Abstract
The purpose of this study was to examine the impacts of incorporating commercial mixture phytobiotic/probiotics as feed additives in terms of performance, survival rate proximate composition, hematological parameters, immunological response and antioxidant enzymes activity of Oreochromis niloticus reared under un-exchanged water system. Nile tilapia with an average beginning weight ranged from 48.49 to 52.50 g and were distributed in concrete ponds (1 m × 1 m × 1 m; L × W × H) at 20 fish / pond. Four treatments were performed as follows: control group (CG) fish fed a basal feed without water exchange and three groups were reared under zero water exchange with adding three different doses of commercial phytobiotic /probiotics on the basal feed (Garlex®, Superimmune®, and Gallipro 200®): Group 1: (200 mg, 500 mg, and 200 mg)/kg diet, Group 2: (400 mg, 1000 mg, and 400 mg)/ kg diet and Group 3: (600 mg, 1500 mg, and 600 mg)/ kg diet. This trial lasted for ninety days. Groups 1 and 2 had the best growth indices and survival rates, and there was not a significant (P ≤ 0.05) distinction between them. Fish fed Groups 1 and 2 showed the greatest improvement in proximate body composition, immunological responses, and antioxidant enzyme activity, including lysozyme, superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH). This study has detected that feed additives of commercial mixture of 200 mg/kg Garlex®, 400 mg/kg Super Immune®, and 200 mg/kg GallPro-200® led to improved growth and physiological status of O. niloticus under un-exchanged water ponds.
Similar content being viewed by others
Introduction
Recently, Egypt’s aquaculture industry has had tremendous success, ranking it first among African nations and sixth globally1. Egypt’s aquaculture represents more than 80.5% of total seafood production, and its superiority in tilapia production has represented more than 88% of aquaculture production2 in the past decade. Nevertheless, the issue of satisfying the need for animal protein has not been adequately addressed. Rather, there is a continuous increase in the price of farmed fish3. The lack of high-quality fresh water is the biggest challenge that limits the expansion of Egyptian aquaculture projects4. Whereas, water problems arise from irrigation drainage that is influenced by agricultural seasons, variations in water levels all year, and that may be polluted with agricultural pesticides5. Therefore, the solutions proposed to solve this problem are to move from traditional farming systems (semi-intensive and intensive systems) to modern farming systems. But the inputs of modern farming systems, whether fixed costs (tanks, pumps, filters, and ventilation devices) or variable costs (feed or energy), are very expensive. Additionally, managing modern aquaculture systems requires a lot of experience6.
The traditional system in Egypt, particularly intensive systems, depends on water that changes7,8. Therefore, the high cost of energy required to run water pumps can occasionally result in a lack of water, a decline in the quality of the water, or an increase in production expenses for the owners of these farms. Water entering and exiting through pumps is essential for some crops. Accordingly, recommending prolonging water change periods through the use of immunostimulants without compromising growth rates is an appropriate solution to confront irrigation water shortages during a certain period, to reduce energy consumption, or to stop changing water due to its low quality of its source.
An immunostimulant is generally understood to be any substance, medication, stressor, or activity that directly interacts with and activates system cells to increase the innate or non-specific immune response. According to9 immunostimulants can be classified as chemical agents, bacterial preparations, polysaccharides, plant or animal extracts, dietary factors, and cytokines. Probiotics help by increasing feed consumption efficiency and by stimulating immune cells, which lowers the rates of pathogen infestation. Prebiotics may benefit by affecting the balance of advantageous gut flora and inducing the release of vital digestion enzymes, which increases fish’s availability of nutrients. Similarly, synbiotics improve survival rates and alter the microbial composition of the gastrointestinal system more effectively than probiotics or prebiotics alone, which raises aquaculture productivity10. Several studies confirmed the positive role for immunostimulants to improve immune functions of fish that reared under optimum conditions such as Oreochromis niloticus11 Micropterus salmoides12 Cyprinus carpio, Acipenser baerii Brandt13,14. However, only a small number of researches have assessed the impact of immune stimulants in stressful conditions, such as rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus)15,16. Besides, medicinal plants are novel feed additives and are generally inexpensive, sustainable, and easily obtainable17,18. Many of them have shown demonstrable benefits for growth, immunoregulation, and antimicrobial capability in various aquatic animals19. According to earlier reports, several medicinal herbs have the ability to increase aquatic animals’ appetites and serve as feeding attractants by chemically influencing fish eating behavior, leading to improved feed utilization, and then reducing water pollution in culture systems20.
In the same context, many species, such as Oreochromis niloticus19,21, Paralichthys olivaceus22, and Catla catla23 showed their responses with beneficial effects on healthy growth of probiotic and herbal combinations, either feed or watery additives.
Interestingly garlic Allium sativum in feeds showed a significant feeding attraction activity for Japanese seabass and then growth and feed efficiency value of fish were significantly increased24,25. Similar results for garlic were also reported in many other aquatic animals’ goldfish Carassius auratus26, Nile tilapia Oreaochromis nilotica27, and Litopenaeus vannamei28.
The objective of this study was to reduce stressful condition on Nile tilapia by supplementing their diets with commercial phytobiotic/probiotic combinations and evaluating how their growth and physiological changes responded to an intense system with un-exchanged water.
Materials and methods
Location of the trial
For ninety days, this experiment was conducted at the National Institute of Oceanography and Fisheries (NIOF), Fayoum, Egypt, in the Fish Nutrition Laboratory and Fish Research Station.
Fishes, experimental groups
A 240 Nile tilapia Oreaochromis niloticus juvenile with an average initially weight of 50.27 g ± 0.88 (SE) was brought from a commercial farm in Fayoum, Egypt. Fish quickly transported in oxygenated water using tank (1m3) at Fish Nutrition Lab. and acclimatized for 10 days. After that fish were randomly distributed in 12 indoor concrete ponds (1 m × 1 m × 1 m; L × W × H) containing well-aerated water through air stone diffusers. Fish were stocked at rate of 20 fish/pond. Four groups were performed as the follows: control group (CG) fish fed a basal diet without water exchange and three groups were reared under zero water exchange with adding three various concentrations of commercial phyto/probiotics on the basal feed (Garlex®, superimmune®, and Gallipro 200®): Group 1: (200 mg, 500 mg, and 200 mg)/kg diet, Group 2: (400 mg, 1000 mg, and 400 mg)/kg diet and Group 3: (600 mg, 1500 mg, and 600 mg)/kg diet. Treatments were applied in triplicate.
Dietary supplements and Preparation of a tested diets
The ingredients of the basal feed were formulated to contain 30% crude protein, it was milled and well mixed without the supplementary diet for control groups and with adding dietary supplements (Garlex®, Superimmune®, and Gallipro 200®) for the treatments Group 1, Group 2 and Group 3 (Table, 1). The mixture was piloted by a pellet mill with a 3 mm die. Pellets were dried at 30 ˚C in an oven and stored at 5 ˚C in Refrigerator.
Water quality indicators
The water physiochemical indicators were monitored, statistically analyzed, and recorded in Table 2. Water temperature, pH, and oxygen dissolved (DO) were measured daily by an Orion digital pH meter and an oxygen meter (Cole Parmer model 5946). Ammonia, and nitrite were measured every week by chemical methodology29, and unionized ammonia was calculated by the relationship between temperature and pH according to the data of30.
Biological indicators
Total weight gain (WG), average weight gain/day (ADG), Relative weight gain (RWG, %), specific growth rate (SGR), Feed conversion Efficiency (FCE), survival rate (SR), and condition index (CF).
These parameters were calculated according the following equations:
\({\text{WG, g}}\,{\text{=}}\,{\text{final weight }}\left( {{\text{W2}}} \right){\text{--initial weight }}\left( {{\text{W1}}} \right)\)
ADG, g/day = average weight gain, g/experimental period, day,
\({\text{RWG, \% = [(W2--W1) / W1]}} \times 100\)
SGR, %/day = [(ln W2-ln W1)/days] × 100 whereas ln: is the natural log.
\({\text{FCE, \% = }}\left( {{\text{WG, g / Feed intake, g}}} \right) \times 100\)
\({\text{SR\% = }}\left( {{\text{Number of fishes at end/ Number of fishes at start}}} \right) \times {\text{100}}\)
CF, % = (W2/L3) × 100 whereas L: is the final length of fish in cm.
Sampling
At the finish of the trial, blood samples were taken at random from five fish each replication (15 fish per treatment). Clove oil was used to anesthetize fish at a concentration of 0.1 ml/l. Nine fish were used to sampling blood and six fish were euthanized by being frozen (−5 C) after being anesthetized, as previously mentioned for analyzing the body composition31.
Blood sampling
Blood was drawn from the caudal vein using a 3-ml syringe. Each blood sample was emptied in two micro tubes one of them contained EDTA to prevent coagulation and immediately examined for hematology analysis and other micro tubes did not contain EDTA to measure the serum parameters30.
Hematological and serum biochemical assay
Red blood cells (RBCs), White blood cell (WBCs), Hemoglobin (Hb) and Hematocrit (Hct) were analyzed using fully automatic hematological analyzer using commercial test kits (Bio-diagnostic, Egypt), serum biochemicals such as plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum glucose, total protein, urea, and creatinine were measured by calorimetric assay32,33.
Immunological-antioxidant indices
Commercial ELISA kits available from Biosource Inc. (San Diego, California, USA) were used to detected plasma lysozyme activity (Catalog No.: MBS725718), Immunoglobulin M (IgM; catalog No.: CSB-E07978r) and Complement C3 (MD1102094) were quantitatively determined using turbidimetric assay as described by34,35. Additionally, interleukin 10 (IL-10- catalog No. MBS269138)36, and Nitric oxide (NO) was estimated according to37. In the same manner calorimetric methods have been used to measure the amount of malondialdehyde. (MDA; catalog No.: MBS268427), superoxide dismutase (SOD; catalog No.: CSB-E08555r), catalase (CAT; catalog No.: MB2600683), and Glutathione (GSH: Catalog No. CSB-E12144r)38.
Body composition analysis
Fish carcasses and feeds were chemically analyzed in accordance with39. The coefficients 23.62, 39.5, and 17.56 KJ/g for CP, EE, and carbs, respectively, were used to estimate the gross energy (GE) of formulated diets40.
Statistical analysis
Shapiro-test Wilk’s was undertaken to ensure that the collected data were normal and analyzed by one-way analysis of variance (ANOVA) at a 95% confidence limit, using SPSS software, version 16. Duncanʼs Multiple Range 23 test was employed to compare means when F-values from the ANOVA were significant (P < 0.05).
Results
Water quality super
Table 2 presents the averages of the water physiochemical indices which ranged as follows: the water temperature (26.55–26.75 °C) pH levels (8.87–9.07), DO mg/L (3.35–3.80 mg/L), unionized ammonia (0.08–0.13 mg/L), and NO2 (1.69–1.80 mg/L). All this indicator was not significantly (P ≤ 0.05) between all treatments.
Fish growth performance
Growth indices are shown in (Table 3) the statistical analysis appeared significant differences (P ≤ 0.05) among all treatments with all parameters exclusion CF. Survival rate significantly varied in treatments. The growth, feeding utilization, and survival percentage of the treated groups that received a diet enriched with phytobiotics/probiotics were better than those of the control group. Group 2 exhibited the largest growth, with a weight gain percentage of 143.3; Group 1 and Group 3 had weight gain percentages of 142.9 and 80.85%, respectively (Fig. 1).
Weight gain % of Nile tilapia fed supplementary diet of phytobiotcs/probiotics under unchanged water for 90 days CG: control was fed a non-supplemented basal diet; Group 1, Group 2, and Group 3: fed a basal diet fortified with (200 mg, 500 mg, and 200 mg; 400 mg, 1000 mg, and 400 mg; 600 mg, 1500 mg, and 600 mg of Garlic mix, Superimmune, and Gallipro 200) kg-1 diet respectively.
Indicators of hematological and biochemical
Results of the blood hematology are displayed in (Table 4) and indicate a considerable variation among groups, where the highest values of WBC, Hb, RBC and Hct. were obtained with groups that administered dietary phytobiotics/probiotics especially Group2.
Blood biochemistry is presented in Table 5. Data showed noticeable differences (P < 0.05) among treatments in creatinine, Urea, ALT, AST, glucose, and total protein. Treated-groups had the best indicators of serum assay compared to untreated group. Group 2 recorded the lowest values in all indicators except total protein had the highest from the other groups.
Immunological indicators and antioxidant properties
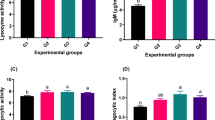
Table (6) showed that treated-fish had considerably greater in values of lysozyme activity, Complement C3, interleukin 10, Nitric oxide, malondialdehyde, superoxide dismutase (SOD), catalase (CAT), and Glutathione (GSH) than control group. Where Group 2 was the highest in these parameters and recorded the lowest MDA.
Chemical composition of carcass
At the end of trial duration, fish carcass was chemically analyzed, and their results presented in (Table 7). Carcass content of protein significantly changed between treatments, where the highest values were recorded with Group 1, 2 and 3 respectively in comparison with control. Group1 and 2 had the lowest fat content compared to group 3 and control. On the other side, Ash values are not significantly different between Groups 1,2 and 3, but they were considerably lower than control group.
Discussion
Results in this experiment indicate that a commercial mixture of Phytobiotic/probiotics enhanced growth, survival percentage, carcass composition, blood indices, immunity, and antioxidant enzyme activities in Oreochromis niloticus, under an un-exchanged water system. Although Table 2 showed that water temperature, pH, DO, and mg/l were within acceptable limits for tilapia growth as recorded by30,41 all treatments deteriorated severely, especially unionized ammonia and nitrite. Whereas42,43 suggested that increased NH3 above 0.05 or 0.068 ppm has a negative effect on biological parameters44, found that red tilapia can die from exposure to unionized ammonia (NH3) at concentrations between 0.11 and 0.15 if prolonged. Another study by45 proposed that NH3 levels below 0.1 mg/l are safe for aquaculture. In our study, the means of NH3 ranged between 0.0855 and 0.125 ppm and led to decreased growth rate, with an increase in the mortality rate of the control group in comparison with the treated groups with photobiotic/probiotics. There is a role for aeration in reducing the severity of ammonia toxicity under unchanged water conditions, as was suggested by46. There are factors impacting water quality that are primarily related to fish waste and feed47. Therefore, it’s important to investigate certain nutritional alternatives in order to enhance fish feed consumption and reduce water fouling, in addition to improving growth performance. Additionally, aeration and natural immunostimulants (phytobiotic/probiotics) can help reduce the stress caused by exposure to high ammonia concentrations. In partial similarity with our study48,49 using the probiotics in recirculating aquaculture system (RAS) improved water quality, immune responses, and feed efficiency of culture species.
In the same trend, phytobiotics or medical plants help to decrease the stress of water pollution in culture systems through improved feeding utilization, immunoregulation, and antimicrobial capability in various aquatic animals50.
The commercial mixture of phytobiotic/probiotics supplements used in our study included garlic as a natural growth promoter, immunostimulant, and antioxidant. Also, commercial probiotics include purified yeast (Saccharomyces cerevisiae), Beta-glucans, Mannans, silicate, and Bacillus subtilis. By using probiotics, the fish’s ability to feed, ingestion, digestion, absorption, metabolism, immunological function, and health improved. Prebiotics may benefit by affecting the balance of advantageous gut flora and inducing the release of vital digestive enzymes, which increases the availability of nutrients for fish. Enzymes involved in digestion (carbohydrates, phosphatases, esterase, lipases, peptidases, cellulases, and proteases) are produced by gut microbes in fish, including some species commonly used as probiotics51. Increased synthesis of amylase, lipase, and protease was observed in the intestine of tilapia fed diets containing Bacillus subtilis and unknown “photosynthetic bacteria”52. According to53,54 probiotics can also be used in fish feeds to enhance the amount of high-cellulose carbohydrate sources as a result of increasing digestive enzyme activity. This tactic may assist fish overcome the low digestibility and anti-nutritional properties of plant-based substitute meal ingredients. Additionally, it had been found that probiotic treatment considerably enhanced the length and density of microvillus in the mid-intestine of tilapia55.
Besides, garlic supplements can boost immunity, stimulate appetite, encourage growth, and enhance the quality of fresh produce56. Allicin, a powerful flavor found in garlic, may increase appetite and facilitate better digestion. Also, allicin can enhance digestion by stimulating the intestinal flora and suppressing harmful bacteria, so enhancing the overall well-being of fish raised in culture57. Furthermore, by promoting the release of bile acids, garlic has been demonstrated to enhance the regulation of digestion58.
In the same context59, found that Japanese seabass fed a diet containing garlic oil boost had the best digestion efficiency. Emphasizing the importance of garlic, there are many biologically active substances found in garlic, such as sugars, amino acids, and their equivalents that are soluble in oil60.
Numerous studies have shown that S. cerevisiae and Bacillus subtilis impact the growth of a number of fish species, such as rainbow trout (Oncorhynchus mykiss)61, common carp (Cyprinus carpion62, and Nile tilapia63,64.
In our study, dietary supplements (Garlex®, Superimmune®, and Gallipro 200®) significantly enhanced the growth (WG, SGR) and feed conversion efficiency (FCE) of Oreochromis niloticus compared to the control. Wherein, WG% after 90 days increased in Group 2 by 84.15% than un-treated group. Similarly65, reported an increase in the daily growth rate by 33% of Oreochromis niloticus fed on Biogen® (a commercial product that contains B. subtilis). Also25, mentioned that dietary garlic greatly increased the feed efficiency of Japanese seabass, enabling them to satisfy body growth requirements and reduce the loss of nutrients into water, which in turn contributes to reducing pollution. In the current study, the decreased growth performance observed in Group 3, as the dosage of phytobiotics and probiotics increased, could have negatively affected the animals’ immune or physiological status, ultimately leading to a decline in growth performance. These findings are consistent with those of66 who reported that fish fed high concentrations of baker’s yeast exhibited slower growth. Another study showed that Rainbow trout had significantly lower growth when fed on a commercial probiotic containing Saccharomyces cerevisiae and Saccharomyces elipsoedas at 1.5% and above61. In the same vein25, found that garlic did not significantly increase the SGR and WG of Japanese seabass as compared with the control. Generally, it is difficult to draw concrete conclusions and provide specific recommendations on the effects of dietary probiotics or garlic on the growth performance of fish. Wherein, the studies vary widely about fish species, fish age and size, stocking density, diet composition, concentration of doses, feed allowances, feeding duration, and, of course, type and source of probiotic. Fish health, physiological and pathological conditions are measured using blood parameters, which are an effective and sensitive indicator67. Results of effects of the commercial mixture of phytobiotic/probiotics as feed supplementary feeds on hematological and serum biochemical of fish illustrated improvements in the physiological status of treated fish groups especially Group 2 which had higher WBCs, RBCs, Hb and Hct comparable to the control group. It is well-known that these features of cultured fish are commonly used to identify stress and disease68. The increasing WBCs may be a result of increased feed protein utilization, which in turn may have increased leucocyte synthesis in the kidney and spleen tissue, and they are a defense against infections. Fish with higher RBCs, Hb, and Hct levels are more active. The ability of blood to bind oxygen is directly correlated with increasing these markers69,70. Furthermore, treated groups had the best blood serum indices, especially Group 2, which recorded the lowest blood glucose, AST, ALT, and the highest total protein. It is believed that when fish are kept in stressful environments, their body physiology and metabolic activity rise. In order for fish to recuperate energy during stressful situations, a higher level of plasma glucose may be needed25. High glucose levels often signify increased stress in fish, and plasma glucose is considered a key stress indicator in these animals71. Increased levels of AST and ALT enzymes may suggest the degeneration and/or destruction of the liver. Additionally, according to72, these enzymes may be used to evaluate the toxicity of supplements and/or diets compared to the probiotic-free control group and increased levels of AST and ALT enzymes may suggest the degeneration and/or destruction of the liver. The probiotic-supplemented groups in the current study showed lower values of these parameters, indicating that probiotics produced optimal physiological function and improved liver health state in fish. Noteworthy that levels of total protein in blood are correlated with liver-synthesized protein, while elevated blood protein levels may be linked to a stronger innate immune response73.
Numerous studies agreed with our findings74. reported that garlic acts as a natural antibiotic, considering its allicin content, and has been shown to decrease blood pressure, cholesterol, and blood sugar. According to69 juvenile starlet sturgeon fed garlic extracts showed a substantial decrease in blood glucose levels. Furthermore75, discovered that garlic may enhance rainbow trout’s (Oncorhynchus mykiss) antioxidative status; however, the amount of garlic in the feed needs to be well adjusted. In the same positive track, dietary probiotics reduced plasma GOT (or aspartate aminotransferase, AST) and GPT (or alanine aminotransferase, ALT) levels, which are often used for the evaluation of liver enzymes76.
Notably and unmistakably diets supplemented with probiotics or phytobiotics have significant effects on stress tolerance capacity, immunity of fish and have a potential efficacy in diminishing oxidative stress among aquatic organisms, including fish and shellfsh77,78. In this study, several systemic, non-specific immune functions are enhanced by dietary Photobiotic/probiotic supplementation, including lysozyme activity, IgM, Complement C3, IL-10, and Nitric oxide (NO). lysozyme activity peripheral, blood immune cell counts, alternative complement activity, phagocytic ability of leucocytes, neutrophil migration and adherence, plasma bactericidal activity, respiratory burst, myeloperoxidase, and superoxide dismutase activities. According to79, there appears a correlation between elevated lysozyme levels in fish blood and phagocyte, or WBC, production. The ability of β-glucan to stimulate phagocytic cells to produce antimicrobial compounds, including lysosomal enzymes, the complement system, and reactive oxygen species (ROS), is commonly acknowledged and has played a vital role as a chemical signaling molecule within the organism. Also, treated Zebrafish with probiotics showed lower levels of oxidative stress, which may indicate improved hepatic stress tolerance80. Furthermore, fish blood has a high concentration of the primary systemic isotype, IgM, which is essential for pathogen neutralization and opsonization81.
Actually, antioxidant enzymes like SOD, CAT, and GSH protect fish cells against the harmful effects of several free radicals, including superoxide and hydrogen peroxide82. However, oxidative stress caused a number of fatty acids to be oxidized, which results in the production of malondialdehyde (MDA).
MDA and SOD have opposing antioxidant activity, and MDA is an indicator of lipid peroxidation and cell injury83. All immunological indices and antioxidant activity were higher in the treated groups, especially Group 2, rather than the control group, which had the highest MDA level, according to our data. Fish welfare can therefore be protected by a well-developed antioxidant system that guards against the oxidation of such fatty acids. Several studies reported that different dietary probiotics or phytobotics can increase lysozyme and improve the fish antioxidant system as well as ameliorate oxidative stress84. Similar effects have also been observed in several fish species that showed enhanced immunity by applying probiotic, prebiotic, and symbiotic feeding85. Major mechanisms of action of probiotics and phytopiotcs include enhancement of epithelial barrier function, improved adhesion to intestinal cells and pathogen inhibition by occupying adhesion sites, production of antibacterial substances, and regulation of the immune function86.
Carcass proximate analysis showed that fish fed a dietary phytobiotic/probiotics (Groups 1and 2) supplemented diets had significantly higher protein content and lower fat content compared with the other treatments. This increase in protein content could have resulted from increased nutrient deposition. This is consistent with studies on probiotic-fed Oeochromis niloticus65,87,88. However, other research has shown that probiotics had no influence on the amount of protein, fat, or ash89. According to90 there was no discernible impact of probiotics or herbs on the overall body composition of young fish. Similarly91, found that garlic extract supplementation had no effect on the whole-body amino acid composition of juvenile sterlet sturgeon (Acipenser ruthenus). Whatever, the production of fish with more protein and less fat is more suitable for consumers, as recorded by64,92.
Conclusion
In general, feed additives of commercial mixture phytobiotic/probiotic at doses of 200 Garlex®, 400 Superimmune®, and 200 GallPro-200® mg kg⁻¹ lead to improved fish growth performance, feed efficiency, and boosted immunological and antioxidant enzyme activities of O. niloticus under unexchanged water. Future research should focus on the sustainability and optimal use of water resources. In cases of fish culturing under unchanged water, the average water quality should be measured over short periods and correlated with growth rates, stress resistance indicators, and gene expression. Hence, when growth rates decline, it should be intervened in by partially altering the water. Through changes in gene expression, more resilient strains to deteriorating water quality can be developed.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
FAO. The state of world fisheries and aquaculture 2022. Towards blue transformation. Rome https://doi.org/10.4060/cc0461en (2022).
GAFRD. General Authority for Fish Resources Development. Annual Fishery Statistics Report (Cairo, 2020).
Ita, B. N., Essien, J. P. & Ebong, G. A. Heavy metal levels in fruiting bodies of edible and non-edible mushrooms from the Niger delta regionof Nigeria. J. AgricSocSci. 2 (2), 84–87 (2006).
Eltholth, M., Fornace, K., Grace, D., Rushton, J. & Häsler, B. Characterisation of production, marketing and consumption patterns of farmed tilapia in the nile delta of Egypt. Food Policy. 51, 131–143 (2015).
Khalil, H. S. & Nasr-Allah, A. Comparative study on the effect of raceway and In-Pond raceway systems on different nile tilapia, Oreochromis niloticus strains fed diets replacing soybean meal by poultry byproduct meal on: water quality, growth performance and production efficiency. Aquacult. Int. 33 (4), 258 (2025).
Zimmermann, S., Kiessling, A. & Zhang, J. The future of intensive tilapia production and the circular bioeconomy without effluents: Biofloc technology, recirculation aquaculture systems, bio-RAS, partitioned aquaculture systems and integrated multitrophic aquaculture. Reviews Aquaculture. 15, 22–31 (2023).
Shaalan, M., El-Mahdy, M., Saleh, M. & El-Matbouli, M. Aquaculture in egypt: insights on the current trends and future perspectives for sustainable development. Reviews Fisheries Sci. Aquaculture. 26 (1), 99–110 (2018).
Kaleem, O. & Sabi, A. F. B. S. Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquaculture Fisheries. 6 (6), 535–547 (2021).
Sakai, M. Current research status of fish immunostimulants. Aquaculture 172 (1–2), 63–92 (1999).
Islam, S. M., Rohani, M. F. & Shahjahan, M. Probiotic yeast enhances growth performance of nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquaculture Rep. 21, 100800 (2021).
Abdel-Aziz, M., Bessat, M., Fadel, A. & Elblehi, S. Responses of dietary supplementation of probiotic effective microorganisms (EMs) in Oreochromis niloticus on growth, hematological, intestinal histopathological, and antiparasitic activities. Aquacult. Int. 28, 947–963 (2020).
Yusuf, A. et al. Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, micropterus salmoides. Aquaculture Rep. 17, 100383 (2020).
Jasim, S. A. et al. Dietary Quercetin improved growth, body composition, haematology, immunity and resistance to Aeromonas hydrophila infection in common carp (Cyprinus carpio). Aquac. Res. 53 (18), 6910–6920 (2022).
Mocanu, E. E., Savin, V., Popa, M. D. & Dima, F. M. The effect of probiotics on growth performance, haematological and biochemical profiles in Siberian sturgeon (Acipenser baerii Brandt, 1869). Fishes 7 (5), 239 (2022).
Ayyat, M. S. et al. Effects of a blend of herbal feed supplements on growth, associated blood indices and body chemical analysis in nile tilapia reared under high stocking density. Aquac. Res. 53 (16), 5475–5485 (2022).
Abdel-Aziz1, M. F. et al. Growth performance, feed utilization, hematological parameters, and histological features of nile tilapia (Oreochromis niloticus) fed diets with supplementary herbal extracts under prolonged water exchange ann. Anim. Sci. 23 (4), 1147–1157 (2023).
Adel, M., Pourgholam, R., Zorriehzahra, J. & Ghiasi, M. Hemato–Immunological and Biochemical parameters, Skin Antibacterial activity, and Survival in Rainbow Trout (Oncorhynchus mykiss) Following the Diet Supplemented with Mentha Piperita against Yersinia Ruckeri Vol. 55, 267–273 (Fish & shellfish immunology, 2016).
El-Dakar, Y. et al. Assessing chamomile and marjoram meals as feed additives on growth indices and haematological parameters of nile tilapia (Oreochromis niloticus) reared under Biofloc system. Sci. Afr. 21, e01755 (2023).
Abarike, E. D. et al. Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 82, 229–238 (2018).
Callier, M. D. et al. Attraction and repulsion of mobile wild organisms to finfish and shellfish aquaculture: a review. Reviews Aquaculture. 10 (4), 924–949 (2018).
Saleh, H. H. et al. Evaluation of some commercial water additives of probiotics and Yucca on water quality, blood biochemistry, sex hormones, reproductive and fry performance of nile tilapia (Oreochromis niloticus) that were reared under unchanged water. Italian J. Anim. Sci. 24 (1), 1224–1238 (2025).
Harikrishnan, R., Balasundaram, C. & Heo, M. S. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317 (1–4), 1–15 (2011).
Bhatnagar, A. & Saluja, S. Role of Zingiber officinale and autochthonous probiotic Bacillus coagulans in feeds of Catla Catla (Hamilton, 1822) for growth promotion, immunostimulation, histoprotection, and control of DNA damage. Fish Physiol. Biochem. 47, 2081–2100 (2021).
Sahu, S., Das, B. K., Mishra, B. K., Pradhan, J. & Sarangi, N. Effect of allium sativum on the immunity and survival of Labeo Rohita infected with Aeromonas hydrophila. J. Appl. Ichthyol. 23 (1), 80–86 (2007a).
Xu, A. et al. Effects of Garlic powder on feedingattraction activity, growth and digestive enzyme activities of Japanese seabass, lateolabrax japonicus. Aquacult. Nutr. 26, 390–399 (2020).
Ji, J., Lu, C., Kang, Y., Wang, G. X. & Chen, P. Screening of 42 medicinal plants for in vivo anthelmintic activity against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol. Res. 111, 97–104 (2012).
Metwally, M. A. A. Effects of Garlic (Allium sativum) on some antioxidant activities in tilapia Nilotica (Oreochromis niloticus). World J. fish. Mar. Sci. 1 (1), 56–64 (2009).
Amoah, K. et al. Effects of varying dietary black Garlic supplementation on the growth, immune response, digestive and antioxidant activities, intestinal microbiota of Litopenaeus vannamei and its resistance to vibrio parahaemolyticus infection. Aquacult. Nutr. 27 (5), 1699–1720 (2021).
APHA. Standard Methods for the Examination of Water and Waste Water 19th edn (NewYork, 1995).
Abdel-Aziz, M. F. et al. Unchanged Water Stress Induces Growth retardation, Histopathological alterations, and antioxidant-immune Disruptions in Oreochromis Niloticus: the Promising Role of Dietary Organic Acids 1–22 (Aquaculture International, 2024).
Feldman, B. F., Zink, J. G. & Jain, N. C. Schalm’s Veterinary Hematology 5th edn (Lippincott Williams and Wilkins, 2000).
Dacie, J. V. & Lewis, S. M. Practical Haematology. In: Lewis, S.M., B.J. Bain, I. Bates, (Eds.), 9th Edn., Churchill Livingstone, Harcourt Publishers Limited, London, pp: 444–451. (2001).
Hamid, N. et al. An integrated assessment of ecological and human health risks of per-and polyfluoroalkyl substances through toxicity prediction approaches. Sci. Total Environ. 905, 167213 (2023).
Sahu, S. et al. Effect of magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo Rohita fingerlings. Fish Shellfish Immunol. 23 (1), 109–118 (2007b).
Dunier, M. & Siwicki, A. K. Effects of pesticides and other organic pollutants in the aquatic environment on immunity of fish: a review. Fish Shellfish Immunol. 3 (6), 423–438 (1993).
Li, X. et al. IL-35 is a novel responsive anti-inflammatory cytokine—a new system of categorizing anti-inflammatory cytokines. PloS One. 7 (3), e33628 (2012).
Montgomery, H. A. C. & Dymock, J. F. The rapid determination of nitrate in fresh and saline waters. Analyst 87 (1034), 374–378 (1962).
Mirghaed, A. T., Fayaz, S. & Hoseini, S. M. Dietary 1, 8-cinoele affects serum enzymatic activities and immunological characteristics in common carp (Cyprinus carpio) exposed to ambient ammonia. Aquac. Res. 50 (1), 146–153 (2019).
AOAC. Association of Official Analytical Chemists. International Official methods of Analysis, Washington, DC, USA, Official methods 925.09, 923.03, 979.09, 962.09, 4.5.01, and 923.05, Vol. II, 17thedition. (2000).
NRC. (National Research Council) Nutrient Requirements of Fish and Shrimp (National Academy, 1993).
Putra, I. et al. Effect of different Biofloc starters on ammonia, nitrate, and nitrite concentrations in the cultured tilapia Oreochromis niloticus system. F1000Res 9, 293 (2020).
El-Shafai, S. A. et al. Gijzen. Chronic ammonia toxicity to duckweed-fed tilapia (Oreochromis niloticus). Aquaculture 232(1–4), 117–127 (2004).
El-Sherif, M. S. & El-Feky, A. M. Effect of ammonia on nile tilapia (Oreochromis niloticus) performance and some hematological and histological measures. (2008).
Constantino, R. V., Templonuevo, R. M. C. & Fajardo, L. J. Stress responses of red tilapia (Oreochromis spp.) to high ammonia levels. Int. J. Fisheries Aquat. Stud. 7(6), 89–93 (2019).
John, A. H. & Semra, K. Effects of dial un-ionized ammonia fluctuation on juvenile hybrid striped bass, channel catfish, and blue tilapia. Aquaculture 195, 163–181 (2001).
da Silva, F. J. R., Lima, F. R. S., do Vale, D. A. & do Carmo, M. V. High levels of total ammonia nitrogen as NH4 + are stressful and harmful to the growth of nile tilapia juveniles. Acta Scientiarum Biol. Sci. 35 (4), 475–481 (2013).
Ballester-Moltó, M., Sanchez-Jerez, P., Cerezo-Valverde, J. & Aguado-Giménez, F. Particulate waste outflow from fish-farming cages. How much is uneaten feed? Mar. Pollut. Bull. 119 (1), 23–30 (2017).
Rurangwa, E. & Verdegem, M. C. Microorganisms in recirculating aquaculture systems and their management. Reviews Aquaculture. 7 (2), 117–130 (2015).
Zibiene, G. & Zibas, A. Impact of commercial probiotics on growth parameters of European catfish (Silurus glanis) and water quality in recirculating aquaculture systems. Aquacult. Int. 27 (6), 1751–1766 (2019).
Francis, G., Makkar, H. P. & Becker, K. Quillaja saponins—a natural growth promoter for fish. Anim. Feed Sci. Technol. 121 (1–2), 147–157 (2005).
Nayak, S. K. Role of Gastrointestinal microbiota in fish. Aquac. Res. 41 (11), 1553–1573 (2010).
Hongsheng, M. Effects of probiotics a on activities of digestive enzyme for tilapia. J. South. China Normal Univ. (Natural Sci. Edition), 1(2). (2010).
Abd El-Rhman, A. M., Khattab, Y. A. & Shalaby, A. M. Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of nile tilapia, Oreochromis niloticus. Fish. Shellfish Immunol. Aug. 27 (2), 175–180 (2009). Epub 2009 Apr 8. PMID: 19361560.
Yılmaz, E. et al. Combined effects of dietary probiotic Lactobacillus sp. and prebiotic glycyrrhizic acid on growth Performance, feed utilization and digestive enzymes activities of nile tilapia (Oreochromis niloticus) juveniles. Aquaculture Stud., 23(3), AQUAST1228 (2022).
Pirarat, N. et al. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 91 (3), e92–e97 (2011).
Lee, S. et al. Effects of extruded pellet and moist pellet on growth performance, body composition, and hematology of juvenile Olive flounder, Paralichthys Olivaceus. Fisheries Aquat. Sci. 19, 1–6 (2016).
Khalil, R. H., Nadia, B. M. & Soliman, M. K. Effects of biogen and Levamisol Hcl on the immune response of cultured Oreochromis niloticus to Aeromonas hydrophila vaccine. Beni-Suef Veterinary Med. J. Egypt. 11, 381–392 (2001).
Elkayam, A., Mirelman, D., Peleg, E., Wilchek, M., Miron, T., Rabinkov, A., … Rosenthal,T. (2003). The effects of allicin on weight in fructose-induced hyperinsulinemic,hyperlipidemic, hypertensive rats. American journal of hypertension, 16(12), 1053–1056.
Gill, C. Herbs and Plant Extracts as Growth Promoters 20–23 (Feed international, 1999).
Putnik, P., Gabrić, D., Roohinejad, S., Barba, F. J., Granato, D., Mallikarjunan,K., … Kovačević, D. B. (2019). An overview of organosulfur compounds from Allium spp.:From processing and preservation to evaluation of their bioavailability, antimicrobial,and anti-inflammatory properties. Food chemistry, 276, 680–691.
Adel, M., Lazado, C. C., Safari, R., Yeganeh, S. & Zorriehzahra, M. J. Aqualase®, a yeast-based in-feed probiotic, modulates intestinal microbiota, immunity and growth of rainbow trout Oncorhynchus Mykiss. Aquac Res. 48, 1815–1826 (2017).
Yanbo, W. & Zirong, X. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim. Feed Sci. Technol. 127, 283–292 (2006).
Abdel-Tawwab, M., Abdel-Rahman, A. M. & Ismael, N. E. M. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for fry nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture 280, 185–189. 10.1016/j (2008).
Hassaan, M. S., Soltan, M. A., Jarmołowicz, S. & Abdo, H. S. Combined effects of dietary malic acid and Bacillus subtilis on growth, gut microbiota and blood parameters of nile tilapia (Oreochromis niloticus), Aquac. Nutr 24, 83–93 (2018).
El-Haroun, E. R., Goda, A. S. & Kabir Chowdhury, M. A. Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of nile tilapia Oreochromis niloticus (L). Aquac. Res. 37 (14), 1473–1480 (2006).
Goda, A. M. A., Mabrouk, H. A. H. H., ,Wafa, M. A. E. H. & El-Afifi, T. M. Effect of using baker’s yeast and exogenous digestive enzymes as growth promoters on growth, feed utilization and hematological indices of nile tilapia, Oreochromis niloticus fingerlings. J. Agric. Sci. Technol. 2 (1), 939–1250 (2012).
Hossain, M. S. et al. Nucleoside by-product dietary supplementation influences blood chemistry, immune response, oxidative stress resistance and intestinal morphology of juvenile amberjack, Seriola Dumerili. Aquacult. Nutr. 23 (6), 1390–1400 (2017).
Campbell, T. Clinical chemistry of Fsh and amphibians. In Veterinary Hematology and Clinical Chemistry (eds Thrall, M. et al.) 499–517 (Lippincott Williams & Wilkins, 2004).
Lee, J., Choi, I. C., Kim, K. T., Cho, S. H. & Yoo, J. Y. Response of dietary substitution of fishmeal with various protein sources on growth, body composition and blood chemistry of Olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Fish Physiol. Biochem. 38, 735–744 (2016).
Montero, D., Izquierdo, M. S., Tort, L., Robaina, L. & Vergara, J. M. High stocking density produces crowding stress altering some physiological and biochemical parameters in Gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 20, 53–60 (1999).
Eslamloo, K., Falahatkar, B. & Yokoyama, S. Effects of dietary bovine lactoferrin on growth, physiological performance, iron metabolism and non-specific immune responses of Siberian sturgeon acipenser Baeri. Fish Shellfish Immunol. 32 (6), 976–985 (2012).
Bhardwaj, S., Srivastava, M. K., Kapoor, U. & Srivastava, L. P. A 90 days oral toxicity of Imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem. Toxicol. 48 (5), 1185–1190 (2010).
Wiegertjes, G. F., Stet, R. M., Parmentier, H. K. & van Muiswinkel, W. B. Immunogenetics of disease resistance in fish: a comparative approach. Dev. Comp. Immunol. 20 (6), 365–381 (1996).
Olusola, S. E., Emikpe, B. O. & Olaifa, F. E. The potentials of medicinal plant extracts as bio-antim.icrobials in aquaculture. (2013).
Mohebbi, A., Nematollahi, A., Dorcheh, E. E. & Asad, F. G. Influence of dietary Garlic (A Llium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss). Aquac. Res. 43 (8), 1184–1193 (2012).
Xia, Y., Wang, M., Gao, F., Lu, M. & Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile nile tilapia (Oreochromis niloticus). Anim. Nutr. 6 (1), 69–79 (2020).
Addo, S. et al. Effects of Bacillus subtilis strains on growth, immune parameters, and Streptococcus iniae susceptibility in nile tilapia, Oreochromis niloticus. J. World Aquaculture Soc. 48 (2), 257–267 (2017).
Dawood, M. A., Koshio, S., Ishikawa, M. & Yokoyama, S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, pagrus major. Fish Shellfish Immunol. 45 (1), 33–42 (2015).
Engstad, R. E., Robertsen, B. & Frivold, E. Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol. 2 (4), 287–297 (1992).
Gioacchini, G. et al. The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish 11 (2), 98–106 (2014).
Cuesta, A., Meseguer, J. & Esteban, M. A. Total serum Immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata). Vet. Immunol. Immunopathol. 101 (3–4), 203–210 (2004).
Yousefi, M. et al. The protective effects of dietary Garlic on common carp (Cyprinus carpio) exposed to ambient ammonia toxicity. Aquaculture 526, 735400 (2020).
Tang, Z., Sun, H., Chen, T., Lin, Z., Jiang, H., Zhou, X., … Yu, X. (2017). Oral delivery of Bacillus subtilis spores expressing cysteine protease of Clonorchis sinensis to grass carp (Ctenopharyngodon idellus): induces immune responses and has no damage on liver and intestine function. Fish & shellfish immunology, 64, 287–296.
Yousefi, M. et al. Effects of dietary malic acid supplementation on growth performance, antioxidant and immunological parameters, and intestinal gene expressions in rainbow trout, Oncorhynchus Mykiss. Aquaculture 563, 738864 (2023).
Adloo, M. N., Soltanian, S., Hafezieh, M. & Ghadimi, N. Effects of long term dietary administration of β-Glucan on the growth, survival, and some blood parameters of striped catfish, Pangasianodon hypophthalmus (Siluriformes: Pangasiidae). Iran. J. Ichthyol. 2 (3), 194–200 (2015).
Rijkers, G. T., Bengmark, S., Enck, P., Haller, D., Herz, U., Kalliomaki, M., … Antoine,J. M. (2010). Guidance for substantiating the evidence for beneficial effects of probiotics:current status and recommendations for future research. The Journal of nutrition,140(3), 671S-676S.
Lara-Flores, M. & Olvera-Novoa, M. A. The use of lactic acid bacteria isolated from intestinal tract of nile tilapia (Oreochromis niloticus), as growth promoters in fish fed low protein diets. Lat Am. J. Aquat. Res. 41, 490–497 (2013).
Opiyo, M. A., Jumbe, J., Ngugi, C. C. & Charo-Karisa, H. Different levels of probiotics affect growth, survival and body composition of nile tilapia (Oreochromis niloticus) cultured in low input ponds. Sci. Afr. 4, 40 (2019).
Merrifield, D. L., Bradley, G., Baker, R. T. M. & Davies, S. J. Probiotic applications for rainbow trout (Oncorhynchus Mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac Nutr. 16, 496–503 (2010).
Güroy, D. et al. Interaction of Dietary Garlic (Allium sativum), Onion (Allium cepa), and Probiotic on the Growth Performance and Health Status of Juvenile Rainbow Trout (Oncorhynchus mykiss) 1–14 (Aquaculture International, 2024).
Lee, J. Y. & Gao, Y. Review of the application of garlic, allium sativum, in aquaculture. J. World Aquaculture Soc. 43 (4), 447–458 (2012).
Bagheri, T., Hedayati, S. A., Yavari, V., Alizade, M. & Farzanfar, A. Growth, survival and gut microbial load of rainbow trout (Onchorhynchus mykiss) fry given diet supplemented with probiotic during the two months of first feeding. Turk. J. Fish. Aquat. Sci. 8, 43–48 (2008).
Acknowledgements
Authors would like to thank all staff members of Aquaculture Department, Faculty of Aquaculture and Marine fisheries, Arish University, Egypt for supporting this research. Also, authors would like to acknowledge all members and staff of Water Resources Research Station, National institute of Oceanography and Fisheries NIOF in Fayoum, Egypt for their help and support in completing this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed F. Abdel-Aziz, Rabab M. Alkaradawe, Hamed H. E. Saleh, Fatma Ragab Abouel Azm, Mohamed A. Elokaby, Abdel-Moneim M. Yones, Dalia S. Hamza and Naglaa R.A. Kasem : Conceptualization, Methodology, Formal analysis, Supervision, Investigation, Resources, Writing – review, editing. Mohamed F. Abdel-Aziz: Writing – original draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All research protocols of this work were ethically approved by the National Institute of Oceanography and Fisheries’ (NIOF, Egypt) Committee for Ethical Care and Use of Animals/Aquatic Animals (NIOF-IACUC) with code number (NIOF-AQ4-F-24-R-022). The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations and that the study is reported in accordance with the ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aziz, M.F., Alkaradawe, R.M., Saleh, H.H.E. et al. Growth, immunity, and antioxidant activity responses of phytobiotics and probiotics incorporated into Oreochromis niloticus diets under stressors of unchanged water. Sci Rep 15, 44668 (2025). https://doi.org/10.1038/s41598-025-30248-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-30248-2