Abstract
Nasal vaccines characterized by secretory IgA (SIgA) induction are effective in preventing mucosal infection. SIgA induction is potentiated by mucosal adjuvants, and the exploration of new safe and effective candidates is important. Nanosized hydroxyapatites (HAs) have the potential to enhance immune responses. The membrane vesicles (MVs) released from Streptococcus mutans UA159, a cariogenic bacterium, contain immunogenic glucosyltransferases (GTFs). We evaluated HAs made from eggshell waste, including nanosized particles, as a mucosal adjuvant against nasally administered MVs. Scanning electron microscopy revealed that the MVs were adsorbed to the eggshell-derived HA surface, and these complexes formed various sizes of aggregates. The coadministration of eggshell-derived HAs reinforced the MV-specific mucosal IgA and systemic IgG response, with levels nearly to those induced by the coadministration of polyriboinosinic polyribocytidylic acid, known as a mucosal adjuvant. MV-only immunization also induced MV-specific antibody responses, although the levels were lower. Immunization with MVs with or without eggshell-derived HAs induced IFN-γ/IL-17 secretion. Biofilm formation by the S. mutans UA159 gtfBgtfC double-deficient strain with wild-type-derived MVs containing GTF-B and GTF-C was inhibited by the induced IgGs via MV neutralization. These results suggest that eggshell-derived HAs are potentially effective mucosal adjuvants against S. mutans MVs coadministered nasally.
Similar content being viewed by others
Introduction
Artificial hydroxyapatites (HAs) are used as dental materials and in bone tissue engineering, as HAs are inorganic components of natural bones and teeth and thus are highly biocompatible. HAs have a hexagonal crystal structure system1. HAs are mainly composed of calcium, phosphate, and hydroxide ions2. The Ca ions are arranged in columns and in a spiral shape around the hydroxyl groups on the screw axis of the crystal1. HAs have strong protein adsorption properties because they have large surface areas, high porosities, and charges on their surfaces3,4,5. Furthermore, nanosized HAs are efficiently taken up by antigen (Ag)-presenting cells such as dendritic cells (DCs)6. Nanoscale but not microscale HAs enhance inflammatory cytokine secretion in response to coadministration of lipopolysaccharide (LPS)7. Nanoscale HAs can be synthesized from inexpensive industrial waste, including eggshells, which are useful sources of calcium phosphates8,9,10.
Mucosal IgA immunity plays key roles in preventing mucosal infection in which pathogens enter the host body through the mucosal surface. The Ag-specific secretory IgA (SIgA) is contained in the exocrine secretions covering the mucosal surface. SIgA exhibits neutralization activity against viruses, bacteria, and toxins and inhibits the adhesion and colonization of microorganisms to the mucosal surface11. For the induction of Ag-specific SIgA responses, it is preferable that Ags are administered via the mucosal route. In particular, intranasal immunization elicits Ag-specific SIgA responses in the upper and lower respiratory mucosa including saliva and nasal secretions through the common mucosal immune system12. Mucosal surfaces are harsh environments for the initiation of immune responses. They are protected by host protective barriers. Tolerance is preferentially induced to maintain homeostasis at the mucosal surface13. Thus, coadministration of an effective, potent mucosal adjuvant or Ag delivery system is needed for robust induction of Ag-specific SIgA responses.
Various potential candidates for mucosal adjuvants include bacterial endotoxins and several pattern recognition ligands, which contribute to the activation of innate immunity. The synthetic double-stranded RNA polyriboinosinic polyribocytidylic acid [poly(I:C)] is an agonist of Toll-like receptor 3 and is known as a potent mucosal adjuvant14,15. Furthermore, nanoparticles, including virus-like particles, liposomes, and nanogels may be applicable as mucosal adjuvants for Ag delivery systems16. However, currently, there are few licensed mucosal adjuvants available for use in humans16,17. Thus, it is essential to explore new safe, effective candidates for mucosal adjuvants. In this context, a previous study showed that nanosized HAs enhanced specific antibody induction in the systemic compartment against biparentally coadministered Ag18,19. In this study, we hypothesized that HAs made from inexpensive eggshell waste, including nanosized particles, could work as mucosal adjuvants.
The eggshells were heated at a temperature above 900 °C to produce calcium oxide20. Because eggs contain sulfur compounds, hydrogen sulfide (H2S) is formed by heat treatment of eggs21. Upon further oxidation of H2S, sulfurous acid and eventually sulfate are generated22. A previous study revealed that the amount of metal present in aluminum hydroxide adjuvants catalyzes sulfite autoxidation, which may be associated with the degradation of protein antigens23. These findings suggest that sulfite contamination in eggshell-derived HAs may cause instability of protein antigens. Repeated exposure of the sulfate contained in eggshell-derived HA to topical nasal mucosa may be harmful to host tissues. Thus, in this study, sulfur compound-free eggshell-derived HAs were generated and used as mucosal adjuvant candidates.
Bacteria release membrane vesicles (MVs) for their own or host–bacteria interactions24. These vesicles contain various molecules, including pathogenic factors and factors needed for their survival25. One of the major cariogenic bacteria, Streptococcus mutans, is a gram-positive oral bacterium in humans. It also releases MVs composed of functional molecules26,27. S. mutans MVs mainly consist of nucleic acids26, protein antigen c (PAc)28, glucan-binding proteins26, and enzymes such as glucosyltransferases (GTFs)26,27 and fructosyltransferase (FTF)29, both of which are known cariogenic factors30,31. S. mutans produces three types of GTFs, GTF-B, GTF-C, and GTF-D, which are glucan synthases31. S. mutans MVs containing GTFs cause sucrose-dependent biofilm formation27. Classical studies have shown that immunization with GTFs derived from Streptococcus sobrinus, another cariogenic bacterium in humans, or from S. mutans induced GTF-specific antibody responses32,33. Nasal immunization of mice with S. sobrinus GTF-I protein elicited anti-GTF-I mucosal immune responses34. Nasal immunization of mice with a combination of S. mutans MVs and poly(I:C) elicited MV-specific mucosal SIgA responses28. Thus, S. mutans MVs containing GTF Ags exhibited immunogenicity and thus could be used as model Ags for mucosal vaccines.
In this study, we evaluated the efficacy of eggshell-derived HAs with low sulfate content as a mucosal adjuvant against the coadministered model Ag, S. mutans MVs containing immunogenic GTFs. We performed nasal immunization of mice with S. mutans MVs plus eggshell-derived HAs multiple times and assessed the Ag-specific immune responses induced in both the mucosal and systemic compartments. We also assessed the trend of immune responses and neutralization effects of induced antibodies against S. mutans biofilm formation.
Results
Evaluation of MVs purified from S. mutans and complexes consisting of MVs and eggshell-derived HAs
First, we evaluated purified MVs from S. mutans UA159, which were used as the model Ag in this study. SDS‒PAGE analysis of the purified MVs revealed predominant bands at approximately 150 kDa, corresponding to GTF-C, and at 165 kDa, corresponding to GTF-B, which are glucan synthase and the main components of MVs (Fig. 1a)29. These bands exhibited glucan-synthesizing enzyme activities toward the substrate sucrose, and the glucans were visualized as white bands (Fig. 1b). Approximately 90 kDa band was also observed and exhibited enzyme activity. In contrast, MVs purified from S. mutans UA159 gtfB and gtfC double deletion strain (UA159 gtfBC-), which is unable to produce GTFs, failed to produce the glucans29. Furthermore, the protein bands corresponding to GTFs, and 90 kDa band, reacted with antiserum taken from mice immunized with MVs derived from S. mutans UA159 plus poly(I:C) (Fig. 1c)28. When the MVs derived from UA159 gtfBC- were subjected to western blotting, the bands corresponding to GTFs were not detected by the antiserum taken from mice immunized with MVs derived from S. mutans UA159 plus poly(I:C)28.
Evaluation of MVs purified from S. mutans UA159. The MVs were subjected to 12.5% SDS‒PAGE followed by CBB staining (a), incubation in acetate buffer containing 3% sucrose for zymography (b), and western blotting with anti-MV serum and HRP-conjugated anti-mouse IgG (c). Representative images from more than three independent experiments are shown. Line 1, size marker; line 2, CBB staining; line 3, zymography; line 4, western blotting. The blots and gels were cropped. Original blots and gels are presented in Supplementary Fig. 1.
In this study, we designed a nasal vaccine candidate consisting of MVs and eggshell-derived HAs. To confirm the adsorption of MVs onto the surface of eggshell-derived HAs, mixtures of MVs and eggshell-derived HAs were probed with anti-MV antiserum and colloidal gold-conjugated anti-mouse IgG, and the mixtures were observed by scanning electron microscopy (SEM). The signals of colloidal gold particles were detected from the MV-only sample (Fig. 2a) and the surface of eggshell-derived HAs in the mixture sample (Fig. 2b) but not from the eggshell-derived HA-only sample (Fig. 2c). Most signals from MVs were not detected, as shown in the image of MVs alone. Furthermore, the MVs adhered to glass surfaces coated with poly-L-lysine (Fig. 2d). The eggshell-derived HAs formed aggregates in the presence of MVs. The aggregates were immobilized on the surface of the poly-L-lysine-coated glass surfaces. These results indicate that MVs worked as an adhesive between eggshell-derived HAs and glass surfaces (Fig. 2e). By contrast, most eggshell-derived HAs did not remain on these surfaces in the absence of MVs (Fig. 2f). Thus, the MVs were actually bound to the surface of eggshell-derived HAs, and these two materials formed aggregates of various sizes ranging from approximately 0.1 to 6 µm in diameter (Fig. 2e).
SEM observations of MVs and eggshell-derived HAs. (a–c) MVs were detected using anti-MV serum and 10 nm colloidal gold-conjugated anti-mouse IgG (indicated by arrows; magnification, ×100,000). MVs (a), MVs and eggshell-derived HAs (b), and eggshell-derived HAs (c). (d–f) SEM images of aggregations (magnification, ×10,000), MVs (d), MVs and eggshell-derived HAs (e), and eggshell-derived HAs (f). Representative images from two independent experiments are shown.
Eggshell-derived HAs enhanced humoral immune responses to nasally coadministered MVs
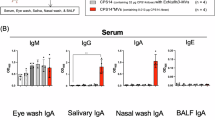
Next, we analyzed MV-specific humoral immune responses induced in mice immunized nasally with MVs plus eggshell-derived HAs. Figure 3a,b presents the immunization schedule and schematic of the complex of Ags plus eggshell-derived HAs. Among the 1, 10, and 100 µg doses of eggshell-derived HAs, MVs formulated with 100 µg of eggshell-derived HAs induced the greatest MV-specific SIgA responses in saliva (data not shown). The SIgA induction levels in mice administered MVs and 100 µg eggshell-derived HAs were significantly greater than those in mice immunized with MVs alone, even though immunization with MV alone elicited detectable levels of SIgA responses after multiple immunizations (Fig. 3c). As expected, no MV-specific SIgA responses were noted in the mice given eggshell-derived HAs alone. In contrast, the mice administered MVs plus poly(I:C) presented the best SIgA response among the experimental groups (Fig. 3c). Anti-MV SIgA production by the coadministration of MVs with eggshell-derived HAs reached levels near those of the coadministration of MV with poly(I:C) after multiple immunizations. The GTF-C-specific salivary SIgA response was markedly enhanced by coadministration of eggshell-derived HAs, compared with immunization with MVs alone (Supplementary Fig. 3). MV-specific SIgA induction in nasal secretions tended to be increased by coadministration of eggshell-derived HAs (Fig. 3d). Anti-GTF-B and anti-GTF-C SIgA responses in nasal secretions were significantly enhanced by coadministration of eggshell-derived HAs (Supplementary Fig. 3). These results suggest that the use of eggshell-derived HAs has the potential to increase the immunogenicity of coadministered Ags, leading to increased specific mucosal SIgA induction after multiple doses.
Ag-specific antibody responses induced by nasal immunization with MVs plus eggshell-derived HAs. Schematic of the experimental procedure (a) and drawing of the complex of MVs and eggshell-derived HAs (b). Seven-week-old female BALB/c mice were randomly divided into 4 groups. The mice were immunized with eggshell-derived HAs, MVs, MVs plus eggshell-derived HAs, or MVs plus poly(I:C) 5 times. Three weeks after priming, the groups of mice were boosted at 2-week intervals. Saliva and serum samples were collected before booster and 2 weeks after the last immunization. Nasal wash samples were collected 2 weeks after the last immunization. MV-specific mucosal SIgA responses in saliva (c) and nasal washes (d), and MV-specific systemic IgG responses (e). MV-specific antibody productions were determined by enzyme-linked immunosorbent assay (ELISA). The data are presented as the means ± SEM (n = 4/group). Representative data from three independent experiments are shown. A significant difference between each group, excluding poly(I:C)-administered group, was determined by repeated-measures ANOVA (c,e) or one-way ANOVA (d) with Bonferroni correction. Assessment of the production of GTF-specific antibodies in immunized mice (f,g). Serum and saliva samples, collected from mice immunized with MVs plus eggshell-derived HAs 2 weeks after the last immunization, were subjected to assessment. The MVs were subjected to 12.5% SDS‒PAGE followed by western blotting with a combination of collected serum and HRP-conjugated anti-mouse IgG (f) and a combination of saliva and HRP-conjugated anti-mouse IgA (g). Representative images from two independent experiments are shown. The blots were cropped. Original blots are presented in Supplementary Fig. 2. EHA; eggshell-derived HAs.
Anti-MV IgGs and anti-GTF-C IgGs were apparently produced in the serum of mice nasally immunized with MV alone (Fig. 3e and Supplementary Fig. 3). Compared with MV alone, coadministration of eggshell-derived HAs significantly enhanced MV-specific IgG responses (Fig. 3e). Increased GTF-B-specific serum IgG responses were induced by the inclusion of eggshell-derived HAs (Supplementary Fig. 3). Eggshell-derived HA immunization failed to induce anti-MV IgG responses. The combination of MVs and poly(I:C) induced the greatest MV-specific IgG responses in the serum (Fig. 3e). These results suggest that a certain level of Ag-specific IgG responses was induced by MVs alone immunization, although the inclusion of eggshell-derived HAs further promoted IgG production to some extent.
The reactivities of the IgG and SIgA induced in the serum and saliva of immunized mice, respectively, to MVs were confirmed by western blot analysis (Fig. 3f,g). The serum and saliva samples from mice immunized with MVs and eggshell-derived HAs were used as primary antibodies. Approximately 150 and 165 kDa proteins contained in MVs corresponding to GTFs were detected (Fig. 3f,g). Whereas these bands corresponding to GTF proteins were not detected when serum and saliva samples from mice immunized with MVs derived from UA159 gtfBC- were used as primary antibodies28. These findings indicate that the serum and saliva of wild-type MV-immunized mice contain anti-GTF antibodies.
Nasal immunization with MVs with or without eggshell-derived HAs induces Th1/Th17 responses
We examined the activation status of DCs in the cervical lymph nodes (CLNs) of immunized mice. CLNs are draining lymph nodes of Ag that is taken up by M cells in the nasopharynx-associated lymphoid tissue and those that penetrate the nasal epithelium35. The frequencies of costimulatory molecule (CD40) and major histocompatibility complex class II (MHC II) expressing CD83+ (activation marker of DCs36) cells in CD11c+ DCs were significantly increased in mice given eggshell-derived HAs (Fig. 4). The frequencies of activated DCs expressing CD80 or CD86, which are also costimulatory molecules, tended to increase in these mice. MV immunization alone tended to increase CD86 and MHC II expression in activated DCs. MVs formulated with eggshell-derived HAs did not markedly increase the frequency of activated DCs (Fig. 4). Taken together, these results may indicate that nanosized HA that did not form aggregates with MVs was the most effective at stimulating DCs.
Expression of costimulatory molecules in activated DCs from CLNs. Mice were immunized with PBS, eggshell-derived HAs, MVs or MVs plus eggshell-derived HAs. One week after immunization, the CLNs were isolated, and mononuclear cells were prepared and subjected to flow cytometry. Representative flow cytometry plots (upper panels) and the frequencies of costimulatory molecules (CD40, CD80, and CD86)- or MHC class II (I-Ad)-expressing CD83+ cells within the CD11c+ DCs (lower panel) are presented. Bar graph data are presented as relative frequencies normalized to those of the PBS-immunized control group. The gating strategy is detailed in Supplementary Fig. 4. A significant difference (exact p-values) between experimental groups and the PBS-treated control group was determined by the unpaired nonparametric Mann‒Whitney U test. EHA; eggshell-derived HAs.
We next determined the trend of T-cell responses induced by nasal immunization with MVs alone or a mixture of MVs plus eggshell-derived HAs. CD4+ cells were purified from the spleens of these groups together with eggshell-derived HA-immunized group. These primed CD4+ cells and whole splenocytes were restimulated with MVs in vitro. Compared with those from eggshell-derived HA-immunized mice, MV-stimulated CD4+ splenic cells from mice given MVs alone or MVs plus eggshell-derived HAs presented greater gamma interferon (IFN-γ) production, although the difference was not significant (Fig. 5a). Whole splenocytes from mice given MVs produced IFN-γ and the coadministration of eggshell-derived HAs enhanced IFN-γ production (Fig. 5b). Interleukin 17 (IL-17) was also produced by splenocytes, including CD4+ T cells, isolated from mice given MVs alone or MVs plus eggshell-derived HAs compared with those from eggshell-derived HA-immunized mice (Fig. 5c,d). In contrast, IL-4 production was low in the splenocytes of all the groups (eggshell-derived HAs alone; 13.38 ± 9.00 pg/ml, MVs alone; 11.0 ± 3.20 pg/ml, MVs and eggshell-derived HAs; 31.6 ± 12.7 pg/ml). Conversely, MVs alone and MVs with eggshell-derived HA immunization elicited MV-specific IgG1 and IgG2a responses in the serum. The IgG1 levels produced by these two types of immunization were almost equal to those produced by MVs with poly(I:C) immunization (Fig. 5e). In contrast, IgG2a production in MV- and MV plus eggshell-derived HA-immunized mice was significantly lower than that in MVs plus poly(I:C)-immunized mice (Fig. 5e). These results indicated that the combination of S. mutans MVs and eggshell-derived HAs induced Th1/Th17 responses. In parallel, certain levels of Th2 responses were also induced.
Cytokine production by splenocytes isolated from groups of immunized mice and IgG subclass induction. Splenic CD4+ cells (a,c) and whole splenocytes (b,d), which were isolated from mice immunized with eggshell-derived HAs, MVs, or MVs plus eggshell-derived HAs, were restimulated with or without MVs in vitro. IFN-γ and IL-17 productions in the culture supernatant were analyzed by ELISA. (e) IgG subclass induction in immunized mice. Serum samples collected 2 weeks after the last immunization were subjected to analysis. The data are presented as the means ± SEM (n = 4/group). Representative data from two independent experiments are shown. (a–d) A significant difference between each group, was determined by repeated-measures ANOVA with Bonferroni correction. (e) A significant difference between each group, was determined by one-way ANOVA with Bonferroni correction. †††p < 0.001, ††††p < 0.0001 compared with EHA in the IgG1 data, ***p < 0.001, **** p < 0.0001 compared with EHA in the IgG2a data and p = 0.0026 [MVs vs MV + poly(I:C)], p = 0.0021 [MVs + EHA vs MV + poly(I:C) ]in the IgG2a data. EHA; eggshell-derived HAs.
MV-specific serum IgG inhibited sucrose-dependent biofilm formation mediated by S. mutans MVs
We examined the neutralization activity of anti-MV IgGs induced in mice nasally immunized with MVs with or without eggshell-derived HAs. The MVs were pretreated with total IgG purified from the serum of immunized mice and further incubated with the UA159 gtfBC- in the presence of 0.25% sucrose. Biofilm formation by GTFs contained in MVs was significantly inhibited by treatment with purified total IgGs from MV-immunized mice. This inhibition was dose-dependent. In contrast, IgGs from mice immunized with eggshell-derived HAs alone exhibited no inhibitory effect (Fig. 6a). Compared with MV alone, IgGs derived from mice given MVs plus eggshell-derived HAs had a greater inhibitory effect on biofilm formation.
Neutralizing activity of MV-specific IgGs induced in immunized mice. MVs (a) or S. mutans UA159 (b) were pretreated with various doses of IgGs purified from the pooled serum of immunized mice. MVs mixed with S. mutans UA159 gtfBC- and S. mutans UA159 were subsequently cultured in TSB supplemented with 0.25% and 1% sucrose, respectively. The biofilms were stained with safranin, and the absorbance was measured at 492 nm. The data are presented as the means ± SEM of relative % to the positive control (S. mutans UA159 gtfBC- plus MV with sucrose or S. mutans UA159 with sucrose), which was 100%. Representative data from two independent experiments are shown. A significant difference between each group, was determined by repeated-measures ANOVA with Bonferroni correction. EHA; eggshell-derived HAs.
Furthermore, treatment of wild-type S. mutans UA159 with total IgGs from mice given MVs with eggshell-derived HAs in the presence of 1% sucrose slightly but significantly suppressed biofilm formation compared with treatment with IgGs induced by eggshell-derived HA immunization (Fig. 6b). IgGs from mice given MVs also slightly suppressed biofilm formation by S. mutans cells. These results indicate that MV-specific IgG induced in immunized mice has neutralization activity against MVs, which is associated with significant suppression of biofilm formation mediated by MVs. Whereas, these MV-specific IgGs had a relatively small effect on inhibition of biofilm formation by S. mutans cells.
Discussion
In this study, we found that highly biocompatible and eco-friendly eggshell-derived HAs serve as a potent mucosal adjuvant. Our results showed that the induction of anti-MV and anti-GTF SIgA was significantly enhanced by coadministration with eggshell-derived HAs. The adjuvant effect of eggshell-derived HAs was more clearly evident in the induction of SIgA against individual protein Ags included in the multivalent Ag. Whereas, although some adjuvant effects were observed in systemic IgG induction, MVs alone immunization stimulated splenocytes to produce substantial amounts of cytokines and induced a certain level of IgG responses against MVs, including GTFs. These IgGs effectively functioned as neutralizing antibodies. In support of our findings, SIgA induction in vaginal washes against nasally administered herpes simplex virus type 2 Ags was enhanced by coadministration of calcium phosphate nanoparticles37.
Eggshell consists of calcium carbonate (94%), calcium phosphate (1%), organic matter (4%) and magnesium carbonate (1%)10. Eggshell-derived HAs contain calcium, phosphorus, and trace minerals such as magnesium and sodium, similar to natural bone. As a results, their biocompatibility is greater than that of commercially available high-purity HA. However, in the process of manufacturing calcium sources from eggshell waste containing sulfur-containing amino acids, sulfur compounds such as sulfites and sulfate are generated. These salts may be detrimental to protein antigens and host tissues23. Indeed, when mice were immunized with MVs plus unmodified synthetic eggshell-derived HAs containing sulfate, no potent adjuvant effects were observed, even when coadministered with 100 µg of eggshell-derived HAs (data not shown). We used low sulfur compound-containing HAs as the mucosal adjuvant candidate. These low-sulfated eggshell-derived HAs did not cause inflammation or adverse reactions in surrounding tissues after implantation into the rat calvarial bone38. Since calcium was obtained from eggshells by calcination at 1,000 °C, any allergenic proteins potentially present in the eggshell-derived HAs were decomposed. Previous study has revealed that calcium phosphate exhibits adjuvant activity without inducing IgE responses39. Further studies are required to evaluate the safety of eggshell-derived HAs combined with MVs and to investigate their long-term effects on the central nervous system, nasal tissue, and the whole body40.
More than 5 repeated administrations were required to induce SIgA responses by MVs adjuvanted with eggshell-derived HAs at nearly the same level as those induced in combination with poly(I:C)28. Generally, three intranasal doses are sufficient to induce adequate antibody responses28,41. Thus, the adjuvant activity of eggshell-derived HAs was weaker than that of poly(I:C). However, poly(I:C) is not yet licensed due to its toxicity42. Therefore, eggshell-derived HAs may be safer for use in living bodies. Nakamura et al. reported that the magnitude of induction did not differ between MV dosages of 1 and 5 µg, both formulated with poly(I:C)28. The optimal dosage and number of doses of eggshell-derived HAs and MVs, without adverse effects, need to be further evaluated for maximal immunogenicity.
SEM observations revealed that the complex consisting of MVs and eggshell-derived HAs formed aggregates with sizes ranging from the nanoscale to the micron scale. Nanoscale HAs naturally agglomerate due to excessive van der Waal forces in the aqueous phase43. Nanosized primary particles naturally agglomerate to form secondary particles in the micron size scale, and in our study, MVs mediated the aggregation of secondary particles at a size of approximately 100 nm to 6 µm. In this context, a previous study showed that the size of the particles used as an Ag delivery system was associated with the induction of Ag-specific systemic IgG responses. That study revealed that greater IgG responses were induced when mice were intranasally immunized with BSA-containing particles made from poly(D,L-lactic-co-glycolic acid) (PLGA) at a size of 1 µm than with 0.2 or 0.5 µm particles44. The Ag delivery particles formulated from poly(L-lactic acid) and PLGA with sizes ranging from 700 to 1000 nm are more appropriate than 500 nm for the generation of robust Ag-specific mucosal antibody responses45. The longer HA nanorods induced depolarization of the cell membrane and were captured by macrophages, which was associated with increased Ag-specific systemic IgG responses46. Although nanosized HAs enhanced IL-1β secretion in response to LPS, 5 µm HAs were the most potent at inducing IL-1β secretion7. In contrast, a study showed that HA sizes ranging from 100–400 nm, but not those of smaller (40 nm) and larger (1–5 µm) sizes, resulted in the greatest generation of Ag-specific systemic IgG responses18. According to these previous studies, the optimal Ag size for the proper induction of immune responses likely varies across studies. The increased surface area of secondary particles consisting of many smaller nanosized HAs potentially allows for the adsorption of many Ags, although our SEM observations revealed low MV adsorption. Eggshell-derived HAs might bind to the host epithelial cell membrane, which is negatively charged41 via Ca2+ ions on the surface of HAs. Taken together, eggshell-derived HAs may increase Ag immunogenicity through the formation of various sizes of MV-HA complexes, the absorption of many Ags, and the retention of Ags at the site of immunization. Whereas our results revealed that MV-HA complexes did not facilitate DC activation, rather, discrete particles of eggshell-derived HAs promoted DC activation. Individual MVs might also stimulate DCs. It appears that the MV-HA complexes may have mutually masked each other’s property. In this regard, previous studies have reported that smaller HA particles, such as 100 nm, are more readily internalized by DCs, leading to DC maturation. These HAs are immediately transported to the lymph nodes. However, as particle size increases, the HAs are retained at injection sites47. Nanosized particles are most effective in activating DCs6,48. These findings suggest that nanoscale size may be required for efficient DC activation. Our vaccine solution may contain discrete nanosized particles of eggshell-derived HAs, which could contribute to DC activation.
S. mutans releases MVs containing various molecules, including antigenic virulence factors, GTFs27. A previous study reported that priming by intranasal administration with two homologous boosting of MVs failed to induce MV-specific mucosal SIgA responses28. However, our present results showed that nasal administration of S. mutans MVs to mice induced certain levels of MV-specific systemic and mucosal antibody responses without the use of adjuvants. Although, the magnitude of antibody responses was lower than that induced by coadministration with adjuvants. These findings suggest that S. mutans MVs exhibit self-adjuvant ability in eliciting humoral immunity. S. mutans MVs are insoluble particles and may contain some molecules that stimulate innate immunity. Previous study has shown that the extracellular vesicles produced from Staphylococcus aureus may work as delivery systems for immunogens to induce protective immunity49. Thus, S. mutans MVs may also serve as a potential vaccine platform. Privous study demonstrated that nasal administration of GTF-I protein derived from S. sobrinus elicited substantial systemic and mucosal anti-GTF-I antibody responses34. However, the amount of GTF protein in the inoculum used in that study was 10 times greater than the amount of MV Ag used in this study, and the absolute GTF amount contained in MVs was much lower. These findings suggest that GTF itself is immunogenic to some extent. In contrast to this previous study in which protein Ags were used, a smaller amount of Ags was employed in this study, and the immunogenicity might be potentiated by the immunostimulatory effect of both MVs and eggshell-derived HAs.
Our results revealed that MVs alone immunization initiated substantial IFN-γ, Th1-type cytokine, production by splenocytes. The coadministration of eggshell-derived HAs somewhat enhanced IFN-γ production, but MVs administration was mainly responsible for IFN-γ production. Moreover, the production of the Th17-type cytokine, IL-17, was increased in the splenocytes of MV- and MV/eggshell-derived HA-administered mice. In this context, a previous study showed that both IFN-γ and IL-17 were simultaneously induced in response to S. aureus, Candida albicans, and mycobacterial infection as well as S. aureus MV immunization. IFN-γ and IL-17 are associated with the recruitment and activation of phagocytes, which are essential for combatting these pathogens, while IL-17 production is regulated by IFN-γ50,51,52,53. In line with these studies, S. mutans MVs also stimulated the production of both IFN-γ and IL-17, although the IFN-γ produced might limit IL-17 production. Our results showed that MV immunization induced substantial IgG1 antibodies, which are typically associated with IL-4 induction, even though the MVs elicited only low levels of IL-4. The reason for this discrepancy is not known. HA nanoparticles elicited a Th2-biased immune response when administered with OVA19. Another study showed that HA nanoparticles without Ags stimulated the secretion of both IFN-γ and IL-4 from bone marrow DCs in vitro6. In this context, our results revealed that the levels of Th1-type IFN-γ and Th2-related molecules, IL-4 and IgG1, tended to be slightly increased by coadministration of eggshell-derived HAs. Taken together, HAs potentially stimulated the production of both Th1- and Th2-type cytokines to some extent, but a Th1-dominated response was induced by MV immunization. Nasal administration of MVs plus eggshell-derived HAs has the potential to induce both humoral and cellular immunity.
Our results showed that the IgG anti-S. mutans MVs, produced in the blood of immunized mice, neutralized the enzymatic activity of the GTFs contained in the MVs. This led to a decrease in GTF-mediated biofilm formation by UA159 gtfBC-. These IgG anti-S. mutans MVs inhibited biofilm formation by wild-type S. mutans, although the decrease was not drastic. These findings suggest that MV-specific IgGs are partly bound to the surface of S. mutans cells or to secreted MVs but that newly released MVs may not be masked by MV-specific IgGs, resulting in insufficient blockade of GTF activity. S. mutans MVs also contain a small amount of PAc, a surface Ag of S. mutans28. Our neutralization results suggest that the potentially induced PAc-specific IgG may have a limited role in neutralizing S. mutans. In this context, previous reports have shown that the biofilms formed by S. mutans or S. sobrinus are significantly suppressed by the IgGs induced in the sera of mice immunized with higher doses of GTF-B54 or GTF-I protein34, respectively. These findings suggest that total serum IgGs might contain enough GTF-B- or GTF-I-specific IgGs to neutralize GTF activities. Therefore, more specific IgGs are likely required for effective neutralization of S. mutans.
S. mutans MVs contain FTF, an another enzyme that catalyzes the biosynthesis of fructan from sucrose, with a molecular weight of approximately 90 kDa29,55. In this study, a band of approximately 90 kDa was also detected by electrophoresis and western blotting of the MVs. Although we did not confirm whether this band corresponded to FTF, it is most likely to be FTF based on its enzymatic activity. A previous study showed that fructan contributes to biofilm formation to some extent56. Thus, FTF may have potential as a vaccine Ag. Thus, one possible strategy to enhance the neutralizing effect is to construct MVs as platform that overexpresses polyvalent bacterial Ags, including PAc, GTFs, and FTF. This may be a promising approach to increase both immunogenicity and neutralizing activity57.
SIgA and IgG may exhibit different neutralizing activities, as more bands were detected by western blotting with salivary IgA than with serum IgG. This may be attributed to the polyreactivity of SIgA58. Limitations of this study include the lack of detailed characterization of SIgA reactivity, the absence of neutralization assays using SIgA due to the lack of murine IgA purification methods, and the absence of in vivo challenge experiments to evaluate the protective efficacy of this vaccine candidate.
In summary, the combination of S. mutans MVs and eggshell-derived HAs resulted in the formation of various sizes of aggregates. Compared with MV alone, nasal administration of S. mutans MVs plus eggshell-derived HAs significantly increased Ag-specific systemic IgG and mucosal SIgA responses. This findings indicate that eggshell-derived HAs are potent mucosal adjuvants. S. mutans MVs showed self-adjuvanticity, suggesting that they also function as adjuvants against Ags bound to MVs. Nasal administration of S. mutans MVs plus eggshell-derived HAs induced Th1/Th17 responses. The induced IgGs neutralized GTF activity, resulting in a reduction in sucrose-dependent biofilm formation by UA159 gtfBC-. Although more administrations are required for robust specific antibody induction than achieved with coadministration with poly(I:C), nasal administration of S. mutans MVs plus eggshell-derived HAs could be safe potential candidates for Ag carriers and mucosal cross adjuvants.
Materials and methods
Bacterial strains and culture conditions
The S. mutans laboratory strains UA159 and UA159 gtfBC-59 were maintained and grown in brain heart infusion broth (BHI, BD Bacto, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 37 °C with a 5% CO2 aerobic atmosphere (Gas pack: Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). Tryptic soy broth without dextrose (TSB, BD Bacto, BD) supplemented with 0.25% or 1% sucrose was used for biofilm formation.
Synthesis of eggshell-derived HAs with a low sulfate concentration
Eggshell-derived HAs with a low sulfate concentration (0.0014 M; BioApatite-Medical®, Bioapatite, INC., Shiga, Japan) were used in this study. HCl (3 M) was added to an aqueous solution of calcium hydroxide made from eggshell waste (148 g/500 mL), and the mixture was stirred until a clear solution was obtained. Seven milliliters of barium chloride (1 M) was added, and the mixture was stirred until it became cloudy. The precipitate, which included barium sulfate, was removed by vacuum filtration. Tris–HCl (1 M) was added to the filtrate to adjust the pH to 7, and the pH of the filtrate was subsequently adjusted to 11 with 4 M sodium hydroxide. Dibasic potassium phosphate (0.6 M) was added to the filtrate, and the mixture was stirred for more than 2 h while keeping the pH above 9. The products were collected by vacuum filtration, washed with large amounts of water to remove salt, dried and crushed with a jet mill.
Purification of MVs
MVs were purified as described previously28. Briefly, S. mutans UA159 was cultured in 1,000 ml of BHI broth at 37 °C for 24 h in an atmosphere containing 5% CO2. The cells were removed by centrifugation (6,000 × g for 20 min), and the supernatant was collected. The supernatant was concentrated to > 50 kDa by ultrafiltration (Amicon Ultra 4, Merck KGaA, Darmstadt, Germany or VIVASPIN 20, Sartorius, Stone House, United Kingdom). The concentrated supernatant was filtered through polyvinylidene difluoride (PVDF) filter membranes (Merck kGaA) with pore sizes of 0.45 and 0.22 µm and then ultracentrifuged twice (150,000 × g for 2 h). The pellets were resuspended in PBS and used as MVs. The protein content of MVs was quantified using a Bio-Rad protein assay kit (Bio-Rad Laboratory, Inc., CA). The purity of MVs was assessed by SDS‒PAGE and western blotting.
SDS‒PAGE and western blotting
The purified MVs and sample buffer (0.06 M Tris–HCl, pH 6.8, 20% glycerol, 1% SDS, 1% 2-mercaptoethanol, 0.0012% bromophenol blue) were mixed in equal volumes and heated in boiling water for 5 min. The denatured samples were subjected to 12.5% SDS‒PAGE. The separated proteins were transferred to immobilon PVDF membranes (Clear Blot Pt Membrane; ATTO, Tokyo, Japan). After blocking with 2% skim milk in Tris-buffered saline with 0.05% Tween 20 (TBS-T), antiserum including IgGs specific for GTFs, which were collected from mice immunized with MVs purified from S. mutans UA159 plus poly(I:C)28, was added as the primary antibody, followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG antibody (Invitrogen, Carlsbad, CA, USA) as the secondary antibody to probe specific proteins. The specific bands were visualized by enhanced chemiluminescence (ECL Plus western blotting Substrate, Thermo Scientific, Southfield, MI) followed by exposure to X-ray film (FUJI FILM, Kanagawa, Japan).
SEM
Fifty-microliter aliquots of PBS containing a mixture of 10 µg of MVs and 500 µg of eggshell-derived HAs or each of them were dropped on glass surfaces coated with poly-L-lysine to attach them and left at room temperature (RT) for 3 h. The glasses were washed with PBS, followed by blocking with 5% skim milk in 40 mM HEPES at RT for 1 h. The glasses were probed with the anti-GTF antisera described above at 4 °C overnight and with 10 nm gold-conjugated goat anti-mouse IgG (EM BBI solutions, Maine, USA) for 1 h. After washing with PBS, the glasses were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in PBS. The glasses were washed again and dehydrated in a graded acetone series ranging from 50% acetone to pure acetone (> 99.5%), dried by critical point drying, coated with osmium vapor using an osmium plasma coater, and visualized by SEM (SU8600, HITACHI High-Tech, Hitachi, Japan).
Mice
Six-week-old female BALB/c mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). The mice were maintained in an experimental facility on sterile food and water under pathogen-free conditions; the mice were 7 weeks old at the beginning of the experiments. All animal protocols (AP21MAS003-2) were approved by the institutional Animal Care and Use Committee of Nihon University. All the mice were used in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Nihon University School of Dentistry at Matsudo. We complied with the ARRIVE guidelines.
Nasal immunization
Groups of mice were anesthetized with midazolam, propofol and butorphanol and then intranasally immunized with 10 µl of PBS containing 2 µg of MVs alone or with 100 µg of eggshell-derived HAs or with 1 µg of poly(I:C). Each nostril was injected with 5 µl of Ag solution. The second immunization was performed after 3 weeks, and the third, fourth, and fifth immunizations were performed at 2-week intervals. Saliva and serum were collected before boosting and 2 weeks after the last immunization to determine Ag-specific antibody responses. Then, the mice were euthanized via CO2 inhalation, and their organs and nasal washes were collected at 2 weeks after the last immunization.
Detection of MV-specific antibody responses
MV-specific antibody responses were determined by ELISA as described previously28. Briefly, plates were coated with MVs (15 µg/ml) and blocked with 1% skim milk in PBS-T. Eightfold dilutions of saliva or nasal washes or sixty-four-fold dilutions of serum in 0.5% skim milk in PBS-T were added to the wells in duplicate. Following a 1-h incubation at 37 °C, the plates were washed with PBS-T, and then HRP-labeled goat anti-mouse IgG, IgG1, IgG2a or IgA-specific Abs (Southern Biotechnology Associates, Inc. [SBA] Birmingham, AL, USA) were added to the appropriate wells. After 1 h of incubation at 37 °C, the plates were washed with PBS-T and incubated with tetramethylbenzidine (TMB) solution [0.2 M citric acid buffer (pH 4.0), 41 mM TMB solution, and 30% hydrogen peroxide] for 10 min. Then, the reaction was stopped with 3 N sulfuric acid, and the ODs at 450 nm were measured with a microplate reader.
DC analysis
DC-enriched populations from CLNs were purified as described elsewhere. Briefly, CLNs were isolated from the mice one week after immunization and digested with 400 Mandl units/mL collagenase D (Roche Diagnostics, Basel, Switzerland) and 100 mg/mL DNase I (Roche) for 1 h at 37 °C. EDTA was added (10 mM final concentration), followed by an additional 5 min of incubation. DC-enriched populations were obtained by Percoll density gradient centrifugation (density: 1.055 g/mL; Cytiva)60. These populations were preincubated with an anti-CD16/CD32 monoclonal antibody to block nonspecific binding. The cells were then stained with FITC-conjugated anti-CD40 (HM40-3), anti-CD80 (16-10A1), anti-CD86 (GL1), anti-I-Ad (39–10-8), PE-labeled CD11c (N418), and APC-tagged CD83 (Michel-19) monoclonal antibodies. Seven-amino-actinomycin D was used for the exclusion of nonviable cells. The samples were then subjected to FACS analysis (BD Accuri C6 Plus, BD Biosciences). The data were analyzed with FLOWJO software (BD Biosciences, Franklin Lakes, NJ, USA).
Analysis of cytokine production
The levels of IFN-γ, IL-17, and IL-4 produced from whole splenocyte or CD4+ T cells isolated from spleens were determined using IFN-γ -, IL-17-, and IL-4-specific Quantikine ELISA kits (R&D System, Minneapolis, MN, USA). The CD4+ T cells from the spleens were purified using the magnetic cell separation system (IMag system, BD Biosciences) according to the manufacturer’s instructions. CD4+ T cells (2 × 106 cells/ml) were cultured with T-cell-depleted, mitomycin-treated splenic feeder cells (2.5 × 106 cells/ml) from naïve mice in complete medium. MVs (final concentration of 10 µg/ml) were added for restimulation of Ag-specific CD4+ T cells. After being incubated for 72 h at 37 °C in 5% CO2 in air, the supernatants were subjected to cytokine ELISA.
Inhibition of biofilm formation
Total IgGs, including specific IgGs against MVs induced in immunized mice, were purified using a Hitrap protein G HP column (Cytiva, Tokyo, Japan). The protein content of the purified IgGs was quantified using a Bio-Rad protein assay kit. Purified IgGs were preincubated with MVs at 37 °C for 30 min. Overnight cultures of UA159 gtfBC- (200-fold dilution) and 0.25% sucrose in TSB were added to the MV reaction mixtures, which were then placed in a 96-well flat-bottom plate (MS-8096F, Sumitomo Bakelite, Tokyo, Japan) and cultured for 16 h at 37 °C under anaerobic conditions. In another experiment, purified IgGs were preincubated with S. mutans UA159, followed by the addition of 1% sucrose in TSB. After 16 h of incubation, the plates were washed with PBS and stained with safranin for 15 min. The plates were subsequently washed with distilled water, 70% ethanol was added, and the plates were shaken for 1 h to solubilize the safranin. The absorbance at 492 nm was measured with a microplate reader (MTP-450, Corona Electrics, Co., Ltd., Ibaraki, Japan).
Statistics
The data are presented as the mean ± the SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) or repeated-measures ANOVA with post hoc Bonferroni correction and the unpaired nonparametric Mann‒Whitney U test with SPSS (IBM SPSS statistics 29, IBM Corporation, Armonk, NY). Values of p < 0.05 were considered statistically significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ma, G. & Liu, X. Y. Hydroxyapatite: Hexagonal or monoclinic?. Cryst. Growth Des. 9, 2991–2994 (2009).
Hoveidaei, A. H. et al. Nano-hydroxyapatite structures for bone regenerative medicine: Cell-material interaction. Bone 179, 116956 (2024).
Tiselius, A., Hjerten, S. & Levin, O. Protein chromatography on calcium phosphate columns. Arch. Biochem. Biophys. 65, 132–155 (1956).
Hayakawa, S. et al. Structural characterization and protein adsorption property of hydroxyapatite particles modified with zinc ions. J. Am. Ceram. Soc. 90, 565–569 (2007).
Wang, T. et al. Hydroxyapatite and its composite in heavy metal decontamination: Adsorption mechanisms, challenges, and future perspective. Chemosphere 352, 141367 (2024).
Wang, X. et al. Rod-shaped and substituted hydroxyapatite nanoparticles stimulating type 1 and 2 cytokine secretion. Colloids Surf. B Biointerfaces 139, 10–16 (2016).
Lebre, F. et al. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 7, 2922 (2017).
Rivera, E. M. et al. Synthesis of hydroxyapatote from eggshells. Mater. Lett. 41, 128–134 (1999).
Bouyer, E., Gitzhofer, F. & Boulos, M. I. Morphological study of hydroxyapatite nanocrystal suspension. J. Mater. Sci. Mater. Med. 11, 523–531 (2000).
Siva Rama Krishna, D., Siddharthan, A., Seshadri, S. K. & Sampath Kumar, T. S. A novel route for synthesis of nanocrystalline hydroxyapatite from eggshell waste. J. Mater. Sci. Mater. Med. 18, 1735–1743 (2007).
Russell, M. W., Kilian, M., Mantis, N. J. & Corthésy, B. Chapter 21—Biological activities of IgA. In Mucosal Immunology 4th edn (eds Mestecky, J. et al.) 429–454 (Academic Press, 2015).
Tsai, C. J. Y., Loh, J. M. S., Fujihashi, K. & Kiyono, H. Mucosal vaccination: onward and upward. Expert Rev. Vaccines 22, 885–899 (2023).
Correa, V. A., Portilho, A. I. & De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 167, 124–138 (2022).
Ichinohe, T. et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79, 2910–2919 (2005).
Zou, J. et al. Poly IC triggers a cathepsin D- and IPS-1-dependent pathway to enhance cytokine production and mediate dendritic cell necroptosis. Immunity 38, 717–728 (2013).
Newsted, D., Fallahi, F., Golshani, A. & Azizi, A. Advances and challenges in mucosal adjuvant technology. Vaccine. 33, 2399–2405 (2015).
Lavelle, E. C. & Ward, R. W. Mucosal vaccines - fortifying the frontiers. Nat. Rev. Immunol. 22, 236–250 (2022).
Hayashi, M. et al. Optimization of physiological properties of hydroxyapatite as a vaccine adjuvant. Vaccine 34, 306–312 (2016).
Zeng, Q. et al. Hydroxyapatite nanoparticles drive the potency of Toll-like receptor 9 agonist for amplified innate and adaptive immune response. Nano Res. 15, 9286–9297 (2022).
Kim, S. R. et al. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 24, 1389–1398 (2003).
Germs, A. C. Hydrogen sulphide production in eggs and egg products as a result of heating. J. Sci. Food Agric. 24, 7–16 (1973).
Zhang, J.-Z. & Millero, F. J. The products from the oxidation of H2S in seawater. Geochim. Cosmochim. Acta. 57, 1705–1718 (1993).
Schlegl, R. et al. Influence of elemental impurities in aluminum hydroxide adjuvant on the stability of inactivated Japanese Encephalitis vaccine, IXIARO(R). Vaccine 33, 5989–5996 (2015).
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24 (2019).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015).
Liao, S. et al. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 196, 2355–2366 (2014).
Senpuku, H. et al. Effects of complex DNA and MVs with GTF extracted from Streptococcus mutans on the oral biofilm. Molecules 24, 3131 (2019).
Nakamura, T. et al. Roles of membrane vesicles from Streptococcus mutans for the induction of antibodies to glucosyltransferase in mucosal immunity. Microb. Pathog. 149, 104260 (2020).
Iwabuchi, Y. et al. Effects of pH on the properties of membrane vesicles including glucosyltransferase in Streptococcus mutans. Microorganisms 9, 2308 (2021).
Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22–29 (2018).
Lemos, J. A. et al. The biology of Streptococcus mutans. Microbiol. Spectr. 7 (2019).
Taubman, M. A. & Smith, D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J. Immunol. 118, 710–720 (1977).
Childers, N. K., Zhang, S. S., Harokopakis, E., Harmon, C. C. & Michalek, S. M. Properties of practical oral liposome-Streptococcus mutans glucosyltransferase vaccines for effective induction of caries protection. Oral Microbiol. Immunol. 11, 172–180 (1996).
Watanabe, K., Hashizume, T., Kurita-Ochiai, T., Akimoto, Y. & Yamamoto, M. Nasal administration of glucosyltransferase-I of Streptococcus sobrinus without adjuvant induces protective immunity. J. Vaccines Vaccin. 1 (2010).
Davis, S. S. Nasal vaccines. Adv. Drug Deliv. Rev. 51, 21–42 (2001).
Li, Z. et al. CD83: activation marker for antigen presenting cells and its therapeutic potential. Front. Immunol. 10, 1312 (2019).
He, Q., Mitchell, A., Morcol, T. & Bell, S. J. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin. Diagn. Lab. Immunol. 9, 1021–1024 (2002).
Hirota, M. et al. Development of a sustainable bone regeneration material using apatite paste derived from eggshell waste. J. Funct. Biomater. 16, 201 (2025).
Vassilev, T. L. Aluminium phosphate but not calcium phosphate stimulates the specific IgE response in guinea pigs to tetanus toxoid. Allergy 33, 155–159 (1978).
Sasaki, E. et al. A novel vaccinological evaluation of intranasal vaccine and adjuvant safety for preclinical tests. Vaccine 35, 821–830 (2017).
Nochi, T. et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat. Mater. 9, 572–578 (2010).
Zhao, T. et al. Vaccine adjuvants: mechanisms and platforms. Signal. Transduct. Target. Ther. 8, 283 (2023).
Morsy, R. Synthesis and physicochemical evaluation of hydroxyapatite gel biosorbent for toxic Pb(II) removal from wastewater. Arab. J. Sci. Eng. 41, 2185–2191 (2016).
Gutierro, I., Hernandez, R. M., Igartua, M., Gascon, A. R. & Pedraz, J. L. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine 21, 67–77 (2002).
Thomas, C., Rawat, A., Hope-Weeks, L. & Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 8, 405–415 (2011).
Zhang, L. et al. Engineered hydroxyapatite nanoadjuvants with controlled shape and aspect ratios reveal their immunomodulatory potentials. ACS Appl. Mater. Interfaces 13, 59662–59672 (2021).
Wang, X. et al. Rod-scale design strategies for immune-targeted delivery system toward cancer immunotherapy. ACS Nano 13, 7705–7715 (2019).
Mathaes, R., Winter, G., Siahaan, T. J., Besheer, A. & Engert, J. Influence of particle size, an elongated particle geometry, and adjuvants on dendritic cell activation. Eur. J. Pharm. Biopharm. 94, 542–549 (2015).
Wang, X., Thompson, C. D., Weidenmaier, C. & Lee, J. C. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat. Commun. 9, 1379 (2018).
Lin, L. et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 5, e1000703 (2009).
Kim, M. R. et al. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy 67, 1271–1281 (2012).
Cruz, A. et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177, 1416–1420 (2006).
Martinez, G. J., Nurieva, R. I., Yang, X. O. & Dong, C. Regulation and function of proinflammatory TH17 cells. Ann. N. Y. Acad. Sci. 1143, 188–211 (2008).
Yuzawa, S., Hashizume, T., Kobayashi, R., Kurita-Ochiai, T. & Yamamoto, M. Glucosyltransferase-I is capable of functioning as an adjuvant to promote immune responses against the co-administered protein glucosyltransferase-B. Int. J. Oral-Med. Sci. 10, 353–361 (2012).
Rozen, R., Steinberg, D. & Bachrach, G. Streptococcus mutans fructosyltransferase interactions with glucans. FEMS Microbiol. Lett. 232, 39–43 (2004).
Nagasawa, R., Sato, T. & Senpuku, H. Raffinose induces biofilm formation by Streptococcus mutans in low concentrations of sucrose by increasing production of extracellular DNA and fructan. Appl. Environ. Microbiol. 83 (2017).
Yu, H., Nakano, Y., Yamashita, Y., Oho, T. & Koga, T. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect. Immun. 65, 2292–2298 (1997).
Overton, E. T. et al. Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans. Vaccine 32, 5490–5495 (2014).
Senpuku, H., Tuna, E. B., Nagasawa, R., Nakao, R. & Ohnishi, M. The inhibitory effects of polypyrrole on the biofilm formation of Streptococcus mutans. PLoS ONE 14, e0225584 (2019).
Liu, L. et al. An optimized method for the induction and purification of mouse bone marrow dendritic cells. J. Immunol. Methods. 495, 113073 (2021).
Acknowledgements
We thank Dr. Masanori Saito for valuable comments. This manuscript was edited by the American Journal Experts.
Funding
This work was supported by funding from BIOAPATITE, INC., with which the authors are affiliated, and Nihon University Research Grant for emerging infectious diseases (Representative researcher; Satoshi Hayakawa, Co-researcher; Hidenobu Senpuku 2022-23). This work was supported in part by JSPS KAKENHI Grant Number JP22K09950 to T.H.-T. and JP20K10286 and JP24K02661 to H.S.
Author information
Authors and Affiliations
Contributions
T.H-T. designed the study, performed the experiments and data analysis, imprinted the results, prepared figures, and wrote and reviewed the manuscript. H.K. carried out SEM and reviewed the manuscript. C.M., Y.S. and K.N. reviewed the manuscript. H.S. conceived and supervised the study, imprinted the results, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
This work was supported by a funding from BIOAPATITE, INC. to which the authors belong.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hashizume-Takizawa, T., Mochizuki, C., Sakai, Y. et al. Nasal immunization with Streptococcus mutans membrane vesicles and eggshell-derived hydroxyapatites elicits enhanced antigen-specific mucosal IgA responses. Sci Rep 15, 35947 (2025). https://doi.org/10.1038/s41598-025-19334-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-19334-7