Abstract
This study investigates the correlation between chest computed tomography (CT) radiomics features and breast density classification, and aiming to develop an automated radiomics model for breast density assessment using chest CT images. The diagnostic performance was evaluated to establish a CT-based alternative for breast density classification in clinical practice. A retrospective analysis was conducted on patients who underwent both mammography and chest CT scans. The breast density classification results based on mammography images were used to guide the development of CT-based breast density classification models. Radiomic features were extracted from breast regions of interest (ROIs) segmented on chest CT images. The diagnostic performance was evaluated to establish a CT-based alternative for breast density classification in clinical practice. Following dimensionality reduction and selection of dominant radiomic features, four four-class classification models were established, including ① Extreme Gradient Boosting (XGBoost), ② One Vs Rest Classifier-Logistic Regression, ③ Gradient Boosting, and ④ Random Forest Classifier. The performance of these models in classifying breast density using CT images was then evaluated. A total of 330 patients, aged 23–79 years, were included for analysis. The breast ROIs were automatically segmented using a U-net neural network model and subsequently refined and calibrated manually. A total of 1427 radiomic features were extracted, and after dimensionality reduction and feature selection, 28 dominant features closely associated with breast density classification were obtained to construct four classification models. Among the tested models—XGBoost, One-vs-Rest Logistic Regression, Gradient Boosting Classifier, and Random Forest Classifier—the XGBoost model achieved the best performance, with a classification accuracy of 86.6%. Analysis of the receiver operating characteristic curves showed Area Under the Curve (AUC) values of 1.00, 0.93, 0.93, and 0.99 for the four breast density categories, along with a micro-averaged AUC of 0.97 and a macro-averaged AUC of 0.96. Chest CT scans, combined with imaging radiomics models, can accurately classify breast density, providing valuable information related to breast cancer risk stratification. The proposed classification model offers a promising tool for automated breast density assessment, which could enhance personalized breast cancer screening and clinical decision-making.
Similar content being viewed by others
Introduction
Since the 21st century, there has been a notable rise in both the rates of breast cancer diagnosis and the associated mortality. Globally, breast cancer ranks as the most frequent malignancy among women. In China, the number of breast cancer incidence and deaths is projected to increase by 36.3% and 54.0% by 2030, respectively1, imposing a substantial disease and economic burden. Therefore, it is crucial to accurately risk-stratify the occurrence of breast cancer and select appropriate imaging screening programs to effectively address this challenge.
Breast density, defined as the ratio of glandular to adipose tissue, is classified into four categories according to the fifth edition of the Breast Imaging-Reporting and Data System (BI-RADS)2. Dense breast tissue is strongly associated with an increased risk of developing breast cancer, with a substantial proportion of breast cancer cases being attributed to this risk factor3. In view of this risk factor, legislation exists in developed countries such as the United States requiring that information about breast density be communicated to patients undergoing mammograms4. Women with dense breast tissue (classified as the two highest BI-RADS categories) are commonly advised to undergo adjunctive screening modalities, such as ultrasonography or magnetic resonance imaging (MRI), to enhance the early detection of breast cancer3,4,5,6.
Although prior studies have demonstrated that annual screening mammography starting at age 40 can significantly reduce mortality and extend life expectancy7, compliance with mammography screening remains suboptimal among Chinese women. The feasibility of assessing breast density using CT has been well-established in a 2014 study by Salvatore et al.8.
CT scans, primarily performed for indications such as lung cancer screening, frequently yield additional diagnostic insights into comorbid conditions, including cardiovascular diseases9,10. Therefore, many women who have undergone chest CT but not mammography may potentially benefit from the additional breast cancer risk stratification information, provided that breast density can be reliably assessed from CT images7,8,11. Breast density assessment is critical due to its strong association with increased breast cancer risk3,12,13,14, and adjunctive screening for women with dense breasts has been shown to improve cancer detection rates4,15,16,17. However, breast density evaluation is not only influenced by factors such as false positives in ultrasound15,18 but also suffers from inter-observer variability19. Prior studies have highlighted the poor reproducibility of mammography-based breast density assessments16, which can adversely impact subsequent screening strategies and clinical protocols.
The objective of this study was to evaluate the concordance between chest CT and mammography in breast density assessment via radiologist image interpretation and to develop an automated breast density classification method for chest CT using imaging radiomics, thereby facilitating a novel approach to breast cancer screening.
Data and methods
Study participant
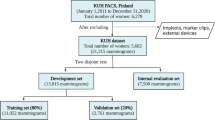
Retrospectively, we analyzed the medical records of breast-healthy patients who underwent mammography and breast ultrasound imaging with negative results in our hospital from January 2021 to December 2022 and underwent chest CT scans within 12 months of the mammography. Initially, A total of 399 cases were included, and 6 cases were excluded due to image quality issues, resulting in 393 female patients for final analysis. Inclusion criteria: ① negative mammography and ultrasound results; ② chest CT performed at our hospital within one year. Exclusion criteria: ① breast lesions detected in any examination; ② incomplete image data; ③ image noise/distortion incompatible with machine learning models.
This study was approved by the Ethical Committee of the First Hospital of Jilin University (Approval Number: [2024-1027]) with informed consent waived due to its retrospective nature. De-identified data were accessed on October 10, 2024, and individual participant identities were not retrievable during or after data collection. The research plan and experiments were conducted in accordance with the provisions of the clinical research project at the First Hospital of Jilin University.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Data is provided within the supplementary information files.
Data collection and modelling
Mammography and non-contrast chest CT scan images were collected, and two radiologists independently classified breast density into four categories using a blind method. Only patients with consistent breast density classifications between the two radiologists (based on mammography images) were included in the dataset.
Following layer-by-layer delineation and verification of four ROIs (left breast, right breast, left axilla, and right axilla) on non-contrast chest CT sequence images, feature extraction, screening, and dimensionality reduction were performed to identify dominant features. The dominant feature dataset was then used to construct multiple four-class prediction models, evaluate their predictive performance, and compare results to determine the optimal model.
Image acquisition
Patient imaging data from the First Hospital of Jilin University were downloaded from the hospital’s Picture Archiving and Communications System (PACS). Image acquisition utilized multiple CT scanners, including Siemens Sensation64, Siemens Definition, NeuViz128, GE Revolution CT256, and Philips Brilliance CT.
Given the retrospective nature of the datasets, CT acquisition protocols varied across patients. Acquisition parameters were as follows: tube voltage of 120kV, tube current adjusted according to the actual situation, matrix of 512×512, slice thickness/ interval of 5-7 mm, reconstructed slice thickness of 1.0 mm, window width (WW) of 300–500 Hu, and window level (WL) of 30–50 Hu.
Scans were performed with subjects in the supine position, arms positioned at the sides, and completed in a single breath hold. The scanning range extended from the lung apex to the adrenal glands.
The mammography machine used in this study was Hologic Lorad M-I. The parameters for the scanning process were established as follows: 2D Mammography. Tube voltage (kV): 22–35 kV, typical 28 KV. Tube current (mA): 10 mA–100 mA, typical 50 mA. Exposure time (sec): 0.1–10 s, typical 0.5 s. Focus size: 0.1 mm for microfocus, 0.3 mm for conventional focus. Image size: Maximum film size: 18 cm × 24 cm.
Image analysis and feature extraction
Image analysis
In accordance with BI-RADS2, each patient’s mammogram or non-contrast chest CT image was visually categorized into one of four classes using a rough quartile evaluation method. Specifically, Category A denoted breasts composed predominantly of fat; Category B indicated scattered fibroglandular tissue; Category C signified heterogeneous breast density; and Category D represented extremely dense breast tissue.
To assess the consistency of breast density evaluation, the Weighted Kappa Statistic was employed. This statistical measure was used to evaluate the agreement in breast density assessment between the two different imaging modalities for each radiologist, as well as the interobserver agreement for each examination independently.
Only patients with concordant four-category breast density classification results, as determined by two radiologists based on mammography images (serving as the criteria), were included in the dataset for developing the four-category breast density classification model.
Breast region of interest segmentation
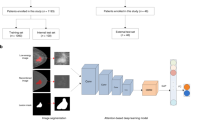
In this study, the in-house developed RIASEG platform was used to complete the automatic delineation of the ROI, and the self-developed RMIT software was employed for image preprocessing. Subsequently, the RIASEG platform software was utilized to perform slice-by-slice segmentation (automatic delineation) and manual refinement/verification of bilateral breast ROIs on non-contrast chest CT sequence images. These software programs, which include functions such as image annotation, DICOM file preprocessing, image segmentation, and data processing, have been validated in prior studies12.
Due to the indistinct boundaries between the breast and surrounding structures on CT images, this study integrated imaging interpretation experience with the anatomical theory of the breast superficial fascial system13 to establish breast ROI delineation criteria: for unilateral breasts, the upper boundary was defined as the first rib at the midclavicular line, the lower boundary as the sixth rib at the midclavicular line, the inner boundary as the median line, and the lateral boundary as the posterior axillary fossa. This delineation protocol ensured excellent consistency and reproducibility in ROI selection.
Following slice-by-slice segmentation and manual verification of all enrolled chest CT images, the software computed the Dice coefficient (Dice Similarity Coefficient) using automatic segmentation results and manually refined outcomes to assess segmentation performance. Dice coefficients for the four labeled regions were as follows: 0.941 for the right breast, 0.942 for the left breast, 0.913 for the right axilla, and 0.921 for the left axilla. All labels exhibited Dice coefficients > 0.9, demonstrating excellent segmentation accuracy.
Following model-based segmentation of non-contrast chest CT images, two radiologists performed layer-by-layer refinement and validation of bilateral breast ROIs. The bilateral ROIs were then merged into an entire breast ROI for subsequent feature extraction. Representative illustrations of the slice-by-slice delineation and validation results for the entire breast ROI are shown in Fig. 1.
Breast ROI region feature extraction and dimensionality reduction
Morphological features, texture features, first-order statistical features, filtered features, and other radiomic features were extracted from the entire breast ROI using Pyradiomics software (http://pyradiomics.html). Feature screening was initially performed by calculating the intra-class correlation coefficient (ICC), followed by a t-test, and further refined using a lasso-cv regression model.
Feature testing involved fitting a univariate logistic regression model (relating single feature variables to classification outcomes) and analyzing model summary statistics to determine if pre-screened features significantly influenced classification results (p < 0.05).
Establishment of imaging radiomics models and efficacy assessment
The data samples exhibited severe class imbalance in breast density classifications. Specifically, the dataset included 26 cases (7.88%) of class 0 (fatty), 133 cases (40.30%) of class 1 (fibroglandular), 149 cases (45.15%) of class 2 (heterogeneously dense), and 22 cases (6.67%) of class 3 (extremely dense). This pronounced imbalance posed risks for modeling, including potential overfitting and compromised evaluation metric validity.
Thus, the SMOTE function from the imblearn package in Python 3.6 was employed to up-sample the four-class dataset and address class imbalance. Following this processing, the resulting dataset comprised 596 samples, with 179 instances per class label. Considering that ensemble learning algorithms (XGBoost, Gradient Boosting, Random Forest) have strong dimensionality reduction capabilities and noise resistance for high-dimensional radiomic features, and show stronger robustness against feature distribution shifts caused by differences in CT equipment parameters (such as tube voltage and slice thickness). Additionally, compared with deep learning (e.g., CNN) or Support Vector Machine (SVM), they have better generalization performance and higher computational efficiency in the case of small and medium sample sizes. Therefore, this paper establishes four breast density classification models: XGBoost, Gradient Boosting, Random Forest, and One Vs Rest Classifier-Logistic Regression. ROC curve and DCA were applied to evaluate the prediction efficiency of the models for each classification. The calibration curve was used to assess the prediction accuracy of the models for each classification. The overall performance of the models was evaluated using macro-average and micro-average.
Breast density staging criteria
In BI-RADS version 5, specific percentage-based quartile criteria for breast density evaluation were replaced by a radiologist-driven visual assessment using rough quartile categorization2,14. Mammography, widely used for 40 years in asymptomatic women for early breast cancer screening, remains the imaging gold standard15. Thus, in this study, breast density types determined by mammographic images were adopted as the reference standard for patient classification.
Statistical analysis
Statistical analyses were performed using Rstudio 4.4.0. For continuous measurements (i.e., data for each characteristic), the data were stratified into four groups according to breast density classification labels for ANOVA, with statistical significance defined as P < 0.05. Each group’s data underwent normality testing and were described as (x ± s) if conforming to a normal distribution and exhibiting homogeneous variance, or as (median [interquartile range]) otherwise. Inter-radiologist consistency for breast density assessments across the two imaging modalities, as well as intra-modality interobserver agreement, were evaluated using the Weighted Kappa Statistic (WKS). Radiomic feature consistency was assessed via intraclass correlation coefficients (ICCs), with ICC ≥ 0.75 denoting good reliability. Model efficacy was evaluated using multiple metrics, including ROC curves with AUC, DCA curves, precision-recall curves, accuracy, specificity, sensitivity, F1 scores, positive/negative predictive values, and micro/macro-averaged statistics.
Results
Extraction of imaging radiomics features and construction of classification models
Feature extraction
A total of 1427 image features were extracted from the delineated breast regions in non-contrast CT images. Feature screening commenced with inter- and intra-observer consistency analyses (ICCs): 1338 features with ICC ≥ 0.75 (indicating reliable consistency) were retained for further analysis, while 89 features with ICC < 0.75 were excluded. A t-test was then applied to the 1338 retained features for significance screening, identifying 757 discriminative features critical for classification. The LASSO algorithm was used for subsequent feature selection: as the hyperparameter α varied, the mean square error (MSE) of the fitted model’s loss function changed (Fig. 2), along with the feature weight coefficients (Fig. 3). When α was optimized to 0.0202 (minimizing MSE), 729 features had zero weight coefficients (removed as non-important), leaving 28 features with non-zero weights—the final dominant features. The filtered features and their weights are shown in Fig. 4, with category-specific statistical data listed in Table 1.
Establishment and evaluation of four-classification imaging radiomics prediction model
XGBoost classification model
XGBoost algorithm parameter tuning was conducted, with optimal hyperparameters identified through 823,543 iterations of random parameter search. The XGBoost model was trained using the training dataset and applied to classify the test dataset. The training dataset comprised 417 cases: 106 in class 0, 104 in class 1, 101 in class 2, and 106 in class 3. The test dataset included 179 cases: 43 in class 0, 45 in class 1, 48 in class 2, and 43 in class 3.
When verified on the test set data, the precision rates for the prediction of each classification label from 0 to 3 are 0.91, 0.87, 0.77, and 0.98 respectively. The sensitivity rates are 0.95, 0.67, 0.85, and 0.98 respectively. The F1-scores are 0.93, 0.72, 0.81, and 0.98 respectively. The overall accuracy ACC = 0.86 (the overall accuracy of each classification label from 0 to 3), and the Cohen’s Kappa coefficient = 0.814. AUC of the receiver operating characteristic (ROC) curves for the four classification labels, as well as their micro-average and macro-average values, are 1.00, 0.93, 0.93, 0.99, 0.97, and 0.96 respectively (as shown in Fig. 5).
Finally, the calibration curves for the XGBoost model performing the four-class classifications of breast density on the test set data was plotted, as shown in Fig. 6. and the decision curve was plotted, as shown in Fig. 7.
Comparison of four four-classification models
Four four-classification models were constructed after parameter optimization using the same tuning method described above: One Vs Rest-Logistic Regression (Kernel), Gradient Boosting, Random Forest, and XGBoost. These models were validated using the identical test set data mentioned earlier, and their performance metrics are compared in Table 2.
The confusion matrices derived from each model’s classification results are shown in Fig. 8. The AUC values for classes 0-3, along with their micro-averaged and macro-averaged ROC curves (as shown in Fig. 9), were as follows: Model 1: 0.71, 0.58, 0.72, 0.57, 0.56, 0.65; Model 2: 0.99, 0.91, 0.94, 0.99, 0.97, 0.96; Model 3: 0.99, 0.92, 0.95, 0.99, 0.97, 0.96; Model 4: 0.99, 0.93, 0.96, 1.00, 0.97, 0.97.
An independent validation dataset comprising 300 samples was constructed using feature data extracted from images of different devices. For the four classification models described above, 50 classification experiments were conducted using the Bootstrap resampling method: 70% of the samples were randomly selected as the training set and 30% as the test set in each experiment, and the classification accuracy was calculated. The results of 50 tests were finally aggregated to obtain the average accuracy and 95% confidence interval of each model. The ACC values (mean ± standard deviation) and their 95% confidence intervals (CIs) for the four models were: 0.247 ± 0.027 [95% CI 0.239–0.255], 0.810 ± 0.029 [95% CI 0.802–0.818], 0.822 ± 0.025 [95% CI 0.815–0.829], and 0.841 ± 0.028 [95% CI 0.823–0.868]. These results indicated that among the models established in this study, three breast density classification models had exhibited good robustness for plain chest scan images derived from different CT devices, and these models had possessed strong generalization ability.
A comprehensive comparison of the four models revealed: the combined classifier using the One Vs Rest multi-classification strategy showed a very low classification accuracy (< 50%), whereas the self-constructed Gradient Boosting, Random Forest, and XGBoost classifiers all achieved accuracies exceeding 80%. The parameter-tuned XGBoost model demonstrated the best predictive performance, making it well-suited for modeling and predicting breast density classification.
Discussion
This study aimed to automatically classify breast density types using CT images and, given that chest CT examinations far outnumber mammograms, provide breast density—a key breast cancer risk factor—as an additional metric via routine chest CT scans. This information can inform more personalized breast cancer screening strategies. The results showed that chest CT images, combined with an imaging radiomics model, can accurately assess breast density. The automated model effectively categorized breast density into four BI-RADS types (fatty, fibroglandular, heterogeneously dense, and extremely dense), with the XGBoost classifier demonstrating optimal performance: 86% overall accuracy, and excellent sensitivity/specificity for all categories. These findings validate CT as a viable alternative for breast density assessment, particularly for women who have undergone chest CT but not mammography, thereby enhancing risk stratification and screening efficacy.
After blinded interpretation of 330 chest CT-mammography images, demonstrated that CT was consistent with mammography for breast density type judgement and that CT had higher inter-observer agreement. This finding is consistent with previous studies using breast-specific 3D imaging techniques such as digital breast tomosynthesis digital breast tomosynthesis (DBT)20,21, which may indicate that the inter-observer agreement for judging breast density on 3D images is higher than on 2D images. Therefore, if CT can determine breast density, it has important implications for optimizing the process of early breast cancer detection.
The ambiguous delineation of breast ROI in chest CT has long posed a critical challenge for related research. Owing to the lack of distinct anatomical boundaries, the determination of ROI scope prioritizes consistency over absolute accuracy. This study establishes a standardized breast ROI delineation protocol based on the anatomical theory of breast superficial fascia22 and cumulative experience in imaging interpretation. Although the defined range may involve slight expansion, findings demonstrate that this protocol not only ensures consistent ROI delineation across cases but also maintains the stability and accuracy of the delineated results.
In this study, the LASSO algorithm was applied to screen the obtained RFs with ICC≥0.75, to exclude redundant features, and finally 28 image radiomics features that are very closely related to the type of breast density were obtained. Among them, feature quantities such as the original grey covariance matrix MCC with larger weight coefficients describe the texture nature of the image, features such as the logarithm of the inverse variance of the grey covariance matrix describe the uniformity of the image texture, and features such as the logarithm of the first-order skewness describe the degree of grey-scale skewness of the image; in general, the vast majority of the dominant features belong to the texture nature of the image, which is in accordance with the definition of breast density.
During the model training process, a variety of four-classification imaging radiomics prediction models were trained. The AUC values of the optimal model, XGBoost, for each classification (0–3) and its micro-averaged and macro-averaged ROC curves were 0.97, 0.97, 0.99, 0.93, 0.96, and 1.00 respectively. This demonstrated that the model had a good evaluation efficacy.
In addition to the four four-class radiomics models described in this paper, this study conducted an in-depth investigation of the combined classifiers based on the One Vs Rest Classifier multiclass classification strategy. One-Vs-Rest constructs four binary classifiers (each classifier takes one class as the positive class and the remaining three classes as the negative class). For these binary classifiers, methods such as Logistic Regression, Support Vector Machine (SVM), and Decision Tree can be adopted, and we have carried out experimental studies on all of them. However, their classification accuracies are all less than 50%, indicating that the classifiers using the One-Vs–Rest strategy have unsatisfactory accuracy in classifying breast density based on CT images.
With respect to each classification category ranging from 0 to 3, the classification performance of all evaluated models for class 1 was notably inferior, followed by that for class 2. This phenomenon can potentially be attributed to the high degree of similarity in the breast density image features between class 1 (fibroglandular) and class 2 (heterogeneously dense). These two categories are widely recognized as significant challenges within the domain of breast density classification. Consequently, artificial intelligence models are prone to misclassify class 1 and class 2, leading to a substantial disparity in classification performance, with these two classes demonstrating markedly poorer results compared to the other classes (class 0 and class 3).
While the classification model developed in this study demonstrates promising predictive utility, several limitations warrant consideration. First, the study’s sample size was limited, and the absence of independent multi-center external data for validation may introduce selection bias. Second, the relatively small dataset used for model development exhibited skewed class distribution, potentially affecting model training efficiency. Finally, this research focused solely on BI-RADS-defined four-class breast density classification using chest CT ROIs, without segmenting breast glands to quantify percent breast density. The clinical utility of this additional metric remains undefined.
Conclusions
This study demonstrates that automated segmentation and manual correction of breast regions of interest (ROIs) on chest CT images, combined with an imaging radiomics model, enables effective breast density classification. The proposed method reliably discriminates different breast density categories, presenting a viable alternative to mammography for breast cancer risk assessment—a critical component of risk stratification. These findings lay the groundwork for developing precise, personalized breast screening protocols, offering a valuable tool to enhance clinical decision-making and optimize early detection strategies.
Drawing on anatomical theories of the superficial mammary fascia and prior imaging interpretation experience, this study innovatively introduces a criterion for defining breast ROIs. When radiomic feature data derived from this delineation criterion were applied to the breast density classification model, it achieved exceptional performance. This validation confirms the criterion’s efficacy and feasibility, offering a valuable reference for similar studies.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Data is provided within the supplementary information files.
References
He, S. et al. Age distribution and change trend of female breast cancer disease burden in the world and China. Chin. Tumors 32(01), 1–7 (2023).
Spak, D. A., Plaxco, J. S., Santiago, L., Dryden, M. J. & Dogan, B. E. BI-RADS® fifth edition: A summary of changes. Diagn. Interv. Imaging 98(3), 179–190 (2017).
Boyd, N. F. et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 356(3), 227–236 (2007).
Hooley, R. J. et al. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09–41. Radiology 265(1), 59–69 (2012).
Allajbeu, I. et al. Introduction of automated breast ultrasound as an additional screening tool for dense breasts in the UK: a practical approach from the BRAID trial. Clin. Radiol. 79(5), e641–e650 (2024).
Yamashita, M. W. et al. Comparison of mammography and mammography with supplemental whole-breast US tomography for cancer detection in patients with dense breasts. Radiology 311(3), e231680–e231694 (2024).
Hendrick, R. E. & Helvie, M. A. United states preventive services task force screening mammography recommendations: Science ignored. AJRAm J. Roentgenol. 196(2), W112-116 (2021).
Salvatore, M. et al. Breast density: Comparison of chest CT with mammography. Radiology 270(1), 67–73 (2014).
Ichikawa, K. et al. Thoracic versus coronary calcification for atherosclerotic cardiovascular disease events prediction. Heart 110(14), 947–953 (2024).
Groen, R. A. et al. Evaluation of clinical applicability of coronary artery calcium assessment on non-gated chest computed tomography, compared with the classic agatston score on cardiac computed tomography. Am. J. Cardiol. 208, 92–100 (2023).
Duncan, A. M. et al. Anatomy of the breast fascial system: A systematic review of the literature. Plast. Reconstr. Surg. 149(1), 28–40 (2022).
Li, M. et al. Development and assessment of an individualized nomogram to predict colorectal cancer liver metastases. Quant. Imaging Med. Surg. 10(2), 397–414 (2020).
Tari, D. U., Santonastaso, R., De-Lucia, D. R., Santarsiere, M. & Pinto, F. Breast density evaluation according to BI-RADS 5th edition on digital breast tomosynthesis: AI automated assessment versus human visual assessment. J. Pers. Med. 13(4), 609–615 (2023).
Cömert, D., van Gils, C. H., Veldhuis, W. B. & Mann, R. M. Challenges and changes of the breast cancer screening paradigm. J. Magn. Reson. Imaging 57(3), 706–726. https://doi.org/10.1002/jmri.28495 (2023).
Mariapun, S. et al. Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: A case control study. Breast Cancer Res. 26(1), 79. https://doi.org/10.1186/s13058-024-01829-2 (2024).
Kim, E. Y. et al. Mammographic breast density, its changes, and breast cancer risk in premenopausal and postmenopausal women. Cancer 126(21), 4687–4696 (2020).
Tran, T. X. M., Moon, S. G., Kim, S. & Park, B. Association of the interaction between mammographic breast density, body mass index, and menopausal status with breast cancer risk among Korean women. JAMA Netw. Open 4(12), e2139161 (2021).
Hooley, R. J. et al. Screening US in patients with mammographically dense breasts: Initial experience with Connecticut Public Act 09–41. Radiology 265(1), 59–69. https://doi.org/10.1148/radiol.12120621 (2012).
Portnow, L. H. et al. International interobserver variability of breast density assessment. J. Am. Coll. Radiol. 20(7), 671–684 (2023).
Bakic, P. R. et al. Breast percent density: Estimation on digital mammograms and central tomosynthesis projections. Radiology 252(1), 40–49. https://doi.org/10.1148/radiol.2521081621 (2009).
Gastounioti, A. et al. Fully Automated volumetric breast density estimation from digital breast tomosynthesis. Radiology 301(3), 561–568 (2021).
Graca Neto, L. & Graf, R. M. Anatomy of the superficial fascia system of the breast: A comprehensive theory of breast fascial anatomy. Plast. Reconstr. Surg. 145(1), 193e–194e (2020).
Acknowledgements
The study was supported by National Natural Science Foundation of China (No. 12226003).
Author information
Authors and Affiliations
Contributions
Weipeng Zhou is the scheme designer of this paper, and is responsible for collecting relevant data, conducting data analysis and modeling, writing and revising the manuscript. Qi Yang is responsible for the data acquisition. Huimao Zhang is the corresponding author of the paper, responsible for scheme design and paper revision. This paper is funded by the fund project hosted by Huimao Zhang. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, W., Yang, Q. & Zhang, H. Enhanced value of chest computed tomography radiomics features in breast density classification. Sci Rep 15, 29367 (2025). https://doi.org/10.1038/s41598-025-14190-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14190-x