Abstract
Anorexia nervosa (AN) is a severe, potentially life-threatening psychiatric disorder characterized by an intense fear of weight gain, a distorted body perception and an extern food restriction leading to an abnormally low body weight. In AN patients, malnutrition is often associated with hepatic cytolysis. A growing body of evidence support an association between AN and auto-immunity. In this exploratory study, we revealed for the first-time the presence of autoantibodies targeting hypoxia-inducible factor 1 alpha (HIF1a) in AN. We evidenced the presence of autoantibodies against HIF1a in 22% of AN patients, which were absent in patients with other metabolic disease as type-I diabetes or in healthy subjects. In addition, we found that HIF1a autoantibodies was associated with hepatic cytolysis in 80% of AN patients and that their levels significantly correlated with those of ALT (alanine aminotransferase). In-vitro experiments demonstrated that HIF1a autoantibodies induced hepatocyte lysis, suggesting a potential link between these autoantibodies and liver dysfunction in AN patients. Altogether, our data reveal an implication of HIF1a autoantibodies in AN with hepatic cytolysis. Future investigation will explore their potential as a diagnostic or a prognostic marker in AN, but also other diseases since dysregulated hypoxia pathway has been implicated in other pathologic conditions.
Similar content being viewed by others
Introduction
Anorexia nervosa (AN) is a severe psychiatric disorder, internationally recognized as a key priority for the improvement of health care1. It mainly affects girls (at least 80% of cases) with peak onset of the disease between 13–14 years and 16–17 years, but it can appear in childhood or adulthood2. Integrating physical and mental health, AN is characterized by starvation and malnutrition, intense fear of gaining weight, distorted perception of body image, high incidence of coexisting psychiatric conditions, treatment resistance, and suicidal risk. The two subtypes of anorexia nervosa are the restricting subtype, which is characterized by dietary restriction, and the binge-eating/purging subtype, in which restriction is accompanied by binge eating, purging, or both3. In AN patients, severe malnutrition and/or refeeding can cause severe liver injury revealed by an increase of liver enzymes. Although the mechanism is unclear, autophagocytosis of liver cells has been described in the process of this hepatic cytolysis4. Hepatic cytolysis is characterized by the destruction of liver cells (hepatocytes), typically manifesting as an elevation in blood levels of liver enzymes, particularly transaminases (ALT and AST). The management of patients is complex and despite multidisciplinary approaches including somatic, psychiatric and nutritional aspects, the rates of relapse and mortality remains high. Thus, the improvement of AN management remains a challenge and the possibility of developing new therapies requires better knowledge about the etiologies of the disease5.
Growing body of evidence supports an association between immune system dysfunction and AN. In a cohort of 2.5 million individuals of which 12,000 diagnosed with an AN, Hedman et al. showed a strong, bidirectional relationship between autoimmune diseases and AN. Indeed, the diagnosis in one illness increases the risk of the other6. A higher prevalence of autoimmune diseases such as type 1 diabetes and Crohn’s disease was observed among patients with AN7. Fetissov et al. have described autoantibodies against α-MSH (Alpha-Melanocyte Stimulating Hormone, ACTH (AdrenoCorticoTropic Hormone), and LHRH (Luteinizing Hormone Releasing Hormone) appetite-regulating neuropeptides8. All these data led us to hypothesize an autoimmune- underlying mechanism in AN.
The study was initiated after observing a strong and unusual signal following an indirect immunofluorescence staining of Hep2 cells, liver, kidney and stomach (LKS) substrates with serum sample from a patient diagnosed with AN, suggesting the presence of a new autoantibody. To validate our hypothesis, we performed a study to (1) verify the presence of this autoantibody in a population of AN patients, (2) identify the target of the autoantibody, and (3) analyze its pathogenic potential.
Results
An intriguing immunofluorescent pattern observed in patients with AN
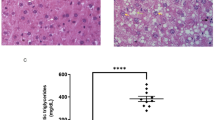
The serum of Miss K, a 15-years-old woman with a history of AN was sent to the immunology laboratory for autoantibodies screening on Hep2 and LKS substrates. We found variable speckled immunofluorescence intensity on Hep2 substrate (Fig. 1) as well as an intriguing immunofluorescent pattern on LKS substrate characterized by graded labeling of renal sections, with occasional nuclear labeling of cortical tubules contrasting with nuclear labeling on the set of medullary tubules (Fig. 1). To complete the immunofluorescence pattern profile, we tested on rat brain sections. Immunofluorescence experiments show a remarkable staining on the granular area of the cerebellum (Fig. 1).
An intriguing immunofluorescent pattern observed in the patients with AN. Immunofluorescence revealed by the serum of patient K, by the serum of patient I and by a serum of a healthy patient on HEP2 cells (× 40), kidney cortex (× 40), kidney medulla (× 40) and granular area of the cerebellum of rat (× 200). Scale bars, 20 μm.
To confirm the presence of this autoantibody in other patients, we constituted a cohort of patients with AN referred to our laboratory for routine screening of autoantibodies (Table 1). A total of 18 patients was obtained, 94% of them were women (17 women and 1 man). The mean age was 21.8 ± 12, 3 years, ages ranging from 8 to 56 years old. The mean of BMI was 13.7 kg/m2, range was 10.1 to 18.6 kg/m2. According to the subtypes of the disease, 15 patients presented a restrictive form and 3 presented a purging one. Hepatic cytolysis was observed in 5 patients presenting ALT level twice the normal value.
We then performed immunofluorescence labeling on HEp-2 and LKS substrates and evidenced another patient (patient I) with the characteristic immunofluorescent pattern as that of patient K, showing nuclear staining in Hep2 substrate or on renal and brain tissues as observed in Fig. 1. Other tests including the screening for ds-DNA, ENA and anti-transglutaminase were negative for all patients (data not shown).
Identification of anti-HIF1a auto-antibodies in patients with AN
As we noticed a strong nuclear immunofluorescent stain associated with regions of hypoxia regulation both in the renal and cerebral tissues, we hypothesized that hypoxia-inducible factor 1 alpha (HIF1a), the major transcriptional factor involved in the regulation of low oxygen concentrations, could be the potential autoantigen. Besides, immunofluorescence experiments using commercial anti-HIF1a antibody showed similar pattern of fluorescence staining on sections of rat kidney9 and on granular area of the cerebellum10 as that obtained with patient’s serum.
To confirm this hypothesis, we screened for anti-HIF1a autoantibodies in the sera of 18 patients with AN using different in-vitro approaches.
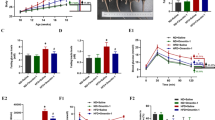
First, western blot (WB) experiments were conducted. Recombinant HIF1-a was run on SDS-PAGE and transferred to nitrocellulose membrane. The membranes were then separately probed with the sera of the 18 AN patients. Results showed a high reactivity to recombinant HIF1a in 4 AN patients (Fig. 2b). As a negative control, the sera of either healthy donors or sera of patients with another metabolic disease (type-I diabetes, Table 2) were used. Recombinant anti-HIF1a antibody served as a positive control (Fig. 2a).
Screening for anti-HIF1a auto-antibodies by Western blot. (a) As control, reactivity of healthy patient sera, patients with Type 1 diabetes sera and anti-HIF1a monoclonal antibody to recombinant HIF1a. (b) Reactivity of the patient to recombinant HIF1a. Band intensity was quantified and is represented in the adjacent graph. c. Reactivity of the patients’sera before and after immunodepletion to recombinant HIF1a.
Next, to validate the specificity of the band observed by WB, we immunodepleted patients’ sera from HIF1a autoantibodies. Our results show that the reactivity of patient sera to recombinant HIF1a is completely abolished after HIF1a autoantibodies depletion (Fig. 2c).
These results confirm the presence of HIF1a autoantibodies in AN patient serum.
Second, as HIF1a is a crucial subunit of HIF1 transcriptional factor, we investigated whether the autoantibodies found in patients sera recognizes the active form of the molecule essentially present in the nucleus. To this end we fractionate cytoplasmic and nuclear proteins from the renal carcinoma cell line 786-O and run on SDS-PAGE. Results revealed strong bands in the nuclear protein fraction when probed with sera of patients I, K, N, P (Fig. 3). Thus, the autoantibodies in the 4 AN patients recognize active HIF1a.
Third, flowcytometry experiments were performed using 18 AN patient’s sera. Briefly, the serum of each patient was used to stain fixed and permeabilized HUH-7 hepatocellular carcinoma cells. Results revealed an increase in the detected mean florescence intensity (MFI) in cells incubated with sera of the 4 AN patients I, K, N, P, as compared to sera of healthy donors (Fig. 4). Of remark, these results confirmed that obtained by WB (Fig. 2b).
Therefore, among the 18 AN patients tested, HIF1a auto-antibodies were detected at high levels in 4 patients (22%). Notably, after 4-months, we were able to re-collect sera from two patients out of these four and reproduced the experiments performed. Same results were obtained which emphasizes their positivity to anti-HIF1a autoantibodies. We also tested for a correlation between HIF1a autoantibodies and other clinical manifestations in AN disease. We didn’t find any correlation between these autoantibodies and BMI, a malnutrition severity indicator (Fig. 5b), or with the subtype of the disease, restrictive or purging or between BMI and ALT levels (Fig. 5c). In contrast, among patients with hepatic cytolysis (n = 5), 4 were significantly positive for HIF1a autoantibody (4/5, 80%, p = 0.0016). Moreover, a significant correlation existed between ALT levels and HIF1a autoantibodies in AN patients with hepatic cytolysis (r = 0.7; p = 0.015) (Fig. 5a).
Characteristics of patients. Correlations analysis were determined using the unpaired, 2-tailed, Pearson correlation coefficient test. Dotted lines correspond to the 95% confidence interval. (a) Correlation between levels of ALT and band quantification of anti-HIF1a autoantibodies. (b) Correlation between BMI and band quantification of anti-HIF1a autoantibodies. (c) Correlation between BMI and levels of ALT.
Pathogenic potential of auto-antibodies anti-HIF1a in hepatic cytolysis
In light of the results obtained, it was indispensable to validate in vitro if the sera of patients with anti-HIF1a autoantibodies have a potential cytolytic effect on hepatocytic cell line, HUH-7. Our results showed that cytolysis was induced in HUH-7 cells only when incubated with sera of patients positive for HIF1a autoantibodies (patients I, K, N, P) but not with sera having minimal or no autoantibodies (Healthy donors or patients H, M, Q) (Fig. 6a). To verify that this effect was specifically mediated by the autoantibodies, immune depletion of the sera from HIF1a autoantibodies was performed and the cytolysis experiment was repeated. Results revealed that serum depletion of patients’ I, K, N, P from HIF1a autoantibodies robustly decreased its cytolytic activity on HUH-7 cells (Fig. 6b).
Discussion
This study reveals for the first time the presence of autoantibodies against hypoxia-inducible factor 1 alpha (HIF1a). Our work constitutes the first documented evidence for the existence of such autoantibodies in AN, shedding light on a previously unknown aspect of autoimmunity in the context of AN. We detected anti-HIF1a autoantibodies in over 20% of AN patients. These autoantibodies were found to be associated with hepatic cytolytic activity in 80% of AN patients presenting hepatic cytolysis. Furthermore, in vitro experiments confirmed their hepato-cytolytic effects, suggesting a potential link between autoantibodies against HIF1a and liver dysfunction in patients with AN. Hence, in the context of AN physiopathology, anti-HIF1a autoantibodies could assist in patient stratification.
Hypoxia-inducible factor 1 alpha is a pivotal transcription factor that orchestrates cellular responses to hypoxic conditions, playing a crucial role in cellular adaptation to low oxygen environments. HIF is a dimeric molecule constituted of a stable alpha subunit and a beta subunit which is constitutively expressed. When oxygen is sufficient, prolyl hydroxylase domain (PHD) hydroxylates HIF1a subunit, promoting their ubiquitination and degradation in the proteasome11. Under hypoxic conditions, PHD is inactivated which allows HIF1a stabilization and its subsequent translocation into the nucleus, where it binds to hypoxia responsive elements (HREs) in target genes promoter.
The discovery of autoantibodies targeting HIF1a in individuals with anorexia nervosa sheds light on a novel aspect of autoimmune dysregulation in this disease. HIF1a represents a striking molecular target in anorexia nervosa because many metabolic factors, such as glucose and lipid, regulate its expression12. Moreover HIF1a potently impacts hypothalamus function which affects nutrition sensing, hormone induction, and appetite regulation13. The role of HIF1a in regulating body weight, liver metabolism and glucose homeostasis is documented12. As documented in the literature, HIF1a influences body weight through multiple regulatory pathways. It is expressed in pro-opiomelanocortin (POMC) neurons, and astrocytes in the arcuate nucleus of the hypothalamus, where it exerts influence on both appetite and energy spending14. Futhermore, HIF1a mRNA and protein levels are additionally enhance in adipose tissue in diet-induced obesity models. In the liver, HIF1a serves as a key regulator of genes associated with peroxisomal fatty acid oxidation. Additionally, HIF1a plays also a crucial role in glucose metabolism, it promotes glucose uptake by upregulation glucose transporters15. Orexin, a neuropeptide that regulates appetite, mediates HIF1a activation leading to higher glycolysis activity and increased glucose uptake16.
In this study, we were unable to detect HIF1a autoantibodies either in healthy patients or in patients with other metabolic disease such as type-I diabetes. Interestingly, we found that HIF1a autoantibodies were associated to a subgroup of patients with AN, who presented hepatic cytolysis. Importantly, patients with a history of exposure to hepatitis A, B, C or those with a documented history of alcoholism were excluded in this study. The observed hepato-cytolysis induced by HIF1a autoantibodies in vitro provides mechanistic insight into their potential role in hepatocellular damage. These findings support the hypothesis that autoantibodies against HIF1a directly contribute to liver dysfunction in patients with AN, possibly through the disruption of hypoxia response pathways and cellular homeostasis.
The liver is a central organ in nutrition sensing and in glucose and lipid metabolism. HIF1a regulation is crucial for normal liver function. In fact, its implication in mediating liver homeostasis has been described in various liver diseases such as in in alcoholic liver disease, nonalcoholic fatty liver disease, viral hepatitis, or drug-induced liver injury17. One possible mechanism of hepatic cytolysis in AN is starvation-induced autophagy4. In fact, a mutual relationship exists between the transcription factor HIF1 and autophagy18. It has been shown that autophagy can promote autoimmunity19. Autophagy plays important roles in the immune system including T cell development, cell activation/differentiation and secretory pathway. Dysregulation of autophagy can lead to increased production of pro-inflammatory cytokines which can exacerbate autoimmune responses and potentially activated autoreactive T cells20. Recently, Chen et al. reported that a decrease in HIF1 transcriptional activity is associated with a suppression in Th17 cells expansion but an enhanced Treg development. Therefore, HIF1a is involved in regulating the balance between Th17/Treg cells21. In turn, the ratio of TH17 to Tregs is crucial in regulating development of autoimmune diseases22. HIF1a plays a crucial role in regulating the Th17/Treg balance, which is important in the development of autoimmune diseases. In several autoimmune diseases, such as lupus erythematosus or rheumatoid arthritis, we observed an overexpression of HIF1a causing its recognition as a foreign antigen by the immune system. Also, HIF1α's involvement in pro-inflammatory cytokine production and immune cell proliferation, coupled with potential post-translational modifications in pathological conditions, could amplify the autoimmune response23.
In summary, as illustrated in Fig. 7, we propose that the disruption of immune tolerance to HIF1a in AN may result from an imbalance in Th17/Treg regulation, increased production of pro-inflammatory cytokines, autophagy dysregulation and post-translational modifications that enhance HIF1a overexpression or its immunogenicity. These mechanisms collectively contribute to the development of autoantibodies against HIF1a. Regarding the hepato damage induced HIF1a autoantibodies in vitro, several pathways could be further explored such as complement activation24, pro-inflammatory cytokine release, apoptosis, or oxidative stress induction. Nevertheless, our findings presents novel insights into the pathophysiology of anorexia nervosa and may aid in patient stratification.
Proposed Model of Anti-HIF1α Autoantibodies and Hepatic Cytolysis in Anorexia Nervosa. This figure summarizes hypothesis for the development of anti-HIF1α autoantibodies in anorexia nervosa (AN) and their contribution to hepatic cytolysis. Disruption of immune tolerance to HIF1a in anorexia nervosa (AN) may be due to autophagy dysregulation, imbalance in Th17/Treg regulation, production of pro-inflammatory cytokines, or post-translational modifications, enhancing HIF1a overexpression and/or immunogenicity. Hepato-cytolysis-induced HIF1a autoantibodies may involve: complement activation, pro-inflammatory cytokine release, apoptosis or oxidative stress induction. Hepatic cytolysis could also contribute to autoantibody generation, creating a potential feedback loop.
. It is important to note that this study is exploratory in nature, conducted on a limited sample size. Furthermore, we lacked detailed demographic and clinical data for the healthy control serum samples obtained from the biobank. This limitation restricts our ability to perform more comprehensive comparisons between the AN patients and healthy controls. While our findings provide valuable initial insights into the presence of HIF1a autoantibodies in AN patients, the small cohort size limits the generalizability of our results. However, this exploratory study has paved the way for more comprehensive investigation and future investigations should explore the potential triggers to the development of autoantibodies against HIF1a. Also, the molecular mechanisms by which these autoantibodies triggers hepatocytes damage should be elucidated. Finally, their potential as a diagnostic or prognostic marker for liver dysfunction in AN should be studies.
Material and methods
Patients
Sera of patients with AN were routinely referred to the laboratory of Immunology at the Public Assistance—Marseille hospital (AP-HM) between April and October 2023, for searching autoantibodies such as anti-transglutaminase, antinuclear autoantibodies (ANA) or autoantibodies related to liver autoimmune disease (AL), in a routine immunological practice. Diagnosis of AN was based on criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)25. Clinical and biological data were collected from the day of sampling. They included the type of AN (restricting or binge-eating/purging subtype), duration of the disease, body mass index (BMI), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and albumin. Patient’s exclusion criteria included prior exposure to hepatitis A, B, or C done by serological screening and a history of alcoholism.
Serum samples from three healthy individuals, obtained from a biobank, were used as a control group. This control group consisted of blood donors under the age of 60, with a composition of 1/3 men and 2/3 women. However, due to regulatory restrictions, detailed demographic and clinical data for these healthy controls were not available, which may limit the depth of comparative analyses. Additionally, serum samples from patients with type 1 diabetes, a metabolic and autoimmune disease, were included as an alternative control group.
Sex as biological variable
Female and male subjects were included in this study. 94% of the cohort is female. Anorexia nervosa (AN) mainly affects girls (at least 80% of cases)2.
Ethical statement and informed consent
Serum samples were part of the Marseilles Biobank (registered as DC 2012_1704), and the study was declared to the medical evaluation board and health data committee of Assistance Publique-Hôpitaux de Marseille (AP-HM), Marseille, France under F75PA7 registration number and fulfilled local requirements in terms of data collection and protection. This retrospective study exclusively analyzed data issuing from healthcare, adhering to local General Data Protection Regulation requirements (PADS24-139) and the need for informed consent was waived by Ethics Committee of Assistance Publique Hôpitaux de Marseille with the registration number CSE24-4. All methods were performed in accordance with the relevant guidelines and regulations (In accordance of Helsinki declarations). All experimental protocols were approved by Ethics Committee of Assistance Publique Hôpitaux de Marseille.
Cell culture
The renal cell carcinoma 786-O cells and the hepatocellular carcinoma HUH-7 cells were confirmed to be mycoplasma free. 786-O cells were cultured in DMEM supplemented with 7% heat inactivated FCS while HUH-7 cells were cultured in DMEM enriched with 10% FCS. All media contained 100 U/ml penicillin, 100 µg/mL streptomycin and 2 mM L-glutamine. Cells were maintained in 5% CO2 at 37 °C.
Autoantibodies identification by indirect immunofluorescence
ANA and liver-related autoantibodies in patient’s sera were detected by commercial indirect immunofluorescence (IIF) assays. Automated instrument (AFT-4000, HTZ™, London, United Kingdom) was used for IIF slide preparation. Samples diluted in phosphate-buffered saline were incubated for 30 min at room temperature (RT) on fixed HEp-2 cells (Kallestad™ HEp-2 Cell Line Substrate, 12 wells slides, Bio-Rad™ Laboratories, Hercules, CA) or rodent multi-organ (liver, kidney, and stomach) substrate panel (8 wells slides, Theradiag™, Marne la Vallée, France). After washing, ANA were detected by incubation with fluorescein isothiocyanate (FITC) conjugated sheep anti-human immunoglobulin (Kallestad™ FITC conjugate, Bio-Rad™ Laboratories, Hercules, CA) and liver-related autoantibodies by FITC conjugated anti-human IgG (Theradiag™, Marne la Vallée, France). Subsequently, slides were washed, embedded with a polyvinyl alcohol mounting medium with DABCO™ anti-fading (Sigma-Aldrich™, St Louis, USA) and examined under fluorescence microscope (Leica™ DM-2000, Wetzlar , Germany).
Anti-double stranded DNA (ds-DNA) and anti-extractable nuclear antigen (ENA) antibody levels were measured in sera with an automated fluorescence-enzyme immunoassay (EliA™, Immunocap™250 Phadia, Uppsala, Sweden). Anti-transglutaminase autoantibody levels were measured in sera with commercial ELISA (Eurospital™, Trieste, Italy).
Flow cytometry
Flow cytometry experiments were conducted using Gallios Flow cytometer (Beckman Coulter, CA, USA). Briefly, detached HUH-7 cells were labeled for 15 min with LIVE/DEAD™ Fixable Aqua dye (Invitrogen) to exclude dead cells from analysis. Cells were then washed twice with ice-cold PBS, fixed and permeabilized using Cyto-Fast Fix-Perm Buffer Set (BioLegend). The sera were then diluted 1:50 in PBS and cells were stained for 30 min at 4 °C. After washing, cells were incubated with 2 µg/mL goat anti-human Alexa Fluor™ 488 secondary antibody (Invitrogen) for 30 min at 4 °C. Samples were then washed and resuspended in PBS. Sera of healthy donors served as control. Results were analyzed with Kaluza software (Beckman Coulter, CA, USA). n = 3 experiments.
Western blotting
Briefly, 5 million cells were collected and washed twice with PBS. Cells were lysed in 500 µL Hypotonic Lysis Buffer, HLB (20 mM Tris–HCL; 10 mM NaCL; 3 mM MgCL2) for 15 min on ice. The homogenate was centrifuged at low speed (1000 G) for 10 min and the supernatant containing cytoplasmic proteins were collected. The pellet corresponding to the nuclear fraction was washed twice with HLB and then resuspended in 50 µL of Cell Extraction Buffer, CEB (10 mM Tris; 100 mM NaCL; 1% Triton-X100; 1 mM EDTA; 0.1% SDS; 0.5% deoxycholate; 10% glycerol). Following 30 min incubation on ice, the homogenate was centrifuged at high speed (11, 000G) for 25 min. The supernatant (nuclear fraction) were collected and stored at − 80 °C. Pierce™ BCA protein assay kit was used to quantify protein concentration. 20 µg of cytoplasmic and nuclear proteins were subjected to SDS-PAGE (NuPAGE™ 4–12%, Invitrogen), and then transferred to a nitrocellulose membrane using iBlot2 system, Invitrogen. The membranes were blocked in TBST-5% BSA for 1 h at room temperature and then probed separately with anti-HIF1 alpha (abcam, ab179483) or patient’s sera (Dilution 1:1000) overnight at 4 °C. The blots were finally incubated with HRP-conjugated secondary antibody for 1 h at room temperature and protein bands were revealed using ECL substrates (Invitrogen).
For recombinant HIF1a, 1.5 µg of the protein (abcam, ab154478) were subjected to SDS-PAGE (NuPAGE™ 4–12%, Invitrogen), transferred to a nitrocellulose membrane, and blocked in TBST-5% BSA and then probed separately with anti-HIF1a (abcam, ab179483) or patient’s sera (Dilution 1:1000) overnight at 4 °C. The blots were finally incubated with HRP-conjugated secondary antibody for 1 h at room temperature and protein bands were revealed using ECL substrates (Invitrogen). n = 3 experiments.
Immune depletion
For the immune depletion of HIF1-α autoantibodies from sera, 1 µg or recombinant HIF1α (abcam, ab154478) was allowed to adsorb on 96-well plate overnight at 4 °C. The plate was then washed three times with cold PBS/ 0.01% Tween20. The plate was blocked in PBS-BSA 5% for 2 h at room temperature. Then, 200 µL of patient sera was added in to wells in a consecutive manner and incubated for 30 min at room temperature. After a series of 5 incubations, serum was collected and used in downstream functional assays. In this exploratory study, only samples showing a signal increase of more than 20% compared to healthy patients were selected for immunodepletion analysis which allow to focus on the most robust positive results. n = 3 experiments.
Cytolytic assays
HUH-7 cells were seeded in 96 well plate in DMEM. After 24 h, media was replaced with DMEM basal medium with the addition of 10% AN patient’s sera. Cells were cultured for additional 72 h and 10 µL of WST1 reagent (Merck) was finally added in to each well. The plate was incubated for 20 min at 37 °C and OD was measured at 450 nm. The cytolytic effect of patient’s sera is translated to a decrease in cell number and thus a decrease in OD. n = 3 experiments.
Statistical analysis
Statistical analysis was performed using Prism software (GraphPad Software Inc., San Diego, CA, USA) or SPSS ® v22. The variance between the different groups to be compared was estimated before statistical analysis. Significant differences between two groups were determined using the unpaired, 2-tailed, Mann–Whitney test. A value of P ≤ 0.05 was considered to be significant.
Data availability
Anonymized data will be made available upon requests directed to the corresponding author. Proposals will be reviewed and approved by the authors based on scientific merit.
References
Naylor, C. Bringing together physical and mental health within primary care: A new frontier for integrated care. J. R. Soc. Med. https://doi.org/10.1177/0141076816665270 (2016).
van Eeden, A. E., van Hoeken, D. & Hoek, H. W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 34, 515–524 (2021).
Mitchell, J. E. & Peterson, C. B. Anorexia nervosa. N. Engl. J. Med. 382, 1343–1351 (2020).
Rautou, P.-E. et al. Acute liver cell damage in patients with anorexia nervosa: A possible role of starvation-induced hepatocyte autophagy. Gastroenterology 135, 840-848.e3 (2008).
Muratore, A. F. & Attia, E. Current therapeutic approaches to anorexia nervosa: State of the art. Clin. Ther. 43, 85–94 (2021).
Hedman, A. et al. Bidirectional relationship between eating disorders and autoimmune diseases. J. Child Psychol. Psychiatry 60, 803–812 (2019).
Raevuori, A. et al. The increased risk for autoimmune diseases in patients with eating disorders. PLoS ONE 9, e104845 (2014).
Fetissov, S. O. et al. Autoantibodies against alpha -MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc. Natl. Acad. Sci. U.S.A. 99, 17155–17160 (2002).
Schietke, R. et al. Renal tubular HIF-2α expression requires VHL inactivation and causes fibrosis and cysts. PLoS ONE 7, e31034 (2012).
Stroka, D. et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 15, 2445–2453 (2001).
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Gaspar, J. M. & Velloso, L. A. Hypoxia inducible factor as a central regulator of metabolism—Implications for the development of obesity. Front. Neurosci. 12, 813 (2018).
Du, D., Zhang, Y., Zhu, C., Chen, H. & Sun, J. Metabolic regulation of hypoxia-inducible factors in hypothalamus. Front. Endocrinol. 12, 650284 (2021).
Zhang, H., Zhang, G., Gonzalez, F. J., Park, S. & Cai, D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 9, e1001112 (2011).
Nagao, A., Kobayashi, M., Koyasu, S., Chow, C. C. T. & Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 20, 238 (2019).
Sikder, D. & Kodadek, T. The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 21, 2995–3005 (2007).
Chu, Q., Gu, X., Zheng, Q. & Zhu, H. Regulatory mechanism of HIF-1α and its role in liver diseases: a narrative review. Ann. Transl. Med. 10, 109 (2022).
Zhu, H. et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int. 13, 119 (2013).
Dj, W. & Ie, A. Autophagy and autoimmunity. Clin. Immunol. Orlando Fla 176, 55–62 (2017).
Yin, H. et al. The therapeutic and pathogenic role of autophagy in autoimmune diseases. Front. Immunol. 9, 1512 (2018).
Dang, E. V. et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146, 772–784 (2011).
Chen, Y. et al. Methyltransferase Setd2 prevents T cell-mediated autoimmune diseases via phospholipid remodeling. Proc. Natl. Acad. Sci. USA 121, e2314561121 (2024).
Tang, Y.-Y., Wang, D.-C., Wang, Y.-Q., Huang, A.-F. & Xu, W.-D. Emerging role of hypoxia-inducible factor-1α in inflammatory autoimmune diseases: A comprehensive review. Front. Immunol. 13, 1073971 (2022).
Mueller-Buehl, A. M. et al. Hypoxic processes induce complement activation via classical pathway in porcine neuroretinas. Cells 10, 3575 (2021).
Diagnostic and Statistical Manual of Mental Disorders: DSM-5. (American psychiatric association, 2013).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all of the following: (i) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (ii) drafting the article or revising it critically for important intellectual content, (iii) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Joshkon, A., Reytier, C., Bertin, D. et al. Identification of autoantibodies against HIF1a in patients with anorexia nervosa and their potential role in hepatic cytolysis. Sci Rep 15, 21347 (2025). https://doi.org/10.1038/s41598-025-05138-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05138-2