Abstract
Selenium is an essential trace element involved in antioxidant defense and immune regulation, yet its clinical role in critically ill patients remains uncertain. In this prospective single-center observational study, we evaluated 144 ICU patients between March 2022 and October 2023. Serum selenium levels were measured, and eligible patients received intravenous selenium supplementation (1000 µg/day for 5 days). Clinical outcomes, including ICU mortality, were analyzed in relation to selenium status and response. Selenium levels < 70 µg/L were observed in 27.8% of patients and were associated with higher severity scores, inflammatory markers, and longer hospital stay. Among 67 patients receiving supplementation, those with a post-treatment selenium increase > 50 µg/L had significantly lower ICU mortality. Multivariate analysis identified SOFA score, FiO2, and selenium increase as independent predictors of ICU mortality. Lower selenium levels were associated with greater illness severity, and adequate selenium repletion may be linked to improved outcomes. However, the study is limited by its non-randomized, single-center design and relatively small sample size.
Similar content being viewed by others
Introduction
Selenium is an essential trace element critical for various physiological processes, including immunomodulation, antioxidant defense1regulation of endocrine and metabolism2. Selenium is the major cofactor of selenoproteins, which are involved in antioxidant and immunomodulatory functions, and its activity is related to selenium level3,4. Selenium also influences innate im-munity, adaptive immunity and the balance between type 2 and type 1 helper T cells5. Selenium deficiency exacerbates oxidative damage and is correlated to immune dysregulation6,7,8.
Decreases of selenium level in critically ill patients are common and linked to lower antioxidant activity, higher disease severity scores and increased mortality9,10. Considering the immunomodulation and antioxidant of selenium, selenium ad-ministration in critically ill patients has been explored as a potential therapeutic intervention. Previous research showed selenium supplements in critically ill patients decrease serum level of C-Reactive Protein (CRP)11,12, interleukin-6 (IL-6) and interleukin-1 beta (IL-1β)13. Selenium supplements were also found to enhance antioxidant effects, alleviate oxidative stress and increase prealbumin level14.
Selenium is absorbed in the gastrointestinal tract mainly as selenomethionine, selenocysteine, selenite, or selenate, all with high oral bioavailability under normal conditions. Selenomethionine, primarily from plant sources, is taken up via methionine transporters and stored in body proteins rather than used directly for selenoprotein synthesis15,16. In contrast, inorganic forms like selenite are metabolized more directly into selenide for the synthesis of functionally active selenoproteins17. In critically ill patients, gastrointestinal dysfunction can impair selenium absorption, making enteral supplementation unreliable. Intravenous administration bypasses these barriers, ensuring rapid and consistent delivery for effective selenoprotein synthesis.
The European Society for Clinical Nutrition and Metabolism (ESPEN) highlights the importance of selenium supplementation in critically ill patients, as it supports antioxidant defense and immune function during oxidative stress18. Given that gastrointestinal function is often impaired in ICU patients, ESPEN suggests considering parenteral selenium supplementation when enteral absorption may be suboptimal. This route offers the advantage of more predictable bioavailability and enables faster correction of selenium deficiency19.
Clinical trials investigating the effects of selenium supplements on mortality and clinical outcomes in critically ill patients have yielded mixed results. Some studies demonstrated benefits, including reduced mortality20,21,22. A recently published randomized control trial revealed that high dose selenium treatment reduces the mortality in septic patients with serum selenium level < 80 µg/L23. However, previous meta-analysis studies showed inconclusive findings. Early reviews by Alhazzani et al. (2013) and Manzanares et al. (2016) found no significant mortality benefit or consistent improvement in clinical outcomes23,24. The Cochrane review by Allingstrup and Afshari (2015) suggested a potential mortality reduction, though evidence quality was low25. Kong et al. reported reduced all-cause mortality and shorter hospital stays, but no effect on 28-day mortality or ICU length of stay (LOS)26. Most recently, an umbrella review by Cortes-Puentes et al. concluded that selenium supplementation shows limited or uncertain benefit across pooled meta-analyses, with considerable heterogeneity and methodological variation among included trials27. Collectively, these findings highlight the lack of definitive evidence and underscore the need for targeted research to clarify patient selection, dosing strategies, and therapeutic timing. Our study aimed to assess the effect of selenium supplement on clinical outcomes in patients hospitalized in the ICU.
Materials and methods
Participant selection
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Chi Mei Medical Center (No. 11304-011, date: 25 Apr 2024). All participants provided informed consent prior to participation.
The trial was conducted in accordance with national guidelines and the principles of the Declaration of Helsinki. The trial protocol was approved by an institutional review board, and informed consent was obtained from all participants. The study was de-signed as a prospective single center study of adult patients in one adult ICU (19 beds) at Chi-Mei Medical Center from March 2022 through October 2023 to assess the effect of selenium administration on clinical outcomes in patients hospitalized in the ICU. Patients were eligible for inclusion in the trial if they met the following criteria: admission to ICU and age ≥ 20 years. Exclusion criteria included known selenium allergy, selenium supplementation (> 500 µg/day) within the previous month, lack of informed consent, signed Do Not Resuscitate orders, and language barriers.
Selenium supplementation
Serum selenium level was obtained after the patient was included. Patents met below criteria were enrolled into the treatment group: serum selenium level < 70 µg/L, serum selenium level < 150 µg/dL with sepsis/septic shock, cancer, stroke/traumatic brain injury, total parenteral nutrition use and dialysis dependent. The patient in the treatment group received a daily intravenous infusion of 1000 µg selenium (Zelnite®) on days 1–5. Zelnite® contains sodium selenite, an inorganic form of selenium (Supplementary Fig. 1). On days 6, serum selenium levels of the patients in the treatment group were obtained again. Patients included in the trial were treated with standard care according to guidelines.
Statistical analysis
Demographic and clinical information, laboratory results, comorbidities, severity scores, mortality, and LOS for both ICU and hospital were surveyed. The descriptive statistic was used to compare the all-cause mortality between groups. Student’s t-test and chi-squared test were applied to assess the difference of factors interested between different groups. Multivariate logistic regression was used to identify the risk factors of selenium insufficiency and evaluate the factors of survival.
Results
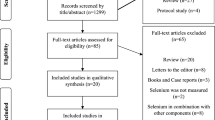
A total of 144 patients underwent serum selenium assessment, the mean age was 68.6 years, with more males (63.2%) than females. 40 patients (27.8%) exhibited serum selenium levels < 70 µg/L, while 67 patients (46.5%) met the trial criteria and received selenium administration (Fig. 1), with a dose of 1000 µg/day for 5 days.
Demographic and clinical characteristics
No significant differences in demographic characteristics or comorbidities were observed between patients with selenium levels < 70 µg/L and those with levels ≥ 70 µg/L (Table 1). Table 2 summarizes baseline disease severity, inflammatory markers, and vitamin D status according to serum selenium levels. Patients with selenium deficiency demonstrated higher severity scores, higher CRP concentrations, and lower plasma 25-hydroxyvitamin D levels compared with those with selenium levels ≥ 70 µg/L. While ICU survival, ICU LOS, and hospital survival did not differ significantly between the two groups, there was a trend toward higher survival rates and shorter ICU stays among patients with higher selenium levels.
Figure 2 compares clinical and laboratory parameters between patients with serum selenium levels < 70 µg/L and those with levels ≥ 70 µg/L. Patients in the selenium-deficient group had significantly higher severity scores: APACHE II scores were 24.7 ± 9.3 compared to 20.1 ± 7.8 in the normal selenium group (p = 0.003), SOFA scores were 8.6 ± 4.3 vs. 6.6 ± 3.9 (p = 0.012), and TISS scores were 31.4 ± 9.4 vs. 28.1 ± 7.0 (p = 0.023), respectively. In terms of biochemical markers, selenium-deficient patients had significantly lower plasma 25-hydroxyvitamin D levels (16.0 ± 7.5 ng/mL vs. 22.6 ± 9.3 ng/mL, p < 0.001) and markedly higher serum C- CRP levels (162.4 ± 99.2 mg/L vs. 82.1 ± 81.0 mg/L, p < 0.001). These findings suggest that lower selenium levels are associated with greater disease severity, higher systemic inflammation, and coexisting micronutrient deficiencies.
Comparison of clinical and laboratory parameters between patients with serum selenium levels < 70 µg/L and ≥ 70 µg/L. (a) APACHE II score was significantly higher in the selenium-deficient group (24.7 ± 9.3) compared to those with normal selenium levels (20.1 ± 7.8), p = 0.003. (b) SOFA score was higher in selenium-deficient patients (8.6 ± 4.3 vs. 6.6 ± 3.9), p = 0.012. (c) TISS score was elevated in patients with selenium < 70 µg/L (31.4 ± 9.4 vs. 28.1 ± 7.0), p = 0.023. (d) Plasma 25-hydroxyvitamin D concentration was lower in the selenium-deficient group (16.0 ± 7.5 ng/mL vs. 22.6 ± 9.3 ng/mL), p < 0.001. (e) Serum CRP was significantly higher in selenium-deficient patients (162.4 ± 99.2 mg/L) compared to those with selenium ≥ 70 µg/L (82.1 ± 81.0 mg/L), p < 0.001.
Selenium administration in patients with selenium insufficiency
Among the 40 patients with serum selenium levels < 70 µg/L, 23 received intravenous selenium supplementation while 17 did not. The baseline demographic characteristics and comorbidity profiles were generally comparable between the two groups. However, patients in the selenium-treated group were older, with a higher proportion aged over 65 years (78.3% vs. 47.1%, p = 0.041), suggesting that clinicians may have favored supplementation in more vulnerable individuals. There were no statistically significant differences in the prevalence of chronic diseases such as diabetes mellitus, hypertension, cancer, or end-stage renal disease between groups. The mean baseline selenium levels were also similar (53.9 ± 12.8 µg/L vs. 57.9 ± 12.0 µg/L, p = 0.404), indicating comparable initial selenium status. These findings suggest that selection for selenium administration was not based on markedly different clinical profiles (Table 3).
In terms of clinical outcomes (Table 4), patients with selenium deficiency who received supplementation demonstrated a trend toward improved survival and comparable clinical severity at ICU admission. Although severity scores—APACHE II (24.5 ± 8.5 vs. 25.1 ± 10.5, p = 0.956), SOFA (8.0 ± 3.9 vs. 9.3 ± 4.9, p = 0.483), and TISS (31.7 ± 8.4 vs. 30.9 ± 10.8, p = 0.602)—were not significantly different between the selenium-treated and untreated groups, patients who received selenium had a notably higher ICU survival rate (91.3% vs. 64.7%, p = 0.053), approaching statistical significance. There were no significant differences in hospital survival (56.5% vs. 52.9%, p = 0.822), ICU LOS (13.8 ± 11.4 vs. 10.8 ± 8.0 days, p = 0.365), or total hospital LOS (31.3 ± 22.2 vs. 31.6 ± 29.0 days, p = 0.593). These findings suggest a potential benefit of selenium supplementation on short-term ICU outcomes, particularly survival, in selenium-deficient patients, though the modest sample size limits definitive conclusions.
Selenium administration and clinical outcome
Within 67 patients receiving selenium administration, 58 patients had ICU survive. There was no significant difference between demographic characteristics of ICU survival group and non survival group, but non survival group had higher percentage of sepsis and septic shock (Table 5). Non survival group had higher SOFA scores (10.7 vs. 7.1, p = 0.007), TISS (33.6 vs. 28.1, p = 0.020), white blood cell count (22.0 vs. 12.3, p = 0.024) and FiO2 (58.3 vs. 33.0, p = 0.000). Non survival group also had lower P/F ratio (198.4 vs. 401.9, p = 0.001). Increase of serum selenium level after selenium administration (ΔSe) was also analysis. 45 patients (77.6%) in survival group had ΔSe > 50 µg/L, while 3 patients (33.3%) in non survival group had ΔSe > 50 µg/L, the survival group had significant higher rate of ΔSe > 50 µg/L (p = 0.012) (Table 6).
To identify independent predictors of ICU mortality among patients who received selenium supplementation (n = 67), we first conducted univariate analyses using clinical and laboratory variables shown in Tables 5 and 6. Variables with statistical significance (p < 0.05) in the univariate analysis were then entered into a multivariate logistic regression model. As shown in Table 7, three variables emerged as independent predictors of ICU mortality. First, a post-supplementation ΔSe > 50 µg/L was strongly associated with reduced risk of ICU mortality (odds ratio [OR] = 0.036; 95% CI 0.002–0.793; p = 0.035). Second, the SOFA score remained an independent predictor of mortality (OR = 1.727; 95% CI 1.029–2.897; p = 0.039). Lastly, higher FiO2 at admission was also associated with increased mortality risk (OR = 1.124; 95% CI 1.026–1.232; p = 0.012).
Discussion
This study corroborates previous findings that selenium deficiency in critically ill patients is associated with higher disease severity and poorer clinical outcomes. Lower serum selenium level (< 70 µg/L) was associated with higher APACHE II, SOFA, CRP, FiO2, percentage of septic shock, and extent hospital LOS, as well as a lower MAP, 25OHD, and P/F ratio. Selenium deficiency is correlated to poor clinical outcome, however, a meta-analysis conducted by Manzanares et al., including 21 randomized trials, concluded that selenium supplement had no effect on mortality, ICU and hospital LOS, or ventilator days; these studies also indicated the importance of pharmacokinetic and pharmacodynamic data on dosing strategy24,28.
A recently published randomized control trial of intravenous high dose selenium supplements in sepsis and septic shock patients implied that high dose selenium treatment reduces the mortality in certain patient groups29. This study revealed that sepsis and septic shock patients with selenium level constantly lower than 80 µg/L had high mortality rate (41–50%), but the mortality rate decreased to 21–30% when selenium level increased beyond 110 µg/L after received intravenous high dose selenium supplements. The crucial role of increase of selenium level was also found in our study; the increase in serum selenium > 50 µg/L following treatment was correlated to ICU survival. Our study also showed a trend of higher survival in patients with selenium levels < 70 µg/L and receiving selenium administration. These findings suggest selenium supplements may reduce mortality in severe selenium deficiency when selenium level increases to a certain level following treatment.
Alteration of micronutrients status in critically ill patients has been reported in previous studies28,30. Redistribution of micronutrients and decreased circulating carrier proteins are possible underlying mechanisms and related to systemic inflammatory response syndrome (SIRS)30,31. Vitamin D, a micronutrient with functions of immunomodulation and lung protection in sepsis, its deficiency was also found in critically ill patients and linked to poor clinical outcome32. In our study, patients with serum selenium levels < 70 µg/L also had lower plasma vitamin D concentration, along with higher disease severity scores, CRP and percentage of septic shock, this indicated that the interaction between SIRS and micronutrients has a profound impact on clinical outcome.
Study of intravenous selenium supplement in patients with acute respiratory distress syndrome (ARDS) revealed that patients receiving selenium supplement had lower airway resistance and higher pulmonary compliance; higher serum selenium level in this study was correlated with higher serum concentrations of glutathione pe-roxidase 3 (GPx-3) and lower serum concentrations IL-6 and IL-1β13. Animal models also indicated selenium deficiency induced oxidative stress and inflammation lead to lung fibrosis33. Our study showed higher FiO2 and lower P/F ratio in critically ill patients with serum selenium levels < 70 µg/L. Selenium is likely to play a crucial role in the respiratory system of critically ill patients through antioxidant activity and immunomodulation.
Alteration of micronutrients status in critically ill patients has been reported in previous studies24,25. Redistribution of micronutrients and decreased circulating carrier proteins are possible underlying mechanisms and related to systemic inflammatory response syndrome (SIRS)25,26. Vitamin D, a micronutrient with functions of immunomodulation and lung protection in sepsis, its deficiency was also found in critically ill patients and linked to poor clinical outcome27. In our study, patients with serum selenium levels < 70 µg/L also had lower plasma vitamin D concentration, along with higher disease severity scores, CRP and percentage of septic shock, this indicated that the interaction between SIRS and micronutrients has a profound impact on clinical outcome.
Study of intravenous selenium supplement in patients with acute respiratory distress syndrome (ARDS) revealed that patients receiving selenium supplement had lower airway resistance and higher pulmonary compliance; higher serum selenium level in this study was correlated with higher serum concentrations of glutathione pe-roxidase 3 (GPx-3) and lower serum concentrations IL-6 and IL-1β16. Animal models also indicated selenium deficiency induced oxidative stress and inflammation lead to lung fibrosis28. Our study showed higher FiO2 and lower P/F ratio in critically ill patients with serum selenium levels < 70 µg/L. Selenium is likely to play a crucial role in the respiratory system of critically ill patients through antioxidant activity and immunomodulation.
This study enrolled a heterogeneous population of critically ill patients and systematically collected a wide range of clinical data, including disease severity scores, laboratory markers, and ICU outcomes. By analyzing changes in serum selenium levels following supplementation, we identified a significant association between an increase in selenium levels and reduced ICU mortality. However, several limitations must be acknowledged. The observational, non-randomized design, single-center setting, and relatively small sample size—particularly in the subgroup receiving selenium—limit the generalizability and causal interpretation of our findings. Moreover, we did not include mechanistic biomarkers such as glutathione GPx3, IL-6, or IL-1β, which are important for assessing oxidative stress and immune modulation. These omissions restrict our ability to directly link selenium supplementation to biological effects. Future prospective, multicenter randomized trials incorporating pharmacokinetic data and relevant biomarkers are warranted to clarify the therapeutic role of selenium in critically ill patients and to define optimal dosing strategies.
Conclusion
In this prospective observational study, lower serum selenium levels (< 70 µg/L) in ICU patients were associated with greater disease severity, elevated inflammatory markers, impaired oxygenation, and longer hospital stays. Among patients receiving selenium supplementation, an increase in serum selenium levels greater than 50 µg/L was independently associated with improved ICU survival. While these findings suggest a potential clinical benefit of selenium repletion in critically ill patients, causality cannot be confirmed due to the non-randomized design and limited sample size. Future multicenter randomized trials are needed to validate these associations, explore optimal dosing strategies, and incorporate mechanistic biomarkers to better understand the immunomodulatory and antioxidant effects of selenium in critical illness.
Data availability
The data that support the findings of the current study may be requested from the corresponding author upon reasonable request.
References
Hosnedlova, B. et al. A summary of new findings on the biological effects of selenium in selected animal Species—A critical review. Int. J. Mol. Sci. 18, 2209. https://doi.org/10.3390/ijms18102209 (2017).
Winther, K. H., Rayman, M. P., Bonnema, S. J. & Hegedüs, L. Selenium in thyroid disorders — essential knowledge for clinicians. Nat. Rev. Endocrinol. 16, 165–176. https://doi.org/10.1038/s41574-019-0311-6 (2020).
Pei, J., Pan, X., Wei, G. & Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 14, 1147414. https://doi.org/10.3389/fphar.2023.1147414 (2023).
Zhang, F., Li, X. & Wei, Y. Selenium and selenoproteins in health. Biomolecules 13, 799. https://doi.org/10.3390/biom13050799 (2023).
Xia, X. et al. Toward improved human health: efficacy of dietary selenium on immunity at the cellular level. Food Funct. 12, 976–989. https://doi.org/10.1039/d0fo03067h (2020).
Mahmoodpoor, A. et al. The effects of selenium supplementation on inflammatory markers in critically ill patients. SN Appl. Sci. 4, 326. https://doi.org/10.1007/s42452-022-05208-4 (2022).
Xu, J. et al. Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol. Trace Elem. Res. 194, 237–243. https://doi.org/10.1007/s12011-019-01775-7 (2019).
Hariharan, S. & Dharmaraj, S. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 28, 667–695. https://doi.org/10.1007/s10787-020-00690-x (2020).
Herrera-Quintana, L., Vázquez-Lorente, H., Molina-López, J., Gamarra-Morales, Y. & Planells, E. Selenium Levels and Antioxidant Activity in Critically Ill Patients with Systemic Inflammatory Response Syndrome. Metabolites. 12, 274. https://doi.org/10.3390/metabo12040274 (2022).
Forceville, X. et al. Rather than glutathione peroxidase, as a potential marker of septic shock and related syndromes. Eur. Surg. Res. 43, 338–347. https://doi.org/10.1159/000239763 (2009).
Valenta, J., Brodska, H., Drabek, T., Hendl, J. & Kazda, A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 37, 808–815. https://doi.org/10.1007/s00134-011-2153-0 (2011).
Alikiaii, B., Mousavi, S., Hashemi, S. T. & Abdollahi, M. Effect of selenium supplementation on CRP levels and incidence of delirium in critically ill patients. DOAJ (DOAJ: Directory Open. Access. Journals) (2018).
Mahmoodpoor, A. et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol. Investig. 48, 147–159. https://doi.org/10.1080/08820139.2018.1496098 (2018).
Mishra, V. et al. Effect of selenium supplementation on biochemical markers and outcome in critically ill patients. Clin. Nutr. 26, 41–50. https://doi.org/10.1016/j.clnu.2006.10.003 (2006).
Fairweather-Tait, S. J. et al. Selenium in human health and disease. Antioxid. Redox. Signal. 14, 1337–1383. https://doi.org/10.1089/ars.2010.3275 (2011).
Burk, R. F. & Hill, K. E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 35, 109–134. https://doi.org/10.1146/annurev-nutr-071714-034250 (2015).
Suzuki, Y. et al. Dynamic pathways of selenium metabolism and excretion in mice under different selenium nutritional statuses. Metallomics. 2, 126–132. https://doi.org/10.1039/b915816b (2010).
Singer, P. et al. Espen guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 38, 48–79. https://doi.org/10.1016/j.clnu.2018.08.037 (2019).
Berger, M. M. et al. Espen micronutrient guideline. Clin. Nutr. 41, 1357–1424. https://doi.org/10.1016/j.clnu.2022.02.015 (2022).
Vašková, J., Kočan, L., Firment, J. & Vaško, L. Correction to: Restoration of antioxidant enzymes in the therapeutic use of selenium in septic patients. Wien. Klin. Wochenschr. 135, 214. https://doi.org/10.1007/s00508-023-02191-7 (2023).
Angstwurm, M. W. A. et al. Selenium in intensive care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock*. Crit. Care Med. 35, 118–126. https://doi.org/10.1097/01.ccm.0000251124.83436.0e (2006).
Brodska, H. et al. Biomarkers in critically ill patients with systemic inflammatory response syndrome or sepsis supplemented with high-dose selenium. J. Trace Elem. Med Biol. 31, 25–32. https://doi.org/10.1016/j.jtemb.2015.02.005 (2015).
Alhazzani, W. et al. The effect of selenium therapy on mortality in patients with sepsis syndrome. Crit. Care Med. 41, 1555–1564. https://doi.org/10.1097/ccm.0b013e31828a24c6 (2013).
Manzanares, W. et al. High-dose intravenous selenium does not improve clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit. Care. 20, 356. https://doi.org/10.1186/s13054-016-1529-5 (2016).
Allingstrup, M. & Afshari, A. Selenium supplementation for critically ill adults. Cochrane Libr. 2018 (3703). https://doi.org/10.1002/14651858.cd003703.pub3 (2015).
Kong, L., Wu, Q. & Liu, B. The impact of selenium administration on severe sepsis or septic shock: a meta-analysis of randomized controlled trials. Afr. Health Sci. 21, 277–285. https://doi.org/10.4314/ahs.v21i1.36 (2021).
Jaff, S. et al. The effect of selenium therapy in critically ill patients: an umbrella review of systematic reviews and meta-analysis of randomized controlled trials. Eur. J. Med. Res. 28, 104. https://doi.org/10.1186/s40001-023-01075-w (2023).
Berger, M. M. & Shenkin, A. Selenium in intensive care: probably not a magic bullet but an important adjuvant therapy*. Crit. Care Med. 35, 306–307. https://doi.org/10.1097/01.ccm.0000251943.86292.87 (2006).
Chen, C. M. et al. Effects of High-Dose selenium on mortality of sepsis and septic shock patients with severe selenium deficiency in Taiwan. Med. Res. Arch. 12, 5044. https://doi.org/10.18103/mra.v12i2.5044 (2024).
Belsky, J. B., Wira, C. R., Jacob, V., Sather, J. E. & Lee, P. J. A review of micronutrients in sepsis: the role of thiamine,l-carnitine, vitamin C, selenium and vitamin D. Nutr. Res. Rev. 31, 281–290. https://doi.org/10.1017/s0954422418000124 (2018).
Duntas, L. H. & Benvenga, S. Selenium: an element for life. Endocrine 48, 756–775. https://doi.org/10.1007/s12020-014-0477-6 (2014).
Amrein, K., Papinutti, A., Mathew, E., Vila, G. & Parekh, D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr. Connections. 7, R304–R315. https://doi.org/10.1530/ec-18-0184 (2018).
Fu, Y. X., Wang, Y. B., Bu, Q. W. & Guo, M. Y. Selenium deficiency caused fibrosis as an oxidative stress-induced inflammatory injury in the lungs of mice. Biol. Trace Elem. Res. 201, 1286–1300. https://doi.org/10.1007/s12011-022-03222-6 (2022).
Acknowledgements
This study represents the collaborative efforts of numerous investigators and healthcare professionals, whose contributions are sincerely appreciated. We extend our deepest gratitude to all the patients who generously participated in this research.
Funding
This research received funding from Chi Mei Medical Center (CMFHR114091).
Author information
Authors and Affiliations
Contributions
C.-M.C.: Conceptualization, methodology, data curation, formal analysis and writing—original draft preparation. W.C.: conceptualization, review and editing. H.-H.T.: writing—review and editing. Y.-C.L.: writing—review and editing. Y.-C.S.: writing—review and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We declare that the work described has been carried out in accordance with the Declaration of Helsinki of the World Medical Association revised in 2013 for experiments involving humans Consent for publication. The study involving human participants were approved by the Institutional Review Board of Chi Mei Medical Center (No. 11304-011, date: 25 Apr 2024). All participants provided informed consent prior to participation.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tan, HH., Liang, YC., Shao, YC. et al. Impact of selenium status and supplementation on outcomes in critically ill patients. Sci Rep 15, 35478 (2025). https://doi.org/10.1038/s41598-025-19315-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19315-w