Abstract
Gum Arabic (GA) (Acacia senegal var. senegal) is an edible tree exudate and dietary fibre shown to improve health in humans and animals. We tested the ramifications of GAon organismal health across the microbiota-gut-brain axis by supplementing female and male zebrafish (Danio rerio) with two concentrations(6% or 60%) of GA (Acacia senegal var. senegal) for two weeks. We assessed the effects on the gut microbiome composition, intestinal and brain metabolic profiles, reproductive fitness, locomotion, and brain gene expression. GA supplementation induced a relative decrease in Proteobacteria and a relative increase in Fusobacteria, with a rise in the beneficial genus Cetobacterium. In the GA-supplemented fish, we detected increased intestinal glucose metabolism, evidenced by reduced glucose retention levels. Additionally, high levels of acetate were detected in the brain. Interestingly, the gene cart1, involved in appetite and hunger control, was significantly downregulated in female brains only. Consistently, we detected increased locomotion in GA-supplemented fish compared to Control fish. Interestingly, GA supplementation had a negative effect on female reproductive fitness and a positive effect on male reproductive fitness. .Our results emphasise the significance of evaluating the impact of dietary fibre at a systemic level to develop relevant nutritional guidelines that consider the different nutritional requirements of each sex.
Similar content being viewed by others
Introduction
Diet modulates the dynamic nature of the gut microbiome, influencing not only its composition but also its function and diversity. Different diets vary in their nutritional profile, whole-food composition, caloric value, and dietary fibre (DF) content, all of which can alter the microbial architecture, leading to either positive or negative health ramifications1. A diet rich in DFs is essential for optimal health and well-being by preventing various non-communicable diseases due to its putative positive effects on cardiovascular, metabolic, and cognitive health in addition to weight management, appetite regulation, and inflammation1,2,3,4,5. DFs constitute a wide range of heterogeneous compounds that resist digestion, and the gut microbiome plays a key role in breaking them down to expose their beneficial properties to the host organism6,7. This bidirectional interaction between the gut microbiome and DFs not only influences intestinal health and metabolism but also mediates host behaviour and fitness via the gut-brain axis8,9.
Gum Arabic (GA) is an edible tree gum exudate obtained by the incision of the trunks and the branches of Acacia senegal (L.) Willdenow, native to the arid and semi-arid regions of Africa. Also known by several names like gum Acacia, Senegal gum, and Sudan Arabic gum, GA is chemically composed of highly branched and high molecular weight glycoproteins and polysaccharide complexes of which 90% is arabinogalactan, a polymer of arabinose (17–34%) and galactose (32–50%) with prebiotic properties, in addition to some amino acids and minerals10,11. It is a key additive (E-Number 414) in the food industry, mainly used as an emulsifier and a stabiliser, but its potential benefits as a DF have been largely overlooked12. Traditional medicine has long suggested health benefits from its oral consumption, particularly with reports indicating positive effects on vital organ functions, such as the kidneys13, the brain14, the heart15, and the liver16 in both humans and animals. Although not primarily consumed in the Western world as a source of DF, people in sub-Saharan regions like Sudan have incorporated GA into their day-to-day life for both medicinal and alimentary purposes long before it was considered safe for human consumption by regulatory bodies like the Food and Drug Administration (FDA) and the Food and Agriculture Organization (FAO) in the 1970s17,18,19.
GA displays a range of potential health benefits. A randomised clinical trial revealed that after 12 weeks of receiving 20 g/day of GA, participants showed a significant decrease in systolic and diastolic blood pressure and fasting blood glucose20. Similarly, the administration of GA was associated with improved renal function in patients with progressive chronic kidney disease (CKD)13, and the oral administration of 30 g GA per day had antioxidant and anti-inflammatory effects in patients undergoing haemodialysis21. A recently published systematic review of 29 clinical trials utilising GA as a therapeutic agent for various human diseases and conditions indicated that GA improves oral health, gastrointestinal functions, and supports healthy weight management in addition to alleviating the adverse effects of sickle cell anaemia and rheumatoid arthritis22. Beneficial effects have also been identified in mice where the administration of GA mitigated the expression of key pro-inflammatory plasma cytokines: IL-6, IL-1β, and TNF-α; despite being fed an inflammatory high-fat diet23. Furthermore, GA improved semen quality in rats24 and ovarian function in mice25 while exhibiting no teratogenic effects26; and in rats, GA enhanced cognitive ability whilst demonstrating neuroprotective properties14,27. Additionally, GA has prebiotic and modulatory effects on the gut microbiome10,28,29,30 via its fermentation by bacteria into microbial-derived metabolites, mainly short-chain fatty acids (SCFAs)29,31,32. This microbiota-gut-brain axis is directly influenced by the diet, which in turn can affect the cognitive behaviour and immunity of the organism33,34,35. The broad range of health benefits associated with GA supplementation suggests that its effects are likely to occur at various levels along the gut-brain axis; however, the exact mechanisms are not yet fully understood.
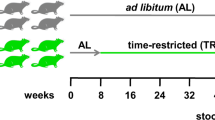
The nutritional requirements of DFs in zebrafish (Danio rerio) are underexplored and under-characterised, with few studies linking DF supplementation to whole-organism phenotypes. In this study, we assessed the health implications of Acacia senegal var. senegal (hereafter referred to as GA*) supplementation at multiple levels, from the gut microbiome to brain function, including reproductive behaviour, by experimentally manipulating the dietary supplementation of GA in zebrafish. Various human clinical trials13,17 have supported the health claims of GA consumption; however, these are not widely accepted in clinical practice due to the lack of mechanistic insight. We conducted a preclinical in vivo study using zebrafish, a non-mammalian vertebrate model, to elucidate and highlight both the benefits and potential trade-offs of dietary fibres, with particular focus on GA. We exposed female and male adult zebrafish (approximately 1 year old) to two GA-supplemented diets (6% or 60% GA*) for two weeks and compared them to Control fish fed a standard diet. No washout period was used. Immediately after the feeding period, we assessed reproductive fitness and locomotory behaviour, then collected the intestine and brain for gut microbiome profiling, tissue metabolomics, and brain transcriptomics (Fig. 1).
Overview of the experimental design for both experiments using 6% and 60% GA* supplementation. The total number and sex (F: female, M: male) of the fish used in the two independent experiments and the sample size for each health assessment are shown. †Two fish died, *Controls were pooled from both experiments. The figure was created in BioRender.com.
Results
The effects of GA on the gut microbiome
To assess the effects of GA on the gut microbiome and its potential prebiotic properties, we performed 16S rRNA sequencing of the gut microbiome in fish with and without GA* supplementation. In line with previous findings, the dominant microbes in the zebrafish belong to two major phyla, Proteobacteria and Fusobacteria36 (Fig. 2A). GA* supplementation reduced the relative abundance of Proteobacteria in favour of an increase in Fusobacteria when compared to Control fish and the strength of the effect was similar in 6% and 60% GA* supplemented fish. However, the effects were stronger in females compared to males (Fig. 2B). The relative abundance of the main genus Cetobacterium in the Fusobacteria increased in females in both experiments whereas in males, we detected an increase only in the 6% GA* cohort (Fig. 2C and Fig. S2 in SI Appendix). This genus is of particular interest since it is beneficial for the health of various teleost species, including zebrafish, where it has been shown to enhance immunity against pathogens, to reduce inflammation, and to produce SCFAs37,38,39,40.
Microbiome composition stratified according to experimental conditions and sex. Abundance plot showing the two major phyla, Proteobacteria and Fusobacteria (A) in all zebrafish used in this analysis and (B) in the two sexes separately. We observed a general drop in the ratio of Proteobacteria/Fusobacteria in GA*-supplemented fish compared to Control. (C) Abundance of Cetobacterium in males and females, showing an increase in GA*-supplemented fish compared to Control fish, with a stronger effect in females.
We found no significant changes in α-diversity in either sex in the 6% GA* experiment as indicated by the Observed Operational Taxonomic Unit (OTU) richness and the Shannon diversity index (Fig. 3A and B). However, the OTU index (but not the Shannon index) indicated a decrease in microbial diversity when comparing female fish from the 60% GA* experiment to female Control fish (Dunn post-hoc after Kruskal–Wallis, Benjamini–Hochberg False Discovery Rate (FDR)41 p adj = 0.003), but no such effect was apparent in males (Fig. 3A and B). This suggests that 60% GA* can potentially reduce the number of gut bacteria in females without affecting their relative abundance. In 6% GA*-supplemented fish, the unweighted and weighted UniFrac β-diversity were more uniform compared to Control fish as indicated by the tight clustering of samples and the narrow ellipses (Fig. 3C and D), and we found a significant effect of supplementation (PERMANOVA: F = 1.32, DF = 1, p = 0.043 and F = 3.3, DF = 1, p = 0.014) but no sex differences (PERMANOVA: F = 1.18, DF = 1, p = 0.1 and F = 2.24, DF = 1, p = 0.062), for unweighted and weighted UniFrac respectively. In fish under 60% GA* supplementation, both females and males showed changes in the microbial communities compared to Control fish as indicated by the unweighted UniFrac β-diversity index (Fig. 3C), as a direct effect of the 60% GA* dosage (PERMANOVA: F = 1.42, DF = 1, p = 0.021) but not sex (PERMANOVA: F = 1.02, DF = 1, p = 0.348). In contrast, the weighted UniFrac β-diversity index did not significantly differ between fish under 60% GA* and Control (Fig. 3D; PERMANOVA for treatment: F = 1.09, DF = 1, p = 0.357 and PERMANOVA for sex: F = 1.60, DF = 1, p = 0.17).
Microbial diversity assessed by α and β diversity. (A, B) Observed and Shannon indices of the gut microbiome based on 16S analysis. Under 6% GA*, no statistically significant changes in the α-diversity between the samples were detected. Under 60% GA*, only female fish displayed a significant drop (Kruskal–Wallis, * denotes p adj = 0.003) in α-diversity compared to the Control indicated by the Observed index. Box plots represent the median, interquartile range, minimum, and maximum values with each dot representing a sample. (C) Unweighted UniFrac β-diversity based on 16S analysis, where 6% and 60% GA* supplementation resulted in a significant decrease, especially in females (PERMANOVA, p < 0.05). (D) Weighted UniFrac β-diversity based on 16S analysis where 6% GA* supplementation caused a decrease in both males and females (PERMANOVA, p < 0.05). However, we found no significant effects of 60% GA* supplementation.
The effects of GA on the metabolic profile of the intestine and the brain
We performed 1H NMR (nuclear magnetic resonance) metabolic analysis to investigate the effects of GA supplementation on the metabolome, with a focus on the gut-brain axis. We identified and quantified 61 metabolites (SI Appendix, Table S2, Table S3, and Fig. S3) where 39 metabolites were detected in the intestinal samples and 22 metabolites were found in the brain (Fig. 4). Interestingly, acetate, one of the key SCFAs, was highly concentrated in the brain tissues of both female and male fish under 60% GA* supplementation compared to Control fish. In the brains of GA*-supplemented fish, we also observed a gradual increase in metabolites compared to the Control in both sexes, with most cerebral metabolites being detected in the 60% GA* fish. These detected brain and intestinal metabolites were most abundant in the 60% GA*-supplemented female fish.
The effects of GA on organismal fitness
To test the effects of GA supplementation on organismal fitness, we assessed reproductive fitness in the experimental adult fish and their offspring (Fig. 5A–D). At the end of the two-week dietary intervention, both Control and GA* fish were crossed with non-experimental ABWT fish by natural spawning. By producing the eggs, female zebrafish have a direct physiological contribution to the clutch, whereas male zebrafish contribute indirectly by providing the sperm needed for egg fertilisation and by initiating the courtship and mating behaviour42,43. We found no statistically significant effects on clutch production (YES/NO) in fish under 6% GA* supplementation (binomial Generalised Linear Mixed-Effects Models (GLMER): \(\upchi\)2 = 0.40, z = 0.64, DF = 1, p = 0.52) nor 60% GA* supplementation (binomial GLMER: \(\upchi\)2 = 0.54, z = -0.73, DF = 1, p = 0.46) when compared to Control (Fig. 5A). Interestingly, the marginally non-significant interaction between 60% GA* and sex may indicate a subtle increase in reproductive success in males compared to females (binomial GLMER: \(\upchi\)2 = 3.18, DF = 1, z = 1.8, p = 0.075) (Fig. 5A). Similarly, neither 6% GA* (Poisson GLMER: \(\upchi\)2 = 0.10, z = -0.56, DF = 1, p = 0.76) nor 60% GA* supplementation (Poisson GLMER: \(\upchi\)2 = 0.13, z = -2.5, DF = 1, p = 0.72) had an impact on the total number of eggs produced (size of clutch) compared to the Control group (Fig. 5B). However, under 60% GA*, there was a significant interaction effect between treatment and sex (Poisson GLMER: \(\upchi\)2 = 12.56, z = 3.54, DF = 1, p < 0.001), indicating a sex-specific effect where GA*-supplemented females produced significantly smaller clutches compared to 60% GA* males (Poisson GLMER: Treatment:Sex interaction: z = − 3.11, DF = 1, p = 0.01) (Fig. 5B). Additionally, Control females produced significantly larger clutches compared to the 60% GA*-supplemented females (Poisson GLMER: Treatment:Sex interaction: z = 2.54, DF = 1, p = 0.05) (Fig. 5B).
Scatter plots displaying measures of reproductive fitness obtained from spawning events between experimental and non-experimental fish. The centre bold dots and error bars indicate mean ± standard error and colours indicate males (purple) and females (yellow). (A) Clutch production during spawning (YES/NO) showed only a significant effect of GA under 60% GA* supplementation indicated by a significant interaction between Treatment:Sex. (B) The total number of eggs (clutch size) did not show any significant effects of 6% GA* supplementation, but we found a strong interaction effect between Treatment:Sex under 60% GA* supplementation, where GA*-supplemented females produced significantly fewer eggs per clutch compared to GA*-supplemented males. (C) Fertilisation success is measured as % unfertilised eggs. Data are plotted on a logarithmic scale for better visualisation. 6% GA* supplementation had no significant effects on the % of unfertilised eggs at 2 hpf, but 60% GA* supplemented females produced significantly more unfertilised eggs compared to Control females and more unfertilised eggs were detected when comparing 60% GA* females to the 60% GA* males. (D) Regarding embryo survival at 24 hpf, 60% GA* supplementation removed the sex-specific drop observed in the Control. The *** denote significance levels of comparisons based on statistical tests with p < 0.001, and ns signifies non-significance.
Whereas 6% GA* supplementation had no significant effects on fertilisation success rate (binomial GLMER: \(\upchi\)2 = 0.08, z = -0.328, DF = 1, p = 0.774) (Fig. 5C) or embryo survival at 24 hpf (binomial GLMER: \(\upchi\)2 = 0.55, z = 1.5, DF = 1, p = 0.15) (Fig. 5D), 60% GA* supplementation resulted in a greater ratio of unfertilised eggs in females but not in males compared to Control fish (binomial GLMER: Treatment: \(\upchi\)2 = 4.52, z = -0.2, DF = 1, p = 0.03; Treatment:Sex interaction: \(\upchi\)2 = 2.92, z = -1.7, DF = 1, p = 0.09) (Fig. 5C). Also, 60% GA* females produced significantly more unfertilised embryos in comparison to the 60% GA* males (binomial GLMER: Treatment:Sex interaction: z = 2.7, DF = 1, p = 0.03) (Fig. 5C). Surprisingly, despite the 60% GA* males displaying better fertilisation rates, embryo survival at 24 hpf was no different than the ones derived from the crossing of 60% GA* females (binomial GLMER: Treatment:Sex interaction: z = 0.8, DF = 1, p = 0.86) (Fig. 5D). Refer to Table S4 in the SI Appendix for the full statistical models. Overall, it appears that while GA* has some potentially beneficial effects on male reproductive fitness, the effects on female reproductive fitness, especially at high dosages, are detrimental.
When assessing the activity of the adult fish by measuring locomotion using artificial intelligence of video recordings of shoaling fish, we focused on 60% GA* supplementation to associate this with the previously reported effects on reproductive fitness. We found that 60% GA*-supplemented fish covered a significantly shorter total distance (mm) compared to Control fish (Gaussian Linear Mixed-Effects Models (LMER): \(\upchi\)2= 30.49, t = -5.5, DF = 1, p < 0.0001) (Fig. 6A), but they swam faster (mm/s) (Gaussian LMER: \(\upchi\)2 = 15.53, t = 3.9, DF = 1, p < 0.0001) (Fig. 6B). This increase in activity in the GA*-supplemented fish could be a possible explanation for the contrasting effects on the reproductive fitness detected in females and males.
The effects of GA on gene expression in the brain
Due to our observation of the substantial effects of 60% GA* supplementation on the gut microbiome and reproductive fitness, we performed whole transcriptome analysis on brain tissues in female and male fish under 60% GA* supplementation and tested for Differentially Expressed Genes (DEGs) (Table 1). When running a complete model with treatment and sex as factors, we found no DEGs when comparing 60% GA* to Control fish. However, when comparing gene expression in the brain between all the fish under 60% GA* supplementation and all Control fish without sex as a covariate, we found that cart1 was downregulated, whereas snora74 and clec3ba were upregulated in the 60% GA*-supplemented fish compared to the Control fish. When comparing Control females and Control males, we identified three statistically significant DEGs (ier2a, egr1, and egr4), that were upregulated in females compared to males, indicating a difference in brain function between the sexes. Two genes, cart1 and slc1a2A, were significantly downregulated in 60% GA* females compared to Control females, but we found no DEGs when running the same analysis in males, further supporting a sex-specific response to GA* supplementation44. The gene cart1 plays a role in feeding behaviour and appetite regulation45. The detected downregulation of the cart1 gene in females suggests a sex-specific regulation of hunger in the brain. In this study, we employed a mixed-sex design (females and males housed together) and therefore both female and male fish were given the exact same amount of food (Control or 60% GA*). Although brain gene expression indicated sex-specific hunger regulation, neither females nor males fed 60% GA* showed statistically significant weight loss compared to sex-matched controls (lmer; emmeans contrasts, Tukeypadj > 0.05) (Fig. S1 in the SI Appendix). Notably, the positive effect of GA* on brain function was supported by the upregulation of clec3ba, which is orthologous to human CLEC3B encoding for tetranectin, a protein with potential neuroprotective and immunomodulatory effects46,47.
Discussion
Nutritional science using animal models recognises that no single animal model perfectly recapitulates human biology nor replicates every nuance of human nutrition. Zebrafish is a pertinent translational model for nutrition, offering valuable cross-species insight48. Thus, it complements, but not replace, mammalian models such as mice and rats. The dosages chosen for this study were based on other animal models, including rats (Rattus sp.) and Nile tilapia (Oreochromis niloticus). In rats, the GA dosages ranged between 4000 mg/kg and 20,000 mg/kg49, which is equivalent to a human equivalent dose (HED) based on body surface area (BSA) of 649—3243 mg/kg50. This translates to 2 mg of GA/fish/day (i.e. 4000 mg/kg body weight when assuming fish weight = 0.0005 kg) in our 6% GA experiment. In Nile tilapia and other fish species, a ratio of GA ≤ 10% (total food weight) showed beneficial health effects51,52,53,54, which translates into the HED of 6% GA in zebrafish (i.e. 4000 mg GA/kg body weight) being the equivalent of 280,000 mg GA per day in a 70 kg human adult. Similarly, 60% GA (i.e. 40,000 mg GA/kg) in zebrafish is the equivalent of 2,800,000 mg GA in a 70 kg human adult. However, when applying the BSA normalisation method, the HEDs are around 256 mg/kg in the 6% GA and 2564 mg/kg in the 60% GA experiments, respectively. The Km factor of a 70 kg human adult with a BSA of 1.81 m2 is 3955. On the other hand, the Km factor of zebrafish is around 2.5 (weight = 0.0005 kg and BSA = 2 × 10–4 m2; Table S5 in the SI Appendix). In summary, the total HED of 256 mg/kg is around 18 g of GA (number of servings = 4 tsp) and around 180 g (number of servings = 36 tsp) of GA for 2564 mg/kg in a 70 kg human adult. Evidently, even the HED of 60% GA is impractical and not realistic. Therefore, results at the higher exposure (60% GA) are unlikely to generalise to typical human intakes, and extrapolation from zebrafish to humans should be made with caution.
Our results demonstrate that GA* supplementation affects female and male zebrafish at multiple levels, spanning from the gut microbiome to metabolic turnover in the gut and brain, and ultimately, overall fitness. The strength of the effects was dosage dependent, where 6% GA* supplementation had positive effects on the gut microbiome in both females and males, with no further implications on reproductive fitness, whereas the effects of 60% GA* supplementation went beyond the microbiome and affected the metabolic rates in the intestines and overall brain activity, potentially at the cost of reduced reproductive fitness in females. The microbiota-gut-brain axis is increasingly recognised to play a key role in organismal health. Changes in the gut microbiota are associated with a range of neurological conditions such as Alzheimer’s, Parkinson’s disease, autism spectrum disorders (ASD), and potentially even mood56,57. GA has previously been shown to enhance cognitive abilities and, more recently, to be anti-epileptic in rats58. Our results suggest that the substantial changes in gut microbiota in GA*-supplemented fish after just two weeks may have downstream effects and ramifications from gut health to brain function and behaviour. Due to the highly dynamic nature of the gut microbiome, dietary changes and the availability of nutritional substrates influence its composition and architecture. DFs have been thoroughly documented to exhibit gut modulatory effects in humans and animals, which contribute to their positive health benefits59,60. In line with previous findings on other DFs, we demonstrated that GA* can favourably shift the Proteobacteria/Fusobacteria (P/F) ratio towards the more beneficial Fusobacteria, and consequently, Cetobacterium (Fig. 2A–C and Fig. S2). In zebrafish, a drop in Fusobacteria and a corresponding increase in the P/F ratio is directly linked with gut dysbiosis61. In our study, 6% GA* was sufficient to generate beneficial effects on the gut microbiome over just two weeks. Our findings suggest a possible difference in gut microbiome composition according to sex but primarily highlight the sex-specific response to GA* supplementation.
The role of sex in shaping the gut microbiome is contested and inconsistent62,63,64,65,66. However, research has shown that sex-specific hormones can alter the gut microbiome composition by influencing the host-microbiome interactions. At the same time, the sex hormones differentially affect the physiology and behaviour of the two sexes, which in turn affects the microbiome62,67,68. However, variation in diet has been consistently shown to exert sex-specific changes on the gut microbiome across different species, including the zebrafish69,70,71. In line with the concept of a sex-specific microbiome, we observed that the increase in the relative abundance of Cetobacterium was more pronounced in females across both GA* treatments (Fig. S2). However, the higher dosage of 60% GA* only reduced the Observed α-richness in females (Fig. 3A). Since the zebrafish display sexual dimorphism in anatomical, physiological, and behavioural aspects72,73,74. We speculate that this sex-specific effect of GA* on the microbiome is due to diet-host interactions. Cetobacterium, a prominent bacterium in the Fusobacteria phylum, is known to produce acetate40 which can cross the blood–brain barrier to control appetite and glucose metabolism40,75. Acetate, like all SCFAs, is primarily produced in the colon due to microbial fermentation of dietary fibres. In mammals, acetate can be endogenously produced by the liver, albeit to a lesser extent76,77. In the zebrafish, the current data are suggestive but still scarce, with almost all papers highlighting and emphasising the role of the gut microbiome in generating acetate as the primary source.
Our metabolic profiling analysis revealed abundant levels of glucose in the intestinal tissue of the Control group, in contrast to negligible amounts in the GA*-supplemented fish (Fig. 4). Interestingly, this suggests that GA* can regulate glucose metabolism and appetite through the microbiota-gut-brain axis, particularly via microbial-derived metabolites. Acetate, a key SCFA, modulates immune responses by interacting with immune cell receptors and influencing cytokine production78. This modulation, in turn, impacts several metabolic pathways like tryptophan metabolism and its broader health implications include metabolic fitness and cognitive performance79,80. We propose that the acetate generated by Cetobacterium in response to GA supplementation induced insulin secretion by stimulating the parasympathetic nervous system40 (Fig. 7). Subsequently, insulin reduces blood glucose levels by stimulating the glycolytic activity of the fish gut; thus, using up intestinal glucose for metabolism81 (Fig. 7). Our metabolomics data do not provide information on insulin levels, most likely because insulin is measured in the plasma and pancreatic tissues using immunoassay techniques82. Although insulin was not available in our dataset, tissue glucose was quantified using NMR in both the brain and the intestine. Additionally, acetate plays a role in appetite and feeding behaviour, which subsequently regulates metabolism via interactions with neuropeptides and gut hormones; however, these contrasting effects are dependent on the species, route of administration, and acetate source75,83,84,85. Notably, high levels of acetate were detected in the brain tissues of both females and males under 60% GA* supplementation (Fig. 4). These findings indicate that GA* can support metabolic health and regulate appetite in both sexes.
Gum Arabic affects general fitness via the microbiota-gut-brain axis in a sex-specific manner with prominent health ramifications detected under 60% GA supplementation. A schematic model displaying our proposed mechanism of action where (\(\uparrow\)) denotes an increase, (\(\downarrow\)) denotes a decrease, (\(\to\)) denotes a causal effect, (\(\leftrightarrow\)) denotes a dynamic relation, and (?) denotes a plausible effect. The figure was created in BioRender.com.
Our results provide further evidence for a positive effect of GA on the microbiota-gut-brain axis. The observed increase in the relative abundance of Cetobacterium under 60% GA* in female zebrafish may be linked to the downregulation of the cart1 gene, an anorexigenic gene, in female brain tissues via an acetate-dependent pathway. The effects of acetate on the regulation of the cart1 gene remain inconsistent with some studies indicating no significant change86 whilst others show an up-regulation85 in gene expression. This discrepancy emphasises that the acetate-mediated effects on metabolism and appetite can differ depending on whether the source is microbial-derived or exogenous83. From a mechanistic perspective, our study suggests that the microbial-derived acetate from the GA* fermentation can potentially counter the anorexigenic effects of the cart1 gene, particularly in females (Fig. 7). Similarly, the gut microbiota has a direct influence on the hypothalamic–pituitary–adrenal axis (HPA), which in turn regulates the organism’s response to metabolic, physiological, and psychological stimuli87. Interestingly, there is also a sex-specific link between the cart1 gene and the HPA axis, particularly in the hypothalamus, with potential downstream effects on the glucocorticoid hormone cortisol and subsequently, stress88. In female zebrafish, the observed downregulation of cart1 may relate to activity in the hypothalamic-pituitary-interrenal (HPI) axis, the teleost homologue of the mammalian HPA axis89, with possible ramifications on stress-related behaviours and appetite regulation90. However, cortisol levels were not measured, and we can only propose, but not corroborate the exact mechanisms. Since the brain acetate levels were mostly abundant in the females of the 60% GA*-supplemented cohort (Fig. 4), the activation of the HPA axis via acetate might also be dosage-dependent. Furthermore, cortisol and insulin have antagonistic effects on metabolism91. We suggest that the stimulation of insulin via the parasympathetic nervous system in response to the microbial-derived acetate has a sex-specific impact on cortisol regulation potentially influencing the contrasting phenotypic data (Fig. 7). The mechanism of the sex-dependent effects of acetate on metabolism and hunger control involves a crosstalk between the microbiota-gut-brain axis and the endocrine system, which warrants further investigation. Additionally, de novo vitamin B12 biosynthesis can be derived from Cetobacterium38 which is essential for maintaining proper brain functioning and supporting the nervous system92. Thus, Cetobacterium-derived B12 can potentially play a role in zebrafish development and influence its locomotion92,93,94. Under 6% and 60% GA*, the raw abundance and relative abundance of Cetobacterium was higher in females; however, this apparent increase was only evident in males under 6% GA* supplementation (Fig. 2C and Supplementary Fig. S2). The impact of vitamin B12 on reproductive fitness and embryo quality is scarce. From our data, any reproductive fitness under 60% GA* supplementation was primarily evident in male fish and not in females (Fig. 5). After conducting a differential abundance analysis, LEfSe (Linear Discriminant Analysis Effect Size) identified Cetobacterium enrichment in females under 60% GA* vs Control (p < 0.05); however, effect size was small (LDA score < 2.5). On the other hand, in males, Cetobacterium was slightly higher in the Control group compared to the 60% GA* group. However, neither the p nor the LDA effect size reached conventional thresholds95. For this reason, any contribution of Cetobacterium-derived B12, if present, to reproduction or locomotion remains uncertain and warrants further investigation. Separately, DFs have been shown to exhibit immunomodulatory effects on the gut and to shape the homeostasis of the immune system96,97. We detected the upregulation of the gene clec3ba in 60% GA*-supplemented fish compared to the Control fish (Table 1), which is in line with the idea that GA may have an immunomodulatory effect and increase the expression of C-type lectins in innate immune cells, namely, macrophages/microglia located in the zebrafish brain98,99. Incorporating GA into the zebrafish diet can potentially activate defence mechanisms against infections.
Overall health is often directly linked with reproductive fitness100,101. We found potentially beneficial effects of GA* supplementation on male reproductive fitness, particularly at high dosages, whereas the effects were detrimental in females. In animals, the implications of DFs on the reproductive parameters of both sexes have been promising. In boars, the incorporation of inulin and wheat bran during the pre-puberty period enhanced testosterone and semen production102. Also, in pigs, the incorporation of a variety of DFs primarily before mating, enhanced reproductive fitness and offspring survival by improving oocyte maturation103. The health implications of DFs on human reproduction are currently understudied as most research has focused on metabolic health, but a high-fibre diet can reduce the reabsorption of oestradiol in the gut and subsequently be detrimental to the menstrual cycle in women due to the diet’s influence on the hypothalamic-pituitary axis, supporting the potential role of the gut-brain axis in reproduction104,105. Moreover, female gonadal hormones can have contrasting effects; therefore, a low intake of DF may negatively affect male fertility106. A possible explanation for the positive impact of GA on male reproductive fitness in our study is that it improves sperm quality as previously reported in mammals24,107,108. Also, GA can influence the steroid hormones governing gametogenesis108,109,110 and exhibit antioxidant activity to protect the gametes from damage25,27,107,110 in both males and females. Despite potentially displaying some adverse effects under specific conditions, DFs play a crucial role in safeguarding metabolic health which in turn can have positive ramifications on fecundity and reproductive nutrition111,112. The association between DFs and reproduction is not linear and likely dosage-dependent, which warrants further investigation. Our study highlights the importance of considering differences in female and male physiology when making dietary recommendations and the potential role of the microbiota-gut-brain axis has on reproductive fitness67,113,114,115.
One possible reason for the associations detected between the 60% GA* dosage and reproductive fitness may be the behavioural changes we identified. We observed that fish supplemented with 60% GA* exhibited increased speed (mm/s) but without swimming a longer distance (mm) compared to Control fish (Fig. 6A and B). This is indicative of a burst swimming pattern rather than a sustained movement. We propose three different possible explanations for the increased speed in GA*-supplemented fish. The first theory is that this hyperactivity may benefit males during courtship and spawning activities, where the males rapidly chase females while nudging their flanks with their snouts and attempting to lead them to the spawning site116. The second theory relates to foraging and prey-capture behaviour117,118, which could be linked to the downregulation of the cart1 gene (anti-anorexigenic effect) in the 60% GA*-supplemented females and may induce an increased desire for food or a change in feeding behaviour119,120,121 (Table 1). The increase in activity under GA* supplementation could, in turn, explain the reduced reproductive fitness in females caused by their energy allocation strategy, which favours somatic growth over ovarian growth and reproduction122,123,124. Thirdly, since the HPA axis might be involved, this increase in locomotion observed in the GA*-supplemented fish, specifically the rapid burst in swimming (speed) without increased exploration (distance) might be related to the role of cortisol in response to stress or anxiety125. Additional studies investigating the possible behavioural and physiological phenotypes of stress and anxiety in zebrafish fed a high-dietary fibre diet are warranted. According to UK guidelines, human adults should aim to consume 30 g of dietary fibre daily for optimal health benefits126. On average, most human clinical trials evaluating the effects of GA on different medical diseases provide around 25–30 g GA per day, which is the equivalent of 357—429 mg per kg body weight22,49. Assuming an adult human weighs 70 kg, a typical human dosage would be approximately 350 mg/kg which is equivalent to around 5 teaspoons (1 tsp = approx. 5 g) of GA powder per day. Interestingly, the European Food Safety Authority (EFSA) suggests that a numerically Acceptable Daily Intake (ADI) for GA is not required as even oral intake of large amounts (430—760 mg/kg per day) is tolerated and nontoxic; albeit, with potential gastrointestinal discomfort127.
Conclusion
A diet rich in DFs such as GA yields downstream health benefits on the gut microbiome, metabolic, and cognitive functions. Despite that, the optimal daily nutritional requirements are still highly debated128,129,130 and we are just beginning to understand the effects of DFs on overall fitness131,132. Additionally, sex differences are often not considered, and varying results of mixed-sex cohorts are typically reported133,134. Our results suggest that GA* can positively affect the gut microbiome composition and subsequently influence brain gene expression and functions. However, we also found negative effects on female reproductive fitness at very high dosages. It is essential to understand when and how DFs exhibit optimal benefits and to enhance our understanding of the interplay between nutrition and health. We employed a holistic approach and demonstrated that a change in GA*, even over a short period, has substantial effects on the microbiota-gut-brain axis and even reproduction, but in a dosage-specific manner. However, further in-depth investigation and mechanistic studies are warranted to verify our findings. Finally, we anticipate follow-up studies in mammalian models, considering sex as a biological variable, to be carried out to help establish sex-specific guidelines for optimal dietary fibre intake.
Materials and methods
Animal ethics statement
AB wild-type zebrafish were obtained from the European Zebrafish Resource Center (EZRC) in Karlsruhe, Germany. The fish were maintained at a 1:1 sex ratio under standard conditions, in compliance with UK Home Office guidelines, in the Controlled Environment Facility (CEF) at the School of Biological Sciences, University of East Anglia (UEA). Experiments were performed with approval from the UK Home Office under project licence: P0C37E901. The work was thoroughly reviewed; ethical approval was granted (ETH2223-0289) through UEA’s AWERB (Animal Welfare and Ethical Review Body) before being approved by the Home Office. Additionally, all experimental methods were performed in accordance with relevant university guidelines and regulations, as well as the ARRIVE guidelines (https://arriveguidelines.org/).
While mammalian animal models like mice or rats are physiologically closer to humans, zebrafish is a well-established model species for studying human conditions, ranging from neurological diseases to cancer and is also a well-established model in food research48,135,136. Sharing > 70% genetic homology with the human genome and all fundamental metabolic pathways with mammals makes them a valuable model for studying these pathways in a species with a lower sentient threshold, as issued by the Home Office (https://www.gov.uk/guidance/research-and-testing-using-animals). Moreover, zebrafish have the advantage of high fecundity, external fertilisation, and rapid development allowing easy monitoring of fitness-related traits in early offspring in a completely non-invasive manner. Also, in vivo experiments using zebrafish benefit from the ability to utilise a larger sample size compared to mice. Finally, the zebrafish model can also provide insights into gut-microbiota-host interactions as it shares structural and functional similarities with the human gut135,136,137,138.
Zebrafish husbandry
All the experimental fish used in this study were healthy adult fish (approximately 1 year old). We excluded any fish with visible morphological and behavioural impairment, such as bad posture, surface lesions, an abnormal caudal fin, and impaired swimming behaviour. Eight adult fish were housed in 3.5 L tanks (6% GA experiment) and 16 adult fish in 8 L tanks (60% GA experiment) on a recirculating rack system (ZebTEC rack with Active Blue technology, Tecniplast, UK) at 28 °C and kept on a 14:10 h light: dark cycle. These zebrafish were fed, ad libitum, three (weekend) or four (weekdays) times daily, where the standard dry feed (ZEBRAFEED 400–600 by SPAROS, Área Empresarial de Marim, Lote C, 8700–221 Olhão, Portugal) was given once or twice a day, and the live Artemia franciscana, from the Great Salt Lake, Utah, USA (Sep-Art Artemia Cysts, Ocean Nutrition) enrichment twice per day. Zebrafish husbandry is not fully standardised, and practices vary among institutions. The current recommendation is to feed adult zebrafish between 3 and 5% of their body weight (split into 1 or 2 meals)139,140. We opted for slightly over 3% of body weight per feed ration, as we believe it strikes a good balance between cost efficiency, preservation of good water quality, and proper maintenance of the adult fish. All fish were fed ad libitum from the same batch of standard dry feed, indicated by the lack of active food searching and the presence of residual feed at the bottom of the tanks.
Formulation of diets and the source of gum Arabic
The control diet consisted of the pre-formulated commercially available ZEBRAFEED 400–600 by SPAROS, which meets the nutritional requirements of zebrafish. The manufacturer reports a crude fibre content of 1.8% for the basal diet (Weende method), which only quantifies insoluble fibre, such as cellulose and lignin, and does not capture soluble DFs derived from GA141. The GA* used in the present study was collected in Sudan and taxonomically identified as Acacia senegal var. senegal and is from the same batch previously used in a clinical trial as part of The GARDS Study13. The Gum Arabic Board of Sudan provided it in spray-dried powder format. We used food-grade gum Arabic (E-414) from Acacia senegal var. senegal. Because GA composition, especially protein content linked to emulsifying properties, varies by species and processing, and we did not independently certify this batch against the E-414 specification in Reg. (EU) 231/2012 (https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX%3A02012R0231-20210803)127, such variability should be considered in replication.
The GA* was fed to the fish by supplementing their dry food. Briefly, the desired amount of GA* was weighed and dissolved in a minute amount of distilled water, enough to allow the GA* to be dissolved entirely then mixed with a weighed amount of standard feed at a specific percentage (6 or 60% w/w), creating a paste that was then spread out to form thin sheets and left to air dry. Using a coffee grinder, the dried sheets were broken down into small ingestible particles, then placed in an incubator at 30 °C overnight until they were fully dry. All diets were kept in opaque and airtight containers in the fridge (4 °C) for the duration of the study. Daily rations were pre-weighed and sealed in labelled Eppendorf tubes, which were also kept in the fridge until use.
Experimental design
Two independent experiments were conducted for 6% and 60% GA* supplementation, each with its control group. For both experiments, fish were divided into two treatment groups (Control and GA*) and maintained under Control or GA* supplementation conditions for two weeks. For the 6% GA* supplementation experiment, eight fish were housed in each 3.5 L tank at a 1:1 sex ratio (4 females and 4 males, total n = 142). For the 60% GA* supplementation experiment, 16 fish were kept in an 8 L tank at a 1:1 sex ratio (8 females and 8 males, total n = 128). The sex of adult zebrafish can be easily identified visually and morphologically, especially the AB wild-type strain. For all the fish, sex was confirmed at necropsy by inspection of the gonads (ovary or testes). The stocking density was maintained at 4–10 fish per 1 L of water, consistent with current husbandry recommendations142. The experiments were divided into blocks, with each block consisting of one Control tank and one GA tank (6% or 60% GA*). We performed nine blocks in the 6% GA* experiment (nine tanks per treatment with eight fish each: 72 fish of which 36 were males and 36 were females) and four blocks (four tanks per treatment with 16 fish each: 64 fish of which 32 were males and 32 were females) in the 60% GA* experiment (Fig. 1). All the fish were randomly selected from population tanks composed of fish from multiple clutches (different parents); sibling matings were not used to establish these tanks. All selected fish were from the same age cohort. Within experimental tanks, fish were randomly allocated to either the Control diet or the GA*-supplemented diet. Additionally, in each block, the tanks were randomly assigned a location on the rack system to minimise confounders. The sample size was determined a priori based on previous pilot experiments conducted within the group. In short, a total sample size of n = 80 fish (40 Control and 40 GA-supplemented) of a 1:1 sex ratio was sufficient to detect between-sex differences in reproductive fitness. In this study, we implemented Reduction (3Rs) and prespecified smaller cohorts without compromising power to detect effects on reproductive fitness/phenotypic data. In the 6% GA* experiment, we used total n = 72 fish (36 Control, 36 GA; 1:1 sex ratio) and in the 60% GA* experiment we used total n = 64 fish (32 Control, 32 GA; 1:1 sex ratio) (Fig. 1).
Our reasons for selecting 6% and 60% GA* are outlined at the start of the Discussion. On a body-weight basis, the zebrafish dosages were intentionally higher than typical human intakes because GA is water-soluble and may leach from feed when delivered in water. Higher inclusion helps ensure actual ingestion of the target amount. In both experiments, the Control fish were under the standard feeding regime of dry and live feed described earlier, whereas the GA fish were fed dry feed supplemented with 6% or 60% GA* and live Artemia. To ensure proper nutrition and avoid caloric restriction, all fish were fed ad libitum over 3% of their body weight/day139,140,143. In both experiments, the GA*-supplemented diet was fed to the experimental fish while the control fish received the equivalent amount of dry standard feed without GA supplementation. To compensate for any anticipated differences in macronutrients (protein, lipids, and total carbohydrates) between the Control and GA* diets, particularly the 60% GA*, we made additional efforts during feeding. All fish were provided with concentrated Artemia nauplii (> 225,000 hatched shrimp per gram) twice a day, during weekdays. Artemia is an excellent source of protein (54%) and a significant source of lipids (11%)144. Additional effort was made to ensure that the fish under the experimental diets (6% and 60% GA*) received generous amounts of Artemia nauplii to meet or even exceed their nutritional requirements145. Additionally, we ensured that the water flow was adjusted during Artemia feeding for all tanks to prevent rapid flushing. This compensates for any macronutrient difference between the dry diets and mitigates any confounding effects, in addition to the randomisation in fish tank allocation. In support of this, across all reproductive and developmental outcomes examined, macronutrient levels (protein, lipid, and carbohydrates) did not significantly predict variation in clutch production, egg number, fertilisation, or survival outcomes. In contrast, where significant effects were observed, notably for egg number (p = 0.001) and number dead at 24 hpf (p = 0.01, both under 60% GA* treatment), GA treatment explained more variance and substantially improved model fit, as indicated by lower AIC/BIC (Akaike Information Criterion/Bayesian Information Criterion) values and significant likelihood ratio tests. These results suggest that GA treatment, rather than macronutrient composition, is the primary driver of the observed differences in these reproductive endpoints (Table S6 in the SI Appendix). The dry feed was standardised by pre-weighing and then storing in labelled Eppendorf tubes as follows: 0.133 g for eight fish (6% GA*) and 0.266 g for 16 fish (60% GA*). More specifically, an adult fish weighing 500 mg and being fed over 3% of its body weight is the equivalent of just over 15 mg of dry food/fish. In the 6% GA* experiment, we fed 3.325% of 500 mg which is around 16.625 mg of dry feed (8 fish per tank X 16.625 mg = 0.133 g) twice daily resulting in an average of around 2 mg GA per day per fish. Following the same rationale, we fed each fish in the 60% GA* experiment, with an average of approximately 20 mg GA per day.
Reproductive-fitness assessments were conducted under blinded conditions. Following the dietary intervention, each fish was assigned a unique ID, and breeding was conducted without reference to treatment groups (Control or GA*). Spawned embryos were then linked to their parental IDs; however, the allocation key linking IDs to treatment was withheld from those counting the embryos until all quantifications were complete. Thus, the researchers counting the embryos remained blinded. For tissue analyses, samples were randomly selected to ensure balanced representation across treatment groups and experimental blocks.
Genomic DNA extraction and 16S rRNA sequencing
Genomic DNA was isolated from n = 50 adult zebrafish intestines (6% GA experiment: 14 Control fish and 16 GA fish; 60% GA experiment: 10 Control fish and 10 GA fish) using FastDNA™ SPIN Kit for Soil (MP Biomedical, product code:116560200-CF) as per manufacturer’s recommendations (Fig. 1). This genomic DNA was sent to Source BioScience (UK) in Cambridge (Source Bioscience Sequencing, William James House, Cowley Road, Cambridge, CB4 0WU) for 16S rRNA sequencing (SI Appendix, Table S1) with 250 or 300 base pair-end reads. The sequencing platform used was Illumina MiSeq. The bioinformatic microbiome analysis pipeline QIIME2 was used to attain expression data146. The R package phyloseq 1.44.0147 was used for the downstream analysis.
Metabolites extraction and 1H NMR metabolomic analysis
Due to the small amount of tissue in each organ, we pooled brains and intestines from two or three fish per treatment and sex, resulting in six pooled samples per tissue (SI Appendix, Table S2). The extraction protocol was conducted and optimised in the Le Gall lab as previously described148. High-resolution [1H] NMR spectra were recorded on a 600-MHz Bruker Avance spectrometer fitted with a 5-mm TCI proton-optimized triple resonance NMR inverse cryoprobe and a 24-slot autosampler (Bruker, Coventry, England). All identified metabolites (SI Appendix, Table S3) were quantified using Chenomx NMR suite 8.6 software (Edmonton, AB, Canada)149.
Reproductive fitness and locomotion assessment
The 6% GA experiment included a total of n = 144 fish (72 Control and 72 GA), whilst the 60% GA experiment included a total of n = 128 fish (64 Control and 64 GA) of equal sex ratio (Fig. 1). We assessed reproductive fitness for both 6% GA fish and 60% GA fish, in addition to their respective control groups.
At the end of the two-week experimental period, natural spawning was initiated by pairing each experimental fish with a non-experimental fish of the opposite sex. The fish were placed in external breeding tanks, separated by a divider, the day before spawning. The breeding tanks were also covered with an opaque cloth to ensure darkness until the next day. On the morning of the experiment, after removing the opaque cover and dividers, the breeding tanks were checked hourly for the presence of eggs. In the event of success, the eggs were collected from the tanks and incubated at 28 °C in a 0.01% solution of system water and methylene blue. The resulting clutches were assessed for the different fitness traits at two time points: 2 and 24 hpf (hour post fertilisation).
In the 60% GA experiment, fish were recorded for 60 min in an isolated room whilst being placed in a special tank. All recordings were captured by a GoPro HERO4, and tracking was performed with the idtracker.ai software150. The specific settings for the artificial intelligence (AI) tracking are mentioned in the SI Appendix, Materials and Methods.
RNA extraction and RNA-seq
Brain samples from a total of n = 40 fish (20 Control fish and 20 60% GA fish) were collected by dissection following euthanasia by metomidate hydrochloride overdose (Aqua-
calm by Syndel, 4131 Mostar Rd #9, Nanaimo, BC V9T 6A6, Canada), and kept cool on ice before freezing in liquid nitrogen (Fig. 1). RNA extraction was performed using Quick-RNA™ Miniprep Plus Kit with Zymo-Spin™ IIICG Columns (Capped) & Spin-Away™ Filters (Zymo Research, product code: R1057, Cambridge Bioscience, UK) according to manufacturer’s recommendations. Library construction was performed using a NEBNext Ultra II Direction RNA-seq library kit (poly-A selection, 200-300 bp inserts) by the company Novogene (UK) in Cambridge (25 Science Park, Milton, Cambridge, CB4 0FW). Illumina sequencing PE150 was used, resulting in each sample having a minimum of 300 reads and 6G of raw data per sample. The bioinformatics pipeline “nf-core/rnaseq” version: 3.11.1 (https://nf-co.re/rnaseq/3.11.1)151 was used to analyse the RNA sequencing data obtained from the zebrafish brain samples using the Ensembl reference genome file (Danio rerio.GRCz11.109). The DEG analysis was performed using the DESeq2 package152. The analysis was stratified into five comparisons according to the diet condition and/or sex by changing the design formula of the DESeqDataSetFromMatrix function.
Statistical analyses
R (version 4.3.1) in R Studio 2023.06.0 Build 421 was used for all the analyses. The Kruskal–Wallis test was used for the α-diversity and statistical significance was determined with ap adj < 0.01. Statistical significance in β-diversity was determined using PERMANOVA with a p < 0.05. GLMM and LMER models from the library lme4 were used for the phenotypic and AI data, respectively153154,. For greater detail regarding these analyses, please refer to the SI Appendix, Table S3. Statistical significance for the differentially expressed genes was determined by a log2 fold change with a p adj< 0.05.
Preprint
A previous version of this manuscript was published as a preprint https://doi.org/10.1101/2024.10.04.616708.
Data availability
The datasets generated and/or analysed during the current study are available in the GEO repository (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GEO: GSE245252 and GEO: GSE245568. Also, the R scripts used for statistical analysis and graphical representation are available on GitHub (https://github.com/asdfjohnny1/Gum_Arabic_Manuscript). The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ross, F. C. et al. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 22, 671–686 (2024).
Zhang, F., Fan, D., Huang, J.-L. & Zuo, T. The gut microbiome: Linking dietary fiber to inflammatory diseases. Med. Microecol. 14, 100137 (2022).
Deehan, E. C., Mocanu, V. & Madsen, K. L. Effects of dietary fibre on metabolic health and obesity. Nat. Rev. Gastroenterol. Hepatol. 21, 301–318 (2024).
La Torre, D., Verbeke, K. & Dalile, B. Dietary fibre and the gut–brain axis: Microbiota-dependent and independent mechanisms of action. Gut Microbiome 2, e4 (2021).
Barber, T. M., Kabisch, S., Pfeiffer, A. F. H. & Weickert, M. O. The health benefits of dietary fibre. Nutrients 12, 3209 (2020).
Williams, B. A., Grant, L. J., Gidley, M. J. & Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 18, 2203 (2017).
Cui, J. et al. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr. Rev. Food Sci. Food Saf. 18, 1514–1532 (2019).
Vuong, H. E., Yano, J. M., Fung, T. C. & Hsiao, E. Y. The microbiome and host behavior. Annu. Rev. Neurosci. 40, 21–49 (2017).
Gould, A. L. et al. Microbiome interactions shape host fitness. Proc. Natl Acad. Sci. USA 115, E11951–E11960 (2018).
Al-Baadani, H. H. et al. The use of gum Arabic as a natural prebiotic in animals: A review. Anim. Feed Sci. Technol. 274, 114864 (2021).
Anderson, D. M. W., Brown, D. M., Morrison, N. A. & Weiping, W. Specifications for gum arabic (Acacia senegal): Analytical data for samples collected between 1904 and 1989. Food Addit. Contam. 7, 303–321 (1990).
Montenegro, M. A., Boiero, M. L., Valle, L. & Borsarelli, C. D. Gum Arabic: more than an edible emulsifier. In Products and Applications of Biopolymers (ed. Montenegro, M. A.) (InTech, 2012).
Khalid, S. A. et al. Gum Arabic in renal disease (GARDS study): Clinical evidence of dietary supplementation impact on progression of renal dysfunction. J. Funct. Foods 82, 104501 (2021).
Rajab, E. et al. Gum Arabic supplementation prevents loss of learning and memory through stimulation of mitochondrial function in the hippocampus of type 2 diabetic rats. J. Funct. Foods 87, 104854 (2021).
Gouda, E. & Babiker, F. Gum Arabic protects the rat heart from ischemia/reperfusion injury through anti-inflammatory and antioxidant pathways. Sci. Rep. 12, 1398 (2022).
Kamal, E., Kaddam, L. A. G., Alagib, A. & Saeed, A. Dietary fibres (gum Arabic) supplementation modulates hepatic and renal profile among rheumatoid arthritis patients: Phase II trial. Front. Nutr. 8, 731995 (2021).
Babiker, R. et al. Effects of gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo-controlled, double-blind trial. Nutr. J. 11, 111 (2012).
Mahomoodally, M. F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid. Based Complement. Alternat. Med. 2013, 617459 (2013).
United Nations Conference on Trade and Development. Commodities at a glance: special issue on gum arabic. (UNCTAD, 2018). https://unctad.org/system/files/official-document/suc2017d4_en.pdf.
Jarrar, A. H. et al. The effect of gum Arabic (Acacia senegal) on cardiovascular risk factors and gastrointestinal symptoms in adults at risk of metabolic syndrome: A randomized clinical trial. Nutrients 13, 2975 (2021).
Ali, N. E. et al. Gum Arabic (Acacia senegal) augmented total antioxidant capacity and reduced C-reactive protein among haemodialysis patients: Phase II trial. Int. J. Nephrol. 2020, 4267176 (2020).
Al-Jubori, Y. et al. The efficacy of gum Arabic in managing diseases: A systematic review of evidence-based clinical trials. Biomolecules 13, 1836 (2023).
Ahmed, A. A. et al. Gum Arabic modifies anti-inflammatory cytokines in mice fed a high-fat diet. Bioact. Carbohydr. Diet. Fibre 25, 100261 (2021).
Fedail, J. S. et al. Gum Arabic improves semen quality and oxidative stress capacity in alloxan-induced diabetic rats. Asian Pac. J. Reprod. 5, 434–441 (2016).
Ahmed, A. A., Fedail, J. S., Musa, H. H., Musa, T. H. & Sifaldin, A. Z. Gum Arabic supplementation improves antioxidant status and alters expression of oxidative stress genes in ovary of mice fed a high-fat diet. Middle East Fertil. Soc. J. 21, 101–108 (2016).
Collins, T. F. X., Welsh, J. J., Black, T. N., Graham, S. L. & Brown, L. H. Study of the teratogenic potential of gum arabic. Food Chem. Toxicol. 25, 815–821 (1987).
Almohaimeed, H. M. et al. Gum Arabic nanoformulation rescues neuronal lesions in bromobenzene-challenged rats by its antioxidant, anti-apoptotic and cytoprotective potentials. Sci. Rep. 12, 12015 (2022).
Calame, W., Weseler, A. R., Viebke, C., Flynn, C. & Siemensma, A. D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 100, 1269–1275 (2008).
Rawi, M. H., Abdullah, A., Ismail, A. & Sarbini, S. R. Manipulation of gut microbiota using acacia gum polysaccharide. ACS Omega 6, 17782–17797 (2021).
Khalid, S. A. et al. Manipulating dietary fibre: Gum Arabic making friends of the colon and the kidney. Bioact. Carbohydr. Diet. Fibre 3, 71–76 (2014).
Dauqan, E. & Abdullah, A. Utilization of gum Arabic for industries and human health. Am. J. Appl. Sci. 10, 1270–1279 (2013).
O’Riordan, K. J. et al. Short-chain fatty acids: Microbial metabolites for gut–brain axis signalling. Mol. Cell. Endocrinol. 546, 111572 (2022).
Fung, T. C. The microbiota–immune axis as a central mediator of gut–brain communication. Neurobiol. Dis. 136, 104714 (2020).
Barber, T. M. et al. Dietary influences on the microbiota–gut–brain axis. Int. J. Mol. Sci. 22, 3502 (2021).
Connell, E. et al. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 17, 43 (2022).
Roeselers, G. et al. Evidence for a core gut microbiota in the zebrafish. ISME J. 5, 1595–1608 (2011).
Xie, M. et al. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish Shellfish Immunol. 120, 56–66 (2022).
Qi, X. et al. Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome 11, 135 (2023).
Zhao, Y., Li, S., Lessing, D. J. & Chu, W. The attenuating effects of synbiotic containing Cetobacterium somerae and Astragalus polysaccharide against trichlorfon-induced hepatotoxicity in crucian carp (Carassius carassius). J. Hazard. Mater. 461, 132621 (2024).
Wang, A. et al. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 13, 1–15 (2021).
KleineBardenhorst, S. et al. Data analysis strategies for microbiome studies in human populations—A systematic review of current practice. mSystems 6, 10–128 (2021).
Spence, R. & Smith, C. Mating preference of female zebrafish, Danio rerio, in relation to male dominance. Behav. Ecol. 17, 779–783 (2006).
Eaton, R. C. & Farley, R. D. Spawning cycle and egg production of zebrafish, Brachydanio rerio, in the laboratory. Copeia 1974, 195–204 (1974).
Zhai, G., Jia, J., Bereketoglu, C., Yin, Z. & Pradhan, A. Sex-specific differences in zebrafish brains. Biol. Sex Differ. 13, 31 (2022).
Rogge, G., Jones, D., Hubert, G. W., Lin, Y. & Kuhar, M. J. CART peptides: Regulators of body weight, reward and other functions. Nat. Rev. Neurosci. 9, 747–758 (2008).
Iram, S., Rahman, S., Choi, I. & Kim, J. Insight into the function of tetranectin in human diseases: A review and prospects for tetranectin-targeted disease treatment. Heliyon 10, (2024).
Luo, M. et al. Identification, characterization, and agglutinating activity of a novel C-type lectin domain family 3 member B (CLEC3B) discovered in golden pompano, Trachinotus ovatus. Fish Shellfish Immunol. 140, 108988 (2023).
Caro, M. et al. Zebrafish dives into food research: Effectiveness assessment of bioactive compounds. Food Funct. 7, 2615–2623 (2016).
Alobaidi, S. Therapeutic potential of gum Arabic (Acacia senegal) in chronic kidney disease management: A narrative review. J. Clin. Med. 13, 5778 (2024).
Reagan-Shaw, S., Nihal, M. & Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 22, 659–661 (2008).
Naiel, M. A. E., Abd El-hameed, S. A. A., Arisha, A. H. & Negm, S. S. Gum Arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune-related gene expression in tilapia fish. Aquaculture 556, 738249 (2022).
Soaudy, M. R. et al. The modulatory impact of Arabic gum and lecithin on the efficiency of cold-stressed Nile tilapia (Oreochromis niloticus). Aquac. Rep. 38, 102332 (2024).
Falaye, A. E., Abah, A. & Sule, S. O. Effect of gum Arabic (Acacia senegal) on growth performance, carcass quality and health of Clarias gariepinus juveniles. J. Med. Vet. 7, 163–76 (2024).
Yousefi, M., NaderiFarsani, M., Ghafarifarsani, H. & Raeeszadeh, M. Dietary Lactobacillus helveticus and gum Arabic improves growth indices, digestive enzyme activities, intestinal microbiota, innate immunological parameters, antioxidant capacity, and disease resistance in common carp. Fish Shellfish Immunol. 135, 108652 (2023).
Verbraecken, J., Van De Heyning, P., De Backer, W. & Van Gaal, L. Body surface area in normal-weight, overweight, and obese adults: A comparison study. Metabolism 55, 515–524 (2006).
Liu, L., Huh, J. R. & Shah, K. Microbiota and the gut–brain axis: Implications for new therapeutic design in the CNS. EBioMedicine 77, 103908 (2022).
Simpson, C. A. et al. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 83, 101943 (2021).
Yakmaz, F., Bozkurt, A. S. & Görücü Yilmaz, Ş. PTZ-kindled rat model; evaluation of seizure, hippocampal EGR-1, and Rev-erbα gene regulation, behavioral analysis, and antioxidant capacity of gum Arabic. Mol. Biol. Rep. 51, 279 (2024).
Cai, Y., Folkerts, J., Folkerts, G., Maurer, M. & Braber, S. Microbiota-dependent and -independent effects of dietary fibre on human health. Br. J. Pharmacol. 177, 1363–81 (2020).
Montagne, L., Pluske, J. R. & Hampson, D. J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 108, 95–117 (2003).
Luan, Y. et al. The fish microbiota: Research progress and potential applications. Engineering 29, 137–46 (2023).
Kim, Y. S., Unno, T., Kim, B. Y. & Park, M. S. Sex differences in gut microbiota. World J. Mens Health 38, 48–60 (2020).
Scepanovic, P. et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome 7, 130 (2019).
Worsley, S. F. et al. Assessing the causes and consequences of gut mycobiome variation in a wild population of the Seychelles warbler. Microbiome 10, 242 (2022).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Liang, X., Bushman, F. D. & FitzGerald, G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl Acad. Sci. USA 112, 10479–10484 (2015).
Jaggar, M., Rea, K., Spichak, S., Dinan, T. G. & Cryan, J. F. You’ve got male: Sex and the microbiota–gut–brain axis across the lifespan. Front. Neuroendocrinol. 56, 100815 (2020).
Yan, R. et al. Effect of sex on the gut microbiota characteristics of passerine migratory birds. Front. Microbiol. 13, 917373 (2022).
Bolnick, D. I. et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat. Commun. 5, 4500 (2014).
Ma, Y. et al. Sex-dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ. Sci. Nano 5, 740–751 (2018).
Chen, L. et al. Acute exposure to PBDEs at an environmentally realistic concentration causes abrupt changes in the gut microbiota and host health of zebrafish. Environ. Pollut. 240, 17–26 (2018).
von Hofsten, J. & Olsson, P. E. Zebrafish sex determination and differentiation: Involvement of FTZ-F1 genes. Reprod. Biol. Endocrinol. 3, 63 (2005).
Martyniuk, C. J. et al. Sex-dependent host–microbiome dynamics in zebrafish: implications for toxicology and gastrointestinal physiology. Comp. Biochem. Physiol. D Genomics Proteomics 42, 100993 (2022).
Maritan, E., Quagliariello, A., Frago, E., Patarnello, T. & Martino, M. E. The role of animal hosts in shaping gut microbiome variation. Philos. Trans. R. Soc. B 379, 20230071 (2024).
Frost, G. et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 5, 3611 (2014).
Liu, X. et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175, 502-513.e13 (2018).
Moffett, J. R., Puthillathu, N., Vengilote, R., Jaworski, D. M. & Namboodiri, A. M. Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—Part 1: Acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front. Physiol. 11, 580167 (2020).
Soliman, M. L., Puig, K. L., Combs, C. K. & Rosenberger, T. A. Acetate reduces microglia inflammatory signaling in vitro. J. Neurochem. 123, 555–567 (2012).
Dalile, B., Van Oudenhove, L., Vervliet, B. & Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 16, 461–478 (2019).
He, Q. et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 36, 1998–2014 (2024).
Polakof, S., Panserat, S., Soengas, J. L. & Moon, T. W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 182, 1015–1045 (2012).
Luong, A. D., Roy, I., Malhotra, B. D. & Luong, J. H. T. Analytical and biosensing platforms for insulin: A review. Sens. Actuators Rep. 3, 100028 (2021).
Hernández, M. A. G., Canfora, E. E., Jocken, J. W. E. & Blaak, E. E. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients 11, 1943 (2019).
Byrne, C. S., Chambers, E. S., Morrison, D. J. & Frost, G. The role of short-chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 39, 1331–1338 (2015).
Zhang, H. et al. Effects of dietary sodium acetate on food intake, weight gain, intestinal digestive enzyme activities, energy metabolism and gut microbiota in cultured fish: Zebrafish as a model. Aquaculture 523, 735188 (2020).
Liu, L., Liu, H., Fu, C., Li, C. & Li, F. Acetate induces anorexia via up-regulating the hypothalamic pro-opiomelanocortin (POMC) gene expression in rabbits. J. Anim. Feed Sci. 26, 266–273 (2017).
Sudo, N. The hypothalamic–pituitary–adrenal axis and gut microbiota: A target for dietary intervention? In The Gut-Brain Axis: Dietary, Probiotic, and Prebiotic Interventions on the Microbiota (ed. Sudo, N.) 293–304 (Elsevier, 2016).
Koylu, E. O., Balkan, B., Kuhar, M. J. & Pogun, S. Cocaine- and amphetamine-regulated transcript (CART) and the stress response. Peptides 27, 1956–1969 (2006).
Lee, H. B. et al. Key HPI axis receptors facilitate light adaptive behavior in larval zebrafish. Sci. Rep. 14, 7759 (2024).
Nipu, N., Antomagesh, F., Faught, E. & Vijayan, M. M. Glucocorticoid receptor activation reduces food intake independent of hyperglycemia in zebrafish. Sci. Rep. 12, 15677 (2022).
Bratusch-Marrain, P. R. Insulin-counteracting hormones: Their impact on glucose metabolism. Diabetologia 24, 74–79 (1983).
Mathew, A. R. et al. Vitamin B12 deficiency and the nervous system: beyond metabolic decompensation—Comparing biological models and gaining new insights into molecular and cellular mechanisms. Int. J. Mol. Sci. 25, 590 (2024).
Sloan, J. L. et al. The vitamin B12 processing enzyme, mmachc, is essential for zebrafish survival, growth and retinal morphology. Hum. Mol. Genet. 29, 2109–2123 (2020).
Robea, M. A. et al. Vitamin B12 ameliorates pesticide-induced sociability impairment in zebrafish (Danio rerio): A prospective controlled intervention study. Animals 14, 405 (2024).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Schley, P. D. & Field, C. J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 87, S221–S230 (2002).
Xie, L., Alam, M. J., Marques, F. Z. & Mackay, C. R. A major mechanism for immunomodulation: dietary fibres and acid metabolites. Semin. Immunol. 66, 101737 (2023).
Ewart, K. V., Johnson, S. C. & Ross, N. W. Lectins of the innate immune system and their relevance to fish health. ICES J. Mar. Sci. 58, 380–385 (2001).
Barkeer, S. et al. Gum acacia dietary fiber: Significance in immunomodulation, inflammatory diseases, and cancer. Phytother. Res. 38, 1509–1521 (2024).
Jasienska, G., Bribiescas, R. G., Furberg, A. S., Helle, S. & Núñez-de la Mora, A. Human reproduction and health: An evolutionary perspective. Lancet 390, 510–520 (2017).
Kosova, G., Abney, M. & Ober, C. Heritability of reproductive fitness traits in a human population. Proc. Natl Acad. Sci. USA 107, 1772–1778 (2010).
Lin, Y. et al. Dietary fibre supplementation improves semen production by increasing Leydig cells and testosterone synthesis in a growing boar model. Front. Vet. Sci. 9, 850658 (2022).
Jarrett, S. & Ashworth, C. J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 9, 59 (2018).
Gaskins, A. J. et al. Effect of daily fiber intake on reproductive function: The BioCycle Study. Am. J. Clin. Nutr. 90, 1061–1069 (2009).
Gaskins, A. J., Mumford, S. L., Wactawski-Wende, J. & Schisterman, E. F. Effect of daily fiber intake on luteinizing hormone levels in reproductive-aged women. Eur. J. Nutr. 51, 249–253 (2012).
Salas-Huetos, A., Bulló, M. & Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 23, 371–389 (2017).
Imbabi, T. A. et al. Enhancing semen quality, brain neurotransmitters, and antioxidant status of rabbits under heat stress by acacia gum, vitamin C, and lycopene as dietary supplements: An in vitro and in silico study. Ital. J. Anim. Sci. 22, 321–336 (2023).
Nofal, A. E., Okdah, Y. A., Rady, M. I. & Hassaan, H. Z. Gum acacia attenuates cisplatin toxic effect: Spermatogenesis dysfunction and infertility in rats. Int. J. Biol. Macromol. 240, 124292 (2023).
Mohamed, R. I., Daoud, I. M., Suliman, A. G. & Kaddam, L. Effect of prebiotic dietary supplement Acacia senegal on hormonal and metabolic markers in polycystic ovary syndrome patients: A pilot study. Cureus (2023).
Nasir, O. et al. Comparative efficacy of gum Arabic (Acacia senegal) and Tribulus terrestris on male fertility. Saudi Pharm. J. 28, 1791–1796 (2020).
Willis, S. K. et al. Glycemic load, dietary fiber, and added sugar and fecundability in two preconception cohorts. Am. J. Clin. Nutr. 112, 27–38 (2020).
Chang, J. et al. Revealing the mechanism of fibre promoting sow embryo implantation by altering the abundance of uterine fluid proteins: A proteomic perspective. J. Proteomics 297, 105123 (2024).
Zhang, J. et al. Probiotic Bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut–brain axis. mSystems 4, 10–128 (2019).
Wang, Y. & Xie, Z. Exploring the role of gut microbiome in male reproduction. Andrology 10, 441–450 (2022).
Hu, Y. et al. Chicory fibre improves reproductive performance of pregnant rats involving altering intestinal microbiota composition. J. Appl. Microbiol. 129, 1693–1705 (2020).
Spence, R., Gerlach, G., Lawrence, C. & Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 83, 13–34 (2008).
Marques, J. C., Li, M., Schaak, D., Robson, D. N. & Li, J. M. Internal state dynamics shape brainwide activity and foraging behaviour. Nature 577, 239–243 (2020).
Filosa, A., Barker, A. J., Dal Maschio, M. & Baier, H. Feeding state modulates behavioral choice and processing of prey stimuli in the zebrafish tectum. Neuron 90, 596–608 (2016).
Nishio, S.-I. et al. Fasting induces CART down-regulation in the zebrafish nervous system in a cannabinoid receptor 1-dependent manner. Mol. Endocrinol. 26, 1316–1326 (2012).
Volkoff, H. The neuroendocrine regulation of food intake in fish: A review of current knowledge. Front. Neurosci. 10, 540 (2016).
Guillot, R. et al. Behind melanocortin antagonist overexpression in the zebrafish brain: A behavioral and transcriptomic approach. Horm. Behav. 82, 87–100 (2016).
Maklakov, A. A. & Immler, S. The expensive germline and the evolution of ageing. Curr. Biol. 26, R577–R586 (2016).
Leibold, S. & Hammerschmidt, M. Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS One 10, e0120776 (2015).
Merrill, L. & Collins, P. M. Environment-specific and sex-specific allocation strategies among gonadal, somatic, and immune indices in a marine fish. Can. J. Zool. 93, 207–212 (2015).
Cachat, J. et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 5, 1786–1799 (2010).
Evans, C. E. L. Dietary fibre and cardiovascular health: A review of current evidence and policy. Proc. Nutr. Soc. 79, 61–67 (2020).
Mortensen, A. et al. Re-evaluation of xanthan gum (E 415) as a food additive. EFSA J. 15, e04909 (2017).
Stephen, A. M. et al. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 30, 149–190 (2017).
Watts, S. A. & D’Abramo, L. R. Standardized reference diets for zebrafish: Addressing nutritional control in experimental methodology. Annu. Rev. Nutr. 41, 511–527 (2021).
Watts, S. A., Powell, M. & D’Abramo, L. R. Fundamental approaches to the study of zebrafish nutrition. ILAR J. 53, 144–160 (2012).
Fowler, L. A., Williams, M. B., D’Abramo, L. R. & Watts, S. A. Zebrafish nutrition—moving forward. In The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications (ed. Fowler, L. A.) 379–401 (Elsevier, 2019).
Leigh, S. C., Nguyen-Phuc, B. Q. & German, D. P. The effects of protein and fiber content on gut structure and function in zebrafish (Danio rerio). J. Comp. Physiol. B 188, 237–253 (2018).
Hillman, C., Cooper, A. H., Ram, P. & Parker, M. O. The effect of laboratory diet and feeding on growth parameters in juvenile zebrafish. Lab Anim. (NY) 53, 327–335 (2024).
Genario, R., de Abreu, M. S., Giacomini, A. C. V. V., Demin, K. A. & Kalueff, A. V. Sex differences in behavior and neuropharmacology of zebrafish. Eur. J. Neurosci. 52, 2586–2603 (2020).
Adhish, M. & Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 9, e14557 (2023).
Chia, K., Klingseisen, A., Sieger, D. & Priller, J. Zebrafish as a model organism for neurodegenerative disease. Front. Mol. Neurosci. 15, 940484 (2022).
Kalueff, A. V., Stewart, A. M. & Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 35, 63–75 (2014).
Xia, H. et al. Zebrafish: An efficient vertebrate model for understanding role of gut microbiota. Mol. Med. 28, 161 (2022).
Frederickson, S. C. et al. Comparison of juvenile feed protocols on growth and spawning in zebrafish. J. Am. Assoc. Lab. Anim. Sci. 60, 298–305 (2021).
Purushothaman, K. et al. Protocol for feeding strategy and proteomics analysis of zebrafish Danio rerio using S-trap and iTRAQ techniques. STAR Protoc. 5, 103513 (2024).
Pesti, G. M. A new analytical procedure to replace the outdated Weende proximal feed ingredient analysis paradigm is long overdue. Anim. Prod. Sci. 64, (2024).
Aleström, P. et al. Zebrafish: Housing and husbandry recommendations. Lab Anim. 54, 213–224 (2020).
Lawrence, C. Zebrafish larviculture. In The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications (ed. Lawrence, C.) 365–378 (Elsevier, 2019).
Van Stappen, G., Sorgeloos, P. & Rombaut, G. (eds.) Manual on Artemia production and use. FAO Fisheries and Aquaculture Technical Paper No. 702 (FAO, Rome, 2024). https://doi.org/10.4060/cd0313en
Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 269, 1–20 (2007).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Le Gall, G. Sample collection and preparation of biofluids and extracts for NMR spectroscopy. In Metabonomics: Methods and Protocols (ed. Bjerrum, J. T.) 1277, 15–28 (2015).
Weljie, A. M., Newton, J., Mercier, P., Carlson, E. & Slupsky, C. M. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal. Chem. 78, 4430–4442 (2006).
Romero-Ferrero, F., Bergomi, M. G., Hinz, R. C., Heras, F. J. H. & de Polavieja, G. G. idtracker.ai: Tracking all individuals in small or large collectives of unmarked animals. Nat. Methods 16, 179–182 (2019).
Ewels, P. A. et al. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 38, 276–278 (2020).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Abi Assaf, J. A., et al. Gum Arabic (Acacia senegal) enhances reproduction and modulates the microbiota-gut-brain axis of zebrafish in a sex-specific and dosage-dependent manner. Preprint at https://doi.org/10.1101/2024.10.04.616708 (2024).
Acknowledgements
We want to express our sincere gratitude to Ms Rispah N. Ng’ang’a for her assistance with the experimental work and data collection. We thank Dr Sarah Worsley and Mr Chuen Lee for their help with the 16S rRNA microbiome analysis. All the bioinformatics tools presented in this paper were carried out on the High-Performance Computing (HPC) Cluster supported by the Research and Specialist Computing Support service at UEA. We would also like to thank the Disease Modelling Unit (DMU) staff at UEA for their assistance with the zebrafish husbandry. Finally, we would like to extend our gratitude to Dr James Mccoll, the imaging manager at UEA. This work was supported by grants from the European Research Council (grant no. SELECTHAPLOID-101001341), the Natural Environment Research Council (grant no. NE/S011188/1) to SI, and a PhD studentship from the Wellcome Trust (grant no. 218467/Z/19/Z) to JAA.
Author information
Authors and Affiliations
Contributions
J.A.A. Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Visualization, Writing—original draft, Writing—review & editing. J.C.D.C. Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Visualization, Writing—review & editing. A.M.G. Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Writing—review & editing. E.R. Investigation, Data curation. J.B. Investigation, Data curation. J.C.K. Investigation, Data curation. K.S. Formal analysis, Investigation, Data curation. Z.C. Investigation, Data curation. G.L.G. Methodology, Software, Validation, Formal analysis, Investigation, Resources. S.S. Conceptualization, Methodology, Resources. S.A.K. Conceptualization, Methodology, Resources, Writing—Review & Editing. S.I. Conceptualization, Methodology, Validation, Formal analysis, Resources, Supervision, Funding acquisition, Writing—Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abi Assaf, J., de Coriolis, JC., Godden, A.M. et al. Gum Arabic modulates the microbiota-gut-brain axis and affects general fitness in zebrafish. Sci Rep 15, 34465 (2025). https://doi.org/10.1038/s41598-025-17665-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-17665-z