Abstract
Despite interest in using industrial hemp (IH) in cattle feed, there are minimal safety data on tissues from exposed cattle. This study sought to describe cannabinoid tissue concentrations, estimate withdrawal times for cattle exposed to IH inflorescence, and generate human exposure estimates for Δ9-tetrahydrocannabinol and cannabidiol. Twenty male Holsteins received IH inflorescence orally at 4.2 mg/kg/d cannabidiolic acid for 14 d. Liver, kidney, muscle, and adipose were collected at 1, 2, 3, 5, and 8 days after IH administration and analyzed for cannabinoids using liquid chromatography mass spectrometry. Withdrawal intervals and target tissues were selected based on the slowest-depleting cannabinoid. ∆9-tetrahydrocannabinol was detected in liver, kidney, and adipose and cannabidiol in all tissues. Withdrawal intervals for liver, kidney, muscle, and adipose were 68, 21, 39, and 154 d, respectively. Adipose was selected as the target tissue and cannabidiol the marker residue. Exposure estimates generated using data from this study revealed that newborns exceeded the lowest published global toxicity threshold of 1 µg/kg Δ9-tetrahydrocannabinol. Additional work should determine safe levels of cannabinoid exposure in vulnerable populations (i.e. children and adolescents). Our results will inform future discussions regarding the inclusion of IH in cattle feed.
Similar content being viewed by others
Introduction
Subsequent to the legalization of cultivation of industrial hemp [IH; Cannabis sativa containing ≤ 0.3% of the psychoactive cannabinoid, Δ9-tetrahydrocannabinol (9-THC)] and its reclassification as a non-controlled substance, interest in IH cultivation and byproduct marketing has continued to increase1. Primary markets for IH products include fiber, seeds and seed products, oils, and cannabinoid extracts (e.g. cannabidiol, or CBD) for medicinal use2. The processing of IH for its primary market product leaves behind plant “waste” material. Cattle and other ruminants are designed to digest and transform plant fiber – undigestible by most species – into tissue products (meat and milk) for human consumption.
The plasma pharmacokinetics of cannabinoids in cattle administered a single dose and multiple doses of IH have been published3,4. Other studies have evaluated tissue concentrations of cannabinoids in cattle fed IH seed meal products, spent hemp biomass (SHB), and unextracted IH leaves and two studies have evaluated the milk residue profile of cattle fed IH silage and extraction byproduct5,6,7,8. Currently, there are no publications evaluating tissue residues following IH inflorescence administration. As the flowers and leaves contain the highest levels of cannabinoids throughout the plant, this is critical information to guide regulatory decisions regarding use of IH byproducts in livestock feed2. While a major goal in using IH as a livestock feed is to improve sustainability of IH production by using plant material remaining following cannabinoid extraction, it is prudent to develop recommendations for animal disposition in a “worst case scenario”, with exposure to unextracted products.
The objectives of this study were to (1) describe cannabinoid concentrations in tissues of cattle administered IH inflorescence, (2) estimate withdrawal intervals (WDI) for cattle exposed to IH, and (3) calculate human exposure estimates for CBD and 9-THC.
Results
No adverse effects were observed in any cattle. Cannabinoid content of IH and average cannabinoid doses are displayed in Table 1. The average administered doses of CBDA and 9-THC were 4.2 mg/kg/d and 0.039 mg/kg/d, respectively.
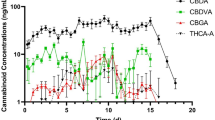
Out of 20 cannabinoids tested (see Supplementary Tables S1-S8), nine were detected in liver: Δ9-tetrahydrocannabinol (9-THC), Δ9-tetrahydrocannabinolic acid (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), cannabidivarinic acid (CBDVA), cannabicyclolic acid (CBLA), (−)−7-nor-7-carboxy cannabidiol (CBD-7-acid), (+)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol glucuronide (THC-acid-glu), and Δ9-tetrahydrocannabinol glucuronide (THC-glu). Six cannabinoids were detected in kidney: Δ8-tetrahydrocannabinol (8-THC), 9-THC, THCA, CBD, CBDA, and (−)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-acid). Four cannabinoids were detected in muscle: CBD, CBDA, CBD-7-acid, and THC-acid. Four cannabinoids were detected in adipose: 9-THC, CBD, CBDA, and CBD-7-acid. The psychoactive cannabinoid 9-THC was found in liver, kidney, and adipose. The bioactive cannabinoid CBD and its precursor, CBDA, were detected in all tissues. Average cannabinoid tissue concentrations are shown in Fig. 1; Table 2. Withdrawal intervals (WDI) are displayed in Fig. 2; Table 3. Limits of detection (LOD) and quantification are presented in Table 4. Adipose was selected as the target tissue and CBD the marker residue based on the slowest depletion rate (154 days).
Withdrawal times of cannabinoids in liver, kidney, muscle, and adipose tissue of male Holstein cattle administered industrial hemp at 4.2 mg/kg cannabidiolic acid (CBDA) per day for 14 days. To satisfy linearity and homoscedasticity requirements of the model, a withdrawal time was unable to be estimated for all cannabinoids detected in each tissue. Total cannabidiol (CBD): CBD + (0.877*CBDA). Total Δ9-tetrahydrocannabinol (9-THC): 9-THC + (0.877*THCA); THCA: Δ9-tetrahydrocannabinolic acid A. Total cannabinoids: sum of all cannabinoids detected. CBD-7-acid: (−)−7-nor-7-carboxy cannabidiol. THC-acid: (−)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol. THC-glu: Δ9-tetrahydrocannabinol glucuronide.
Human exposure estimates are presented in Tables 5 and 6. Newborns exceeded the European Food Safety Authority’s 1 µg/kg acute reference dose (ARfD) for 9-THC (males: 173.1%, females: 156.7%). No other group reached this threshold, and no group approached the Food Standards Agency’s 150 µg/kg/d (0.15 mg/kg/d) acceptable daily intake (ADI) for CBD9,10. The group with the highest percentage of CBD exposure was newborn males, at 5.7% ADI.
Discussion
This is the first report of cannabinoid tissue residue profiles in cattle administered IH inflorescence. Our target was 5 mg/kg CBDA, based on the 5 mg/kg CBD dose reported for human patients suffering from seizures due to Lennox-Gastaut and Dravet syndromes; this was the target dose for our previous studies3,4. Understanding of plasma pharmacokinetics is critical for designing tissue depletion trials; thus, the same IH dose used in those previous studies was selected for the present study. This amount of IH is also easily administered orally via gelatin bolus. The average CBDA and 9-THC doses administered in this study were 4.2 and 0.039 mg/kg/d, respectively.
We detected a total of 11 different cannabinoids, with the widest range found in liver and the slowest depletion in adipose, with an estimated WDI of 154 days for CBD. Thus, adipose tissue was selected as the target tissue and CBD the marker residue. The psychoactive cannabinoid 9-THC was detected in liver, kidney, and adipose tissue, but had a shorter WDI than CBD. The bioactive cannabinoid CBD and its precursor, CBDA, were detected in all tissues. This was expected, as CBDA was the cannabinoid with the highest concentration in the IH material. Using our exposure estimate assumptions and data from the present study, no group reached the 150 µg/kg ADI for CBD, and only newborn males and females exceeded the 1 µg/kg 9-THC ARfD.
Many acidic cannabinoids were detected in liver and muscle. Similarly, previous studies using IH inflorescence, SHB, and IH leaves have consistently reported predominantly acidic cannabinoids in plasma3,4,11,12. In the current study, we detected THC-acid-glu, THC-glu, 8-THC, 9-THC, and THC-acid, which were not detected in plasma in our previous studies3,4. The difference in cannabinoid profile of plasma compared to tissue suggests that there is conversion in the rumen with subsequent distribution to non-central compartments, tissue-specific metabolism resulting in unique tissue cannabinoid signatures, or a combination of both. Cultivar differences across studies may contribute to the variation in cannabinoid profile, but we believe it is unlikely to be responsible for the dramatically increased number of cannabinoids detected in tissues from this study. The spectrum of cannabinoids detected in liver is consistent with its role in cannabinoid metabolism reported for other species. In liver, the cytochrome P450 (CYP) pathway and additional phase II metabolic reactions, predominantly glucuronidation, occur13,14,15,16. Significant first-pass metabolism has been described in humans; presumably, this occurs in cattle, which would prevent some cannabinoids from entering systemic circulation and result in a restricted plasma cannabinoid signature, as we have reported3,4,17. Rumen, microbial, and tissue enzymes responsible for cannabinoid metabolism and the extent of first-pass metabolism have not been described in cattle. Similar to our past studies in plasma, we did not detect THC-11-OH—the bioactive and psychoactive metabolite of 9-THC—in tissue3,4. While the activity of many cannabinoids is unknown in humans or cattle, identifying pharmacokinetic trends can increase our understanding of metabolic pathways.
Three previous studies have reported tissue residues in cattle exposed to hemp products (Table 7). In a study by Smith et al. investigating tissue residue profiles of cattle fed hempseed cake, the authors detected no cannabinoids in muscle and observed varying depletion times and concentrations in liver, kidney and adipose5. The liver and kidney profiles from that study were similar to each other, with detectable levels of various acidic cannabinoids. While no cannabinoids were above the LOD in liver on day 4, CBNA and CBDA/THCA were still detectable in kidney on day 8 in 2 of the 4 sampled animals. In adipose, CBD/9-THC, CBDA/THCA, and CBCA were detected on day 0; only CBD/9-THC was detectable on days 1, 4, and 8 of withdrawal. The CBD/9-THC concentration was 10.1 ng/g on day 0 and 6.3 ng/g on day 8.
Compared to the aforementioned study, we detected higher adipose CBD and 9-THC concentrations but similar depletion rates. The previous study utilized an analytical method that could not differentiate isomeric compounds (CBD vs. 9-THC, CBDA vs. THCA), unlike our method. We detected adipose concentrations of CBD and 9-THC at an average of 224 and 47 ng/g, respectively, on day 1 and 143 and 28 ng/g, respectively, on day 8. These concentrations are approximately 4-22X higher compared to the study by Smith et al.5 This is expected, since inflorescence contains more cannabinoids than seeds2. Comparing the change in adipose CBD and 9-THC concentrations from day 1 to day 8 in our study versus the study by Smith et al., CBD/9-THC concentrations decrease by about 40% for both studies. This suggests that the elimination rate for both studies during this sampling period was similar. In humans and rats, a depot effect in adipose has been observed: cannabinoids become concentrated and are slowly released over time, resulting in prolonged residues18,19. This raises concern for a similar phenomenon in cattle, wherein 9-THC or CBD remain in adipose and redistribute to other organs for an extended period due to a slower terminal elimination phase. Because neither study sampled cattle longer than 8 days, we cannot deny the possibility of a slower terminal elimination half-life for the cannabinoids we measured. Long-term elimination data is also lacking for plasma, as we only sampled through 96 h in our single-dose trial and 108 h in our multi-dose trial3,4.
A study by Irawan et al. investigating the depletion of cannabinoids in tissue and milk from cows fed SHB sheds some light on long-term cannabinoid disposition12. In that study, Jersey cows were fed 7.5% SHB for 28 days followed by a 28 d withdrawal and a routine dry-off period. There was minimal accumulation of cannabinoids in muscle and the highest concentration in liver. Similar to the present study, the proportions of CBD and 9-THC in adipose were greater than in SHB. The doses in the study by Irawan et al. were approximately 20-fold greater for 9-THC (0.81 vs. 0.039 mg/kg) and approximately 36-fold greater for CBD (15.6 vs. 0.43 mg/kg) than in our study. Based on visual inspection of the data from the prior study, 9-THC and CBD levels in adipose on the final day of SHB feeding (similar to our day 1 sample) were approximately 400 and 3,000 ng/g, respectively. This is roughly 8.5-fold greater for 9-THC and 13-fold greater for CBD than our values. This increase in concentration is not as great as the increased dose between studies. The discrepancy in tissue concentrations could be due to altered accumulation based on body fat percentage and other physiologic differences in the study animals. We used Holstein steers for this study, which presumably had higher bodyfat and metabolic differences compared to the lactating Jersey cows used by Irawan et al.12 Even after a 28-day withdrawal from SHB, Irawan et al. reported higher average adipose CBD and 9-THC concentrations (628 ± 828 ng/g and 92 ± 107 ng/g, respectively). While this could be due solely to the increased dose used by Irawan et al., their observations in milk support the depot theory discussed earlier12. Irawan et al. reported higher CBD and CBDA levels in milk at 7 d postpartum (90 d after withdrawal) compared to 28 d after withdrawal. Fatty acids are released during the energy-intensive postpartum period, supporting the idea that cannabinoids are stored and later released from adipose into other compartments. Thus, the levels of CBD and 9-THC that Irawan et al. reported at the end of the 28-d withdrawal period could simply be due to a slow terminal elimination rate in adipose.
Fruge et al. reported similar findings in their study evaluating cannabinoid depletion in Holstein steers fed IH leaves11. They administered IH leaves at a dose of 3.9 mg/kg CBDA once daily for 14 d, which is similar to our dose11. In adipose, THCA was not detected and CBDA was only detected on day 1 of withdrawal. The highest levels of CBD and 9-THC in adipose were detected on day 1 of withdrawal (246.6 ng/g and 14.9 ng/g, respectively) with decreased, but detectable, levels at 6 weeks (42.7 ng/g and 3.4 ng/g, respectively). While the day 1 CBD concentration reported by Fruge et al. is similar to the present study, the 9-THC concentration was much less than the present study11. Fruge et al. did not report individual cannabinoid concentrations in the IH they used, but it is likely that cultivar differences are responsible for the disparity in 9-THC concentrations. Those authors noted large standard deviations in fecal cannabinoid concentrations between animals, suggesting individual variability in digestive and absorptive ability for cannabinoids11. The inconsistency between individuals, cultivars, and IH forms complicates the ability to interpret and apply depletion data for exposed cattle.
Based on the results from this study, WDI for multiple cannabinoids were modeled. The cannabinoid with the longest WDI was CBD in adipose tissue, making CBD and adipose the ideal marker residue and target tissue, respectively. We expected to see a progressive decline in concentrations over time but observed the highest concentrations of cannabinoids in adipose on day 2. This can be explained by higher average doses received by cattle euthanized on day 2 versus day 1. This aberrant temporal trend may have skewed our WDI models towards overestimation, since the model relies on linear depletion kinetics. Our study and prior literature agree that adipose is the tissue with the slowest depletion5,11,12. In humans and rats, 9-THC persists in adipose for several weeks18,19. While improved detection limits could identify a more slowly depleting cannabinoid, data from the present study, prior literature, and communication from regulatory agencies support that CBD and 9-THC are currently the most important cannabinoids. For a majority of cannabinoids in liver, kidney, and muscle, there was little extrapolation from the last data collection point until the estimated WDI. However, for all cannabinoids in adipose and additionally for CBD-7-acid in liver and muscle, tissue concentrations did not appear to have reached a terminal elimination phase by the final collection point. Thus, there was a very large degree of extrapolation in the models, making application of these WDI values invalid. Nonetheless, the prolonged WDI for cannabinoids in adipose is consistent with prior literature and is useful for confirming adipose as the target tissue for IH exposure.
Compared to prior IH literature, we observed different cannabinoid profiles that could be due to variations in the form of IH administered. In the present study, we did not detect CBCA, or CBGA in any tissues, unlike the report by Smith et al.5 Irawan et al. detected CBCA in liver and muscle at the end of the SHB feeding period12. Similar to Smith et al., we detected CBDVA in liver, but WDI model assumptions weren’t satisfied for the present study. In a study by Addo et al. investigating hempseed meal in non-lactating dairy cows, the authors detected no cannabinoids in plasma, rumen fluid, urine, kidney, liver, muscle, or adipose tissues, but did detect CBD and CBDA in the feces of cows receiving either a mix of canola and hempseed meal or hempseed meal alone6. Addo et al. reported similar CBD intake (0.0064 mg/kg CBD) to the Smith study and used an LCMS method with a higher LOD (10 ng/g vs. 0.1 to 3.5 ng/g in the study by Smith et al.).5,6 Smith reported cannabinoid concentrations in liver and adipose exceeding 10 ng/g. Thus, we are unsure of the reason behind the negative cannabinoid finding in the Addo study. At least some of the difference in cannabinoid profiles between the present study and the studies involving hempseed, leaves, and SHB is likely due to differences in cannabinoid composition and other dietary factors influencing absorption (e.g. pH, protein binding in the feed matrix, heat treatment or other processing, hempseed cake vs. meal, etc.).5,6,11,12 A study evaluating cannabinoid composition in ensiled hemp showed increases in neutral and decreases in acidic cannabinoids over time20. Comparative cannabinoid pharmacokinetics across IH forms (e.g. inflorescence, leaves, hempseed products, SHB, silage) and using a consistent dietary inclusion level of IH should be investigated and would be crucial for establishing accurate WDI and feeding recommendations for regulatory agencies and producers.
Human exposure estimates were generated for 9-THC and CBD using assumptions that visualized the “worst-case scenario” (overestimate exposure). Using data from the present study, newborn males and females exceeded the 1 µg/kg ARfD for 9-THC; no group reached the 150 µg/kg/d ADI for CBD. In the report by Smith et al., no group evaluated reached the ARfD for 9-THC5. As in previous studies evaluating tissues and milk, we did not consider THCA or CBDA, the acidic precursors of 9-THC and CBD, due to regulatory agencies’ focus on 9-THC and CBD5,7. Although other studies have evaluated various formulations of IH, inflorescence and leaves contain the highest levels of cannabinoids2. It is possible that feeding IH inflorescence or unextracted whole-plant material at a higher level than the current study could increase tissue concentrations and prolong WDI. Based on our calculations, adipose concentrations of 9-THC and CBD could be as high as 43 ng/g and 6,481 ng/g, respectively, and not exceed the published limits for those cannabinoids in any demographic. In the present study, adipose 9-THC concentrations would need to be several fold higher to reach the most conservative published ARfD of 1 µg/kg 9-THC in most groups9. If using a less strict cutoff for ARfD, such as the 7 µg/kg threshold established in Switzerland and some other countries, adipose 9-THC concentrations would need to be 4-fold higher than in the current study for the highest-exposure group (newborn males)21. Adipose CBD concentrations would need to be approximately 26-fold higher than the present study to reach the 150 µg/kg/d ADI10. This hypothetical situation seems doubtful even at higher dietary IH inclusion levels. Irawan et al. reported CBD concentrations in adipose at the end of a 28-day withdrawal which would not result in intake above the ADI using our calculations, even if using the high end of the CBD estimate (1,456 ng/g). However, 9-THC levels in adipose at the end of the withdrawal period in that study (92 ± 107 ng/g) could still exceed the 1 µg/kg ARfD for some populations (up to 461% ARfD, using 199 ng/g 9-THC) and would represent 66% of a 7 µg/kg ARfD for newborn males (the highest exposure group). Visually estimating 9-THC concentration at the end of the intervention period (no withdrawal) in Irawan’s study to be 500 ng/g, this concentration would result in 165% and 150% of a 7 µg/kg ARfD for newborn males and females, respectively but would not result in exposures above 7 µg/kg in any other demographic. It should be noted that the assumptions made for the estimates in this study result do not account for different fat intake sources. For example, newborns would not be consuming any animal fat, but would rather be consuming fat from breastmilk. However, the assumptions made in the estimates were selected to give the most conservative measure of possible exposure.
Hempseed cake, SHB, or silage with low levels of cannabinoids appear to be safe feed sources for cattle5,7,12,22. Considering the discussion above, it is plausible that a practical withdrawal period is attainable for cattle fed SHB or low levels of unextracted plant material; however, tolerance limits are necessary for these future discussions. Comparison of plasma and tissue cannabinoid dispositions in cattle after exposure to various IH forms would be useful to explore potential bioavailability differences and would inform WDI recommendations. It is possible that dietary fiber, fatty acid, protein content, or dietary and rumen pH could impact cannabinoid absorption.
The tissue sampling schedule for this study, selected based on the published half-life of CBDA (14.1 h), presents some limitations3. While some depot formation was anticipated, the collection timepoints did not allow CBD levels in adipose to reach LOD. The WDI estimates are made assuming linear depletion of residues and the methods used in this study tend to overestimate WDI’s to ensure consumer safety. However, the authors believe there is likely a separate terminal elimination phase for CBD in adipose that was not reached during the sampling schedule in this study. Even if the terminal elimination rate was the same as the depletion rate we were able to model with the data from this study, extrapolating from 8 days after withdrawal to 154 days incorporates uncertainty into our estimates. The FDA recommends sampling to include at least one timepoint where tissue concentrations are detectable but below the tolerance23. Additional studies incorporating a longer sampling window are needed to precisely describe depletion times.
Establishment of federal tolerance limits for cannabinoids is required to generate and apply relevant WDI. The ARfD used in our calculations has been criticized for being excessively strict and not evidence-based compared to other global thresholds for 9-THC exposure21. An analysis by the German Federal Institute for Risk Assessment (BfR) found that some hemp-derived foods may result in exposures beyond 1 µg/kg24. However, in a review of the BfR analysis, authors from the nova-Institute concluded that the proposed ARfD was too stringent and that the safety factors applied to 9-THC were excessive and inconsistent compared with safety factors used for other food contaminants25. The authors cited multiple more recent studies regarding IH-derived foods that had not been considered by the BfR and which indicated the safety of IH products. (Do note that some of the authors of that review have ties to the European Industrial Hemp Association.) As discussed above, if using an ARfD greater than 1 µg/kg, it is possible that tissue concentrations from exposed cattle may be safe even with zero withdrawal. There are multiple other countries that have adopted higher ARfD’s for 9-THC that are worth consideration when establishing tolerance guidelines for cattle in the United States.
Conversely, there is concern that the 1 µg/kg ARfD may not be sufficient to protect vulnerable populations, particularly infants, toddlers, and adolescents. The EFSA assessment did not look at neurodevelopmental toxicity, so it is possible that the current ARfD would not account for those effects9. In fact, it was due to the lack of toxicity data in infants and children that the EFSA applied a higher safety factor to their calculations when deriving ARfD. Cannabinoid exposure in the perinatal (pre- and postnatal) and adolescent periods in rodent models and humans has been shown to result in behavioral and neurologic changes26. Exposure in this period of brain development alters neural plasticity and synaptic function which predispose these individuals to later psychiatric and abuse disorders and decreased cognitive ability. While young populations are particularly at risk, there are long-term cognitive and behavioral changes in adults and potential functional and structural changes with chronic exposure27. In addition, early cannabinoid exposure has been shown to disrupt metabolism and reproductive performance in rodents28. Variable doses in rodent models and exposures from human studies make it challenging to identify a precise dose at which these toxicities occur. Due to the unapproved status of IH or any cannabinoid product in cattle, any detectable level of cannabinoid in feed or tissue is considered an adulterant29,30,31. We anticipate that our data will be useful in re-evaluating the risk associated with IH products in livestock feed. Based on the results presented in this study and data from previous studies in cattle and sheep, regulation of 9-THC and CBD content and total dietary percentage will be needed to ensure inclusion in livestock feed is safe for human consumers5,11,12,32.
In summary, a variety of cannabinoids, including the psychoactive cannabinoid 9-THC and the bioactive cannabinoid CBD, were detected in tissues of cattle administered IH inflorescence. Tissue residue depletion models generated WDI estimates up to 154 d. Human exposure calculations revealed one consumer group (newborns) exceeded the most conservative international toxicity threshold for 9-THC. Further work is needed to determine the toxicity level of cannabinoids in sensitive human populations (i.e. infants, children, and adolescents). Limitations of the study include the short sampling schedule and the single IH dosing regimen evaluated. Future regulatory discussions should focus on defining acceptable risk of exposure to 9-THC and CBD to enable consistent WDI recommendations for exposed cattle.
Methods
Study design
Experimental procedures were approved by the Institutional Animal Care and Use Committee at Kansas State University (IACUC #4326). All study activities were conducted in conformity to requirements from the United States Department of Agriculture, the State of Kansas, and Association for Assessment and Accreditation of Laboratory Animal Care according to The Guide for the Care and Use of Agricultural Animals in Research and Teaching.33 Industrial hemp was grown and handled in keeping with licensing requirements under the Kansas Department of Agriculture Industrial Hemp Research Program (license numbers: KDA-0621466839 and KDA-0302873296). The cultivar used was Otto II Stout, which was grown and harvested by K-State faculty at the John C. Pair Horticultural Center near Wichita, KS during the fall of 2019.
This was a residue depletion study with all animals receiving the same treatment, so no random allocation was required. Sample size was chosen based on FDA guidance recommendations of 4 animals per slaughter timepoint34. The experimental unit was the individual animal. The study was completed in accordance with ARRIVE guidelines.
Subjects and housing
Twenty male Holstein cattle (n = 16 bulls, n = 4 steers), approximately 5–7 mo and weighing (± SD) 294 ± 28 kg were enrolled in the study. Animals were sourced from another investigator at Kansas State University (IACUC #4225); the animals were initially procured from a commercial calf raising operation in South Dakota. Animals were enrolled if they were considered healthy on examination by a veterinarian. Animals had been previously acclimated to the research facility and were group-housed in outdoor dirt pens with access to shelter throughout the study period. Animals were fed a custom grain mix twice daily and had ad libitum access to grass hay and water via an automated watering device during the entire study. None of the animals had received IH previously. The pen area and shelter space supplied per calf exceeded the guidelines established in the Guide for the Care and Use of Agricultural Animals in Research and Teaching.
Industrial hemp dosing and cattle sampling
Prior to study initiation, the CBDA content as a percent of total IH weight was determined using ultra-performance liquid chromatography triple quadrupole mass spectrometry (UPLC-MS/MS) and was used to calculate IH doses on an as-fed basis. (A separate full analysis of the cannabinoid content of the IH cultivar used was performed prior to animal dosing at the Kansas State University Olathe Campus Postharvest Physiology Lab; DEA Registration Number RK0682256.) Cattle received IH at a target dose of 5 mg/kg cannabidiolic acid (CBDA) by oral bolus every 24 h for 14 d in order to achieve steady state. Hemp flower material was finely chopped using a food chopper. Ground hemp material was placed in gelatin capsules and the weight of the capsules (with empty capsule weight tared) was recorded. Each capsule contained approximately 4–8 g of ground IH, depending on animal weight, and each animal received 2 capsules per day. Supplementary Table S9 includes dosing information for each animal. Animals were monitored after IH administration to ensure capsules were consumed (not spit out).
One group of 4 animals was euthanized on each of days 15, 16, 17, 19, and 22 (1, 2, 3, 5, and 8 days after last IH administration). Euthanasia was performed in accordance with AVMA guidelines for euthanasia via penetrating captive bolt and exsanguination. Per humane slaughter guidelines for exsanguination, an incision was made in the ventral neck which included the jugular veins and carotid arteries. Liver, kidney, muscle, and adipose tissues were collected from all euthanized animals. Muscle was collected from the semimembranosus and adipose was collected from the perirenal fat pad.
Tissue cannabinoid analysis
All solvents and chemicals were LC-MS grade and purchased from Fisher Scientific (Hampton, NH). Ultrapure 18Ω water was obtained in-house with a Millipore Synergy UV-R system (Burlington, MA). Cannabinoids standards purchased in individual solutions in methanol or acetonitrile from Cerilliant Corporation (Round Rock, TX) and Cayman Chemicals (Ann Harbor, MI). Cannabinoids included in the panel were: (+)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol glucuronide (THC-acid-glu), (−)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-acid), cannabicyclolic acid (CBLA), (−)−7-nor-7-carboxy cannabidiol (CBD-7-acid), (±)−11-hydroxy-Δ9-tetrahydrocannabinol (THC-11-OH), cannabidivarinic acid (CBDVA), cannabidivarin (CBDV), cannabidiol (CBD), cannabidiolic acid (CBDA), Δ9-tetrahydrocannabinolic acid A (THCA), cannabigerolic acid (CBGA), cannabigerol (CBG), Δ9-tetrahydrocannabinol (9-THC), Δ8-tetrahydrocannabinol (8-THC), cannabichromene (CBC), Δ9-tetrahydrocannabivarin (THCV), cannabichromenic acid (CBCA), cannabinol (CBN), and cannabicyclol (CBL). Δ9-tetrahydrocannabinol glucuronide was purchase from ElSohly Laboratories, Inc (Oxford, MS). The deuterated analogs used as internal standards were: (±)−11-nor-9-carboxy-Δ9-tetrahydrocannabinol-d9 (THC-acid-d9) (±)−11-hydroxy-Δ9-tetrahydrocannabinol-d3 (THC-11-OH-d3), cannabidiolic acid-d3 (CBDA-d3) and Δ9-tetrahydrocannabinolic acid A-d3 (THCA-d3), cannabidiol-d3 (CBD-d3), Δ9-tetrahydrocannabinol-d3 (9-THC-d3), and cannabichromene-d9 (CBC-d9). Negative (control) tissues were sources from the Kansas State University Veterinary Diagnostic Laboratory from regular submissions that had not received IH. The tissues were analyzed prior to spiking to ensure no cannabinoids were detected.
Briefly, tissue samples were spiked with a mixture of internal standards and homogenized with a single-probe homogenizer in a mixture of acetonitrile and formic acid 5% in a ratio of tissue to extraction volume of 1:10 (w/v). All extracts were subsequently cleaned-up by dispersive solid phase extraction (dSPE) using Bond-Elut EMR (Agilent Technologies, Santa Clara, CA) followed by QuEChERS with Bond Elut EMR-Lipid Polish (Agilent Technologies, Santa Clara, CA). Calibration standards for muscle, kidney and liver were prepared by spiking negative control tissues to obtain a final concentration of cannabinoids of 1.25, 2.5, 6.25, 12.5, 25, 62.5, 125 ng/g. Quality controls were prepared by spiking negative control tissues with 2, 10 and 100 ng/g of cannabinoids. Cleaned tissue extracts (2.5 mL) were evaporated under vacuum at 40 °C. Muscle and kidney residues were reconstituted with 0.25 mL of a mixture of acetonitrile-water (1:1) containing 0.1% formic acid, centrifuged, and transferred to an autosampler vial for LC-MS analysis.
To the liver residues, 2mL of water was added before further clean-up by solid-phase extraction (SPE) using Oasis HLB PRIME SPE cartridges, 6 cc, 200 mg (Waters Co., Milford MA). After loading the extract, the cartridge was washed twice with 2 mL of aqueous methanol 25%, let dry for 1 min and cannabinoids were eluted with 2 × 2 mL of acetonitrile-methanol (90:10). The solvent was evaporated with a vacuum concentrator at 40 °C and the residue reconstituted with 0.2 mL of acetonitrile-water (1:1) containing 0.1% formic acid, centrifuged and transferred to an autosampler vial for LC-MS analysis.
For fat tissue samples, matrix-matched calibration standards were prepared by adding 10 µL of cannabinoids stock solutions in acetonitrile (10, 25, 50, 100, 250, 500, 1000 ng/mL) to 80 µL of the negative control fat extract and 10 µL of internal standard solution. Quality controls were prepared at 56.25 ng/g, 112.5 ng/g, 225 ng/g, and 450 ng/g.
If the concentration of a sample was beyond the ULOQ, the extract and highest QC were diluted further with mobile phase; dilution of the QC was performed to ensure accuracy following dilution.
Cannabinoids analysis was performed using an Acquity H UPLC and a TQ-S triple quadrupole mass spectrometer (Waters Corp., Milford, MA). The chromatographic separation was performed with a UPLC column Eclipse Plus C18 100 × 2.1 mm, 1.8 µ (Agilent Technologies, Santa Clara, CA) heated at 55 °C. The flow rate was set at 0.5 mL/min. The mobile phase consisted of a gradient of water containing 0.1% formic acid (A) and acetonitrile (B) as follows: 0 min: 60% B, 6.50 min: 86% B, 7.50–9 min: 100% B, 9.01–12 min: 60% B. The total run time was 12 min. The injection volume was 5 µL. The capillary voltage was 3.0 KV, the source temperature 150 °C, the desolvation temperature 500 °C, the desolvation nitrogen flow at 1000 L/h, and the cone nitrogen flow at 150 L/h. The data acquisition was performed by electrospray ionization (ESI) in positive and negative mode using multiple reaction monitoring (MRM). Data processing was performed using linear regression with a weighing factor of 1/X and was accepted if the coefficient of determination R2 was > 0.99. The complete cannabinoid concentration dataset is available in Supplementary Tables S1-S8. Limit of detection, lower limit of quantification, and upper limit of quantification are summarized in Table 4. The LLOQ was determined by repeated extractions of tissues spiked at low concentrations and was adequate when the deviation from the calculated concentration was < 20% and the relative standard deviation was < 15%. Quality controls were used to calculate the precision and the accuracy of the method for each tissue (Supplementary Tables S10-S13). A full description of MRM parameters is available in Supplementary Table S14. Method validation following FDA guidelines has been performed for adipose tissue and is being prepared for publication.
Tissue withdrawal period estimation
Withdrawal estimates were made using the US FDA WithdrawalApp in R Studio. At least two data values at three or more timepoints were required to estimate a withdrawal interval; thus, a withdrawal interval could not be estimated for all cannabinoids detected in each tissue. Not all data points were included for each cannabinoid WDI model in each tissue, in order to satisfy linearity and homoscedasticity of the model. A summary of tissue cannabinoid concentrations and the data points used for each cannabinoid WDI model are available in Supplementary Reports R1-R24. Per GFI guidelines, A 99th percentile upper tolerance limit and a 95% confidence interval were used23. A tolerance of 10 ng/g was used as this was the LLOQ for 9-THC and CBD in adipose, which are the target compounds and tissue of interest. As there is no legal tolerance level for these compounds, the definition of tolerance is simply the lowest reliably measurable concentration, which is the LLOQ. Withdrawal intervals were rounded up to the next full day. The tissue-specific WDI and the overall WDI were determined based on the slowest-depleting cannabinoid for individual tissues and across all tissues, respectively. Total CBD was calculated by adding the CBD concentration and (0.877*CBDA) for that sample. Total THC was calculated by adding the 9-THC concentration and (0.877*THCA) for that sample. Total cannabinoid levels were calculated by adding all cannabinoids detected in that sample. For individual cannabinoids, all values above LOD were included in the data file loaded into the WithdrawalApp. For calculated values (total CBD, total THC, and total cannabinoids), all values were included.
For liver tissue, withdrawal periods were estimated for CBD, CBDA, total CBD, CBD-7-acid, THCA, total THC, THC-glu, and total cannabinoids. For kidney tissue, withdrawal periods were estimated for CBD, total CBD, THCA, total THC, and total cannabinoids. For muscle tissue, withdrawal periods were estimated for CBDA, total CBD, CBD-7-acid, THC-acid, and total cannabinoids. For adipose tissue, withdrawal periods were estimated for CBD, total CBD, CBD-7-acid, 9-THC, total THC, and total cannabinoids.
Human exposure estimates
Estimates of human exposure to 9-THC and CBD were calculated similarly to a previous report on cannabinoid residues in cattle following administration of hempseed cake5. Exposure estimates were separated by age group and sex, using age groups and fat intake data from the Environmental Protection Agency (EPA) Exposure Factor Handbook35. This table included fat intake data from the top decile of animal fat consumers; data from the 95th percentile of consumers within that decile were used, assuming that 100% of fat intake was from adipose tissue from cattle administered oral IH inflorescence. The EPA total fat intake data are generated from all reported sources of fat, including meat, dairy (human and animal), and vegetable sources. Certain age groups (i.e. newborns and young infants) consume little to no beef fat. However, this assumption was still made in order to generate the most conservative exposure estimates. For ages 71–81 y and 81 + y, there were not separate data for male and female consumers. Bodyweight (BW) data required for calculation of cannabinoid intake and exposure limits were extracted from the Centers of Disease Control (CDC) anthropometric data from 2015 to 2018 (for ages 3 y and older) and from the 2000 growth chart publication (for newborn through 2 y).36,37 When the age ranges from the EPA and CDC publications did not match, the mean BW from the youngest age for the corresponding fat intake group was used (e.g. for fat intake for children aged 2–3 y, the mean 2 y BW was used). For ages 21 y and older, the age ranges of the EPA and CDC publications did not match; the mean BW for the group that was most similar to the fat intake group was used (e.g. for 31–41 y fat intake, mean 30–39 y BW was used). The 1 µg/kg acute reference dose (ARfD) for 9-THC published by the European Food Safety Authority (EFSA) and the 0.15 mg/kg/d (150 µg/kg/d) acceptable daily intake (ADI) for CBD published by the Food Standards Agency were used to calculate allowable exposure limits:9,10
The ARfD represents the threshold of acute toxicity; the value published by the EFSA is the lowest global threshold for 9-THC exposure5,21. Intake of 9-THC or CBD through ingestion of adipose tissue from cattle exposed to IH inflorescence was calculated by the following equation,
where the concentration of 9-THC or CBD represents the 99% CI for concentration on the sampling day with the highest mean concentration (day 2 for both 9-THC and CBD, with concentrations of 74.7 and 371 ng/g, respectively). Finally, the percentages of the allowable exposure limit for 9-THC and CBD represented by the intake values were calculated by:
These estimate procedures for fat, 9-THC, and CBD intake, BW, 9-THC ARfD, and CBD ADI enabled generation of conservative estimates of 9-THC and CBD exposure. While Smith et al. calculated values for the 10th, 50th, and 90th percentiles of fat intake, only the 95th percentile of the top decile of animal fat consumers was used in the present study to demonstrate the scenario with the highest consumer risk5.
Data availability
All data used in preparation of this manuscript are included in the main or supplementary data files.
References
Johnson, R. Comparing Hemp Provisions in the 2014 and 2018 Farm Bills, < (2021). https://www.congress.gov/crs-product/IF11984
Kleinhenz, M. D. et al. Nutrient concentrations, digestibility, and cannabinoid concentrations of industrial hemp plant components. Appl. Anim. Sci. 36, 489–494. https://doi.org/10.15232/aas.2020-02018 (2020).
Kleinhenz, M. D. et al. Plasma concentrations of eleven cannabinoids in cattle following oral administration of industrial hemp (Cannabis sativa). Sci. Rep. 10 https://doi.org/10.1038/s41598-020-69768-4 (2020).
Kleinhenz, M. D. et al. Short term feeding of industrial hemp with a high Cannabidiolic acid (CBDA) content increases lying behavior and reduces biomarkers of stress and inflammation in Holstein steers. Sci. Rep. 12, 3683. https://doi.org/10.1038/s41598-022-07795-z (2022).
Smith, D. J. et al. Excretion and residue depletion of cannabinoids in beef cattle fed hempseed cake for 111 days. Food Addit. Contam. Part. Chem. Anal. Control Expo Risk Assess. 40, 552–565. https://doi.org/10.1080/19440049.2023.2187645 (2023).
Addo, F., Ominski, K., Yang, C. & Plaizier, J. C. Quality and safety of hemp meal as a protein supplement for nonlactating dairy cows. J. Dairy. Sci. 106, 7602–7612. https://doi.org/10.3168/jds.2023-23222 (2023).
Wagner, B. et al. Transfer of cannabinoids into the milk of dairy cows fed with industrial hemp could lead to ∆9-THC exposure that exceeds acute reference dose. Nat. Food. 3, 921–932. https://doi.org/10.1038/s43016-022-00623-7 (2022).
Wang, Y. et al. Replacing alfalfa hay with industrial hemp ethanol extraction byproduct and Chinese Wildrye hay: effects on lactation performance, plasma metabolites, and bacterial communities in Holstein cows. Front. Vet. Sci. 10, 1061219. https://doi.org/10.3389/fvets.2023.1061219 (2023).
EFSA Scientific opinion on the risks for human health related to the presence of tetrahydrocannabinol (THC) in milk and other food of animal origin. Eur. Food Saf. Auth. J. 13 (6), 4141 (2015).
Choudhry, A. & Haynes, B. Safety Assessment: Cannabidiol (CBD) Isolate as a Novel Food for Use in a Range of Food Categories Including Food Supplements (Food Standards Agency, 2024).
Fruge, G. F. et al. Cannabinoid transference and retention in tissues of beef steers fed hemp leaves. Food Addit. Contam. Part. Chem. Anal. Control Expo Risk Assess. 42, 703–715. https://doi.org/10.1080/19440049.2025.2506106 (2025).
Irawan, A. et al. Cannabinoid distribution and clearance in feeding spent hemp biomass to dairy cows and the potential exposure to ∆9-THC by consuming milk. J. Agric. Food Chem. 73, 13934–13948. https://doi.org/10.1021/acs.jafc.5c02827 (2025).
Jiang, R., Yamaori, S., Takeda, S., Yamamoto, I. & Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of Cannabidiol by human liver microsomes. Life Sci. 89, 165–170. https://doi.org/10.1016/j.lfs.2011.05.018 (2011).
Gaston, T. E. & Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 70, 313–318. https://doi.org/10.1016/j.yebeh.2016.11.016 (2017).
Harvey, D. & Brown, N. Comparative in vitro metabolism of the cannabinoids. Pharmacol. Biochem. Behav. 40, 533–540. https://doi.org/10.1016/0091-3057(91)90359-A (1991).
Yamamoto, I., Watanabe, K., Narimatsu, S. & Yoshimura, H. Recent advances in the metabolism of cannabinoids. Int. J. Biochem. Cell. Biol. 27, 741–746. https://doi.org/10.1016/1357-2725(95)00043-o (1995).
Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 42, 327–360. https://doi.org/10.2165/00003088-200342040-00003 (2003).
Johansson, E., Norén, K., Sjövall, J. & Halldin, M. M. Determination of delta 1-tetrahydrocannabinol in human fat biopsies from Marihuana users by gas chromatography-mass spectrometry. Biomed. Chromatogr. 3, 35–38. https://doi.org/10.1002/bmc.1130030109 (1989).
Gunasekaran, N. et al. Reintoxication: the release of fat-stored ∆9-tetrahydrocannabinol (THC) into blood is enhanced by food deprivation or ACTH exposure. Br. J. Pharmacol. 158, 1330–1337. https://doi.org/10.1111/j.1476-5381.2009.00399.x (2009).
Klevenhusen, F., These, A., Weiß, K., Gusovius, H. J. & Pieper, R. Ensiling conditions and changes of cannabinoid concentration in industrial hemp. Arch. Anim. Nutr. 78, 242–253. https://doi.org/10.1080/1745039X.2024.2383216 (2024).
Beitzke, B. & Pate, D. W. A broader view on deriving areference dose for THC traces in foods. Crit. Rev. Toxicol. 51, 695–722. https://doi.org/10.1080/10408444.2021.2008867 (2021).
Irawan, A. et al. Feeding spent hemp biomass to lactating dairy cows: effects on performance, milk components and quality, blood parameters, and nitrogen metabolism. J. Dairy. Sci. 107, 258–277. https://doi.org/10.3168/jds.2023-23829 (2024).
FDA CVM. Guidance for Industry #3: General Principles for Evaluating the Human Food Safety of New Animal Drugs Used in Food-Producing Animals (Food and Drug Administration Center for Veterinary Medicine, 2022).
BfR. Tetrahydrocannabinol Levels Are Too High in Many hemp-containing foods - health Impairments Are Possible: BfR Opinion No 034/2018 (German Federal Institute for Risk Assessment Bundesinstitut für Risikobewertung, (2018).
Skoczinski, P., Carus, M., Grotenhermen, F., Beitzke, B. & Kruse, D. Evaluation of Limit and Guideline Values of THC (tetrahydrocannabinol) in in Hemp Foods (nova-Institut GmbH, 2019).
Hurd, Y. L. et al. Cannabis and the developing brain: insights into its Long-Lasting effects. J. Neurosci. 39, 8250–8258. https://doi.org/10.1523/jneurosci.1165-19.2019 (2019).
NIDA. Cannabis (Marijuana), (2024). https://nida.nih.gov/research-topics/cannabis-marijuana
Martínez-Peña, A. A. et al. The impact of early life exposure to cannabis: the role of the endocannabinoid system. Int. J. Mol. Sci. 22, 8576. https://doi.org/10.3390/ijms22168576 (2021).
FDA. Section 348(a) - Food additives in United States Code, 2006 Edition, Supplement 5, Title 21 - FOOD AND DRUGS (Food and Drug Administration, 2011).
FDA. Section 342(a)(2)(C) - Adulterated food in United States Code,. Supplement 1, Title 21 - FOOD AND DRUGS. (Food and Drug Administration, 2019). (2018).
AAFCO. Hemp as a Feed Ingredient; A National Discussion, (2022). https://www.aafco.org/news/hemp-as-a-feed-ingredient-a-national-discussion/
Irawan, A. et al. Cannabinoid residuals in tissues of lambs fed spent hemp biomass and consumer’s exposure assessment. Food Chem. Toxicol. 191 https://doi.org/10.1016/j.fct.2024.114848 (2024).
PSA. Guide for the Care and Use of Agricultural Animals in Research and Teaching 4th edn (ADSA, ASAS, 2020).
FDA CVM. Guidance for industry #207 (VICH GL48(R)): studies to evaluate the metabolism and residue kinetics of veterinary drugs In Food-Producing Animals: Marker Residue Depletion Studies To Establish Product Withdrawal Periods. (Food and Drug Administration Center for Veterinary Medicine, (2015).
EPA. Update for Chap. 11 of the Exposure Factors Handbook: Intake of meats, Dairy products, and fats. EPA/600/R-17/485F Edn (Environmental Protection Agency, 2018).
Fryar, C. D., Carroll, M. D., Gu, Q., Afful, J. & Ogden, C. L. Anthropometric Reference Data for Children and Adults: United States, 2015–2018 Vol. 3 (National Center for Health Statistics - Vital and Health Statistics, 2021).
Kuczmarski, R. J. et al. 2000 CDC growth charts for the United States: Methods and development. Vol. 11 138 (National Center for Health Statistics - Vital and Health Statistics, (2002).
Funding
The live animal portion of this study was supported by the Agriculture and Food Research Initiative project award no. 2020-67030-31479, from the U.S. Department of Agriculture’s National Institute of Food and Agriculture. The method development and validation was partially funded by the U. S. Food and Drug Administration’s Veterinary Laboratory Investigation and Response Network (FDA Vet-LIRN) under grant 1U18FD006915-01. The views expressed in this manuscript are those of the authors and may not reflect the official policy of the Department of Health and Human Services, the U.S. Food and Drug Administration or the U.S. Government.
Author information
Authors and Affiliations
Contributions
BRF – data analysis, manuscript preparation; MDK – funding acquisition, conception, study design, sample collection; GM – LCMS method development and tissue analysis; JJG – funding acquisition, hemp cultivation and harvest; MMW, AKC, MSM, AAL, KEK, BTJ, SAF, SRM – sample collection; AT – LCMS method development and validation; JFC – funding acquisition, conception, study design. All authors participated in manuscript review and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
The authors have no conflicts of interest to disclose.
Additional info (supplementary information line (if any) and corresponding author line)
Raw cannabinoid concentration data, sorted by tissue, can be viewed in Supplementary Tables S1-8, additional dose calculation details in Supplementary Table S9, UPLC-MS quality control data and multiple reaction monitoring parameters in Supplementary Tables S10-13, and FDA WithdrawalApp reports can be viewed in the Supplementary Reports R1-R24.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fritz, B.R., Kleinhenz, M.D., Magnin, G. et al. Tissue residue depletion of cannabinoids in cattle administered industrial hemp inflorescence. Sci Rep 15, 42337 (2025). https://doi.org/10.1038/s41598-025-26448-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26448-5