Abstract
Cu-Cu hybrid bonding is a key technology for fine-pitch interconnections in advanced semiconductor packaging. However, native Cu oxidation and interface instability hinder reliable low-temperature bonding. While noble metal passivation can mitigate oxidation, conventional sputtering-based methods pose challenges for Cu/SiO2 hybrid bonding. Atomic layer deposition (ALD) offers a promising alternative for area-selective metal passivation, enabling precise deposition on Cu surfaces without affecting surrounding dielectrics. In this study, we evaluate the bonding characteristics of ruthenium (Ru) passivation layers deposited using plasma-enhanced ALD (PEALD) and compare them with sputtered Ru passivation layers. While PEALD Ru exhibited comparable thin-film properties, bonding performance was significantly lower, as confirmed by scanning acoustic tomography (SAT) and shear strength measurements. Further analysis revealed that lower crystallinity in PEALD Ru and changes in Cu surface properties due to thermal pre-treatment contributed to reduced bonding strength. These findings provide insights for optimizing ALD-based metal passivation, enabling improved Cu/SiO2 hybrid bonding for advanced semiconductor packaging.
Similar content being viewed by others
Introduction
For decades, semiconductor technology has continuously improved device performance by achieving device miniaturization in accordance with Moore’s Law. However, as concerns about the physical limitations of further scaling increase, enhancing semiconductor performance through system scaling rather than traditional device scaling has gained significant attention1,2. Alongside Heterogeneous Integration (HI), which integrates diverse chips into a single package, 3D packaging technology has emerged as a critical approach for achieving vertical integration between chips. Traditionally, 3D packaging has been performed using solder bump bonding with tin (Sn). However, as the number of I/O connections continues to increase, the limitations of conventional bump processes have become evident, leading to the development of Cu-Cu direct bonding technology. Cu-Cu direct bonding enables ultra-fine pitch interconnections at the micrometer scale while addressing issues related to the formation of intermetallic compounds (IMCs)3,4,5. However, a major drawback is that it requires high temperatures of approximately 400 °C to achieve a stable bonding interface6. To overcome this limitation, extensive research has been conducted on low-temperature Cu-Cu bonding, with Direct Bond Interconnect (DBI) hybrid bonding currently recognized as a key technology for achieving both reliable metal and dielectric bonding7.
Meanwhile, metal passivation technology has proven to be highly effective for low-temperature Cu-Cu bonding by suppressing the formation of native copper oxide through the deposition of noble metal films such as Au or Pt on the Cu surface. This approach has demonstrated excellent bonding characteristics even at temperatures below 200°C8,9,10,11,12. It has also been conducted on its application to Cu/SiO2 structures, given its ability to prevent the formation of copper oxide. However, the conventional metal passivation technology, which relies on sputtering deposition, presents technical challenges when applied to Cu/SiO2 hybrid bonding. Specifically, the metal passivation layer should be selectively deposited on the copper pads without covering the dielectric surface. However, the sputtering deposition method requires additional etching or lithography-based patterning processes, making it unsuitable for fine-pitch interconnections due to its Top-Down process characteristics.
To overcome these limitations, area-selective deposition techniques are essential. Besides ALD, area-selective electroless plating has been demonstrated for passivation layer deposition on damascene Cu structures25. While electroless plating offers the advantage of lower cost by utilizing well-established plating methods, it faces significant challenges for next-generation applications. As the bonding pad pitch shrinks to the nanometer scale, achieving uniform deposition thickness across an entire wafer becomes exceedingly difficult due to the inherent principles of wet chemical plating, which are complex to control with atomic-level precision.
In contrast, area-selective atomic layer deposition (AS-ALD) has been actively investigated as a more promising approach for future nodes. AS-ALD follows a Bottom-Up process and enables nanometer-scale thin film deposition with outstanding conformality and uniformity, making it particularly suitable for advanced device and interconnect fabrication13. As the size of copper pads in hybrid bonding structures continues to decrease, the precise control afforded by AS-ALD becomes critically important.
In this study, we evaluated the bonding characteristics of metal passivation films deposited using PEALD method and confirmed key factors that should be considered for ALD-based metal passivation technology. Ruthenium (Ru) was selected as the passivation material due to its strong oxidation resistance, stable interface with Cu, and compatibility with CMOS processes. Compared to other noble metals such as Pt or Au, Ru can be deposited by ALD with excellent thickness control, making it highly suitable for future area-selective deposition schemes14,15,16,17,18. In addition, PEALD offers better conformality and film uniformity than electroless or CVD methods, which is advantageous for fine-pitch hybrid bonding.
While hybrid bonding studies using selective-area ALD of cobalt have been previously reported, similar approaches employing ruthenium have not yet been demonstrated despite its favorable properties19[,20 To date, research involving Ru-based metal passivation has relied exclusively on sputtering methods. In this study, we aim to evaluate the feasibility of using Ru for future selective-area ALD by comparing the characteristics of sputtered and PEALD-deposited Ru films. We compared the thin film characteristics and bonding performance of Ru passivation layers deposited via PEALD and sputtering methods, aiming to determine the critical factors responsible for differences in bonding characteristics.
Experimental method
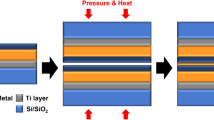
A 700 nm thick silicon dioxide (SiO2) layer was grown on an 8-inch silicon wafer using the wet oxidation process. Subsequently, diced SiO2 samples were prepared through a dicing process (DISCO Corporation, Automatic Dicing Saw DAD 3350). The fabricated samples were sequentially cleaned using acetone, isopropyl alcohol (IPA), and deionized (DI) water before being placed into the sputtering chamber for metal thin-film deposition. Ti (50 nm) and Cu (1 μm) thin films were deposited sequentially via DC sputtering (Sputtering system, Sorona Inc., SRN-110). The base pressure was set to 5 × 10⁻⁵ Torr, and the working pressure was maintained at 3 × 10⁻³ Torr during the process.
For Ru passivation layer deposition, the Cu samples were loaded into a plasma-enhanced atomic layer deposition (PEALD) system, where a 10 nm thick Ru metal thin film was deposited. The PEALD process was carried out at process temperatures of 350 °C and 250 °C using Ru(EtCp)₂ precursor and NH₃ plasma, with purge steps between precursor exposures. To evaluate the impact of deposition temperature on bonding characteristics, we conducted PEALD at both 350 °C and 250 °C. The higher temperature of 350 °C was selected based on conventional ALD conditions for Ru, while 250 °C was chosen to explore the feasibility of low-temperature deposition, which is crucial for advanced semiconductor packaging.
To analyze interdiffusion behavior during the heat treatment of the PEALD Ru passivation samples, the samples were heated on a hot plate at 200 °C for 1 h, followed by X-ray photoelectron spectroscopy (XPS) analysis. It is important to note that ion sputtering during XPS depth profiling can induce atomic mixing, which may influence the apparent interface width; however, for comparative purposes, this effect was consistently applied across all samples. Additionally, atomic force microscopy (AFM) was employed to characterize the surface roughness of the Ru passivation samples.
The bonding process of Ru passivation samples was conducted at the chip level using 5 mm×5 mm samples. The bonding process was carried out under a non-vacuum, atmospheric environment. Following pre-bonding, the bonding step was performed at 200 °C, 15 MPa, for 1 h, followed by an annealing process at 200 °C for 2 h. The long bonding durations in this study were intentionally chosen for our initial mechanistic investigation to ensure sufficient time for diffusion and bonding reactions to occur, allowing us to evaluate the fundamental differences in bonding behavior between ALD and sputtered Ru. It is important to note that the thermocompression bonding method used here differs from the standard industrial hybrid bonding process. In a typical manufacturing flow, a wafer-level dielectric-to-dielectric bond is first formed, after which the bonded pairs undergo batch annealing at 200–300 °C. During this step, the thermal expansion mismatch between Cu and the surrounding dielectric generates significant compressive stress at the Cu interface, which drives Cu diffusion and completes the bond. This stress-induced intermixing suggests that when applied to an industrial hybrid bonding process, our ALD-Ru passivation approach could facilitate reliable bonding with significantly shorter annealing times and lower temperatures than those used in this fundamental study.
Future optimization will target reducing bonding and post-bond annealing times. Strategies include increasing bonding pressure, implementing optimized surface pre-treatments, and improving ALD film quality (e.g., crystallinity, impurity reduction). Recent studies on metal passivation using materials like Au and Ag have already demonstrated robust, low-temperature Cu-Cu bonding with much shorter durations, providing a benchmark for the potential of this technology26. By leveraging the advantages of an industrial hybrid bonding scheme, we anticipate that ALD-Ru passivation can achieve comparable or superior performance, making it a viable solution for high-volume manufacturing. The bonding characteristics of the fabricated samples were analyzed using scanning acoustic tomography (SAT) to evaluate the bonding interface.
To isolate the influence of deposition method on the thin film characteristics of Ru, we intentionally introduced a thermal treatment to the underlying Cu layer, replicating the temperature conditions of the PEALD process. This approach ensured that the Cu layer exhibited consistent properties across both deposition methods, thereby eliminating temperature-induced variations in Cu thin film characteristics. After preheating the Cu layer under vacuum conditions at the same temperature and duration as the PEALD process, a 6 nm Ru passivation layer was deposited via sputtering. The overall fabrication process for these samples is illustrated in Fig. 1.
The crystallinity and grain size of the thin films were examined using grazing incidence X-ray diffraction (GIXRD) and X-ray diffraction (XRD). The bonding process was carried out under identical conditions to the previous samples, with the bonding process at 200 °C, 15 MPa, for 1 h, followed by a post-bonding annealing process at 200 °C for 2 h. SAT analysis was subsequently performed to assess the bonding interface characteristics.
Results & discussion
The interdiffusion behavior between the ruthenium thin film and the copper layer in the ruthenium passivation sample by PEALD method was analyzed. As shown in Fig. 2, the elemental compositions were compared using XPS depth profile analysis for samples under ambient conditions and after annealing at 200 °C for 1 h on a hot plate. In the as-deposited PEALD ruthenium passivation sample, a Cu signal was detected during the early stage of XPS depth profiling. However, cross-sectional TEM and corresponding EDS analysis confirmed that the deposited Ru layer was continuous and did not contain Cu within the film region. Therefore, the observed Cu signal is considered to originate from the Cu/Ru interface, Likely due to the Limited analysis depth of XPS, rather than indicating a non-uniform or intermixed Ru layer. However, after annealing at 200 °C for 1 h, the surface of the same sample exhibited an increased copper content of 43.1%. Meanwhile, ruthenium was detected at a concentration of 2.49% within the thin film after 168 s of ion beam etching. These results indicate that the PEALD ruthenium passivation sample fabricated at a deposition temperature of 350 °C, exhibited significant Cu-Ru interdiffusion even at a low temperature of 200 °C. This interdiffusion behavior was nearly identical to that observed in the sputtered ruthenium passivation sample14. As shown in Table 1, the surface copper content of the sputtered ruthenium passivation sample also increased to 43.9% after the same annealing process. It showed nearly the same level as the PEALD-deposited sample. Therefore, we expected that the PEALD process could also enable low-temperature Cu-Cu bonding.
Similar to the sputtered ruthenium passivation sample, the activation energy for copper diffusion into ruthenium in the PEALD sample was calculated. The activation energy was determined based on the elemental ratios detected via XPS analysis at ambient and 200 °C annealing conditions. The calculated activation energy for Cu diffusion into Ru in the PEALD sample was 3.44 kJ/mol, which is significantly lower than the previously reported value of 6.28 kJ/mol for the sputtered ruthenium passivation sample13. This suggests that the PEALD-deposited ruthenium passivation layer can facilitate a stable copper bonding structure at low temperatures.
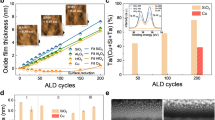
Additionally, the surface roughness of the PEALD ruthenium passivation sample was measured and compared with that of the sputtered sample. As shown in Fig. 3(a), we confirmed that the root mean square roughness (Rq) of the PEALD sample was 6.96 nm through AFM analysis. In contrast, the sputtered ruthenium passivation sample exhibited an Rq value of 9.36 nm, as shown in Fig. 3(b). We included the AFM results of the bare Cu surface (prior to Ru deposition) in Fig. 3(c). The RMS roughness of this underlying Cu seed layer was measured to be approximately 7.6 nm, which is comparable to the value observed after Ru deposition. This strongly suggests that the surface morphology of the deposited Ru film is primarily inherited from the underlying Cu template. Further substantiating this, TEM analysis (Fig. S1 in supplementary information) also indicates that the Ru layer faithfully follows the topography of the Cu surface without significant smoothing or further roughening. These results indicate that the PEALD process produces a smoother ruthenium passivation film than the sputtering process, which is a critical factor for achieving stable copper bonding.
Following analyzing this film characteristics, chip-level bonding experiments were conducted using the PEALD ruthenium passivation sample. The bonding process was performed at 200 °C under 15 MPa for 1 h, followed by post-annealing at 200 °C for 2 h. The bonding interface was analyzed using SAT analysis. As shown in Fig. 4(a), significant void formation was observed at the bonding interface in the PEALD sample, which means an unstable bonding interface. This result contrasts with the SAT result of the sputtered ruthenium passivation sample, as shown in Fig. 4(b), where a more uniform bonding interface was observed. This discrepancy in bonding characteristics was further confirmed through shear strength test. As shown in Fig. 5, the shear strength of the PEALD ruthenium passivation sample was about 1.41 MPa, which is significantly lower than the 17.16 MPa measured for the sputtered passivation sample14. Given that the PEALD ruthenium passivation sample demonstrated sufficient thin film characteristics for low-temperature copper bonding, it was necessary to investigate the underlying cause of the poor bonding performance, despite similarities with previously reported metal passivation technologies.
SAT analysis of the bonding interface in Ru passivation bonded sample (a) PEALD at 350 °C, (b) Sputtering14.
The PEALD process temperature, previously set at 350 °C, was considered a key factor affecting bonding performance compared to the sputtering method. To investigate this effect, an additional PEALD process was conducted at a reduced temperature of 250 °C. Due to the significantly prolonged deposition time at lower temperatures, the Ru passivation layer thickness was adjusted from 10 nm to 6 nm. For comparison, a sputtered Ru passivation sample with the same 6 nm thickness was also prepared.
Furthermore, this study aimed to verify whether the bonding characteristics observed in the 10 nm Ru samples would be consistent in thinner films. Previous studies have suggested that a thinner Ru layer may enhance Cu diffusion, potentially influencing bonding performance15. In addition, to evaluate the impact of Cu thin film property changes during the PEALD process, an additional experiment was conducted by preheating the Cu layer prior to Ru deposition.
The bonding process was carried out under the same conditions as in the previous experiments, with bonding performed at 200 °C under 15 MPa for 1 h, followed by a post-bonding annealing step at 200 °C for 2 h. These systematic modifications were designed to provide deeper insight into the factors governing low-temperature Cu bonding performance.
Figure 6 shows the bonding interface observed in SAT analysis. As shown in Fig. 6(b), the sputtered ruthenium passivation sample exhibited a uniform bonding interface across the entire area. In contrast, significant void formation and an unstable bonding interface were observed in both Fig. 6(a) and Fig. 6(c). Notably, while Fig. 6(a) corresponds to a PEALD-deposited Ru sample, Fig. 6(c) represents a sputtered Ru sample where the underlying Cu layer was preheated prior to deposition. Despite the Ru layer in Fig. 6(c) being deposited via sputtering, the bonding interface exhibited noticeable defects. This suggests that preheating the Cu layer before Ru deposition may negatively impact bonding performance, likely due to changes in Cu grain structure or surface chemistry. These results reinforce the notion that bonding strength is influenced not only by the deposition method but also by pre-treatment conditions affecting Cu-Ru interdiffusion and interface stability.
To investigate the impact of process temperature on the PEALD Ru passivation sample, an additional sample was fabricated by pre-heating the underlying copper layer to 250 °C before depositing a 6 nm-thick ruthenium passivation film. Figure 7 shows the grain size of the Cu layer based on deposition methods through GIXRD analysis. Grain size was calculated based on Scherer Equation through FWHM and X-ray incident angles obtained by GIXRD analysis for each peak. For the Cu(111) orientation, the grain sizes were calculated as 16.24 nm for the PEALD sample at 250 °C, 10.15 nm for the sputtered sample, and 27.17 nm for the sample with the pre-heated copper layer sample. Among the samples, the sputtered sample without additional heat treatment exhibited the smallest grain size, whereas the PEALD sample at 250 °C and the sample with the pre-heated underlying copper layer showed significantly increased grain sizes. Larger grain sizes generally lead to reduced diffusivity in metal interdiffusion. Therefore, the increased grain size observed in the two high-temperature processed samples suggests a potential drawback for Cu bonding in metal passivation applications, compared to the sputtered sample10,21. These findings indicate that process temperature plays a crucial role in determining the microstructural properties of the ruthenium passivation layer and its influence on bonding performance.
To further analyze the impact of pre-heated underlying copper layer, the grain size was compared for samples where only the copper layer was deposited and annealed without the ruthenium passivation layer. Figure 8 presents the XRD patterns of copper samples subjected to different preheating conditions. The heat treatment process was conducted in a vacuum chamber for 6 h at each specified temperature. As shown in Fig. 8(a), the intensity of the Cu(111) peak increased with higher pre-heating temperatures. Also a comparison of grain sizes in the Cu(111) orientation revealed an increasing trend with temperature in Fig. 8(b). We confirmed that 27.74 nm at room temperature, 28.63 nm at 150 °C, and 29.04 nm at 250 °C. The trend was consistent with the previously identified bonding characteristics observed in the 6 nm thick ruthenium passivation samples based on the deposition methods. Therefore, we inferred that changes in the film characteristic of the heated underlying copper layer due to heat treatment during the PEALD process could also influence the bonding characteristics.
While the PEALD Ru in this study was deposited at 350 °C and 250 °C to evaluate its bonding characteristics, we acknowledge that for future industrial integration and to minimize material degradation, further reduction in deposition temperature is highly desirable. Recent advancements in ALD chemistry have shown promise in this regard. For instance, Kim et al. successfully demonstrated the deposition of conformal Ru thin films via ALD at temperatures as low as 150–200 °C using a novel precursor, T-Rudic, and H2O as a reactant22. This suggests that high-quality, highly conformal Ru films can be achieved at temperatures well below 150 °C with optimized ALD processes, which would significantly simplify integration and prevent potential thermal degradation of underlying Cu layers and other device components. Therefore, future research will focus on exploring such advanced ALD chemistries and process conditions to achieve robust Ru passivation layers at even lower temperatures, thereby fully realizing the potential of ALD for low-temperature Cu-Cu hybrid bonding.
Figure 9 shows the GIXRD patterns of the 6 nm-thick ruthenium passivation samples deposited using different methods. As shown in Fig. 9(a), the PEALD-deposited ruthenium passivation sample at 250 °C exhibited an Ru(111) peak at 41.2°. In contrast, the sputtered ruthenium passivation sample showed Ru(002) and Ru(101) peaks at 42.4° and 44.3°, respectively, as shown in Fig. 9(b). A comparison of the ruthenium peaks between the two samples indicates that the PEALD method resulted in the growth of an FCC-structured ruthenium film, whereas the sputtering method led to the formation of an HCP-structured ruthenium film. PEALD favors the formation of metastable FCC Ru due to limited atomic mobility from sequential precursor reactions, whereas sputtering promotes stable HCP Ru via higher kinetic energy deposition23. Based on the GIXRD results in Fig. 9, the crystallinity of the ruthenium passivation films was compared. The crystallinity was evaluated by calculating the intensity ratio of the main ruthenium peak to the Cu(111) peak for each sample. Table 2 presents the integrated peak areas and the crystallinity values obtained using this method. The PEALD-deposited ruthenium passivation sample exhibited a crystallinity value of 0.923 for the Ru(111) peak. In contrast, the sputtered sample showed a significantly higher crystalline value of 2.090 for the Ru(002) peak.
Previously, we showed that the crystallinity of metal passivation layer has a significant correlation with bonding characteristics24. Regarding this, these calculations confirm that the PEALD-deposited ruthenium passivation film had a considerably lower degree of crystallinity than the sputtered counterpart. This difference can be attributed to the fundamental differences in the deposition mechanisms of these two methods. In sputtering, metal atoms are ejected from a target by high-energy ion bombardment and subsequently condense onto the substrate with relatively high kinetic energy. This high-energy deposition process enhances surface mobility, allowing adatoms to diffuse more freely and rearrange into a more thermodynamically stable crystalline structure, often leading to a preferred orientation and larger grain sizes. In contrast, the lower crystallinity observed in the PEALD-deposited Ru film can be attributed to the self-limiting nature of ALD, which leads to lower surface diffusion of adatoms during film growth. Unlike sputtering, where adatoms arrive with high kinetic energy and can readily rearrange into thermodynamically favorable crystalline structures, PEALD relies on sequential precursor adsorption and surface reactions, limiting adatom mobility. Additionally, the lack of energetic bombardment results in reduced atomic rearrangement, further limiting grain growth and crystallization.
Furthermore, to investigate the potential influence of impurities, we conducted cross-sectional TEM imaging with EDS elemental mapping, as shown in Fig. 10. These analyses showed no meaningful presence of nitrogen or oxygen within the Ru film, suggesting that impurity incorporation is unlikely to be the primary reason for the observed lower crystallinity. However, we acknowledge that residual carbon, nitrogen, or hydrogen from organometallic precursors and plasma reactants are common in ALD processes and can impede grain growth. While a comprehensive impurity analysis was not explicitly performed in this study, meticulously controlling such potential incorporation will be a key focus for future optimization efforts to enhance ALD-based Ru film crystallinity.
These findings suggest that beyond analyzing metal diffusion behavior, optimizing the crystallinity of the metal passivation film and refining ALD process conditions at low temperatures are critical factors for the development of ALD-based metal passivation technology. ALD-based metal passivation technology is expected to be effective for selectively depositing metal passivation films on copper surfaces in Cu/SiO2 hybrid bonding. The results of this study contribute to the optimization of ALD-based metal passivation processes and the enhancement of bonding performance.
Conclusions
Metal passivation technology plays a crucial role in enabling Cu-Cu bonding at low temperatures and holds significant potential for application in Cu/SiO2 hybrid bonding. However, conventional metal passivation techniques using sputtering method shows technical limitations when applied to Cu/SiO2 hybrid bonding. ALD, particularly area-selective atomic layer deposition (AS-ALD) technology presents a promising approach for selectively depositing metal passivation layers on Cu surfaces while preserving the integrity of surrounding dielectrics.
This study analyzed the thin-film characteristics and bonding performance of Ru passivation layers deposited via PEALD and sputtering. Despite comparable film properties, SAT analysis and shear strength measurements showed significantly weaker bonding in PEALD samples. The results suggest that lower Ru crystallinity and Cu surface modifications during thermal pre-treatment were primary factors affecting bonding performance. Notably, even sputtered Ru samples exhibited poor bonding when Cu was preheated, underscoring the importance of Cu surface conditions.
The findings highlight the critical role of deposition temperature and crystallinity in determining bonding performance. By optimizing PEALD conditions, future research can enhance ALD-based metal passivation for advanced packaging applications, addressing the limitations of conventional sputtering techniques. These insights pave the way for more reliable and scalable Cu/SiO2 hybrid bonding solutions in next-generation semiconductor devices.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Moore, G. E. Cramming more components onto integrated circuits, Reprinted from Electronics, volume 38, number 8, April 19, pp.114 ff. IEEE Solid-State Circuits Society Newsletter, 2006. 11(3): pp. 33–35. (1965).
Chau, R. Process and Packaging Innovations for Moore’s Law Continuation and Beyond. in 2019 IEEE International Electron Devices Meeting (IEDM). (2019).
Kim, B. J. et al. Intermetallic compound growth and reliability of Cu pillar bumps under current stressing. J. Electron. Mater. 39 (10), 2281–2285 (2010).
Chen, C. W. et al. The IMC formation and progress in the copper pillar Cu/Sn1.8Ag /OSP-Cu microbump structure upon current stressing. in 2014 IEEE 16th Electronics Packaging Technology Conference (EPTC). (2014).
Suga, T. & Otsuka, K. Bump-less interconnect for next generation system packaging. in 2001 Proceedings. 51st Electronic Components and Technology Conference (Cat. No.01CH37220). (2001).
Chen, K. N. et al. Temperature and duration effects on microstructure evolution during copper wafer bonding. J. Electron. Mater. 32 (12), 1371–1374 (2003).
Paul, E. Metal/Silicon Oxide Hybrid Bonding, in Handbook of Wafer Bondingp. 261–278 (Wiley, 2012).
Liu, D. et al. Demonstration of Low-Temperature Fine-Pitch cu/sio₂ hybrid bonding by Au passivation. IEEE J. Electron. Devices Soc. 9, 868–875 (2021).
Hong, Z. J. et al. Scheme for Multi-Chiplet integration with low thermal budget by asymmetric Cu-Cu bonding with Au passivation bonding structure. IEEE Electron Device Lett. 44 (3), 492-495 (2023).
Liu, D. et al. Investigation of Low-Temperature Cu–Cu direct bonding with Pt passivation layer in 3-D integration. IEEE Trans. Compon. Packag. Manuf. Technol. 11 (4), 573–578 (2021).
Hong, Z. J. et al. Investigation of bonding mechanism for low-temperature CuCu bonding with passivation layer. Appl. Surf. Sci. 592, 153243 (2022).
Hsu, M. P. et al. Enhancement of Low-Temperature Cu-Cu bonding by metal alloy passivation in ambient atmosphere. IEEE Electron Device Lett. 45 (8), 1500–1503 (2024).
Mackus, A. J. M., Merkx, M. J. M. & Kessels, W. M. M. From the Bottom-Up: toward Area-Selective atomic layer deposition with high selectivity. Chem. Mater. 31 (1), 2–12 (2019).
Park, S. W. et al. First demonstration of enhanced Cu-Cu bonding at low temperature with ruthenium passivation layer. IEEE Access. 12, 82396–82401 (2024).
Jeon, C. et al. Ru passivation layer enables Cu–Cu direct bonding at low temperatures with oxidation Inhibition. ACS Appl. Mater. Interfaces. 16 (36), 48481–48487 (2024).
Sondhi, K. et al. Area selective deposition of ruthenium on 3D structures. J. Vacuum Sci. Technol. A 40 (6), 062412 (2022).
Kotsugi, Y. et al. Atomic layer deposition of Ru for replacing Cu-Interconnects. Chem. Mater. 33 (14), 5639–5651 (2021).
Brady-Boyd, A. et al. The role of atomic oxygen in the decomposition of self-assembled monolayers during area-selective atomic layer deposition. Appl. Surf. Sci. 586, 152679 (2022).
Li, M. J. et al. Cu–Cu bonding using selective Cobalt atomic layer deposition for 2.5-D/3-D chip integration technologies. IEEE Trans. Compon. Packag. Manuf. Technol. 10 (12), 2125–2128 (2020).
Breeden, M. et al. Proximity effects of the selective atomic layer deposition of Cobalt on the nanoscale: implications for interconnects. ACS Appl. Nano Mater. 4 (8), 8447–8454 (2021).
Jiang, Q., Zhang, S. H. & Li, J. C. Grain size-dependent diffusion activation energy in nanomaterials. Solid State Commun. 130 (9), 581–584 (2004).
Kim, H. M. et al. Area-selective atomic layer deposition of ruthenium using a novel Ru precursor and H2O as a reactant. Chem. Mater. 33, 4353–4361 (2021).
Zhao, M. et al. Ru octahedral nanocrystals with a Face-Centered cubic structure, {111} facets, thermal stability up to 400°C, and enhanced catalytic activity. J. Am. Chem. Soc. 141 (17), 7028–7036 (2019).
Jeong, M. S. et al. Unraveling diffusion behavior in Cu-to-Cu direct bonding with metal passivation layers. Sci. Rep. 14 (1), 6665 (2024).
Hsu, M. P. et al. Low-temperature area-selective metal passivation bonding platform for heterogeneous integration. IEEE Electron Device Lett. 45, 1273–1276 (2024).
Liu, Y. L. et al. 10-second cu/polymer hybrid bonding using Area-selective metal passivation for 3D integration. IEEE Electron Device Lett. 46 (5), 833–836 (2025).
Acknowledgements
This work was supported by the National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2022M3I7A4072293) and by the National Nano Fab Center (NNFC) grant funded by the Korea government (MSIT). (No. RS-2024- 00440244).
Author information
Authors and Affiliations
Contributions
As the first author, S. W. Park contributed to the analysis of all results and wrote a paper. H. Han, C. W. Ahn contributed to the sample fabrication process through the PEALD process. As a corresponding author, J. K. Park contributed to the overall experimental idea and data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, S.W., Han, H., Ahn, C.W. et al. Influence of deposition conditions on ALD based Ru passivation for Cu-Cu hybrid bonding. Sci Rep 15, 36620 (2025). https://doi.org/10.1038/s41598-025-20296-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-20296-z