Abstract
Cellular senescence plays a critical role in the relapse of acute myeloid leukemia (AML), yet the underlying mechanisms remain incompletely understood. Here, we investigated the function of ribosomal protein L5 (RPL5) in mediating cellular senescence and its impact on AML relapse. In relapsed AML patients, the proportion of senescent cells and the expression of RPL5 were elevated, compared to patients at newly diagnosised or in complete remission, suggesting a potential link between RPL5 and AML relapse. Following chemotherapy induction, a cellular senescence model was constructed using KG-1 A cells, with RPL5 expression significantly elevated. Knockdown of RPL5 suppressed cellular senescence and enhanced apoptosis, possibly due to different regulatory mechanisms for cell proliferation and senescence. Moreover, downregulation of RPL5 mitigated chemotherapy-induced senescence and improved the response of AML cells to chemotherapy drug. Mechanistically, RPL5 regulated cellular senescence via the p53-p21-pRb pathway and its downregulation led to a reduction in senescence-related protein expression levels. These findings suggest that RPL5 plays a critical role in mediating cellular senescence and chemotherapy response in AML, providing insights into novel therapeutic strategies for overcoming chemotherapy resistance and preventing disease relapse. Future studies may explore RPL5-targeted therapies and assess their clinical applicability in AML.
Similar content being viewed by others
Introduction
Acute myeloid leukemia (AML) encompasses a diverse array of malignancies characterized by the uncontrolled proliferation of hematopoietic stem cells within the bone marrow1. Although the majority of patients initially have responde to induction chemotherapy and achieve complete remission (CR), 40–50% of younger and most elderly patients eventually relapse. Several mechanisms of relapse have been identified, including the expression of drug-resistant proteins or enzymes, pre-existed or acquired mutations, and cellular senescence phenotype2.
Chemotherapy-induced stress triggers cellular senescence3,4, which was initially thought to be a stable cellular state and a tumor-suppressive mechanism5. In addition, cellular senescence has also been found to be more dynamic and reversible, particularly in relation to the evasion and recurrence of malignant tumors3,6,7. Common hallmarks of cellular senescence include increased cell volume, cell cycle arrest, heightened activity of senescence-related beta-galactosidase (SA-β-gal), and elevated expression of senescence-associated cellular signaling proteins like p53, p21, p16, and Rb8. Among the various stress signals, p53 is referred to as the “genome guardian”, and plays a pivotal role in regulating growth arrest, DNA repair, apoptosis, and cellular senescence5.
Ribosomal protein L5 (RPL5), a component of the ribosome involved not only in binding to ribosomal RNA (rRNA) for protein synthesis but also in several extra-ribosomal functions9,10,11. RPL5 has been shown to interact with mouse double minute 2 (MDM2), thereby insulating its E3 ligase activity and stabilizing and activating p5312. Moreover, RPL5 has been implicated in the development and progression of various tumors, including breast cancer, liver cancer, melanoma and non-small cell lung cancer13,14,15,16. However, it remains uncertain whether RPL5 contributes to AML relapse by modulating cellular senescence. Therefore, this study aims to explore the expression of RPL5 in AML and ascertain whether it regulates cellular senescence via p53-p21-pRb pathway to mediate relapse.
Materials and methods
Materials
Human AML cell lines KG-1 A were purchased from Shanghai Cell Bank, Chinese Academy of Sciences. Cytarabine (Ara-c) was purchased from the Pfizer Pharmaceuticals Ltd, New York, USA. The primary antibodies used in western-blotting are listed as follows: RPL5 (Thermo Fisher Scientific, #PA5-106584), p53 (Cell Signaling Technology, #9282S), p21 (Cell Signaling Technology, #2947S), p16 (Cell Signaling Technology, #18769S), Rb (Cell Signaling Technology, #9309S), pRb (Cell Signaling Technology, #8516S), β-actin (Cell Signaling Technology, #3700).
Patients’ characteristics
We collected 53 patients with AML (non-acute promyelocytic leukemia) of different disease states and 8 patients with iron deficiency anemia (IDA) as controls from our center as study subjects (Table 1). Then, we collected their bone marrow specimens, which were collected after the diagnosis of the disease and before the first chemotherapy for all 18 patients in the newly diagnosed (ND) group, and after the assessment of the disease and before receiving treatment for all 18 patients in complete remission (CR), 17 patients in relapse and 8 patients with IDA. Stratification of molecular genetic risk in all patients according to the ELN (European Leukemia Net) 2017 guidelines. All methods were carried out in accordance with relevant guidelines and regulations. All experiments involving human participants and/or human tissue samples were conducted in compliance with relevant ethical guidelines and regulations, which was approved by the Medical Research Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (No.2012991). Informed consent was obtained from all participants and/or their legal guardians prior to their inclusion in our study.
Wright-Giemsa and senescence-associated β-galactosidase staining
Bone marrow specimens were collected from AML and IDA that were used to make bone marrow smear and to extract bone marrow mononuclear cells (BMMCs). The bone marrow smear were stained using the Wright-Giemsa (W-G) staining kit (Baso, China, according to the manufacturer’s instructions) to observed leukaemia cell morphology under the microscope (ZEISS, Germany). The BMMCs were subjected to senescence-associated β-galactosidase (SA-β-gal) staining using a commercial kit (Beyotime, #C0602, according to the manufacturer’s instructions) and blue-stained cells were visualized under bright-field microscopy (Olympus, Japan). Blue-stained cells were visualized under bright-field microscopy, and the percentage of SA-β-gal–positive cells was calculated from five randomly selected fields per sample.
Cell culture
KG-1 A cells were cultured in RPMI-1640 medium (Hyclone, USA) supplement with 10% fetal bovine serum (FBS, Giboco, USA). The cells were cultured in humidified 5% CO2 incubator at 37 °C.
Cellular senescence model construction
The Ara-C powder was dissolved in a 0.9% NaCl aqueous solution to create a stock solution at a concentration of 100µM. The stock solution was diluted in RPMI-1640 supplemented with 10% FBS to achieve a final concentration of 10µM. Then, 4 × 106 KG-1 A cells were treated with 10µM Ara-C for 3 days to construct cellular senescence model, which was the experimental group (Ara-c), and the untreated group was served as the control (Con).
Cell transfection
Short hairpin RNA (ShRNA) mediated downregulation of RPL5 was performed from Genepharma (Shanghai, China) and used following the standard protocol. Three distinct shRNA sequences targeting different regions of the RPL5 transcript:
RPL5-Homo-841: AAGCCCAAGAAAGAAGTTAAA
RPL5-Homo-210: CACACCCAAATACAGGATGAT
RPL5-Homo-450: GACTGGTGATGAATACAATGT
KG-1 A cells were transfected by sh-RPL5 or negative control lentivirus, then sieved out stable RPL5 knockdown cells line by puromycin. We established a stable RPL5 knockdown leukemia cells line as RPL5-shRNA group (Sh), the lentiviral empty vector group as the negative control (NC), whereas the non-transduced group as the blank control (BLANK), confirmation of successful establishment of RPL5 stable knockdown cell lines was achieved through expression analysis at both mRNA and protein levels.
Flow cytometry detection of cellular senescence and cell cycle distribution
The Con and Ara-c group KG-1 A cells were gathered and subjected to cellular senescence staining via SA-β-gal activity sssay kit (Cell Signaling Technology, #35302). Fluorescence intensity was analyzed on a flow cytometer (Cytoflex, Beckman Coulter, USA), while cellular G1/S/G2 distribution was evaluated using Cell Cycle Assay Kit (Multi Sciences, #CCS01) analyzed on flow cytometer (Navios Flow Cytometry, Beckman Coulter, USA). Apoptotic cells were identified by double staining with APC-Annexin V and PI (Bestbio, #BB-41033) and the experimental data were analyzed using CytExpert for DxFLEX 2.0 (URL link: https://www.beckman.com/flow-cytometry/research-flow-cytometers/cytoflex/software).
Cell proliferation analysis
Cell proliferation was measured by the CCK-8 Kit (Beyotime, #C0038). Cells were cultured with 1640 in 96-well plates (3 × 104 cells per well), and then were incubated with CCK-8 solution at 0 h, 24 h, 48 h, 72 h for 2 h, and absorbance at 450 nm was measured by microplate reader.
Immunoblotting analysis
Total protein was extracted using RAPI lysis buffer (Beyotime) containing 10 μm PMSF (Beyotime, #ST505) and quantified by the BCA kit (Beyotime, #P0010). Equal quality protein per sample was separated by SDS-PAGE (Beyotime, #P0012AC) gel and transferred to PVDF Transfer Membrane (Millpore), membranes were blocked with 5% nonfat milk solution for 2 h then incubated overnight at 4 °C with primary antibodies, followed by incubation with HRP-coupled secondary antibody (Thermo Fisher Scientific). We used the BeyoColor™ (15–120 kD; #P0078) and Thermo Fisher Scientific (10-180kD; #26616) Pre-stained Protein Molecular Weight Marker. Immunoreactive bands were visualized by the Enchanced Chemiluminescence Kit (Thermo Fisher Scientific, #A38556) using the Fine-Do×6 Chemiluminescent Imaging system (Tanon).
qRT-PCR analysis
Total RNA was purified using RNA Quick purification Kit (YEASEN)and used as cDNA synthesis template for reverse transcription with the RevertAid RT kit (Thermo Fisher Scientific, #K1691). qRT-PCR was performed by LightCycle96 real-time PCR system (Roche, China) using SYBR Premix kit (Takara, #CN830S). The primers were synthesized by sangon (Shanghai, China). as follows: GAPDH (Forward: AGCAAGAGCACAAGAGGAAG, Reverse: GGTTGAGCACAGGGTACTTT), RPL5 (Forward: CCGCAGGCTTCTCAATAGG, Reverse: CCTGGCTGACCATCAATGC). Relative expression of mRNA was evaluated by the 2−ΔΔCT method, with GAPDH serving as an internal control.
Immunofluorescence analysis
The cells were grown on glass coverslips and fixed with 4% paraformaldehyde. Cells were permeabilized with 1% Triton X-100 (Sigma, #9002-93-1,) in PBS for 10 min and blocked in 10% goat serum (Solarbio, #SL038) for 30 min at room temperature. Cells were incubated with anti-RPL5 antibodies (Thermo Fisher Scientific, #PA5-106584) dissolved in PBST (Servicebio, G2157-1 L) overnight at 4 °C. After washing thrice with PBST, cells were incubated with goat anti-rabbit IgG (H + L)/AF647 (Bioss, bs-0295G-AF647) antibodies for 2 h at room temperature. The nuclei were stained using DAPI (Beyotime, #C1005). Images were captured using a fluorescence microscope (Axio Scope A1, Zeiss, Germany).
Statistical analysis
Experiment data were analyzed by Graphpad Prism 9.2.0 and represented as the mean ± sd. Two-tailed t-test was used for comparisons between two groups, non-parametric rank and sum test for non-normally distributed data, and the comparison between multiple groups of data is consistent with the normal distribution, one-way ANOVA is used, and when the difference is statistically significant, the LSD method is continued to be used for pair-by-two comparison. If the normal distribution is not present, the Kruskal-Wallis H test is used, and the Mann-Whitney U test is continued to be used for pair-to-pair comparisons when the difference is statistically significant. *represents p < 0.05, **represents p < 0.01, ***represents p < 0.001, and ns represents p > 0.05.
Results
Senescent cell proportion and RPL5 expression level were elevated in relapsed AML patients
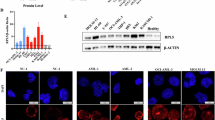
Wright–Giemsa (W-G) staining revealed that leukemic cell morphology and tumor burden were markedly increased in AML patients at relapse compared to patients with iron deficiency anemia (IDA), newly diagnosed (ND) AML, or those in complete remission (CR) (Fig. 1A1-4). Concurrently, SA-β-galactosidase (SA-β-gal) staining showed a significantly higher proportion of senescent cells in the relapse group relative to the IDA, ND, and CR groups (Fig. 1B1-4, quantified in Fig. 1C; p < 0.01). qRT-PCR analysis demonstrated a marked upregulation of RPL5 mRNA expression in relapsed AML patients compared to all other groups (Fig. 1D; *p < 0.05, **p < 0.01), with no significant differences observed among IDA, ND, and CR. Importantly, Western blotting from paired samples of one AML patient at ND, CR, and relapse confirmed that RPL5 protein levels were substantially elevated at relapse, indicating a disease-stage-specific increase (Fig. 1E; Supplementary Fig. S3).
To address heterogeneity, we analyzed RPL5 expression alongside the clinical characteristics of newly diagnosed AML patients. No significant associations were observed between RPL5 levels and age, sex, or bone marrow blast percentage (Supplementary Fig. S1A-C). When stratified by FAB subtype, RPL5 expression was significantly higher in M1 and M2 compared with M5 (Supplementary Fig. S1D), while no significant differences were found across common genetic mutations, including FLT3 and NPM1 (Supplementary Fig. S1E).
Senescent cell proportion and RPL5 expression levels were elevated in relapsed AML patients. (A) W-G staining and (B) SA-β-gal staining of bone marrow smears from patients with IDA or AML patients across ND, CR, and relapse. (C) Quantification of β-gal-positive senescent cells from (B), expressed as the percentage of positive cells per total counted. Values are means ± SEM, based on ≥ 3 fields per sample. (D) Relative mRNA expression of RPL5 in IDA and AML patients across ND, CR, and relapse phases. Data are shown as mean ± SEM; significance was determined using one-way ANOVA. (E) Western blot of RPL5 protein in paired AML samples from ND, CR, and relapse stages from the same patient; β-actin was used as the loading control. * p < 0.05, ** p < 0.01.
Chemotherapy-induced senescence in KG-1 A cells associated with elevated RPL5 expression
To explore the role of RPL5 in senescence, a chemotherapy-induced senescence model in KG-1 A cells using Ara-C (10 µM for 72 h) were established. Morphological SA-β-gal staining demonstrated a significant increase in blue-stained senescent cells following Ara-C treatment compared to untreated controls (Fig. 2A). This was corroborated by flow cytometry, which revealed a rightward shift in fluorescence intensity and a significant increase in the proportion of SA-β-gal-positive cells after Ara-C treatment (Fig. 2B), indicating enhanced cellular senescence. Cell cycle profiling showed a significant accumulation of cells in the G1 phase, with concomitant reductions in S and G2 phase populations in the Ara-C-treated group, consistent with a senescent phenotype (Fig. 2C). Notably, Western blot analysis demonstrated that Ara-C exposure led to a significant increase in RPL5 protein expression compared to the control group (Fig. 2D; Supplementary Fig. S4), suggesting that RPL5 upregulation was a characteristic feature of chemotherapy-induced senescence in AML cells.
In addition, we treated KG-1 A cells with another clinically relevant chemotherapeutic agent, doxorubicin (Dox, 0.8 µM for 48 h)17, and observed similar outcomes: increased SA-β-gal positivity, elevated β-galactosidase activity, and upregulated RPL5 expression (Supplementary Fig. S2 and S10). These findings further support that RPL5 upregulation represents a general cellular response to chemotherapy-induced senescence, rather than a drug-specific effect of Ara-C.
The cellular senescence model was constructed and RPL5 expression was increased in senescent cells. (A) SA-β-gal staining of KG-1 A cells treated with Ara-C (10 µM, 72 h), showing increased senescent cell proportions; right panel shows quantification. (B) Flow cytometry of SA-β-gal–positive cells after Ara-C treatment (top), representative histogram overlay (bottom left), and bar graph quantification (bottom right). (C) Cell cycle analysis via PI staining indicates G1 arrest following Ara-C exposure; right bar graph summarizes G1/S/G2 proportions. (D) Western blot analysis shows elevated RPL5 protein expression after Ara-C treatment; right panel shows densitometric quantification normalized to β-actin. **p < 0.01, ***p < 0.001, ****p < 0.0001, ns: not significant.
RPL5 knockdown enhanced apoptosis, suppressed senescence
To investigate the functional role of RPL5, we established a stable RPL5 knockdown model in KG-1 A cells using shRNA interference. qRT-PCR and Western blot analyses confirmed efficient suppression of RPL5 expression at both mRNA and protein levels in the shRNA-transduced cells, compared to negative control and blank groups (Fig. 3A and B; Supplementary Fig. S5). CCK-8 assays showed that while RPL5 knockdown led to a trend toward reduced proliferation, the difference did not reach statistical significance at 48 h (Fig. 3C). These results were further validated by a longitudinal proliferation assay analyzed using two-way ANOVA, which revealed a significant interaction between group and time (p < 0.05). Although no difference was observed at 0–24 h, divergence became apparent by 48 h, supporting a time-dependent suppression of proliferation in RPL5-silenced cells.
In parallel, Annexin V/PI staining demonstrated a significant increase in apoptosis following RPL5 knockdown (Fig. 3D), indicating that knockdown of RPL5 promotes cell death. Furthermore, both SA-β-gal staining and flow cytometry revealed a marked reduction in the senescent cell population (Fig. 3E and F), highlighting the essential role of RPL5 in maintaining the senescence phenotype in AML cells.
Knockdown of RPL5 increased apoptosis, reduced senescence, and had no effect on proliferation in KG-1 A cells. (A-B) The significant reduction of RPL5 expression at both mRNA and protein levels upon shRNA-mediated knockdown. (C) Downregulation of RPL5 led to decreased cell proliferation capacity, the difference had no statistical significance. (D) RPL5 knockdown resulted in an increase in apoptotic cell proportion, showing significant statistical difference compared to the NC. (E-F) Significantly decreased in senescent cell proportion following RPL5 downregulation, by flow cytometry analysis and senescence staining compared to the NC group. *p < 0.05, **p < 0.01, ***p < 0.001.
Knockdown RPL5 mitigated chemotherapy-induced senescence, enhanced AML cell response to chemotherapy
To further determine the role of RPL5 in chemotherapy-induced senescence, we treated KG-1 A cells with Ara-C (10 µM, 72 h) and compared senescence and apoptosis features between NC and RPL5-knockdown (Sh) cells. SA-β-gal staining revealed a significantly lower proportion of blue-stained senescent cells in the Sh + Ara-C group compared to NC + Ara-C controls (Fig. 4A). This result was corroborated by flow cytometric detection of SA-β-gal activity, which showed a marked decrease in fluorescence intensity and β-gal–positive cell population following RPL5 knockdown (Fig. 4B). Cell cycle analysis showed that RPL5 knockdown relieved Ara-C-induced G1 arrest, reflected by a significant reduction in G1 phase cells and a concomitant increase in S and G2 populations (Fig. 4C). Furthermore, Annexin V/PI staining demonstrated that RPL5 knockdown significantly increased the proportion of apoptotic cells under chemotherapy conditions (Fig. 4D).
Together, these findings indicate that RPL5 is required for the maintenance of chemotherapy-induced senescence and that its suppression facilitates escape from G1 arrest and enhances apoptotic sensitivity, potentially improving therapeutic response in AML.
RPL5 knockdown reversed chemotherapy-induced senescence, but promoted cell cycle and cells apoptosis. (A) SA-β-gal staining shows reduced proportion of senescent cells in the Sh + Ara-C group compared to NC + Ara-C. (B) Flow cytometric analysis of SA-β-gal activity confirms decreased fluorescence intensity and β-gal + cell percentage upon RPL5 knockdown. (C) Cell cycle analysis reveals that RPL5 knockdown reduces G1 phase arrest and increases S and G2 phase fractions after Ara-C exposure. (D) Annexin V/PI staining shows a significant increase in apoptosis rate in Sh + Ara-C compared to NC + Ara-C. Data are presented as mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
RPL5 induced cellular senescence via the p53-p21-pRb pathway
Following chemotherapy induction, a significant increase in the expression of senescence-related proteins, including p53, p21, p16, and pRb, was observed in the Ara-C group compared to the control group (Con)(Fig. 5A, Supplementary Figure S6-7). While Rb expression did not show a significant difference, the upregulation of p53, p21, p16, and pRb was statistically significant, suggesting their involvement in the induction of cellular senescence. Interestingly, knockdown of RPL5 led to a significant reduction in the expression of RPL5, p53, p21, and p16 compared to the NC group (Fig. 5B, Supplementary Figure S8-9). Following chemotherapy treatment, RPL5, p53, p21, and p16 expression levels were markedly increased in the NC group, surpassing those of both the Sh and NC groups. These results suggest that RPL5 plays a crucial role in mediating the upregulation of aging-related proteins induced by chemotherapy. This dysregulation likely contributes to cellular senescence observed after chemotherapy withdrawal, which may lead to disease relapse in AML patients. Our findings highlight the importance of the p53-p21-pRb signaling pathway, modulated by RPL5, in chemotherapy-induced senescence and potential disease relapse in AML.
Chemotherapy led to upregulation of senescence-related proteins. (A) Increased expression of p53, p21, p16 and pRb proteins after chemotherapy in AML cells. (B) RPL5 knockdown decreased their expression, while chemotherapy treatment boosted levels. * represents *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns represents p > 0.05 compared to NC group, ■represents compared to NC: ■p < 0.05, ■■p < 0.01; ▴represents compared to NC + Ara-c: ▴p > 0.05.
RPL5 protein translocated to nucleus regulated senescence-associated proteins in AML cells
To validate whether RPL5 protein in AML cells engages in extraribosomal functions by translocating to the nucleus during the senescence process, thereby influencing the expression levels of the senescence-associated proteins p53/p21 and pRb, we utilized IF to assess the relative cellular localization of RPL5 protein in NC, Sh, NC + Ara-c, and Sh + Ara-c of KG-1 A cells (Fig. 6). It was revealed that in the NC + Ara-c, there was a significant elevation in RPL5 protein expression compared to the NC. Furthermore, the RPL5 protein in the NC + Ara-c group co-localized with the DAPI-stained cell nuclei, indicative of translocation from the cytoplasm to the nucleus relative to the NC group. Notably, such translocation phenomena were absent in the Sh + Ara-c and Sh. These observations underscored that, under the chemotherapy drug Ara-c, RPL5 protein in KG-1a cells exerted extraribosomal functions by translocating into the nucleus, thereby modulated the expression levels of senescence-associated proteins p53/p21 and pRb, ultimately regulated cellular senescence.
RPL5 downregulation suppressed proliferation and senescence in AML cells after chemotherapy
To assess whether RPL5 contributes to AML cell regrowth following chemotherapy-induced senescence, we monitored cell recovery dynamics after Ara-C withdrawal. KG-1 A cells with or without RPL5 knockdown were exposed to Ara-C (10 µM, 72 h), followed by drug withdrawal and extended culture for up to 15 days.
Microscopy at Day 18 revealed marked regrowth and increased cellular density in the NC + Ara-C group (Fig. 7A2), while Sh + Ara-C cells remained sparse with signs of ongoing apoptosis (Fig. 7B2). Consistent with this, SA-β-gal staining showed persistent senescence in NC + Ara-C cells (Fig. 7A3), whereas Sh + Ara-C cells exhibited reduced SA-β-gal positivity (Fig. 7B3), indicating impaired senescence rebound upon RPL5 silencing.
Proliferation assays showed similar OD values between NC + Ara-C and Sh + Ara-C groups during the first 8 days (Fig. 7C). From Day 9 onward, NC + Ara-C cells resumed growth, while Sh + Ara-C cells remained arrested. Two-way ANOVA confirmed a significant group × time interaction (p < 0.01), highlighting divergent recovery patterns. These findings indicate that RPL5 is not required for steady-state proliferation but is critical for AML cell recovery from chemotherapy-induced senescence, suggesting a potential role in relapse.
Downregulation of RPL5 after chemotherapy inhibited both proliferation and senescence in AML cells. (A–B) Microscopy images on Day 3 and Day 18 after Ara-C treatment show robust regrowth and residual senescence in NC + Ara-C group versus impaired recovery and reduced β-gal staining in Sh + Ara-C. (C) Long-term CCK-8 assays reveal divergent proliferation trajectories post-Ara-C withdrawal. NC + Ara-C cells resumed proliferation from Day 9, whereas Sh + Ara-C cells remained growth-arrested (****p < 0.0001 by two-way ANOVA). Data are mean ± SD.
Discussion
Recent studies in leukemia research have underscored the role of cellular senescence not merely as a tumor-suppressive barrier but as a facilitator of relapse in AML18,19. Despite the recognized significance of cellular senescence in AML relapse, the precise underlying pathways remained elusive.
RPL5, a core component of the ribosome, has been increasingly recognized for its involvement in tumor progression through aberrant expression or mutation10,11,16. Here, we identify RPL5 as a key mediator of chemotherapy-induced senescence and subsequent relapse in AML. Patients with relapsed AML exhibited significantly higher levels of senescent cells and RPL5 expression compared to those at diagnosis or remission (Fig. 1), suggesting RPL5 as a relapse-associated biomarker.
We established senescence models using Ara-C and doxorubicin in AML cells, both of which significantly upregulated RPL5 expression (Fig. 2). This indicates that RPL5 acts as a universal responder to genotoxic stress, independent of drug class20,21. Knockdown of RPL5 alleviated senescence, enhanced apoptosis (Figs. 3 and 4), and impaired cellular regrowth after chemotherapy (Fig. 7), underscoring its essential role in post-treatment recovery and relapse.
Mechanistically, our findings align with the well-established RPL5–MDM2–p53 axis. RPL5 can inhibit the E3 ligase activity of MDM2, stabilizing p53 and activating the p53–p21–pRb pathway under nucleolar stress22,23. We further observed nuclear translocation of RPL5 and subsequent pathway activation (Figs. 5 and 6), reinforcing its extraribosomal role. Moreover, only cells with preserved RPL5 expression resumed proliferation after chemotherapy withdrawal, further confirming its relapse-promoting function24,25. Emerging evidence indicates that p53 signaling also modulates immune checkpoints. p53 activation has been shown to upregulate PD-L1 via HIF-1α26 and activate cGAS-STING signaling leading to with CD47 blockade - an immune checkpoint inhibitor - synergistically reduced leukemic burden combining PLK4 inhibition23, suggesting that RPL5 may influence AML immune escape via p53 stabilization-an area meriting further exploration.
Although RPL5 is ubiquitously expressed (Human Protein Atlas), its stress-induced nuclear functions may offer a therapeutic window. Rather than direct inhibition, targeting its nuclear translocation or MDM2 interaction may selectively impair its pro-senescent function while preserving basal translational activity.
In conclusion, our study identifies RPL5 as a pivotal regulator of chemotherapy-induced senescence and relapse in AML via the p53–p21–pRb pathway. These findings propose a novel “senescence–survival axis” and provide a foundation for developing targeted strategies to overcome resistance and recurrence in AML.
Conclusions
Our findings suggested that RPL5 played a pivotal role by activating the p53-p21-Rb pathway in mediating cellular senescence, chemotherapy response, and disease relapse in AML. Additionally, senescent AML cells could resume proliferation after chemotherapy withdrawal, ultimately contributing to disease recurrence in AML patients. It highlighted the potential of RPL5 as a therapeutic target for overcoming chemotherapy resistance and preventing disease relapse.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Pelcovits, A. & Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Island medical journal 2020;103(3):38–40. (2013).
Duy, C. et al. Chemotherapy induces senescence-like resilient cells capable of initiating AML recurrence. Cancer Discov.11(6), 1542–1561 (2021).
te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62 (6), 1876–1883 (2002).
Toussaint, O., Medrano, E. E. & von Zglinicki, T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol.35(8), 927–945 (2000).
Collado, M., Blasco, M. A. & Serrano, M. Cellular senescence in cancer and aging. Cell 130 (2), 223–233 (2007).
Milanovic, M. et al. Senescence-associated reprogramming promotes cancer stemness. Nature 553 (7686), 96–100 (2018).
Saleh, T. et al. Tumor cell escape from therapy-induced senescence. Biochem. Pharmacol.162, 202–212 (2019).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol.28(6), 436–453 (2018).
Goudarzi, K. M. & Lindström, M. S. Role of ribosomal protein mutations in tumor development (Review). Int. J. Oncol.48(4), 1313–1324 (2016).
Fancello, L., Kampen, K. R., Hofman, I. J., Verbeeck, J. & De Keersmaecker, K. The ribosomal protein gene RPL5 is a haploinsufficient tumor suppressor in multiple cancer types. Oncotarget8(9), 14462–14478 (2017).
Kang, J. et al. Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy. Signal. Transduct. Target. Therapy. 6 (1), 323 (2021).
Han, T. et al. Olaparib induces RPL5/RPL11-dependent p53 activation via nucleolar stress. Front. Oncol.12, 821366 (2022).
Tong, D. et al. MECP2 facilitates breast cancer growth via promoting ubiquitination-mediated P53 degradation by inhibiting RPL5/RPL11 transcription. Oncogenesis9(5), 56 (2020).
Jung, J. H. et al. Colocalization of MID1IP1 and c-Myc is critically involved in liver cancer growth via regulation of ribosomal protein L5 and L11 and CNOT2. Cells 9(4), 985. https://doi.org/10.3390/cells9040985 (2020).
Li, Y. et al. WDR74 modulates melanoma tumorigenesis and metastasis through the RPL5-MDM2-p53 pathway. Oncogene 39 (13), 2741–2755 (2020).
Park, J. E. et al. Ribosomal protein L5 mediated Inhibition of c-Myc is critically involved in Sanggenon G induced apoptosis in non-small lung cancer cells. Phytother. Res. 35 (2), 1080–1088 (2021).
Chueahongthong, F., Tima, S., Chiampanichayakul, S., Berkland, C. & Anuchapreeda, S. Co-treatments of edible curcumin from turmeric rhizomes and chemotherapeutic drugs on cytotoxicity and FLT3 protein expression in leukemic stem cells. Moleculeshttps://doi.org/10.3390/molecules26195785 (2021).
Miller, D. et al. Heterogeneity in leukemia cells that escape drug-induced senescence-like state. Cell Death Dis.14(8), 503 (2023).
Tomiyasu, H. et al. FOXO1 promotes cancer cell growth through MDM2-mediated p53 degradation. J. Biol. Chem.300(4), 107209 (2024).
Marmisolle, I. et al. Oncogene-induced senescence mitochondrial metabolism and bioenergetics drive the secretory phenotype: further characterization and comparison with other senescence-inducing stimuli. Redox Biol. 82, 103606 (2025).
Wang, J. et al. Chemotherapy-induced cellular senescence promotes stemness of aggressive B-cell non-Hodgkin’s lymphoma via CCR7/ARHGAP18/IKBα signaling activation. J. Immunother. Cancer 13(1), e009356. https://doi.org/10.1136/jitc-2024-009356 (2025).
de Castillo Duque, N. M. et al. Structure of nascent 5S RNPs at the crossroad between ribosome assembly and MDM2-p53 pathways. Nat. Struct. Mol. Biol. 30 (8), 1119–1131 (2023).
Lindström, M. S., Bartek, J. & Maya-Mendoza, A. P53 at the crossroad of DNA replication and ribosome biogenesis stress pathways. Cell Death Differ.29(5), 972–982 (2022).
Zhang, Y. & Lu, H. Signaling to p53: Ribosomal proteins find their way. Cancer Cell.16(5), 369–377 (2009).
Kim, T. H., Leslie, P. & Zhang, Y. Ribosomal proteins as unrevealed caretakers for cellular stress and genomic instability. Oncotarget5(4), 860–871 (2014).
Hayashi, Y. et al. Antitumor immunity augments the therapeutic effects of p53 activation on acute myeloid leukemia. Nat. Commun.10(1), 4869. (2019).
Acknowledgements
We thank all the patients who gave consent to disclose their medical records and answered our review calls.
Funding
This work was supported by Anhui Natural Science Foundation Project (No.2208085MH217) and Research Fund of Anhui Institute of Translational Medicine (No. 2022zhyx-C51, 2023zhyx-C81, 2023zhyx-C86).
Author information
Authors and Affiliations
Contributions
QS Tao and ZM Zhai conceptualized and designed the study. WQ Zhang and LL Liu were responsible for cell experiments and molecular experiments. RT Chen, HT Yan, Q Zhang collected data and were involved in analysis. XY Ren, HP Wang and WY Xue were responsible for flow cytometry data analysis. WQ Zhang, LL Liu and RT Chen wrote the original draft. QS Tao was responsible for editing the draft. QS Tao and Zhimin Zhai was responsible for review and editing the draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, W., Liu, L., Chen, R. et al. Ribosomal protein L5 induces cellular senescence via p53-p21-pRb pathway to mediate relapse of acute myeloid leukemia. Sci Rep 15, 27649 (2025). https://doi.org/10.1038/s41598-025-12108-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-12108-1