shows superior discriminant performance in predicting NAFLD among patients with type 2 diabetes. Yin et al.30 illustrated a noteworthy, non-linear, positive correlation between IR and AIP. Reduced insulin sensitivity and elevated concentrations are the main features of IR. Insulin resistance as the central pathophysiological phenomenon of metabolic syndrome can lead to dyslipidemia31. Individuals with low insulin sensitivity exhibit increased hepatic cholesterol synthesis, leading to an overproduction of very low-density lipoprotein cholesterol, which are the precursor particles of low-density lipoprotein cholesterol32. Studies have found that knocking out the vascular endothelial growth factor B gene in mice can improve glucose metabolism and insulin resistance, but increase lipid accumulation, which provides new ideas for the treatment of fatty liver33. In a phase 2 randomized trial, the study found that survodutide (a dual glucagon receptor and glucagon-like peptide-1 receptor agonist) can significantly reduce the degree of fatty liver in patients and delay the course of liver cirrhosis compared with placebo34. This result indicated that maintaining the stability of glucose metabolism and lipid metabolism is of great importance for the prevention and treatment of fatty liver disease. AIP is calculated based on the ratio of TG to HDL-C, and can indirectly reflect the status of lipid abnormalities and fat metabolism, which are all core pathological processes of MAFLD35.
Taking together, these results suggest indices related to lipid metabolism and IR could be a new and promising index for predicting the occurrence of MAFLD. However, the calculation of IR is based on the measures of fasting blood glucose and insulin, which severely limits the popularity of this technique, because fasting insulin is not routinely tested for nondiabetic patients. By integrating clinical and laboratory data in the calculation of eGDR, this measure offers a more comprehensive assessment of insulin resistance. Since HbA1c is lower in individuals without diabetes, the eGDR index that combines WC, hypertension, and HbA1c can more effectively evaluate the IR level in normal people. Subgroups such as age, BMI, diabetes status, and hypertension status showed differences in the association between eGDR and MAFLD.
For individuals over the age of 60, factors such as decreased insulin sensitivity, impaired liver function, and increased chronic inflammation make the elderly more prone to insulin resistance, leading to fat accumulation in the liver and subsequently triggering the development of MAFLD36. In overweight individuals, obesity reduces serum adiponectin levels, which contributes to insulin resistance and other metabolic disorders37. Additionally, overweight individuals typically have a higher proportion of body fat, which not only has a negative impact on insulin sensitivity but also releases large amounts of adipokines and pro-inflammatory factors, leading to chronic inflammation and fat accumulation in the liver. People with diabetes often experience insulin resistance, which puts them at an increased risk of developing MAFLD. Endothelial dysfunction caused by high blood pressure can disrupt the normal metabolism of insulin, further exacerbating insulin resistance38. This, in turn, accelerates fat accumulation in the liver, increasing the risk of MAFLD. Additionally, hypertensive patients tend to have elevated levels of inflammatory markers, which can damage liver cells and promote fat buildup in the liver39.
This research holds substantial clinical significance. First, it is the first attempt to comprehensively evaluate the associations between eGDR and MAFLD incidents among American adults. Secondly, this study also explored the relationship between AIP and the incidence of MAFLD, and further discovered the mediating role of AIP in the development of MAFLD in the population with abnormal glucose metabolism. However, some limitations need to be considered. First, as a cross-sectional study, the causal relationship between eGDR and MAFLD could not be elucidated. Prospective research in the future is necessary to better investigate the potential causal relationships. Second, although adjustments have been made for potential covariates, residual confounding remains an inherent challenge in observational studies. In addition, while HbA1c measurements often rely on standardized methods, specific conditions such as anemia, kidney disease and splenectomy may affect the accuracy of the results. Thirdly, the parameters for eGDR were derived from the adults. The parameter estimates may differ when applied to individuals aged < 18 years. Finally, although this research includes 3,585 participants, there remains a risk of insufficient sample size for certain groups. Therefore, further research is needed to include more participants to validate our results.
Conclusion
In the American adult population, the eGDR index was nonlinearly and negatively associated with the prevalence of MAFLD, and this relationship is opposite in AIP and MAFLD. Moreover, AIP plays a mediating role in the relationship between eGDR and MAFLD.
Data availability
The datasets supporting the findings of this article are available in the NHANES website, https://www.cdc.gov/nchs/nhanes/.
Abbreviations
- NHANES:
-
National Health and Nutrition Examination Survey
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- VCTE:
-
Vibration-controlled transient elastography
- CAP:
-
Controlled attenuation parameters
- LSM:
-
Liver stiffness measurement
- eGDR:
-
Estimated glucose disposal rate
- AIP:
-
Atherogenic index of plasma
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
Gamma glutamyl transferase
- ALP:
-
Alkaline phosphatase
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- ER:
-
Endoplasmic reticulum
- DM:
-
Diabetes mellitus
- IFG:
-
Impaired fasting glucose
- ANOVA:
-
Analysis of variance
References
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73, 202–209 (2020).
Tak, J., Kim, Y. S. & Kim, S. G. Roles of X-box binding protein 1 in liver pathogenesis. Clin. Mol. Hepatol. https://doi.org/10.3350/cmh.2024.0441 (2024).
Kanwal, F. et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 71, 808–819 (2020).
Shen, M., Lee, A., Lefkowitch, J. H. & Worman, H. J. Vibration-controlled transient elastography for assessment of liver fibrosis at a USA academic medical center. J. Clin. Transl. Hepatol. 10, 197–206 (2022).
Tanase, D. M. et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J. Diab. Res. 2020, 3920196 (2020).
Vesković, M. et al. The Interconnection between Hepatic Insulin resistance and metabolic dysfunction-associated steatotic liver disease-the transition from an adipocentric to liver-centric approach. Curr. Issues Mol. Biol. 45, 9084–9102 (2023).
Park, S. Y., Gautier, J. F. & Chon, S. Assessment of Insulin secretion and insulin resistance in human. Diab. Metab. J. 45, 641–654 (2021).
Zhang, Z., Zhao, L., Lu, Y., Xiao, Y. & Zhou, X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: findings from a nationwide, population based, prospective cohort study. Cardiovasc. Diabetol. 23, 194 (2024).
Guo, R., Tong, J., Cao, Y. & Zhao, W. Association between estimated glucose disposal rate and cardiovascular mortality across the spectrum of glucose tolerance in the US population. Diab. Obes. Metab. https://doi.org/10.1111/dom.15954 (2024).
Peng, J. et al. Estimated glucose disposal rate for predicting cardiovascular events and mortality in patients with non-diabetic chronic kidney disease: A prospective cohort study. BMC Med. 22, 411 (2024).
Qu, L. et al. Association between atherogenic index of plasma and new-onset stroke in individuals with different glucose metabolism status: insights from CHARLS. Cardiovasc. Diabetol. 23, 215 (2024).
Eslam, M. et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic dysfunction-associated fatty liver disease. Hepatol. Int. 19, 261–301 (2025).
Rinella, M. E. et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835 (2023).
Schiffrin, E. L. & Fisher, N. Diagnosis and management of resistant hypertension. BMJ 385, e079108 (2024).
American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: Standards of care in diabetes-2024. Diab. Care 47, S20–S42 (2024).
Huang, D. Q. et al. Metabolic dysfunction-associated steatotic liver disease in adults. Nat. Rev. Dis. Prim. 11, 14 (2025).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 71, 793–801 (2019).
Li, J. et al. The relationship between the ratio of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol (NHHR) and both MASLD and advanced liver fibrosis: Evidence from NHANES 2017–2020. Front. Nutr. 11, 1508106 (2025).
Chen, Y. & Zhao, X. The mediating role of insulin resistance in the association between inflammatory score and MAFLD: NHANES 2017–2018. Immun. Inflamm. Dis. 12, e70035 (2024).
Grzelka-Woźniak, A. et al. Indirect insulin resistance markers are associated with nonalcoholic fatty liver disease in type 1 diabetes. Pol. Arch. Intern. Med. 133, 16404 (2023).
Vergani, M. et al. Metabolic dysfunction-associated steatotic liver disease, insulin sensitivity and continuous glucose monitoring metrics in patients with type 1 diabetes: A multi-centre cross-sectional study. Diab. Obes. Metab. https://doi.org/10.1111/dom.16333 (2025).
Ichikawa, T. et al. Estimated glucose disposal rate predicts the risk of incident metabolic dysfunction-associated steatotic liver disease. Endocr. Pract. https://doi.org/10.1186/s12902-025-01891-7 (2025).
Ziamanesh, F. et al. Unraveling the link between insulin resistance and non-alcoholic fatty liver disease (or metabolic dysfunction-associated steatotic liver disease): A narrative review. J. Diab. Metab. Disord. 22, 1083–1094 (2023).
Bae, S., George, J. & Qiao, L. From MAFLD to hepatocellular carcinoma and everything in between. Chin. Med. J. (Engl.) 135, 547–556 (2022).
Lee, W. H. et al. Insulin receptor responsiveness governs TGFβ-induced hepatic stellate cell activation: Insulin resistance instigates liver fibrosis. FASEB J. 39, e70427 (2025).
Ajoolabady, A. et al. Endoplasmic reticulum stress in liver diseases. Hepatology 77, 619–639 (2023).
Chen, Y., Lu, C., Ju, H., Zhou, Q. & Zhao, X. Elevated AIP is associated with the prevalence of MAFLD in the US adults: Evidence from NHANES 2017–2018. Front. Endocrinol. (Lausanne) 15, 1405828 (2024).
Li, X. M., Liu, S. L., He, Y. J. & Shu, J. C. Using new indices to predict metabolism dysfunction-associated fatty liver disease (MAFLD): Analysis of the national health and nutrition examination survey database. BMC Gastroenterol. 24, 109 (2024).
Lin, J., Li, H. & Wan, Q. A cross-sectional study of the correlation between the atherogenic index of plasma and nonalcoholic fatty liver disease in patients with type 2 diabetes. Diab. Metab. Syndr. Obes. 15, 2227–2234 (2022).
Yin, B. et al. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: A cross-sectional study. Cardiovasc. Diabetol. 22, 157 (2023).
Neeland, I. J. et al. Metabolic syndrome. Nat. Rev. Dis. Prim. 10, 77 (2024).
Bulum, T., Kolarić, B. & Duvnjak, L. Insulin sensitivity modifies the relationship between thyroid function and lipid profile in euthyroid type 1 diabetic patients. Endocrine 42, 139–145 (2012).
Luo, X. et al. Reducing VEGFB expression regulates the balance of glucose and lipid metabolism in mice via VEGFR1. Mol. Med. Rep. 26, 285 (2022).
Sanyal, A. J. et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N. Engl. J. Med. 391, 311–319 (2024).
Ziolkowska, S., Binienda, A., Jabłkowski, M., Szemraj, J. & Czarny, P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 22, 11128 (2021).
Liang, Z., Huang, R. & Zhang, L. Correlation between hepatic steatosis severity diagnosed by ultrasound and metabolic indexes in elderly patients with MAFLD. Front. Med. (Lausanne) 11, 1467773 (2024).
Parida, S., Siddharth, S. & Sharma, D. Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease. Int. J. Mol. Sci 20, 2519 (2019).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766 (2021).
Yanai, H., Adachi, H., Hakoshima, M., Iida, S. & Katsuyama, H. Metabolic-dysfunction-associated steatotic liver disease-its pathophysiology, association with atherosclerosis and cardiovascular disease, and treatments. Int. J. Mol. Sci. 24, 15473 (2023).
Acknowledgements
The research team extends its gratitude to the contributors of the NHANES datasets for their invaluable participation.
Funding
The authors declare that they have no funding and conflicts of interest related to this research.
Author information
Authors and Affiliations
Contributions
Yuhang Zhou designed the study and conducted the formal analysis. Bang Wang and Miaoxin Wu provided resources. Yuhang Zhou managed software and supervision. Both Gaochao Li and Han Hong were involved in validation and visualization. Yuhang Zhou wrote the original draft. Yuhang Zhou and Minhui Xu revised and edited the manuscript. All authors reviewed and approved the final version of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study used publicly available data from public databases and therefore did not require ethical approval.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Wang, B., Wu, M. et al. Association between estimated glucose disposal rate and the risk of MAFLD in American adults: a cross-sectional study. Sci Rep 15, 19251 (2025). https://doi.org/10.1038/s41598-025-04502-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04502-6