Abstract
Sepsis-induced acute kidney injury (AKI) is associated with a high mortality rate and presents a significant clinical challenge. However, effective treatment measures have not yet been established. The cholinergic anti-inflammatory pathway (CAP), a neural mechanism that regulates immune responses, has been reported to mitigate kidney injury. Although numerous studies have demonstrated the benefits of CAP activation prior to injury, its therapeutic potential after the onset of injury remains insufficiently explored. Therefore, this study aimed to evaluate the renoprotective effects of CAP activation after the onset of lipopolysaccharide (LPS)-induced AKI. LPS (5 mg/kg) was administered to C57BL/6 wild-type mice to induce AKI. Subsequently, GTS-21 (10 mg/kg), a selective α7 nicotinic acetylcholine receptor agonist, was administered to assess its anti-inflammatory and renoprotective effects. In RAW 264.7 and U937-derived macrophages, LPS-induced inflammation was treated with GTS-21, and its effects were evaluated. RNA sequencing was performed to elucidate the underlying molecular mechanisms. Even after the onset of LPS-induced AKI, the administration of GTS-21 demonstrated anti-inflammatory and renoprotective effects. In macrophages, the LPS-induced increase in TNF-α was suppressed by GTS-21 (50 μM) administration. Furthermore, CCL2 expression in macrophages might be involved in mediating these effects.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) has various causes; however, infection is a significant cause of AKI in clinical settings. Septic AKI is a common cause of AKI in patients requiring renal replacement therapy, accounting for 22% of cases1. Furthermore, the in-hospital mortality rate is as high as 60%, indicating a poor prognosis for survival2. Sepsis-induced AKI is a significant clinical problem; however, an effective treatment has not yet been established. Furthermore, unlike AKI induced by surgery or chemotherapy, sepsis-associated AKI cannot be predicted, making preventive interventions difficult. Therefore, the development of an effective treatment for sepsis-induced AKI is of significant clinical importance. Recent research has highlighted that the cholinergic anti-inflammatory pathway (CAP) is a crucial mechanism for modulating inflammation through the nervous system3,4,5. Activation of CAP by vagus nerve stimulation (VNS) and acetylcholine receptor agonists has been shown to have therapeutic effects in inflammatory diseases, such as rheumatoid arthritis, in a collagen-induced arthritis mouse model6,7 and inflammatory bowel disease in dextran sodium sulfate (DDS)-induced colitis mice8. In recent years, with the development of transcutaneous vagus nerve stimulators, many clinical trials on CAP activation have been conducted9,10,11,12. CAP is the efferent arm of the inflammatory reflex, by which the central nervous system suppresses peripheral inflammation. VNS is thought to activate the splenic nerve, which signals to choline acetyltransferase (ChAT)⁺ β₂-adrenergic T cells in the spleen. These T cells release acetylcholine, which inhibits cytokine production by splenic macrophages via α7 nicotinic acetylcholine receptors (α7nAChR). CAP activation suppresses downstream of α7nAChR, leading to reduced TNF-α production and inflammation are suppressed via inhibition of nuclear factor kappa light-chain-enhancer of activated B cells (NF-κB) nuclear translocation13,14,15,16,17, and activation of the Janus kinase 2 (JAK2)/signal transduction and activator of transcription 3 (STAT3) pathway18,19. Moreover, α7nAChR signaling inhibits pyrin domain-containing 3 (NLRP3) inflammasome activation20. However, the mechanisms underlying CAP activation remains unclear. We have previously demonstrated that VNS, nicotine, and pulsed ultrasound stimulation (US) suppress AKI21,22. Using a bilateral ischemia–reperfusion injury (IRI) model, which is a representative animal model of AKI, VNS was administered 24 hours before IRI, and damage assessment was conducted 24 hours afterward. The results confirmed the renoprotective effects, as evidenced by the suppression of cytokines such as TNF-α, a marker of systemic inflammation, a decrease in plasma creatinine levels, and histological improvement in renal tubular necrosis. This renal protective effect is critically dependent on macrophages and α7nAChR21. Furthermore, it has been shown that VNS induces the hairy and enhancer of split-1 (Hes1) gene expression in macrophages, which promotes the transition to an anti-inflammatory macrophage phenotype, thus attenuating renal injury22. Optogenetic stimulation of the vagus nerve and C1 neurons improves renal damages23,24. Although many researchers have shown that CAP activation alleviates renal injury25,26,27,28,29,30,31,32, all of these reports examined interventions prior to kidney injury, and the precise mechanisms underlying these renoprotective effects remain to be fully elucidated. This study aimed to investigate whether the activation of CAP could protect against renal injury even after the onset of sepsis by using an α7nAChR agonist, and to elucidate a novel underlying mechanism.
Results
Following inflammation induction, administration of GTS-21 resulted in its anti-inflammatory and renoprotective effects
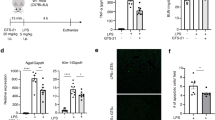
The half-life of GTS-21 has been reported to be less than one hour33, and a single injection of GTS-21 is insufficient to achieve significant anti-inflammatory and renoprotective effects (Supplemental Fig. 1). Therefore, GTS-21 was administered to the mice 4 and 6 hours after LPS injection (Fig. 1A). Plasma TNF-α production induced by LPS was significantly suppressed in the GTS-21 group compared with in that the control group (Fig. 1B). In the kidney, the mRNA expression levels of kidney injury molecule 1 (Kim-1), a marker of acute kidney injury, were also reduced compared to that in the control group (Fig. 1C). Histologically, the renal damage was reduced by GTS-21 treatment (Fig. 1D and E). The relative expression levels of the inflammatory cytokines (Il-1β, Tgf-β, and Ctgf) in the kidney were also reduced in the GTS-21-treated group (Fig. 1F-H).
Administration of GTS-21 after the onset of sepsis suppressed systemic inflammation and acute kidney injury. (A) Experimental design: WT mice were intraperitoneally injected with 5 mg/kg LPS. Four and 6 hours later, they received GTS-21 (10 mg/kg) injection (n = 5–8 in each group). Eight hours after LPS injection, the mice were euthanized and blood, kidney samples were collected. (B) Plasma TNF-α concentrations were analyzed by ELISA. TNF-α levels, which was increased by LPS administration, were significantly decreased by GTS-21 administration (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P = 0.0024). (C) RNA was extracted from whole kidney samples and qPCR was performed. Kim-1 which is a marker gene of AKI, was increased by LPS and decreased by GTS-21 (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P = 0.0012). (D, E) Representative images of PAS staining (E) and tubular injury scores (D) (vehicle-vehicle and LPS-vehicle; P = 0.0007, LPS-vehicle and LPS-GTS-21; P = 0.012). Arrowheads point to the injured tubules (E). A decrease in pathological renal damage was observed with GTS-21. (F–H) In the kidney, cytokine levels were elevated following LPS treatment and were attenuated by GTS-21 ((F) vehicle-vehicle and LPS-vehicle; P = 0.0165, LPS-vehicle and LPS-GTS-21; P = 0.0362, (G) vehicle-vehicle and LPS-vehicle; P = 0.011, LPS-vehicle and LPS-GTS-21; P = 0.0006, (H) vehicle-vehicle and LPS-vehicle; P = 0.0956, LPS-vehicle and LPS-GTS-21; P = 0.0465). Data are shown as mean ± SD. Statistical significance was assessed using two-way ANOVA analysis with Tukey’s multiple comparison test for data B, C, and F–H, and using Mann–Whitney test for data D. n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The anti-inflammatory effects of GTS-21 were attenuated by splenectomy
The spleen is important in the mechanism of CAP as it receives α7nAChR signals21,34. We anticipated that the spleen would also be important even after sepsis onset. Splenectomy was performed five days prior to LPS injection to investigate its involvement (Fig. 2A). Similarly, GTS-21 was administered 4 and 6 hours after LPS injection. In sham-operated mice, the LPS-induced increase in TNF-α was significantly suppressed by GTS-21 treatment, whereas this suppressive effect was diminished in splenectomized mice (Fig. 2B). Although splenectomy itself led to a reduction in TNF-α levels (Fig. 2B), this finding is consistent with previous reports demonstrating that splenectomy suppresses TNF-α production35. Splenectomy alone did not affect basal TNF-α levels (Supplemental Fig. 3), and LPS stimulation still induced TNF-α production in splenectomized mice (Fig. 2B). These results suggest that the anti-inflammatory effect of GTS-21 is attenuated in the absence of the spleen and is likely mediated, at least in part, through splenic mechanisms.
The anti-inflammatory effect of GTS-21 was attenuated by splenectomy. (A) Experimental design: Five days prior to LPS injection, splenectomy was conducted (n = 6 in each group). (B) The reduction in plasma TNF-α by GTS-21 was attenuated in splenectomized mice (LPS-vehicle-sham and LPS-GTS-21-sham; P = 0.0104, LPS-vehicle-SPLX and LPS-GTS-21-SPLX; P = 0.7935). The reduction rate of LPS-GTS-21, calculated in reference to the mean value of LPS-vehicle, has been included (LPS-vehicle-sham and LPS-GTS-21-sham; −30.9%, LPS-vehicle-SPLX and LPS-GTS-21-SPLX; −16.3%). Data are shown as mean ± SD. Statistical significance was assessed using two-way ANOVA analysis with Tukey’s multiple comparison. n.s., not significant, *P < 0.05.
GTS-21 suppressed TNF-α production in macrophages after LPS-stimulation in vitro. (A) Experimental design: 1.0 × 105 RAW 264.7 cells were seeded in 24-well plates for 24 hours. TNF-α was induced by LPS (100 ng/mL). Four and 6 hours later, GTS-21 (50 μM) was administered, and eight hours later, the supernatant was collected. (C) Experimental design: 1.0 × 105 U937 cells were seeded in 24-well plates for 24 hours. TNF-α was induced by LPS (1 μg/mL). Four and 6 hours later, GTS-21 (50 μM) was administered, and 8 hours later, supernatant was collected. (B, D) TNF-α concentrations were analyzed by ELISA. The decrease in TNF-α was observed with GTS-21 administration in both RAW 264.7 (B) (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P < 0.0001) and U937 cells (D) (vehicle-vehicle and LPS-vehicle; P = 0.0003, LPS-vehicle and LPS-GTS-21; P = 0.0093). Relative TNF-α levels were calculated based on the average value of TNF-α increased by LPS stimulation (D). Data are shown as mean ± SD. Statistical significance was assessed using two-way ANOVA analysis with Tukey’s multiple comparison. **P < 0.01, ***P < 0.001,****P < 0.0001.
Administering GTS-21 to macrophages after LPS stimulation in vitro resulted in an anti-inflammatory effect
In CAP, it is believed that α7nAChR in splenic macrophages receives ACh signals21,34. Therefore, we focused on macrophages and conducted in vitro experiments. Inflammation was first induced in the mouse macrophage cell line RAW 264.7 using LPS, followed by the administration of GTS-21 (Fig. 3A). Consequently, the suppression of TNF-α production in the supernatant was confirmed (Fig. 3B). We conducted the same experiments on human macrophages derived from the monocyte cell line, U937 (Fig. 3C). GTS-21 suppressed the production of TNF-α in the supernatant (Fig. 3D). We confirmed that the administration of GTS-21 after inflammation suppressed the production of TNF-α in both mouse and human macrophages.
Candidate genes that exhibited changes upon GTS-21 administration were identified using RNA-Sequencing
RNA-sequencing (RNA-seq) analysis was performed to identify candidate genes related to GTS-21–mediated anti-inflammatory effects in macrophages. Genes that showed substantial changes between treatments were selected to generate a heatmap. These genes were then categorized into five clusters based on their expression profiles using k-means clustering, which facilitated the identification of distinct gene expression patterns (Fig. 4A, B). From this set, 11 genes overlapped between the RAW 264.7 and U937 cells (Fig. 4C). The FPKM values for these 11 genes are shown in Fig. 4D and E. Notably, Ccl2 exhibited especially pronounced changes.
RNA-seq revealed that Ccl2 was significantly altered by GTS-21 in both murine and human macrophages. (A, B) The Clustered heatmap shows selected genes with an absolute log2 fold change of LPS/CTL > 1 and LPS-GTS-21/LPS > 1 (350 genes in RAW 264.7 cells, 146 genes in U937 cells). (C) Venn diagram created by selecting genes with significant expression changes due to drug treatment in RAW 264.7 and U937 cells. The left side shows the selected genes in RAW 264.7 cells, while the right side shows the selected genes in U937 cells. There were 11 overlapping genes between RAW 264.7 and U937 cells. (D, E) Heatmaps showing transformed FPKM of 11 commonly selected genes in RAW264.7 (D) and U937 cells (E) among control, LPS, and LPS + GTS-21.
The administration of GTS-21 decreased CCL2 levels in both macrophages and mouse spleen
Following RNA-seq results showing that GTS-21 reduced Ccl2 expression, we performed quantitative real-time PCR (qPCR) to validate the changes in Ccl2 expression in macrophages. In both RAW 264.7 and U937 cells, Ccl2 mRNA expression increased after LPS and decreased after GTS-21 administration (Fig. 5A, C and D). Changes in the relative expression levels of Ccl2 in the mouse spleen were also evaluated. Ccl2 mRNA expression in the whole spleen, which was elevated following LPS administration, was attenuated after GTS-21 administration (Fig. 5B, E). CCL2 protein expression in the mouse splenic macrophages was confirmed using flow cytometry. The spleen was harvested, and cell suspensions were analyzed using flow cytometry. Splenic macrophages were identified as CD11b and F4/80 double-positive cells, and the expression levels of CCL2 in these macrophages were assessed. Although the difference was not significant, the group treated with GTS-21 after LPS administration showed a tendency for a decrease in the median fluorescence intensity of CCL2 compared with the group treated with LPS alone. (Supplemental Fig. 4). To further investigate the role of CCL2, we assessed whether its inhibition could attenuate LPS-induced TNF-α production. In RAW 264.7 cells, administration of the CCL2 inhibitor Bindarit significantly reduced LPS-induced TNF-α elevation (Fig. 5 F, Supplemental Fig. 5).
CCL2 elevation was decreased by GTS-21, both in vitro and in vivo. (A, B) Experimental design. (C-E) Ccl2 expression was analyzed using qPCR. (F) Effect of CCL2 Inhibition on LPS-induced TNF-α Production in RAW 264.7 cells.Ccl2 expression was increased by LPS and decreased by GTS-21 in RAW 264.7 cells (C) (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P < 0.0001), U937 cells (D) (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P < 0.0001), and murine whole spleen (E) (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-GTS-21; P = 0.0026). (F) RAW 264.7 cells were treated with LPS and the CCL2 inhibitor Bindarit. TNF-α levels were measured after 4 h. Bindarit significantly suppressed TNF-α production (vehicle-vehicle and LPS-vehicle; P < 0.0001, LPS-vehicle and LPS-Bindarit; P < 0.0001). Data are shown as mean ± SD. Statistical significance was assessed using two-way ANOVA analysis with Tukey’s multiple comparison for data A and B, and using one-way ANOVA analysis with Tukey’s multiple comparison for data C. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In this study, we demonstrated that GTS-21 administration after the onset of septic AKI exerted anti-inflammatory and renoprotective effects. Notably, we identified that macrophage-derived CCL2 suppression as a potential downstream mechanism. Globally, 25–75% of AKI cases are related to sepsis or septic shock. Sepsis-induced AKI is associated with longer ICU and hospital stays, higher mortality rates, and a decreased quality of life36. In addition, with the global trend of an aging population, most patients with sepsis are the elderly. As the population ages, the number of patients with sepsis-induced AKI is expected to increase37. The development of AKI not only increases rehospitalization and mortality38, but also increases the risk of developing and progressing to chronic kidney disease39. These factors are expected to further increase healthcare costs associated with sepsis-induced AKI. Therefore, establishing effective treatments for sepsis-induced AKI is important from a healthcare/economic perspective. Various factors, including surgery, chemotherapy, medications, and infections, can induce AKI1. Septic AKI poses unique challenges, as the onset of sepsis is difficult to predict and preventive interventions are not feasible. Furthermore, there are no established treatments for septic AKI, highlighting the need for novel therapeutic approaches.
VNS and acetylcholine receptor activation have been reported to exert renoprotective effects in AKI, primarily through the inflammatory reflex known as CAP21,22,29,30,34. While CAP activation holds promise as a novel therapeutic approach for AKI, most studies to date have focused on its preventive effects administered prior to the onset of injury21,22,29,30,34, and its efficacy in established AKI remains insufficiently explored. To the best of our knowledge, the only evidence supporting the efficacy of CAP following injury is from our previous study on the effectiveness of VNS in cisplatin-induced nephropathy32. Investigating the potential of post-injury CAP activation to ameliorate AKI is critical for its therapeutic application in clinical practice. In the current study, we conducted experiments using a septic AKI model, in which injury is difficult to predict.
CAP is a vagus nerve-mediated mechanism of immune regulation that has been studied in various disease contexts. The concept of CAP was originally proposed based on findings that electrical stimulation of the efferent vagus nerve attenuated TNF-α release in rats with LPS-induced sterile endotoxemia3. However, while these results were foundational, several aspects of the CAP model warrant careful consideration. For instance, the anatomical and functional connectivity between the vagus nerve and the splenic nerve remains controversial, as no direct synaptic connection has been clearly identified40. Furthermore, alternative pathways involving sympathetic nerves and splanchnic innervation have been proposed as parallel or compensatory routes for anti-inflammatory signaling41. Using optogenetics, Tanaka et al. demonstrated that both efferent and afferent vagal pathways contribute to the renoprotective effects of VNS23. However, considering that the anti-inflammatory and renoprotective effects of VNS are complex and mediated by multiple factors, we selected GTS-21, an α7 nicotinic acetylcholine receptor agonist, in the present study to better assess the downstream mechanisms.
We found that renal damage was ameliorated even when treatment was initiated 4 hours after the onset of LPS-induced sepsis. We hypothesized that CAP is involved in renoprotection even post-injury. Given that α7nAChR on macrophages receives CAP signals, we focused on macrophages to elucidate the mechanism of renoprotection. RNA-seq analysis of mouse and human macrophages revealed that inflammation-induced Ccl2 expression was suppressed by GTS-21 treatment (Fig. 4). qPCR confirmed that GTS-21 treatment reduced Ccl2 expression in macrophages (Fig. 5C, D), as well as in the spleen (Fig. 5E).
CCL2, also known as MCP-1, is a member of the CC chemokine family and is a chemotactic factor for monocytes. The major sources of CCL2 include epithelial cells, endothelial cells, smooth muscle cells, monocytes/macrophages, fibroblasts, astrocytes, and microglia. CCL2 is involved in the migration and infiltration of monocytes, microglia, and memory T lymphocytes during injury and infection42. Previous studies on rheumatoid arthritis reported that the administration of GTS-21 involves CAP and leads to a decrease in CCL2 levels in the joints. This reduction in CCL2 expression results in a decrease in the number of infiltrating monocytes and dendritic cells, thereby alleviating arthritis6,43. In cisplatin nephropathy, VNS has been reported to decrease elevated CCL2 levels in the plasma and kidneys and reduce the number of renal macrophages during injury32. These findings support a possible involvement of local CCL2 reduction in the anti-inflammatory effects. In this study, we demonstrated that GTS-21 decreased CCL2 levels in macrophages. Blocking the CCL2/CCR2 axis suppresses the anti-inflammatory polarization of macrophages44. This suggests that reducing CCL2 levels in macrophages might contribute to the mitigation of renal injury. Furthermore, clinical studies have observed elevated CCL2 levels in patients with AKI, suggesting its potential as a biomarker of early-stage AKI45. Consequently, by activating CAP to control elevated CCL2 levels, it may be suppressed not only in sepsis but also in other pathological conditions.
Furthermore, RNA-seq analysis revealed that Apbb1ip and Csf1r displayed expression patterns similar to Ccl2, suggesting that these genes may play potential roles in the inflammatory response in the LPS-stimulated macrophage model. Apbb1ip encodes an adaptor protein that interacts with Rap1 GTPase and mediates integrin activation, a critical step for immune cell adhesion. In macrophages, Apbb1ip has been shown to facilitate the activation of β2 integrins following LPS stimulation46. Csf1r, a receptor tyrosine kinase, is known to promote macrophage survival, proliferation, and chemotaxis through its signaling pathway47. GTS-21 suppressed the expression of these genes, suggesting that it may modulate macrophage migration to injured tissues and contribute to its anti-inflammatory effects under LPS stimulation.
This study has several limitations. First, although GTS-21 was administered relatively early after LPS injection (4 and 6 hours), it remains unclear whether delayed administration would achieve similar therapeutic effects, particularly in the progressive context of sepsis. Second, while GTS-21 reduced Ccl2 expression in vitro (Fig. 5C and D), we could not provide definitive in vivo evidence that CCL2 suppression in splenic macrophages is the main driver of its protective effects. Third, the modest effect size of the CCL2 inhibitor Bindarit suggests that additional signaling pathways may contribute to the observed effects of GTS-21. While Apbb1ip and Csf1r identified by RNA-seq may contribute to the anti-inflammatory effects, previous studies have also implicated other downstream mechanisms, such as Hes-1 upregulation22and enhanced macrophage–macrophage interactions in the spleen34. These alternative mechanisms may also contribute to the effects observed in this study. In our previous experiments examining the effects of GTS-21 prior to LPS-induced injury, we confirmed that the inflammatory response to LPS did not differ significantly between male and female mice34. Accordingly, only male mice were used in this study. However, the potential influence of sex on treatment efficacy remains to be clarified.
In conclusion, further investigations are warranted to elucidate the precise molecular mechanisms underlying the anti-inflammatory and renoprotective effects of GTS-21, as well as to determine the optimal timing and conditions for its therapeutic application in sepsis-associated AKI.
Methods
Animals
C57BL/6 male mice (8–10 weeks of age, 20–25 g) purchased from CLEA (Tokyo, Japan) were used for the animal experiments. Septic AKI mouse models were created by intraperitoneal injection of 5 mg/kg LPS (L4391; Sigma-Aldrich, St Louis, MO, USA). 3-(2,4-Dimethoxybenzylidene)- anabaseine dihydrochloride (GTS-21) (SML0326, Sigma-Aldrich) was used to stimulate α7nAChR. The doses of LPS and GTS-21 administration were determined based on our previous studies, including a report investigating the effects of GTS-21 on LPS-induced acute kidney injury34. Based on our preliminary experiments showing limited efficacy of a single 20 mg/kg post-injury administration of GTS-21 (Supplemental Fig. 1), we modified the protocol to administer GTS-21 at 10 mg/kg twice, at 4 and 6 hours after LPS injection. As TNF-α production peaked around 24 hours in previous studies34, making it difficult to detect treatment differences, we evaluated TNF-α levels at 8 hours in the current study. Four and 6 hours after LPS injection, GTS-21 (10 mg/kg) was administrated intraperitoneally. The mice in the control group received equal volumes of normal saline. Eight hours after LPS injection, the mice were euthanized by intraperitoneal injection of medetomidine (0.3 mg/kg), butorphanol (5 mg/kg), and midazolam (4 mg/kg). Blood and kidney tissues were collected as described our previous study34. Approximately 500 μl blood samples were centrifuged at 7,000 g for 5 min and approximately 250 μl of plasma was collected. All mice were housed under specific pathogen-free (SPF) conditions in an animal facility. Mice were maintained in individually ventilated cages under controlled environmental conditions with a 12-h light/dark cycle, constant temperature (23 ± 2 °C), and humidity (50 ± 10%). Standard rodent chow and water were provided ad libitum. To minimize stress‐related variability, mice were habituated to gentle handling by the same experimenter for four consecutive days prior to experiments. All experiments were conducted by administering LPS at approximately 9:00 a.m., followed by GTS-21 administration at 1:00 p.m. and 3:00 p.m., and euthanasia was performed at 5:00 p.m. All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by Nagasaki University (2006101636). All methods were performed in accordance with the ARRIVE guidelines, and all procedures were carried out by experimenters who had received appropriate training in animal handling and care at the animal facility.
Cell culture and reagents
RAW 264.7 cells, a mouse macrophage cell line, were incubated in D-MEM high-glucose medium (043–30,085; FUJIFILM Wako Chemicals, Osaka, Japan) containing 10% fetal bovine serum (FBS) (10,270–106; Thermo Fisher Scientific, Waltham, MA, USA), 10% L-glutamine (25,030,081; Thermo Fisher Scientific), 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate (169–23,191; FUJIFILM Wako Chemicals). U937 cells (JCRB Cell Bank, Osaka, Japan), a human monocyte cell line, were incubated in RPMI-1640 with L-Glutamine and Phenol Red (189–02,025; FUJIFILM Wako Chemicals) containing 10% FBS, 100 units/mL penicillin G sodium, and 100 μg/mL streptomycin sulfate48. The cells were maintained at 37℃ in a humidified incubator containing 5% CO2. Passages 10–20 of the cell culture were used in this study. U937 cells were differentiated into human macrophages using phorbol 12-myristate 13-acetate (PMA) (S7791; Selleck Chemicals, Houston, TX, USA). For in vitro experiments, 1.0 × 105 cells were seeded in 24-well plates for 24 hours, followed by treatment with LPS alone, GTS-21 alone, or GTS-21 after LPS. RAW 264.7 cells were treated with 100 ng/mL LPS, while U937 cells required a higher concentration (1 μg/mL) due to lower responsiveness in preliminary experiments. GTS-21 was used at 50 μM in both cell types. The LPS dose and timing for RAW 264.7 cells were based on previous studies using 100 ng/mL for 4–24 hours to assess TNF-α production49, in which 100 μM GTS-21 effectively suppressed TNF-α levels. Based on these findings, we adopted a protocol with two equally divided doses of GTS-21.
For the inhibition experiments using Bindarit, a CCL2 inhibitor, RAW 264.7 cells (1.0 × 105 cells) were seeded one day prior to stimulation. LPS (100 ng/mL) and Bindarit (200 μM) were administered simultaneously, and culture supernatants were collected 8 hours later and subjected to ELISA. An equivalent volume of DMSO was used as a vehicle control for Bindarit. The concentration of Bindarit was selected based on previous reports demonstrating its inhibitory effects at concentrations ranging from 100 to 400 μM in mouse monocytes and periodontal macrophages50, as well as its established use at 300 μM in RAW 264.7 cells51.
Kidney histology
One-quarter of the left kidney was fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. Tissues were sliced into 3 μm sections and stained with periodic acid-Schiff (PAS) to evaluate tubular damage52. Tubular injury was defined as tubular dilation, tubular atrophy, formation of tubular casts, detachment of tubular epithelial cells, and loss of the brush border53. Tubular injury scores were evaluated based on the proportion of injured tubules in the total cortex as follows: 0, none; 1, < 25%; 2, 25–50%; 3, 50–75%; and 4, > 75%. Each sample was evaluated based on the average score of four selected fields in the outer medulla54.
Splenectomy
Five days prior to LPS injection, splenectomy was performed under anesthesia. The splenic arteries and veins were ligated, and the spleen was removed through a small left-back incision. Sham-operated mice underwent a skin incision only. General anesthesia was induced by the intraperitoneal injection of medetomidine (0.3 mg/kg), midazolam (4.0 mg/kg), and butorphanol (5.0 mg/kg). This combination provided adequate sedation, muscle relaxation, and analgesia. After completion of the surgical procedure, atipamezole (0.5 mg/kg, i.p.), an α2-adrenergic antagonist, was administered to reverse the effects of medetomidine and facilitate recovery. Although no additional post-surgical analgesia was administered, the anesthetic combination used provides analgesic effects for several hours. All mice were closely monitored during the recovery period, and no signs of pain or distress were observed.
Enzyme-linked immunosorbent assay (ELISA)
Mouse and human TNF-α levels were measured using ELISA with the TNF alpha Mouse Uncoated ELISA Kit with Plates (88–7324-88; Thermo Fisher Scientific) and the TNF alpha Human Uncoated ELISA Kit with Plates (88–7346-88; Thermo Fisher Scientific), according to the manufacturer’s instructions. As the measured concentrations exceeded the assay range of the ELISA kit (analytical sensitivity: 15.6 pg/mL; assay range: 15.6–1000 pg/mL), the samples were diluted 10- to 20-fold before analysis, and the final values were determined by back-calculating from the dilution factors. Synergy LX (BioTek Instruments) was used as the ELISA plate reader.
RNA extraction and qPCR
Total RNA from the kidney tissue was extracted from a quarter of the left kidney (approximately 35- 40 mg) using RNAiso Plus (9109; Takara Bio Inc., Shiga, Japan). Total RNA was extracted from cultured cells using the FastGene RNA Basic Kit (FG-80006; NIPPON Genetics, Tokyo, Japan). The RNA concentration was measured using Synergy LX. The cDNA was generated from the resulting 450 ng RNA using PrimeScript RT Master Mix (RR036A; Takara Bio Inc.), as described by the manufacturer. Relative mRNA expression levels of the genes were assayed using iTaq Universal SYBR Green Supermix (1,725,121; Bio-Rad, Tokyo, Japan). The gene expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. qPCR was performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad) and the data were analyzed using the ΔΔCt method. The maximum difference in Ct values among samples was approximately 0.3, which corresponds to a maximal error of about 1.2 in expression levels. To validate the amplification efficiency of qPCR, standard curves were generated using serial dilutions of cDNA templates for Gapdh and Ccl2. Both assays demonstrated acceptable amplification efficiencies, supporting the validity of the quantification. The primer sequences are listed in Supplemental Table 1.
RNA sequencing and computational analysis
RNA sequencing and analyses were performed as previously described49. RAW 264.7 cells and U937 were seeded for 24 hours, stimulated with LPS, and subsequently treated with GTS-21.Total RNA was isolated using the RNeasy Mini Kit (74,106; Qiagen, Hilden, Germany) and 450 ng of RNA was used. Poly (A)-containing mRNA from the total RNA was converted to cDNA with poly (A) primers using the TruSeq RNA Sample Preparation kit v2 (Illumina). High-throughput mRNA sequencing was performed using the Hiseq2500 (Illumina) system. Sequenced paired-end reads were mapped onto the mouse build mm10 reference genome using HISAT2 with the parameter “-q –dta -cufflinks.” The SAM file was converted into the BAM format. Fragments per kilobase of exon per million reads mapped (FPKM) were calculated as indicators of gene expression using cuffdiff and cummerBund. Genes with FPKM > 0 in all three groups and FPKM > 5 in at least two groups were selected for downstream analyses. Highly variant genes were selected with an absolute log2 fold change of LPS/CTL > 1 and LPS-GTS-21/LPS > 1, and heatmaps were generated by K-means clustering method using the R package ClusterGVis (GitHub-junjunlab/ClusterGVis: One-step to Cluster and Visualize Gene Expression Matrix). The transformed FPKM were calculated using the R package zFPKM55.
Flow cytometry
Spleen suspensions were prepared from the mice. The spleen tissue was mushed with the rubber end of a 2.5-mL syringe plunger, passed through a 40-μm cell strainer, and then centrifuged at 500 g for 10 min at 4℃. The supernatant was discarded, and the cells were resuspended in 5 mL cold of phosphate-buffered saline. After blocking nonspecific Fc binding with anti-mouse CD16/32 (5 μg/mL; 101,302; BioLegend, San Diego, CA, USA), fresh spleen suspensions were incubated with the following antibodies: anti-mouse CD45.2-APC (2 μg/mL; 17–0454-82; Thermo Fisher Scientific), CD11b-eFluor450 (2 μg/mL; 48–0112-82; Thermo Fisher Scientific), and F4/80-FITC (5 μg/mL; 11–4801-85; Thermo Fisher Scientific). Intracellular Fixation & Permeabilization Buffer Set (88–8824-00; Thermo Fisher Scientific) and CCL2-PE (2 μg/mL; 12–7096-82; Thermo Fisher Scientific) were used for CCL2 staining. Fixable Viability-eFluor506 (65–0866-14; Thermo Fisher Scientific) was used to exclude dead cells. Compensation beads (UltraComp eBeads, 01–2222-42; Thermo Fisher Scientific) were used for the compensation. Flow cytometry data were acquired using an Attune NxT Flow Cytometer (Thermo Fisher Scientific) and analyzed using the FlowJo software (version 10.0; Tree Star Inc.). The gating strategy was performed as previously described21 (Supplemental Fig. 2).
Statistical analysis
Two-tailed Student’s t-test or Mann–Whitney U test was used for comparisons between two groups. One-way or two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was used for three or more groups. Statistical significance was defined as P < 0.05. Data are expressed as mean ± standard deviation (SD). All analyses were performed using GraphPad Prism version 9 (GraphPad Software).
Data availability
The raw sequencing data generated during this study have been deposited in the DDBJ Sequence Read Archive under accession numbers DRR699391–DRR699394 for RAW 264.7 cells and DRR699399–DRR699402 for U937 cells. All other data can be obtained from the corresponding author upon reasonable request.
References
Mehta, R. L. et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387, 2017–2025. https://doi.org/10.1016/S0140-6736(16)30240-9 (2016).
Yasuda, H., Kato, A., Fujigaki, Y. & Hishida, A. Incidence and Clinical Outcomes of Acute Kidney Injury Requiring Renal Replacement Therapy in Japan. Ther. Apher. Dial. 14, 541–546. https://doi.org/10.1111/j.1744-9987.2010.00826.x (2010).
Borovikova, L. V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462. https://doi.org/10.1038/35013070 (2000).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859. https://doi.org/10.1038/nature01321 (2002).
Wang, H. et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388. https://doi.org/10.1038/nature01339 (2003).
Liu, D. et al. The effect of the cholinergic anti-inflammatory pathway on collagen-induced arthritis involves the modulation of dendritic cell differentiation. Arthritis Res. Ther. 20, 1–11. https://doi.org/10.1186/s13075-018-1759-9 (2018).
van Maanen, M. A. et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 60, 114–122. https://doi.org/10.1002/art.24177 (2009).
Meroni, E. et al. Vagus Nerve Stimulation Promotes Epithelial Proliferation and Controls Colon Monocyte Infiltration During DSS-Induced Colitis. Front Med (Lausanne) 8, 694268, https://doi.org/10.3389/fmed.2021.694268 (2021).
Kovacic, K. et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. The Lancet Gastroenterology and Hepatology 2, 727–737. https://doi.org/10.1016/S2468-1253(17)30253-4 (2017).
Chapman, S. J. et al. Noninvasive vagus nerve stimulation to reduce ileus after major colorectal surgery: early development study. Colorectal Dis. 23, 1225–1232. https://doi.org/10.1111/codi.15561 (2021).
Muthulingam, J. A. et al. Cervical transcutaneous vagal neuromodulation in chronic pancreatitis patients with chronic pain: A randomised sham controlled clinical trial. PLoS ONE 16, 1–16. https://doi.org/10.1371/journal.pone.0247653 (2021).
Aranow, C. et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann. Rheum. Dis. 80, 203–208. https://doi.org/10.1136/annrheumdis-2020-217872 (2021).
Guarini, S. et al. Efferent Vagal Fibre Stimulation Blunts Nuclear Factor-κB Activation and Protects Against Hypovolemic Hemorrhagic Shock. Circulation 107, 1189–1194. https://doi.org/10.1161/01.CIR.0000050627.90734.ED (2003).
Altavilla, D. et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-κB activation, blunts TNF-α production, and protects againts splanchic artery occlusion shock. Shock 25, 500–506. https://doi.org/10.1097/01.shk.0000209539.91553.82 (2006).
Yoshikawa, H. et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-κB phosphorylation and nuclear factor-κB transcriptional activity through nicotinic acetylcholine receptor α7. Clin. Exp. Immunol. 146, 116–123. https://doi.org/10.1111/j.1365-2249.2006.03169.x (2006).
Sun, P. et al. Involvement of MAPK/NF-κB Signaling in the Activation of the Cholinergic Anti-Inflammatory Pathway in Experimental Colitis by Chronic Vagus Nerve Stimulation. PLoS ONE 8, e69424–e69424. https://doi.org/10.1371/journal.pone.0069424 (2013).
Wang, M. et al. Vagus Nerve Stimulation Ameliorates Renal Ischemia-Reperfusion Injury through Inhibiting NF- κ B Activation and iNOS Protein Expression. Oxid. Med. Cell. Longev. 1–8, 2020. https://doi.org/10.1155/2020/7106525 (2020).
de Jonge, W. J. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851. https://doi.org/10.1038/ni1229 (2005).
Lu, X.-X. et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit Mediates Vagus Nerve Stimulation-Induced Neuroprotection in Acute Permanent Cerebral Ischemia by a7nAchR/JAK2 Pathway. Med. Sci. Monit. 23, 6072–6081. https://doi.org/10.12659/MSM.907628 (2017).
Lu, B. et al. α7 Nicotinic Acetylcholine Receptor Signaling Inhibits Inflammasome Activation by Preventing Mitochondrial DNA Release. Mol. Med. 20, 350–358. https://doi.org/10.2119/molmed.2013.00117 (2014).
Inoue, T. et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J. Clin. Investig. 126, 1939–1952. https://doi.org/10.1172/JCI83658 (2016).
Inoue, T. et al. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Int. 95, 563–576. https://doi.org/10.1016/j.kint.2018.09.020 (2019).
Tanaka, S. et al. Vagus nerve stimulation activates two distinct neuroimmune circuits converging in the spleen to protect mice from kidney injury. Proc. Natl. Acad. Sci. 118, e2021758118–e2021758118. https://doi.org/10.1073/pnas.2021758118 (2021).
Abe, C. et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 20, 700–707. https://doi.org/10.1038/nn.4526 (2017).
Yeboah, M. M. et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74, 62–69. https://doi.org/10.1038/ki.2008.94 (2008).
Sadis, C. et al. Nicotine Protects Kidney from Renal Ischemia/Reperfusion Injury through the Cholinergic Anti-Inflammatory Pathway. PLoS ONE 2, e469–e469. https://doi.org/10.1371/journal.pone.0000469 (2007).
Gigliotti, J. C. et al. Ultrasound Prevents Renal Ischemia-Reperfusion Injury by Stimulating the Splenic Cholinergic Anti-Inflammatory Pathway. J. Am. Soc. Nephrol. 24, 1451–1460. https://doi.org/10.1681/ASN.2013010084 (2013).
Chatterjee, P. K. et al. Nicotinic Acetylcholine Receptor Agonists Attenuate Septic Acute Kidney Injury in Mice by Suppressing Inflammation and Proteasome Activity. PLoS ONE 7, e35361–e35361. https://doi.org/10.1371/journal.pone.0035361 (2012).
Chatterjee, P. K. et al. Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS ONE 12, e0188797–e0188797. https://doi.org/10.1371/journal.pone.0188797 (2017).
Gao, Y. et al. GTS-21 attenuates LPS-induced renal injury via the cholinergic anti-inflammatory pathway in mice. American journal of translational research 9, 4673–4681 (2017).
Kim, H. et al. The proximal tubular α7 nicotinic acetylcholine receptor attenuates ischemic acute kidney injury through Akt/PKC signaling-mediated HO-1 induction. Exp. Mol. Med. 50, 1–17. https://doi.org/10.1038/s12276-018-0061-x (2018).
Uni, R. et al. Vagus nerve stimulation even after injury ameliorates cisplatin-induced nephropathy via reducing macrophage infiltration. Sci. Rep. 10, 1–11. https://doi.org/10.1038/s41598-020-66295-0 (2020).
Jun, T. & Kenji, H. α7 Nicotinic Receptor Agonists: Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer’s Disease. Open Med Chem J. 4, 37–56. https://doi.org/10.2174/1874104501004010037 (2010).
Nakamura, Y. et al. Alpha 7 nicotinic acetylcholine receptors signaling boosts cell-cell interactions in macrophages effecting anti-inflammatory and organ protection. Commun Biol 6, 666. https://doi.org/10.1038/s42003-023-05051-2 (2023).
Huston, J. M. et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628. https://doi.org/10.1084/jem.20052362 (2006).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol 19, 401–417. https://doi.org/10.1038/s41581-023-00683-3 (2023).
Clifford, K. M. et al. Challenges with Diagnosing and Managing Sepsis in Older Adults. Expert Rev Anti Infect Ther 14, 231–241. https://doi.org/10.1586/14787210.2016.1135052 (2016).
Schulman, I. H. et al. Readmission and Mortality After Hospitalization With Acute Kidney Injury. Am J Kidney Dis 82, 63–74 e61, https://doi.org/10.1053/j.ajkd.2022.12.008 (2023).
Cerdá, J. et al. Epidemiology of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 881–886. https://doi.org/10.2215/CJN.04961107 (2008).
Martelli, D., McKinley, M. J. & McAllen, R. M. The cholinergic anti-inflammatory pathway: A critical review. Autonomic Neuroscience: Basic and Clinical 182, 65–69. https://doi.org/10.1016/j.autneu.2013.12.007 (2014).
Occhinegro, A., McAllen, R. M., McKinley, M. J. & Martelli, D. Acute Inhibition of Inflammation Mediated by Sympathetic Nerves: The Inflammatory Reflex. NeuroImmunoModulation 30, 135–142. https://doi.org/10.1159/000531469 (2023).
Singh, S., Anshita, D. & Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol 101, 107598. https://doi.org/10.1016/j.intimp.2021.107598 (2021).
Bai, X. et al. GTS-21 alleviates murine collagen-induced arthritis through inhibition of peripheral monocyte trafficking into the synovium. Int. Immunopharmacol. 122, 110676. https://doi.org/10.1016/j.intimp.2023.110676 (2023).
Li, X. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66, 157–167. https://doi.org/10.1136/gutjnl-2015-310514 (2017).
Puthumana, J. et al. Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest 131, e139927. https://doi.org/10.1172/JCI139927 (2021).
Emmanuelle Caron, Annette J. Self & Hall, A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Current Biology 10, https://doi.org/10.1016/s0960-9822(00)00641-2 (2000).
Stanley, E. R. & Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol 6, https://doi.org/10.1101/cshperspect.a021857 (2014).
Wu, C. H. et al. Activation of α7 nicotinic acetylcholine receptors attenuates monocyte–endothelial adhesion through FUT7 inhibition. Biochem. Biophys. Res. Commun. 590, 89–96. https://doi.org/10.1016/j.bbrc.2021.12.094 (2022).
Umene, R. et al. Induction of tetraspanin 13 contributes to the synergistic anti-inflammatory effects of parasympathetic and sympathetic stimulation in macrophages. Biochem Biophys Res Commun 665, 187–194. https://doi.org/10.1016/j.bbrc.2023.04.118 (2023).
Shen, Z. et al. Inhibition of CCL2 by bindarit alleviates diabetes-associated periodontitis by suppressing inflammatory monocyte infiltration and altering macrophage properties. Cell Mol Immunol 18, 2224–2235. https://doi.org/10.1038/s41423-020-0500-1 (2021).
Mora, E., Guglielmotti, A., Biondi, G. & Sassone-Corsi, P. Bindarit: an anti-inflammatory small molecule that modulates the NFkappaB pathway. Cell Cycle 11, 159–169. https://doi.org/10.4161/cc.11.1.18559 (2012).
Yang, A. et al. Activation of the alpha7nAChR by GTS-21 mitigates septic tubular cell injury and modulates macrophage infiltration. Int Immunopharmacol 138, 112555. https://doi.org/10.1016/j.intimp.2024.112555 (2024).
Dong, Y. et al. Ischemic duration and frequency determines AKI-to-CKD progression monitored by dynamic changes of tubular biomarkers in IRI mice. Front. Physiol. 10, 1–15. https://doi.org/10.3389/fphys.2019.00153 (2019).
Hasegawa, S. et al. Activation of Sympathetic Signaling in Macrophages Blocks Systemic Inflammation and Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 32, 1599–1615. https://doi.org/10.1681/ASN.2020121723 (2021).
Traver Hart, H. K. K. Sarah LaMere, Katie Podshivalova, Daniel R Salomon Finding the active genes in deep RNA-seq gene expression studies. BMC Genomics 14, 778. https://doi.org/10.1186/1471-2164-14-778 (2013).
Acknowledgements
We thank Ryoko Yamamoto, Kurara Nakamura, and Yoko Komori at the Department of Physiology of Visceral Function and Body Fluid, Nagasaki University Graduate School of Biomedical Sciences for the excellent technical support. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This research received financial support from various sources, including the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI grant 22H03090); Japan Agency for Medical Research and Development (AMED PRIME JP22gm6210013); Japan Science and Technology Agency (JST) FOREST (Fusion Oriented Research for disruptive Science and Technology) (JPMJFR210J); MSD Life Science Foundation; Salt Science Research Foundation (No. 1919&22C5); SRF; Takeda Science Foundation; Astellas Foundation for Research on Metabolic Disorders; Suzuken Memorial Foundation; Tokyo Biochemical Research Foundation; Japan Kidney Association and Nippon Boehringer Ingelheim Joint Research Project; The Naito Foundation; Daiichi Sankyo Foundation of Life Science; The Uehara Memorial Foundation; Terumo Life Science Foundation to TI; the Japan Society for the Promotion of Science Grant-in-Aid for Research Activity Start-up (JSPS KAKENHI grant 21K20874); the Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (JSPS KAKENHI grant 23K15251) to YN; and the Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (JSPSKAKENHI grant 22K16107 and 24K19036); Nagasaki University Grant for Co-creation Research to C-HW; the Japan Society for the Promotion of Science Grant-in-Aid for Research Activity Start-up (JSPS KAKENHI grant 24K23464); Suzuken Memorial Foundation; the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care to Ryusuke Umene.
Author information
Authors and Affiliations
Contributions
SM, Rie Uni, BS, and TI convinced and designed research; SM, Rie Uni, C-HW, BS, YN and TI conducted the experiments, analyzed the data; C-HW analyzed the RNA-seq data; SM, Rie Uni, and C-HW prepared figures; SM and YN wrote the manuscript draft; Rie Uni, C-HW, YN, Ryusuke Umene, MN, RI, TN, and TI supervised this study and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsuo, S., Uni, R., Wu, CH. et al. α7 nicotinic acetylcholine receptor agonist attenuates lipopolysaccharide-induced acute kidney injury in mice by reducing CCL2 expression in macrophages. Sci Rep 15, 40005 (2025). https://doi.org/10.1038/s41598-025-23583-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-23583-x