Abstract
This study investigates Basil Seed Gum (BSG), a naturally derived biopolymer, for chemical enhanced oil recovery (CEOR) under high-temperature/high-salinity conditions. BSG, extracted from Ocimum basilicum L., was benchmarked against partially hydrolyzed polyacrylamide (HPAM), xanthan gum (XGU), and guar gum (GGU) via rheological, Fourier-transform infrared (FTIR), adsorption, and core flooding tests. Rheological results confirmed pronounced shear-thinning behavior, viscosity retention up to 100 °C, and tolerance to 100,000 ppm NaCl, with performance comparable to XGU and superior to HPAM and GGU. Adsorption experiments on sandstone indicated lower maximum adsorption for BSG (~ 0.80 mg/g) compared to HPAM (> 1.40 mg/g), especially at high salinity. Modeling showed the Redlich–Peterson isotherm provided the best fit (R² = 0.9958), indicating mixed adsorption mechanisms. In core flooding, seawater injection recovered 30.2% of OOIP, with water breakthrough at 0.50 PV. Subsequent BSG polymer flooding increased recovery to 58%, and chase brine raised final recovery to 72.1%, achieving an incremental oil recovery of 41.9% over seawater flooding alone. Findings suggest BSG offers a combination of thermal/salinity stability, low rock adsorption, and notable recovery gains, supporting its suitability as a natural polymer alternative in CEOR projects for challenging reservoirs.
Similar content being viewed by others
Introduction
In the evolving global energy landscape, marked by the steady rise of renewable, geothermal, and other low-carbon energy sources, the role of Enhanced Oil Recovery (EOR) has shifted from being portrayed solely as a necessity for meeting global energy demand, to being recognized as a competitive and complementary strategy for optimizing the performance of existing hydrocarbon assets. Within this broader context, sustainable EOR approaches that employ cost-effective and environmentally responsible materials can offer significant value by maximizing recovery from mature reservoirs while supporting environmental goals. Among various chemical EOR processes, polymer flooding is a promising technique as it is able to decrease the mobility ratio between injected water and the reservoir oil, which leads to high sweep efficiency and low residual oil saturation1,2.
Synthetic polymers, particularly partially hydrolyzed polyacrylamide (HPAM), are widely applied in EOR projects; however, their performance declines sharply under high-temperature (> 80 °C) and high-salinity (> 30,000 ppm TDS) conditions common in many carbonate and sandstone reservoirs3. At elevated temperatures, HPAM undergoes thermal degradation, resulting in precipitation and up to 80% irreversible viscosity loss at 100 °C due to charge screening in saline environments. In highly saline brines, divalent cations (Ca²⁺, Mg²⁺) further promote polymer chain contraction or precipitation, increasing hydrodynamic resistance within the porous medium. These effects, compounded by the viscosity loss, can lead to pore blockage, formation damage, and higher injection pressures, ultimately reducing polymer injectivity4,5. Laboratory studies have consistently reported severe thermal thinning and poor injectivity when formation water salinity exceeds 20,000 ppm NaCl6,7,8. Field-scale and simulation-based analyses have confirmed that while polymer flooding can achieve substantial incremental oil recovery, sustaining injectivity and viscosity under high-salinity/high-temperature conditions remains a significant operational challenge4.
In response to these limitations, natural biopolymers have gained considerable attention as environmentally friendly alternatives. Biopolymers such as xanthan gum (XGU)5 and guar gum (GGU)6 have demonstrated favorable rheological and viscoelastic properties7, good salt tolerance8, and biodegradability9, making them suitable candidates for EOR applications in moderate conditions10,11. Nevertheless, issues such as thermal instability, microbial contamination, and variability in molecular structure have limited their deployment in high-stress environments10. Recent simulation studies12,13, further reveal that increasing salinity levels can significantly reduce the ultimate oil recovery of biopolymer floods, highlighting the importance of salt-tolerant formulations for realistic field deployment. In Table 1, a list of the most important biopolymers used in enhanced oil recovery in recent years is mentioned, along with a review of the obtained results.
An alternative and promising approach in polymer science has focused on the design and use of stimuli-responsive polymers or especially temperature-viscosifying polymers (TVPs)22. They have the ability to reversibly change their physicochemical characteristics such as solubility, viscosity and hydrophilicity in response to variations of temperature23,24. Such behavior is often driven by coil-to-globule transitions, lower or upper critical solution temperatures (LCST or UCST) and phase separation. In addition, TVPs are particularly attractive in EOR (such as high temperatures EOR), where the temperature of reservoir can be even above 60–120 °C, according to the ability of being designed as the polymers which are maintaining the original apparent viscosity or increasing the viscosity under high temperatures, providing a better sweep efficiency, delayed breakthrough and better conformance control22,25. This concept has already shown promise in laboratory EOR trials where viscosity recovery at elevated temperatures boosts displacement efficiency under harsh conditions26.
In this context, basil seed gum (BSG), a natural biopolymer extracted from Ocimum basilicum L., has emerged as a highly promising material for enhanced oil recovery (EOR) operations. While traditionally employed in food and pharmaceutical formulations for its gel-forming and stabilizing abilities27, recent studies have highlighted its unique temperature-responsive rheological behavior. Specifically, BSG exhibits viscosity enhancement and network restructuring with increasing temperature28. These characteristics, which are also valuable in other fields such as controlled drug delivery29,30,31,32, position BSG as a prime candidate among biopolymers suitable for high-temperature EOR operations.
The present work aims to investigate the possibility of utilizing BSG as a green, temperature-responsive polymer for enhanced oil recovery. The research focuses on: (i) extraction and purification of BSG obtained from the modified hot-water method, (ii) defining the structural and thermal properties of BSG in comparison with HPAM, XGU, and GGU, (iii) estimating the rheological behavior of BSG under different temperature, salinity, concentration, and shear rate conditions, and (iv) determining the adsorption capacity of BSG onto sandstone rock surfaces through batch adsorption experiments and isotherm modeling. In addition, core-flooding experiments were carried out to assess the displacement efficiency of BSG under high-temperature/high-salinity reservoir conditions. This study systematically compares the performance of BSG with conventional polymers under representative reservoir scenarios, thereby enhancing the knowledge base on biopolymer use in chemical EOR and supporting the development of sustainable, responsive materials for subsurface energy applications.
Materials and methods
Materials
All chemicals used in this study were of analytical grade and used as received without further purification. Basil seeds (Ocimum basilicum L.), the primary source for the biopolymer investigated in this work, were procured from the local market in Ahwaz, Iran. Deionized water (resistivity: 18.2 MΩ.cm at 25 °C) from a Milli-Q system (Millipore, USA) was used in all experimental procedures.
Commercial-grade polymers used for performance comparison included partially hydrolyzed polyacrylamide (15 million Da), xanthan gum, and guar gum. Xanthan gum, guar gums and HPAM were purchased from Sigma-Aldrich. Extra pure sodium hydroxide pellets (NaOH, 98% purity) and Hydrochloric acid (HCl) were purchased from SRL Chemicals and were used to alter the pH of the systems.
The sandstone powder used as the adsorbent substrate was obtained from an outcrop of a hydrocarbon-bearing formation in southwestern Iran. after collecting the rock samples, they were transported to the laboratory, and thoroughly cleaned to remove impurities. The cleaned samples were then crushed and ground into a fine powder with a particle size distribution suitable for adsorption studies.
For the core flooding experiments, intact cylindrical sandstone cores were also prepared from the same formation. The cores were cleaned using Soxhlet extraction with toluene and methanol to remove residual hydrocarbons, then dried at 105 °C for 24 h. The crude oil used in these flooding tests was collected from one of the oilfields located in southwestern Iran, ensuring that the oil properties matched the geological and chemical characteristics of the studied formation (Table 2).
In addition to synthetic brines used for salinity control in rheological and adsorption studies, Synthetic formation water (FW) and seawater (SW) were prepared in the laboratory using deionized water and analytical-grade salts. Their chemical compositions are listed in Table 3.
Extraction of basil seed gum (BSG)
Extraction of basil seed gum (BSG) from Ocimum basilicum L. seeds followed a modified hot-water method adapted from previous studies33. Basil seeds were washed and soaked in deionized water (1:60 w/v). The pH was adjusted to 8.0 with 0.01 mol L⁻¹ NaOH, and the mixture was maintained at 68 ± 1 °C under constant stirring (1,000 rpm, 20 min) until complete swelling and mucilage release from the seed coat. The resulting dispersion was dehulled using a mechanical extractor (Pars-Khazar 700 P, Iran), filtered through a 20 μm screen (AMIAD, Australia), and centrifuged (2,300×g, 30 min, 20 °C; Himac CR22GII, Hitachi, Japan) to remove fine particulates. The purified BSG was freeze-dried and stored in sealed containers under cool, dry conditions, yielding a light-colored, water-soluble hydrocolloid with stable viscoelastic and thermal properties. Gravimetric yield analysis confirmed reproducible mucilage recovery above 10% w/w (dry seed basis), aligning with reported values for mild-condition extractions33, supporting both the simplicity and economic feasibility of the process.
Characterization
Characterization of sandstone samples
X-ray diffraction (XRD) analysis of the sandstone sample collected for adsorption experiments, was conducted for determination of mineralogical composition. The XRD patterns were recorded on a PANalytical X’Pert PRO MPD (Malvern PANalytical, Almelo, Netherlands) with Cu-Kα radiation (λ = 1.54 Å) in the 2θ range of 10° to 90°. To make sure of suitability of the substrate for adsorption studies, the obtained data were analyzed to identify the major mineral phases present in the sandstone.
Thermal stability analysis (TGA)
Thermal stability of the four polymer samples was investigated by Thermogravimetric Analysis (TGA). Measurements were carried out on a TA Instruments TGA Q500 (TA Instruments, New Castle, DE, USA) under nitrogen. About 5 mg of each polymer sample was heated between 25 °C and 600 °C with a heating rate of 10 °C/min. The weight loss as a function of temperature was recorded to determine the thermal degradation behavior.
Fourier transform infrared spectroscopy (FTIR)
The chemical composition and functional groups of the polymers were investigated by FTIR measurements. The spectra were measured in the range of 500–4000 cm⁻¹ with a resolution of 4 cm⁻¹ using a Shimadzu IRPrestige-21 (Shimadzu Corporation, Kyoto, Japan). The polymer samples were prepared as KBr pellets for analysis.
Rheological measurements
Rheological characterization is a fundamental step in evaluating polymer solutions for enhanced oil recovery (EOR), since viscosity directly governs mobility control, displacement efficiency, and injectivity under reservoir conditions. Numerous studies show that polymer rheology is influenced by both intrinsic molecular characteristics (e.g., molecular weight, chain flexibility) and extrinsic factors (concentration, salinity, temperature, shear rate)34,35. Previous works have demonstrated that increasing salinity generally compresses polymer coils, especially in the presence of divalent cations, while temperature variations and shear forces can significantly alter viscosity36,37. Additionally, xanthan gum, guar gum, HPAM, and other biopolymers display across wide shear-rate ranges, often well described by power-law models38,39.
In this study, the rheological properties of all evaluated polymer solutions, including BSG, HPAM, XGU, and GGU, were determined using an Anton Paar MCR 302 rotational rheometer (Anton Paar GmbH, Graz, Austria) with suitable geometries. Measurements were systematically conducted to assess the effects of polymer concentration, salinity, temperature, and shear rate on viscosity under reservoir-relevant conditions.
Although many previous studies have reported that polymer concentrations above 0.5 wt% are economically unattractive for chemical enhanced oil recovery40, higher concentrations were included here exclusively to (i) complete the rheological and adsorption characterization, (ii) identify viscosity thresholds and non-linear trends across the concentration range, and (iii) ensure compatibility and validation of the present dataset with results reported in previous literature. These higher-concentration data are intended for scientific completeness rather than field-scale design recommendations.
Effect of concentration on viscosity
The viscosity of the polymer solutions at different concentrations (0–1.2% w/w) was determined using a fixed temperature of 25 °C and shear rate of 10 s⁻¹. This action was performed in an effort to investigate the effect of polymer concentration on the rheological behavior.
Effect of salinity on viscosity
In this step, temperature was set at 25 °C, shear rate to 10 s⁻¹ and concentration to 1% w/w, while the viscosity was measured at different salinities (1–100000 ppm NaCl) with the aim of determining the effect of ionic strength on viscosity.
Temperature dependence of viscosity
The viscosity was obtained within a range of temperature between 25 °C and 100 °C at a constant concentration (1% w/w) and shear rate (10 s⁻¹) in the absence of any salinity and the temperature maintained constant during the measurements in a thermostated water bath.
Shear rate dependence of viscosity
The temperature was kept constant at 25 °C, the concentration was fixed at 1% w/w and the viscosity was determined as a function of the shear rate (in the range 0.001–100 s⁻¹) with distilled water. The upper limit of 100 s⁻¹ was selected in accordance with realistic flow conditions in porous media, as shear rates above this value are generally not encountered even under turbulent flow; this adjustment aligns the rheological test conditions with reservoir-relevant scenarios. This approach was carried out to explore the shear-thinning or sheer-thickening properties of polymer solutions.
making sure about reproducibility of the results, each measurement was repeated three times and the average values were reported. On the basis of the obtained data rheological characteristics of the polymers under various conditions were studied.
The rheological characterization of the BSG polymer solutions was carried out in two steps. First, the experimental shear stress versus shear rate curves were fitted to three fundamental rheological models, Power-law, Bingham Plastic, and Herschel–Bulkley, in order to determine whether the polymer solutions exhibited Newtonian behavior, shear-thinning (pseudoplastic), shear-thickening (dilatant), or plastic flow with a yield stress. The governing equations, fitting parameters, and a concise assessment of the advantages and limitations of these models are summarized in Table 4.
In the second step, viscosity responses were modeled as a function of shear rate, polymer concentration, and temperature using three complementary models, the Power-law model, Ostwald–De Waele model, and Arrhenius model, to capture the combined effects of hydrodynamic conditions and molecular interactions on the apparent viscosity. A detailed description of each model’s governing equation, fitting parameters, applicable range, and limitations is presented in Table 5.
Adsorption measurements
The adsorption of polymers onto the solid phase was studied based on a batch adsorption method, with investigations focusing on the adsorption capacity as a function of polymer concentration and salinity. As mentioned earlier sandstone powder was selected as the solid adsorbent. Before the tests, sandstone sample was pre-treated to remove contaminants by washing with distilled water and acetone, and were subsequently dried at 105 °C for 24 h. The monomer solutions for the study were prepared in synthetic brine at different concentrations, ranging from 200 to 8000 ppm, and also in brines with varying salinity levels, using NaCl solutions of 0, 7000, 20,000, and 50,000 ppm.
Adsorption behavior of polymers on sandstone surfaces was studied through batch adsorption tests. The process was initiated by the addition of a known amount (1 g) of the sandstone sample to 25 mL of the polymer solution inside a closed glass vial in order to prevent evaporation or contamination. The filled vials were put into a shaker incubator (IKA KS 4000i, IKA working, Inc., Wilmington, NC, USA) and shaken at 200 rpm for 24 h at 25 °C, which was found to be enough time according to preliminary experiments to reach adsorption equilibrium, i.e., at that point the polymer–adsorption takes place at a certain rate and the rate of desorption.
Once reaching equilibrium, the solid and liquid phases were decanted for further analysis. The suspension was then centrifuged at 4000 rpm for 15 min with an Eppendorf Centrifuge 5810R (Eppendorf AG, Hamburg, Germany). The sandstone particles were precipitated by centrifugation; thus, the adsorbent was separated from the polymer solution (supernatant). The supernatant was carefully removed without disrupting the solid phase and saved for polymer concentration determination.
The concentration of the polymer in the supernatant was determined using UV-Vis spectroscopy (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan). A calibration curve was prepared using standard polymer solutions to quantify the concentration.
Polymer adsorption on the sandstone substrate was quantified using the following mathematical expression:
In this equation q represents the adsorption capacity (mg/g), Co and Ce denote the initial and equilibrium polymer concentrations (mg/L), respectively, V is the volume of the solution (L) and m refers to the mass of the adsorbent (g).
The adsorption data of BSG solution were analyzed using various isotherm models to understand the adsorption mechanism. A summary of the models used, their equations, parameters, assumptions, and advantages/disadvantages are provided in Table 6.
The adsorption data were analyzed to determine the effect of polymer concentration, salinity, and temperature on the adsorption capacity. The results were fitted to adsorption isotherm models such as Langmuir and Freundlich to understand the adsorption mechanism. Each experiment was conducted in triplicate to ensure reproducibility, and the average values were reported.
Core flood test
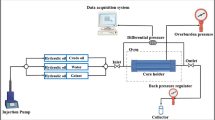
Core flooding tests aimed at evaluating the displacement efficiency of basil seed gum (BSG) were executed on sandstone core plugs in conjunction with the formation water, synthetic seawater, and crude oil specified in Sects. 3 − 1. Initially, the samples were solvent-cleaned in a Soxhlet apparatus using toluene, then oven-dried for 48 h at 40 °C. Afterwards, each plug was installed in a high-pressure core holder and subjected to a confining pressure of 4000 psi. The cores were evacuated for 12 h prior to being saturated with synthetic formation brine, achieved by injecting approximately 10 pore volumes. Crude oil injection followed at 0.2 cc/min until water production ceased, establishing the irreducible water saturation; fluid volumes from this stage allowed estimation of the initial oil content.
To restore reservoir-like wettability, the cores were aged while fully immersed in crude oil for seven days. Secondary recovery was represented by injecting seawater at 0.1 cc/min and 80 °C, terminating when no further oil was displaced. Residual oil saturation after water flooding was determined from the measured volumes. The final phase comprised injection of a BSG solution at its optimum concentration and chase brine, under the same flow rate, while monitoring water cut and recovery factor.
Results and discussion
haracterization of materials
Mineralogical composition of sandstone (XRD analysis)
As shown in Fig. 1, The X-ray diffraction (XRD) pattern of the sandstone sample exhibits a high degree of crystallinity with sharp, observably peaked diffraction peaks extended throughout the 2θ range of 10°–90°. The highest peak is the one at about 26.6°, which corresponds with quartz (SiO₂), being the primary mineral phase of most sandstones52,53. Other major peaks seen at 20.8°, 36.5°, 50.1° and 60.0° are related to other quartz faces, verifying the polycrystalline nature of the same. Minor peaks identified within the 30°–40° and 60°–70° ranges indicate the possible existence of accessory minerals like feldspar and mica, typical of detrital sedimentary rocks.
The high intensity and narrow peaks in the diffraction patterns depict the crystallinity of the framework with negligible amorphous content. The results underscore the mineralogical maturity and diagenetic stability of this sandstone sample, which in turn indicates a major input from quartz-rich source rocks and a long geological history of sedimentary reworking. The main diffraction peaks, their corresponding 2θ angles, relative intensities, and associated mineral phases are summarized in Table 7.
Chemical structure of polymers (FTIR analysis)
Figure 2 exhibits the FTIR spectra of basil seeds gum, xanthan gum, guar gum, along with HPAM, illustrating their characteristic functional groups. All spectra show wide absorption bands at 3200–3600 cm⁻¹ due to O–H stretching vibrations, which are characteristic of hydroxyl groups in polysaccharides.
In the BSG spectrum (Fig. 2.a), a main peak centred around 2930 cm⁻¹ due to C–H stretching, the intense peaks located at 1650 and 1400 cm⁻¹, and the stretching bands of carboxylate groups (COO⁻) are observed, indicating that uronic acids were present27,54. The GGU spectrum (Fig. 2.b) exhibits peaks at 1610 cm⁻¹ and 1020 cm⁻¹ corresponding to galactomannan backbone (C = O stretching) and glycosidic (C-O bound) groups55. The XGU spectrum (Fig. 2.c) exhibits the same characteristics except for low-intensity peaks around 1040 cm⁻¹ assigned to C–O–C stretching vibrations of the glycosidic linkages55. On the other hand, the HPAM spectrum (Fig. 2.d) possesses sharp bands around 1650 cm⁻¹(C = O stretching of amide I) and 1450 cm⁻¹ (N–H bending of amide II), indicating the existence of amide groups. It can be attributed to C–N stretching in HPAM. Thus, these FTIR spectra together reveal the existence of polysaccharidic structures in natural gums and the differences in structure between biopolymers and the synthetic HPAM, which can strongly influence their performance in EOR or viscosity modulation applications55. The characteristic absorption bands and corresponding functional groups observed in the FTIR spectra of the tested polymers are summarized in Table 8 below.
Thermal stability of polymers (TGA analysis)
Figure 3 displays the thermogravimetric analysis (TGA) profiles of BSG, XGU, GGU and HPAM, illustrating their thermal stability and decomposition behavior under a nitrogen atmosphere All the four samples show a typical multistep degradation. The weight loss at less than 120 °C signifies the loss of adsorbed and bound water, and the highest moisture level is present in the XGU, in line with its high hydrophilicity. The primary degradation of the natural gums (BSG, XGU, and GGU) takes place between 220 °C and 350 °C, which can be attributed to decomposition of polysaccharide backbones and depolymerization of glycosidic links. Among the polysaccharides, XGU has a little higher thermal resistance, which is supposed to be similar to the more crosslinked or branched molecular structures. Both BSG and GGU have similar thermal degradation profiles, BSG displays slightly steeper weight loss, which might be attributed to their lower molecular weight fractions or higher uronic acid contents56.
In contrast, HPAM shows an earlier and steeper mass loss starting around 200 °C, which continues rapidly, indicating its lower thermal stability compared to the natural gums. This early degradation is attributed to the decomposition of the amide groups and the polymer backbone. Overall, the higher thermal resistance of natural polysaccharides compared to HPAM suggests their potential advantage in applications requiring elevated thermal endurance, such as high-temperature oilfield operations or thermal EOR processes.
Rheological behavior of polymer solutions
Effect of concentration on viscosity
The variation of steady-shear viscosity of BSG, XGU, GGU and HPAM with concentration (wt%) at 25 °C under a constant shear rate of 10s⁻¹ is presented in Fig. 4. Viscosity in all samples increases non-linearly as a function of polymer concentration, in agreement with entanglement and intermolecular interaction theories45,46.
Viscosity vs. Concentration plot (at 10 s-1, 25οC and no salinity) of basil seed gum (BSG), xanthan gum (XGU), guar gum (GGU), and hydrolyzed polyacrylamide (HPAM). The estimated Critical Association Concentration (CAC) points located at: ≈0.24 wt% (XGU), ≈ 0.27 wt% (BSG), ≈ 0.40 wt% (HPAM), and ≈ 0.60 wt% (GGU, weak indication).
XGU exhibits the highest viscosifying capacity, surpassing 1100 cp. at 1.2 wt%, indicative of its well-known rigid, ordered structure and strong hydration ability. GGU, on the other hand, the lowest viscosity at equivalent concentrations indicating a weaker molecular interaction and network entanglement. The extracted and purified BSG shows better viscosity performance than the commercial HPAM and GGU, especially at concentrations higher than 0.5 wt%, and suggests that it can be a sustainable natural viscosifier as well as an efficient natural thickening agent. However, correspondingly, the synthetic HPAM exhibits moderate thickening performance, indicating that there is still potential to explore natural options, such as BSG and XGU, as additives with potential for rheology modification.
In addition, detailed examination of the viscosity–concentration curves allowed estimation of the polymer concentrations corresponding to the onset of Critical Association Concentration (CAC), defined as the concentration at which polymer chains begin to form extensive intermolecular associations and an interconnected network, leading to a sharp increase in viscosity. Among several reported approaches for CAC determination, one common method is the two-slope fitting technique, where the CAC is identified from the intersection point of two dashed trend lines: the first, with a lower slope, representing the dilute or semi‐dilute regime, and the second, with a higher slope, corresponding to the entangled‐network regime dominated by intermolecular interactions57. Using this graphical method, the CAC values were approximated as 0.24 wt% for XGU, 0.27 wt% for BSG, and 0.40 wt% for HPAM. For GGU, a distinct CAC was not observed; however, a weak indication appeared near 0.60 wt%.
Effect of salinity on viscosity
Figure 5 depicts the effect of brine salinity (0.1–100,000 ppm) on the viscosity of 1 wt% polymer solutions at 25 °C and a constant shear rate of 10 s⁻¹. XGU exhibited the highest salinity stability, retaining nearly 1,050 cp. at 10,000 ppm and showing only a minor decline at even higher salinities. BSG ranked second in performance, maintaining ~ 660 cp. at 10,000 ppm (over 90% of its initial viscosity) and still retaining ~ 450 cp. at 100,000 ppm, more than double that of GGU and almost an order of magnitude higher than HPAM under the same conditions. In contrast, both HPAM and GGU experienced sharp viscosity losses once salinity exceeded 1,000 ppm, limiting their usability in high-salinity reservoirs58.
The robust salinity tolerance of BSG, combined with its natural biopolymeric origin, environmental compatibility, and ease of extraction, supports its potential for CEOR deployment in moderately saline reservoirs. Although XGU remains the benchmark for salinity tolerance, BSG offers distinct advantages over HPAM and GGU, establishing it as a viable alternative for natural polymer flooding, particularly where moderate ionic levels and sustainable field practices are prioritized.
Temperature dependence of viscosity
The viscosity response of the polymer solutions (1 wt%) to the temperature ranging from 25 to 100 °C at a constant shear rate of 10 s⁻¹ is plotted in Fig. 6. All polymers show the usual overall decreasing trend in viscosity with increasing temperature because of greater freedom of mobility of molecules and disturbance of intermolecular forces. Nevertheless, the level of decrease of solution viscosity varies largely between polymers.
XGU displayed exceptional thermal stability, maintaining viscosities above 850 cP up to 90 °C, with a sharp drop at 100 °C likely linked to partial backbone degradation. BSG, extracted in-house from basil seeds, showed a substantial viscosity decrease from ~ 700 cP to ~ 400 cP between 25 °C and 60 °C, but subsequently demonstrated remarkable stability and even partial recovery, reaching ~ 550 cP at 90 °C. This recovery is hypothesized to result from thermally induced structural reorganization supported by FTIR observations that indicate a polysaccharide backbone containing uronic acids, capable of promoting hydrogen bonding and associative network formation at elevated temperatures.
In contrast, HPAM suffered a steep viscosity loss, approaching zero above 90 °C, confirming its thermal limitations, while GGU exhibited moderate stability but inferior retention compared to BSG and XGU. Overall, the temperature-responsive nature of BSG, coupled with its stability in the 60–90 °C range, commonly encountered in CEOR reservoirs, reinforces its suitability as a green, sustainable alternative to synthetic polymers for medium- to high-temperature applications.
Shear rate dependence of viscosity
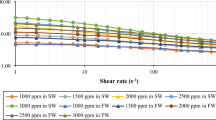
The Fig. 7 indicates that all measured polymer solutions (BSG, XGU, GGU as well as HPAM) were typified by the shear-thinning behavior over a wide band of shear rates (0.001–100 s⁻¹) at which the viscosity is substantially reduced as the shear rate increases, following the polymer chains orientation and disentanglement in flow. It is not surprisingly that XGU remains the most viscous in the whole structure which highlights its known structural rigidity. In comparison, GGU show the slowest response in terms of viscosity, which may indicate less crosslinking55.
Interestingly, BSG shows a relatively but steady rheological behavior, even higher than HPAM and close to XGU, especially at the low-shear stage. This mild behavior could indicate good intermolecular interactions, suggesting its suitability as a native viscosifier which may merit further attention in shear-sensitive applications.
Rheological modeling of Basil seed gum solutions
The shear stress–shear rate behavior of BSG solutions was evaluated using three common rheological models, Power‑law, Bingham‑Plastic, and Herschel–Bulkley, to identify the most representative equation and confirm the pseudoplastic nature of the polymer (Fig. 8).
The fitted curves in Fig. 8 clearly show that the Herschel–Bulkley model provides the closest agreement to the experimental data. The fitted parameters and correlation coefficients (R²) are summarized in Table 9.
Among the models, Herschel–Bulkley achieved the highest R² )R² =0.967), closely followed by the Power-law model (R² = 0.959), while the Bingham-plastic model showed considerably lower accuracy (R² = 0.731). For the Herschel–Bulkley fit, the yield stress was 8.29 Pa, the consistency index k was 10.25 Pa·sⁿ, and the flow behavior index n was 0.1425, confirming the pseudoplastic (shear-thinning) nature of the fluid with a measurable yield stress. This behavior is in agreement with the observed molecular entanglement and network structure inferred from the polymer’s molecular composition.
To further characterize the flow behavior under different physical environments, the viscosity data were fitted to: the Power-law model (shear rate dependent), Ostwald–De Waele model (polymer concentration dependent) and Arrhenius model (temperature dependent), shown in Fig. 9.
The model parameters were estimated via nonlinear regression and are summarized in Table 10. Among the three models, the Power-law and Ostwald–De Waele models provided excellent correlation with the experimental data, while the Arrhenius model captured the general decreasing trend of viscosity with temperature, albeit with minor deviations at higher temperatures, possibly due to structural transitions in the polymer.
Asorption behavior of polymers on sandstone
Effect of polymer concentration and salinity on adsorption
The adsorption of the investigated polymers on sandstone powder is obviously affected by the polymer concentration and water salinity (Fig. 10). Noteworthily, the adsorption capacity of all polymers is higher at higher salinity of the aqueous phase, implying that the polymer is retained on the rock better with a higher ionic strength. Such a trend indicate that salt ions could make the interaction between the polymer chains and the mineral more favorable by shielding the electrostatic repulsion or enhancing the polymer conformation for adsorption. Based on the data of the adsorption in Fig. 10, it can be seen that XGU gives the lowest adsorption under all salinity conditions, and is followed by BSG, both of which demonstrate dramatically lower adsorption when compared with those of GGU and HPAM. Such a lower adsorption tendency is highly beneficial for polymer-flooding because of the lower polymer loss in a reservoir and the higher movement of polymer through porous media.
Furthermore, the adsorption increases with the polymer concentration, but tends to level off at higher concentrations, which is attributed to the saturation of the adsorption sites on the sandstone surface. The moderate adsorption of XGU and BSG even at high salinities demonstrates that they may serve as a high-efficient and economic replacement of traditional synthetic HPAM in the presence of high-salinity reservoirs, in which the polymer retention and degradation become the major issues. These results highlight the need for choosing polymers that not only survive exposure to harsh reservoir conditions, but also have reduced adsorption, which contribute to the efficiency and economics of enhanced oil recovery projects.
To enable direct comparison of the adsorption behavior of the studied polymers at each salinity level, the adsorption capacities of HPAM, XGU, GGU, and BSG were plotted together for the tested concentration range under four salinity conditions: distilled water (a), 7000 ppm NaCl (b), 20 000 ppm NaCl (c), and 50 000 ppm NaCl (d) (Fig. 11).
In all salinities, HPAM exhibited the highest adsorption onto sandstone, followed by GGU, whereas XGU and BSG consistently showed the lowest values. The differences were most pronounced at low concentrations and high salinities, where the adsorption of HPAM exceeded 1.4 mg/g, compared to less than 0.8 mg/g for BSG and XGU. These results confirm the advantage of BSG and XGU in terms of lower polymer loss due to adsorption, which is particularly beneficial for CEOR operations in high-salinity reservoirs. The overall trends also indicate that adsorption increases with polymer concentration for all systems, reaching near-plateau values at higher concentrations, consistent with saturation of available adsorption sites.
Adsorption isotherm modeling
To quantitatively describe the adsorption behavior of the extracted BSG polymer on sandstone surfaces, three commonly used isotherm models, Langmuir, Freundlich, and Redlich–Peterson, were applied to the experimental adsorption data, as depicted in Fig. 12.
The experimental data were fitted to each model to evaluate their applicability and predictive accuracy. The Langmuir isotherm assumes monolayer adsorption onto a surface with a finite number of identical sites and is represented by the following equation:
Where q is the amount of adsorbate adsorbed per gram of adsorbent (mg/g), C is the equilibrium concentration of the adsorbate (g/L), qm is the maximum adsorption capacity (mg/g) and k is the Langmuir constant related to the affinity of binding sites (L/g).
The Freundlich adsorption isotherm, which describes adsorption on heterogeneous surfaces, is expressed by the following empirical equation:
In this equation, denotes the quantity of adsorbate retained per unit mass of the adsorbent, expressed in mg/g. The variable C represents the adsorbate’s equilibrium concentration in the solution (g/L). The constant k reflects the Freundlich isotherm’s adsorption capacity, with units of [(mg/g)/(L/g)¹⁄ⁿ], while n is a dimensionless parameter that indicates the adsorption intensity.
The Redlich–Peterson model combines features of both Langmuir and Freundlich equations and is given by:
Where q is the amount of adsorbate adsorbed per gram of adsorbent (mg/g), C is the equilibrium concentration of the adsorbate (g/L), k, a, and n are empirical constants and n is typically between 0 and 1, indicating the deviation from ideal Langmuir behavior.
The model parameters, obtained from non-linear regression fits, are summarized in Table 11. The high correlation coefficients of the Redlich–Peterson model indicate that it is the most suitable description of the experimental data encompassing both monolayer and heterogeneous adsorption.
Core flood analysis
Table 12 presents the physical and petrophysical properties of the sandstone core used in the flooding experiments, providing the baseline parameters for interpreting the performance trends. The flooding sequence comprised three stages, seawater flooding, BSG polymer slug injection, and chase brine flooding, carried out under constant flow conditions to evaluate both the displacement and sweep efficiencies.
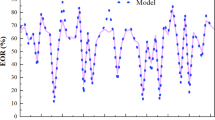
During the initial seawater injection stage (0.00–1.5 PV), oil recovery increased steadily to 30.1% of the original oil in place (OOIP). Water breakthrough occurred early, at approximately 0.50 PV, with a water cut around 89%, followed by a rapid climb to 100% water cut by 1.24 PV. The plateau in oil recovery between 1.24 PV and the end of the seawater stage indicated that displacement had occurred primarily in high-permeability channels, leaving significant volumes of bypassed oil in less accessible regions because of an unfavorable mobility ratio and, consequently, poor sweep efficiency Fig 13
The injection of a 0.4 PV slug of 1500 ppm BSG solution (1.5–1.90 PV) dramatically altered the displacement dynamics. Within the first 0.13 PV of polymer injection, cumulative oil recovery rose from 30.2% to 40.7%, and the water cut dropped to 97%. The polymer’s rheological stability under reservoir-relevant shear and salinity conditions enabled a significant viscosity increase in the injected phase, reducing the water–oil mobility ratio. This improvement in mobility control reduced water channeling and fingering while diverting flow into previously unswept pore spaces. By the end of this stage (1.94 PV), oil recovery had reached 58% OOIP, confirming that flow diversion and the associated expansion of the displacement front had markedly increased the areal and vertical sweep efficiency.
During the chase brine injection phase (1.90–4.00 PV), the incremental recovery trend continued as the residual polymer retained within the pore structure maintained a lower mobility ratio. This sustained mobility control allowed the chase brine to access and displace oil from regions that remained unswept after the polymer slug had passed. Cumulative oil recovery ultimately reached 72.1% OOIP at 3.62 PV, after which production plateaued, indicating that the post-polymer residual oil saturation (Sorp) had been approached. Notably, the water cut remained below pre-polymer levels for a significant portion of the chase stage, reflecting the lasting impact of BSG on controlling water encroachment.
A key consideration in polymer flooding is the ability of the polymer to recover its viscosity after experiencing high shear during injection, a factor critical to maintaining mobility control within the porous medium. To quantitatively evaluate this, a shear rate cycle analysis was performed Fig. 14. Calculations showed that the BSG solution experienced a high apparent shear rate of approximately 45.48 s⁻¹ at the stainless-steel injection needle exit (0.72 mm ID, 0.1 cc/min), where its viscosity was reduced to approximately 2.0 cP, as seen on the up-curve of the rheogram.
Upon entering the sandstone core, the shear rate dropped sharply to about 2.16 s⁻¹, as estimated using the Cannella correlation59. At this lower shear rate, the polymer exhibited substantial structural recovery, with its viscosity increasing more than 10-fold to approximately 25 cP, as confirmed by the down-curve in Fig. 14. This quantitatively confirmed resilience against shear degradation ensures that the polymer regains sufficient thickening power under core-scale flow conditions. This behavior, combined with BSG’s inherent salinity tolerance and low rock adsorption, sustained a favorable mobility ratio, reduced water channeling, and contributed directly to the observed improvement in sweep efficiency and incremental oil recovery throughout the flooding sequence. The lasting effect was evident in the chase brine phase, where the water cut remained below pre-polymer levels for a substantial portion of the run.
Structure–performance relationship of BSG in high-stress EOR environments
Variations in the molecular architecture of biopolymers govern their suitability for deployment in enhanced oil recovery operations, particularly under high-temperature and high-salinity conditions60,61. Structural analysis (FTIR) in this study confirmed that BSG possesses a higher proportion of uronic acids and hydroxyl groups compared with HPAM and GGU. This composition promotes extensive intermolecular hydrogen bonding and enables network restructuring when subjected to thermal stimulation, aiding in viscosity retention or partial recovery at elevated temperatures.
The relatively broad molecular weight distribution of BSG facilitates the formation of polymer entanglements across pore-scale flow channels, improving mechanical stability while mitigating shear-induced chain fragmentation during injection and propagation. Its semi-flexible polysaccharide backbone permits reversible coil–gel transitions, which enhance sweep efficiency and mobility control under variable shear and thermal loads.
Moreover, the lower overall charge density of BSG minimizes susceptibility to divalent-ion bridging and precipitation in saline environments, thereby improving injectivity and long-distance propagation within the reservoir matrix. These structure–property relationships are corroborated by our experimental observations—including thermal stability profiles (TGA), rheological performance under brine salinity up to 100,000 ppm, moderate adsorption capacity with low polymer retention, and improved core-scale displacement efficiency. Collectively, these findings elucidate the molecular basis for BSG’s performance as a green, thermoviscosifying polymer, supporting its potential for deployment in high-stress sandstone reservoirs.
Conclusion and final remarks
This study presents a comprehensive evaluation of basil seed gum (BSG) as a naturally derived salinity-tolerant biopolymer for chemical enhanced oil recovery (CEOR) under conditions representative of high-temperature (up to 90 °C) and high-salinity sandstone reservoirs. Systematic characterization confirmed BSG’s polysaccharide-rich composition, high thermal stability, and pseudoplastic flow behavior with measurable yield stress. Rheological measurements demonstrated superior salt tolerance over HPAM and GGU, retaining more than 450 cP at 100,000 ppm NaCl, and exhibiting partial viscosity recovery at elevated temperatures, indicating potential thermally induced structural reorganization. Adsorption studies revealed moderate rock affinity (~ 0.80 mg/g at 50,000 ppm NaCl) and lower polymer retention compared with HPAM and GGU, with adsorption behavior best described by the Redlich–Peterson model (R² = 0.9958).
Core flood experiments confirmed the lab-scale findings. Seawater flooding yielded 30. % OOIP with early water breakthrough (0.50 PV) due to an unfavorable initial mobility ratio (~ 115). Subsequent injection of a 0.4 PV slug of BSG (1500 ppm) increased recovery to 5%, delaying water encroachment and enhancing sweep efficiency. Chase brine injection further increased recovery to 72. % OOIP, corresponding to 41. % incremental gain over waterflooding.
Overall, BSG demonstrates a balanced combination of rheological robustness, low adsorption, and effective mobility control, translating into significant core-scale recovery improvements. These attributes, combined with its biodegradable and renewable origin, position BSG as a technically viable and environmentally sustainable candidate for CEOR in saline, high-temperature formations. Further research should focus on dynamic adsorption tests using the 1-D injection method to better simulate in-reservoir displacement processes, along with long-term stability assessments and pilot-scale implementations to validate its performance under dynamic reservoir conditions.
Data availability
All data will be available on academic request from the corresponding author.
References
Lakatos, I. & Lakatos-Szabo, J. Global oil demand and role of chemical EOR methods in the 21st century. Int. J. Oil Gas Coal Technol. 1 (1–2), 46–64 (2008).
Firozjaii, A. M. & Saghafi, H. R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 6 (2), 115–122 (2020).
Liang, K. et al. Comparative study on enhancing oil recovery under high temperature and high salinity: polysaccharides versus synthetic polymer. ACS Omega. 4 (6), 10620–10628 (2019).
Olabode, O. et al. Recovery potential of biopolymer (BP) formulation from solanum tuberosum (waste) starch for enhancing recovery from oil reservoirs. Energy Rep. 6, 1448–1455 (2020).
Fu, X. et al. Enhanced oil recovery performance and solution properties of hydrophobic associative Xanthan gum. Energy Fuels. 36 (1), 181–194 (2021).
Elsaeed, S. M. et al. Guar gum-based hydrogels as potent green polymers for enhanced oil recovery in high-salinity reservoirs. ACS Omega. 6 (36), 23421–23431 (2021).
Ghoumrassi-Barr, S. & Aliouche, D. A rheological study of Xanthan polymer for enhanced oil recovery. J. Macromolecular Sci. Part. B. 55 (8), 793–809 (2016).
Xu, L. et al. Temperature/salt tolerance and oil recovery of Xanthan gum solution enhanced by surface-modified nanosilicas. Pet. Sci. 20 (1), 577–589 (2023).
Virginia, E. O. et al. Enhanced oil recovery methods using biodegradable materials in different reservoirs. GSJ, 12(9). (2024).
Agi, A. et al. Natural polymer flow behaviour in porous media for enhanced oil recovery applications: a review. J. Petroleum Explor. Prod. Technol. 8 (4), 1349–1362 (2018).
Ogunkunle, T. F. et al. Comparative analysis of the performance of hydrophobically associating polymers, Xanthan and Guar gum as mobility controlling agents in enhanced oil recovery application. J. King Saud Univ. Eng. Sci. 34 (7), 402–407 (2022).
Olabode, O. et al. Effect of salt concentration on oil recovery during polymer flooding: simulation studies on Xanthan gum and gum Arabic. Polymers 15 (19), 4013 (2023).
Olabode, O. et al. Investigating the effect of salt concentration on oil recovery during Guar gum polymer flooding: A simulation study. Results Eng. 22, 102269 (2024).
Gunaji, R. G., Junin, R. & Bandyopadhyay, S. Application of biopolymer schizophyllan derived from local sources in Malaysia for polymer flooding operation. in AIP Conference Proceedings. (AIP Publishing LLC., 2022).
Castro, R. H. et al. Experimental investigation of the viscosity and stability of Scleroglucan-Based nanofluids for enhanced oil recovery. Nanomaterials 14 (2), 156 (2024).
Mota, G., Guimarães, R. & Pereira The influence of concentration and temperature on the rheological behavior of Diutan gum aqueous solutions. Int. J. Polym. Anal. Charact. 26 (8), 735–753 (2021).
Gussenov, I. et al. Exploring potential of Gellan gum for enhanced oil recovery. Gels 9 (11), 858 (2023).
Serikov, G. et al. Synergistic application of Welan gum and polysaccharides for enhanced oil recovery. J. Petroleum Explor. Prod. Technol. 15 (3), 1–20 (2025).
Sakthivel, S., Sharfan, I. I. B. & Abdulhamid, M. A. Water-soluble Chitosan polymer for enhanced oil recovery in the carbonate reservoir. Int. J. Biol. Macromol. 281, 136528 (2024).
Mohammad, A. F. et al. Hydroxyethyl Cellulose as a Multifunctional Agent for Integrated Brine Desalination, CO₂ Capture, and Enhanced Oil Recovery (Chemical Engineering and Processing-Process Intensification, 2025).
Rellegadla, S., Jain, S. & Agrawal, A. A holistic approach to determine the enhanced oil recovery potential of hydroxyethylcellulose, tragacanth gum and carboxymethylcellulose. J. Mol. Liq. 341, 117334 (2021).
Cao, P. F., Mangadlao, J. D. & Advincula, R. C. Stimuli-responsive polymers and their potential applications in oil-gas industry. Polym. Rev. 55 (4), 706–733 (2015).
Khutoryanskiy, V. & Georgiou, T. Temperature-Responsive Polymers (Wiley Online Library, 2018).
Hoogenboom, R. Temperature-responsive Polymers: properties, synthesis, and Applications, in Smart Polymers and their Applications 13–44 (Elsevier, 2019).
Pu, W. F. et al. Amphoteric hyperbranched polymers with multistimuli-responsive behavior in the application of polymer flooding. RSC Adv. 5 (107), 88002–88013 (2015).
Olabode, O. et al. Experimental investigation of the effect of surfactant–polymer flooding on enhanced oil recovery for medium crude oil. Polymers 16 (12), 1674 (2024).
Naji-Tabasi, S. & Razavi, S. M. A. Functional properties and applications of Basil seed gum: an overview. Food Hydrocoll. 73, 313–325 (2017).
Hosseini-Parvar, S. et al. Steady shear flow behavior of gum extracted from ocimum Basilicum L. seed: effect of concentration and temperature. J. Food Eng. 101 (3), 236–243 (2010).
Vanparijs, N., Nuhn, L. & De Geest, B. G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 46 (4), 1193–1239 (2017).
Schmaljohann, D. Thermo-responsive polymers and hydrogels in tissue engineering. e-Polymers 5 (1), 021 (2005).
Xu, X. et al. Thermoresponsive polymers for water treatment and collection. Macromolecules 55 (6), 1894–1909 (2022).
Gupta, I. et al. Biopolymers: implications and application in the food industry. Biocatal. Agric. Biotechnol. 46, 102534 (2022).
Razavi, S. M. et al. Optimisation study of gum extraction from Basil seeds (Ocimum Basilicum L). Int. J. Food Sci. Technol. 44 (9), 1755–1762 (2009).
Kulichikhin, V. G. & Malkin, A. Y. The role of structure in polymer rheology. Polymers 14 (6), 1262 (2022).
Trachenko, K. et al. Extrinsic and intrinsic effects setting viscosity in complex fluids and life processes: the role of fundamental physical constants. Eur. Phys. J. E. 48 (1), 2 (2025).
de Moura, M. R. V. & Moreno, R. B. Z. L. Concentration, Brine salinity and temperature effects on Xanthan gum solutions rheology. Appl. Rheology. 29 (1), 69–79 (2019).
Kamal, M. S. et al. Rheological properties of thermoviscosifying polymers in high-temperature and high‐salinity environments. Can. J. Chem. Eng. 93 (7), 1194–1200 (2015).
Azad, M. S. Characterization of nonlinear viscoelastic properties of enhanced oil recovery polymer systems using steady-shear rheometry. SPE J. 28 (02), 664–682 (2023).
Zhong, L. et al. Rheological behavior of Xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 244, 160–170 (2013).
AlSofi, A. M. & Blunt, M. J. Polymer flooding design and optimization under economic uncertainty. J. Petrol. Sci. Eng. 124, 46–59 (2014).
Hsissou, R. et al. Rheological behavior models of polymers. Biointerface Res. Appl. Chem. 12, 1263–1272 (2021).
Bird, R. B., Dai, G. & Yarusso, B. J. The rheology and flow of viscoplastic materials. Rev. Chem. Eng. 1 (1), 1–70 (1983).
Raja, A., Wilfert, P. K. & Picken, S. J. Using the Herschel–Bulkley consistency index to characterise complex biopolymer Systems—The effect of screening. Polymers 16 (19), 2822 (2024).
Chauhan, G. et al. Rheological studies and optimization of Herschel-Bulkley flow parameters of viscous Karaya polymer suspensions using GA and PSO algorithms. Rheol. Acta. 57 (3), 267–285 (2018).
Irgens, F. Rheology and non-newtonian Fluids 1 (Springer, 2014).
Marcotte, M., Hoshahili, A. R. T. & Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 34 (8), 695–703 (2001).
Peleg, M. Temperature–viscosity models reassessed. Crit. Rev. Food Sci. Nutr. 58 (15), 2663–2672 (2018).
Alafnan, S. et al. Langmuir adsorption isotherm in unconventional resources: applicability and limitations. J. Petrol. Sci. Eng. 207, 109172 (2021).
Rajahmundry, G. K. et al. Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 276, 130176 (2021).
Proctor, A. & Toro-Vazquez, J. The Freundlich isotherm in studying adsorption in oil processing. J. Am. Oil Chemists’ Soc. 73 (12), 1627–1633 (1996).
Foo, K. Y. & Hameed, B. H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156 (1), 2–10 (2010).
Deer, W. A., Howie, R. A. & Zussman, J. An Introduction To the rock-forming Minerals (Mineralogical Society of Great Britain and Ireland, 2013).
Blatt, H., Middleton, G. V. & Murray, R. C. Origin of sedimentary rocks. (1980).
Samanta, A., Ojha, K. & Mandal, A. The characterization of natural surfactant and polymer and their use in enhanced recovery of oil. Pet. Sci. Technol. 29 (7), 765–777 (2011).
Kumar, G. et al. Enhanced Oil Recovery Using Viscosity-Augmented Guar Gum: A Comparative Study with Xanthan Gum and Partially Hydrolyzed Polyacrylamide 39 1856–1869 (Energy & Fuels, 2025). 4.
Tripathi, D. et al. Basil seed mucilage as a bioadhesive polymer: development of Naproxen sodium microspheres and suppositories with in-vitro and ex-vivo studies. ADMET DMPK. 12 (6), 881–901 (2024).
Mao, J. et al. Novel hydrophobic associating polymer with good salt tolerance. Polymers 10 (8), 849 (2018).
AlQuraishi, A. A. & Alsewailem, F. D. Adsorption of Guar, Xanthan and Xanthan-Guar mixtures on high salinity, high temperature reservoirs. In: Offshore Mediterranean Conference and Exhibition. OMC. (2011).
Berg, S. & van Wunnik, J. Shear rate determination from pore-scale flow fields. Transp. Porous Media. 117 (2), 229–246 (2017).
Rellegadla, S., Prajapat, G. & Agrawal, A. Polymers for enhanced oil recovery: fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 101 (11), 4387–4402 (2017).
Sveistrup, M. et al. Viability of biopolymers for enhanced oil recovery. J. Dispers. Sci. Technol. 37 (8), 1160–1169 (2016).
Author information
Authors and Affiliations
Contributions
Ali YarahmadiExperiments, Writing, Conceptualization, AnalysisGhasem ZargarConceptualization, Analysis, SupervisionSiavash AshooriConceptualization, SupervisionAbbas Khaksar ManshadConceptualization, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yarahmadi, A., Zargar, G., Ashoori, S. et al. A comparative study of the rheological and adsorption behaviors of bio-and synthetic polymers for enhanced oil recovery. Sci Rep 15, 44729 (2025). https://doi.org/10.1038/s41598-025-28455-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-28455-y