Abstract
The prevalence of autoimmune diseases such as inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) is increasing. Glucocorticoid-induced TNFR-related protein (GITR), a TNF receptor superfamily (TNFRSF) member, is activated by GITR-ligand (GITRL). GITR signaling is pathogenic in models of RA and IBD, leading to lymphocyte proliferation and secretion of pro-inflammatory cytokines. Despite promising preclinical data, GITR neutralization in autoimmune diseases remains under-explored, due to challenges in avoiding antibody-mediated GITR activation. Therefore, we developed a human GITR-specific antibody that inhibits GITRL-mediated GITR-signaling, while preserving the GITRL epitope on GITR. The antibody strongly inhibited GITR signaling in the in vitro assays via a novel mechanism of disrupting downstream higher-order structures rather than direct blocking of GITR binding. Even though the antibody did not demonstrate efficacy in an NSG human skin graft transplant model, this general mechanism might be a viable therapeutic intervention for other TNFRSF members relying more significantly on soluble ligands.
Similar content being viewed by others
Introduction
Autoimmune diseases are a major public health concern, illustrated clearly by a 2023 study showing that autoimmune disorders affect at least 10.2% of the UK population1. Furthermore, the study showed that in many autoimmune disorders, such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), and Sjögren’s syndrome (SS), the disease incidence has significantly increased over the last two decades1, suggesting that the prevalence of various autoimmune disorders is on the rise, a finding supported by world-wide epidemiology data2. Tumor Necrosis Factor α (TNF-α) neutralizing biotherapeutics are a mainstay in treatment of many of these autoimmune diseases, where the approved drug modalities are either monoclonal antibodies (e.g., adalimumab and infliximab) or soluble TNF receptor (etanercept). TNF-α is the prototypical member of the TNF superfamily (TNFSF). Its pro-inflammatory effects are thought to arise from signaling via the TNF receptor 1 (TNFR1) through nuclear translocation of the transcription factor NF-κB, resulting in transcriptional induction of multiple pro-inflammatory genes. The TNFSF has 19 members which can bind to 29 members of the TNF receptor superfamily (TNFRSF), and downstream signaling of multiple members of the TNFRSF, in addition to TNFR1, are known to lead to NF-κB activation3,4. Therefore, it is logical that various drug discovery efforts have been focused on identifying therapeutic interventions to additional members of the TNFSF/TNFRSF besides the prototypical TNF/TNFR1 ligand-receptor pair, and several of those efforts are showing early promise in clinical trials, including targeting CD40L/CD40, OX40L/OX40, and TL1A/DR3 ligand-receptor pairs (reviewed in5).
Multiple examples exist of agonistic antibodies designed against the TNFRSF for treatment of cancer, while the scientific community has mostly focused on developing neutralizing antibodies against the ligands, i.e., TNFSF, for the treatment of autoimmune diseases. However, targeting the receptor instead of the ligand can offer benefits for clinical development. These benefits include superior target coverage and a reduced risk of immunogenicity, as there are no immunogenic soluble drug/ligand complexes, which has been observed with TNF inhibitors6. The main reason there is a scarcity of blocking antibodies against TNFRSF members is that most antibodies binding TNFRSF members have propensity to induce signaling downstream of binding7. Nonetheless, in the last few years, studies have shown that it is possible to inhibit TNFRSF with an antibody (Ab) by locking the receptors in a non-signaling conformation as demonstrated for TNFR2 and CD408,9.
Glucocorticoid-induced TNFR-related protein (GITR, encoded by TNFRSF18), a member of the TNFRSF, is activated by its cognate ligand GITR-ligand (GITRL, encoded by TNFSF18). GITR signaling has been demonstrated to be pathogenic in mouse models of RA10,11,12,13 and IBD14,15,16. GITR stimulation leads to induction of lymphocyte proliferation, and secretion of pro-inflammatory cytokines, including IL-9, IL-13, and IL-217,18,19. Furthermore, GITR stimulation has emerged as an intriguing mechanism in immuno-oncology where GITR-agonistic Ab has been shown to deplete GITR + T regulatory cells (Tregs) in a clinical trial20. This mechanism aligns with findings that GITR signaling destabilizes Tregs15. Taken together, GITR is a promising therapeutic target in autoimmune diseases to restore the balance of T cells from T effector cells to Tregs.
TNFSF/TNFRSF signaling in humans involves ligands binding to receptors in a 3:3 stoichiometry, where the ligands are usually homotrimers, and the receptors are homodimers where only one monomer from each receptor dimer can bind each trimeric ligand molecule. This stoichiometry results in a hexagonal honeycomb structure on the cell surface between the ligands and the receptors21. Furthermore, signal transduction requires intracellular trimerization of TNF receptor associated factor (TRAF) proteins, which results in a corresponding intracellular hexagonal honeycomb structure via the dimerization of the RING-domains on the TRAF proteins22,23,24. Interestingly, GITRL is one of two unique TNFSF members that exist as dimers in the mouse in contrast to trimers in humans22. Therefore, linear oligomerization of the mouse GITR (muGITR) results in GITR signaling, while human GITR (hGITR) requires the hexagonal oligomerization for signaling. This difference is of paramount importance for researching this pathway and can make mechanistic discoveries using GITR-antibodies in murine pre-clinical studies challenging to translate to human biology.
In this study, we developed a human hGITR-specific antibody which inhibits GITRL-mediated GITR signaling, while having the unique property of conserving GITRL binding to GITR. This mechanism of inhibiting GITR signaling, and even more broadly, any TNFRSF signaling, is novel. Here we investigated whether such GITR antagonistic mechanism might constitute an effective strategy in suppressing T cell mediated pro-inflammatory responses in vitro and in vivo.
Results
Ligand-non-competitive hGITR-nc-Ab-#1 inhibits GITRL-mediated signaling
We set out to identify a GITR-antagonistic Ab that could bind GITR at an epitope non-overlapping with the GITRL epitope, while still blocking the GITRL-mediated signaling, similarly to what has been described for TNFR2 and CD40 antibodies8,9. Through a mouse immunization and hybridoma Ab campaign we screened and successfully identified multiple antibodies which bind recombinant GITR without blocking subsequent binding of recombinant GITRL, indicating that these antibodies are ligand-non-competitive (Fig. 1A). To determine if any of these antibodies could inhibit GITRL-mediated signaling through GITR, we utilized transgenic GITR-overexpressing Jurkat cells expressing luciferase gene under the control of NF-κB responsive promoter (GITR-Jurkat-NF-κB-Luc), which express luciferase upon GITRL-stimulation (Fig. 1B). Screening of the biochemical hits in this cell assay resulted in the identification of hGITR-nc-Ab-#1 (nc for ligand non-competitive), which inhibited the GITRL-mediated GITR-activation with an IC50 of 0.45 nM (95% CI: 0.34 nM – 0.59 nM) (Fig. 1C). The antagonistic effect was not dependent on the bivalency of the antibody since one-armed variant of hGITR-nc-Ab-#1 was capable in inhibiting GITRL-mediated GITR-activation (Fig. 1D).
Discovery and characterization of ligand non-competitive NFκB neutralizing anti-GITR antibody. (A) Discovery of ligand competitive and non-competitive antibodies from mice immunization campaign measured by antibody mediated % inhibition of human GITRL binding to recombinant human GITR protein via competition ELISA. (B) Recombinant GITRL protein induces NFκB signaling in GITR-NFκB-Luciferase-Jurkat cell line. (C) Bivalent ligand non-competitive anti-GITR antibody (hGITR-nc-Ab-#1) neutralizes recombinant GITRL protein induced NFκB signaling. (D) Monovalent one-armed ligand non-competitive anti-GITR antibody (hGITR-nc-Ab-#1-OA) also neutralizes recombinant GITRL protein induced NFκB signaling.
hGITR-nc-Ab-#1 and #2 prevent the trimeric GITRL from binding GITR in a correct stoichiometry
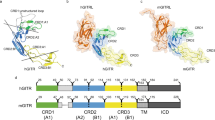
To understand the mechanism of action of hGITR-nc-Ab-#1, we crystallized the hGITR-nc-Ab-#1 Fab fragment with recombinant hGITR extracellular domain (ECD) protein and solved the structure of the complex at 2.86 Å resolution (Fig. 2A; Table 2). Structural analysis of the complex indicates that the hGITR-nc-Ab-#1 epitope centers on GITR cysteine-rich domain (CRD) 2 with peripheral contacts on GITR CRD1 and CRD3 (Fig. 2B). While it was reported that GITRL also interacts with GITR CRD222, the analysis reveals that the hGITR-nc-Ab-#1 epitope does not overlap with the GITRL binding site. Furthermore, structural superposition of the GITR molecules from GITR/hGITR-nc-Ab-#1-Fab and GITR/GITRL complexes suggests that hGITR-nc-Ab-#1 and GITRL can bind GITR simultaneously without structural interferences (Fig. 2B). These findings support our binding data and antibody selection criteria. Interestingly, based on the crystallography data, it becomes apparent that hGITR-nc-Ab-#1 would be unable to bind membrane-associated GITR if the GITR molecule is anteriorly interacting with a GITRL-trimer in the functional 3:3 stoichiometry, due to a steric hindrance from the cell membrane preventing the Ab from binding its epitope (Fig. 2C). Because GITR-GITRL signaling complex is believed to form a higher order honeycomb structure resulting from dimeric GITR binding trimeric GITRL22, we propose a working model to explain the lack of GITRL-mediated signaling observed in the NF-κB assay when hGITR-nc-Ab-#1 was applied (Fig. 2D). We speculate that the binding of hGITR-nc-Ab-#1 disrupts the GITR-GITRL honeycomb structure and leads to the inhibition of GITR-GITRL signaling, even though GITRL would still be able to bind GITR. To experimentally validate the model of possible concurrent binding of hGITR-nc-Ab-#1 and GITRL to GITR on cells, prior to adding trimeric hGITRL, the GITR-expressing primary human T cells were incubated with hGITR-nc-Ab-#2, an affinity-optimized variant of hGITR-nc-Ab-#1 (Table 1) which binds the same epitope. Pre-incubation with recombinant hGITR-nc-Ab-#2 did not inhibit the binding of recombinant soluble GITRL to GITR-expressing cells. Instead, it led to an approximately two-fold increase in the recombinant GITRL-binding signal compared to cells pre-incubated with an isotype control antibody. This indicates that GITRL trimers are unable to simultaneously engage three GITR molecules in the presence of hGITR-nc-Ab-#2, instead binding to one or two, thereby increasing the apparent GITRL: GITR stoichiometry (Fig. 2B, E,F). As a control we included a ligand-competitive (lc) monovalent antibody hGITR-lc-Ab-#3, a single-digit nM affinity Ab (Table 1). As expected, it completely blocked GITRL binding to GITR-expressing cells (Fig. 2E). Taken together, these results suggest that the mechanism of action of the ligand-non-competitive GITR antagonistic antibodies is a disruption of the hexameric GITR signaling network that does not prevent GITRL binding to its receptor. A comprehensive explanation of the proposed mechanism is shown in Fig. 2F.
Crystal structure of the ligand non-competitive anti-GITR antibody. (A) Structure of the hGITR-nc-Ab-#1 Fab (blue) with GITR (green). GITR is colored by domain. (B) Structural superposition of GITR/hGITR-nc-Ab-#1 Fab and GITR/GITRL complexes suggests that hGITR-nc-Ab-#1 and GITRL can bind GITR simultaneously without structural interferences. The amino acids constituting the Ab-epitope on GITR are highlighted in red, while the amino acids for the GITRL-epitope are highlighted in purple. (C) Structural model showing that the cell membrane sterically hinders hGITR-nc-Ab-#1 from binding to GITR if GITR is pre-complexed with GITRL in a 3:3 stoichiometry. (D) GITRL-binding to GITR in a 3:3 stoichiometry is believed to form a higher order honeycomb structure (enforced by extracellular engagement of GITR/GITRL binding and intracellular association of GITR/TRAFs) essential for effective signaling (left). The binding of hGITR-nc-Ab-#1 disrupts the 3:3 stoichiometry of binding between GITR and GITRL, and that prevents formation of honeycomb structures, and thus prevents signaling even in the presence of bound GITRL (right). (E) Experimental validation using activated primary human T cells, showing that blocking the epitope identified with Ab-#1, does not prevent GITRL from binding to the cells, but rather enhances GITRL binding (hGITR-nc-Ab-#2 in blue). A ligand-competitive Ab, hGITR-lc-Ab-#3, is shown in red. (F) Model of mechanism of action of hGITR-non-competitive antibodies.
High-affinity hGITR-nc-Ab-#2 prevents GITRL-mediated cytokine release from primary T cells
Further characterization of hGITR-nc-Ab-#2 showed binding to the GITR-Jurkat-NF-κB-Luc cells with an EC50 of 38 pM (Fig. 3A), where it inhibited GITRL-mediated NF-κB activation with an IC50 of ~ 130 pM (Fig. 3B). Next, using primary cells, we set out to test hGITR-nc-Ab-#2 in a competition assay with hGITRL. Peripheral blood mononuclear cells (PBMCs), stimulated via TCR-activation provide a more physiologically relevant readout than the short-term NF-κB assay, due to being a more relevant cell type for human diseases, and also because GITR surface expression on T cells increases in the course of the experiment. In this assay, hGITR-nc-Ab-#2 successfully inhibited in a dose-dependent manner both IL-9 and IL-13 secretion induced in the presence of hGITRL, with IC50 of 1.0 and 2.2 nM, respectively (Fig. 3C), suggesting that hGITR-nc-Ab-#2 can prevent GITR-activation in primary human immune cells despite a lack of direct competition with GITRL binding.
High affinity anti-GITR antibody hGITR-nc-Ab-#2 neutralizes GITRL signaling and prevents GITRL mediated cytokine release. (A) hGITR-nc-Ab-#2 binds to GITR-Jurkat-NF-κB-Luc cell line with an EC50 of 38 pM by FACS. (B) hGITR-nc-Ab-#2 neutralizes recombinant GITRL mediated NFKb signaling in GITR-Jurkat-NF-κB-Luc cell line with IC50 of ~ 130pM. (C) Human PBMCs were stimulated for 72 h with TCR stimulation, cytokine cocktail, and recombinant GITRL. hGITR-nc-Ab-#2 inhibits GITRL-mediated secretion of IL-9 and IL-13.
GITRL non-competitive GITR-blockade does not alleviate inflammation in an NSG human skin graft transplant model
Having shown that hGITR-nc-Ab-#2 blocks the pro-inflammatory effect of recombinant GITRL in vitro, we explored if the molecule’s in vitro anti-inflammatory properties translated to in vivo anti-inflammatory pharmacology. As previously described, while GITRL forms a trimer in humans, it exists as a dimer in mice. Any GITR-crosslinking in mouse cells by a bivalent antibody would result in the formation of a linear chained structure sufficient for GITR-activation. This is different from GITR on human cells, which require a higher order honeycomb network for receptor activation. Due to these differences in human and mouse GITRL binding stoichiometry and since the hGITR-nc-Ab-#2 is not mouse cross-reactive (data not shown), to examine the hGITR/hGITRL biology, we employed a humanized mouse model. To this end, we adopted a model where NSG MHC I/II KO mice, engrafted with HLA-A2 + human skin, were injected with allogeneic HLA-A2- human PBMCs intravenously, and 4 weeks later the mice were assessed for skin graft rejection, systemic cytokine levels, and cellular composition both in the blood as well as in the spleen. In this mouse model, following human PBMC engraftment, hGITR and hGITRL were present, including hGITR-expressing T cells (Fig. S1). Additionally, the human skin graft is expected to express both hGITR and hGITRL, based on scRNAseq data accessed through The Human Protein Atlas25,26.
To study in vivo pharmacology, the antibodies were injected intraperitoneally twice a week, starting 1 day before the administration of the human PBMCs (Fig. 4A). hGITR-nc-Ab-#2 was dosed at 15 mg/kg, and was compared to a CD28-Ab, a strong inhibitor of T cell proliferation serving as a positive control. Another anti-GITR antibody hGITR-nc-Ab-#4, which binds to the same epitope as the hGITR-nc-Ab-#2, albeit with somewhat weaker affinity for GITR (Table 1), was also administered in the same study, as both the GITR-antibodies were at this time considered candidates for further development. Based on visual scoring described in Table 3, while skin graft rejection was ameliorated in the CD28-Ab treated mice (q < 0.05), no significant impact was observed in animals treated with hGITR-nc-Ab-#2 or hGITR-nc-Ab-#4, as compared to control treatment with an isotype Ab (Fig. 4B). Of note, considerable variability in the human T cell engraftment efficiency in blood (Fig. 4C) and spleen (Fig. 4D) was found in animals treated with hGITR-nc-Ab-#2 and hGITR-nc-Ab-#4, with no apparent difference to isotype control treated animals. In contrast, administration of the CD28 blocking Ab prevented human T cell engraftment. The engraftment variability observed, due to the technically challenging model, sets a demanding requirement in establishing differences between the treatment groups with this endpoint.
GITRL non-competitive hGITR antibodies did not prevent skin graft rejection in an NSG skin transplant model. (A) Schematic showing the NSG human skin graft transplant model. 8–10 weeks old female NSG mice were engrafted with human abdominal skin from HLA-A2 + donor. 8–10 weeks after cell transplantation, mice were injected intravenously with 10 × 106 human PBMCs from an HLA-A2 mismatched donor. 15 mpk isotype control, 15 mpk hGITRL non-competitive blocking antibodies (hGITR-nc-Ab-#2, and -#4), and 5 mpk anti-CD28 antibody were given i.p. on day − 1 before PBMC injection followed by twice per week injection for 4 weeks. 4 weeks after the PBMC injection, visual scores were taken for the human skin graft. Blood and spleen samples were harvested for human leukocyte engraftment analysis by flow cytometry. (B) Visual scores assessing the skin graft dryness, scabbing, depression, cracking and color change were documented for the mice. The anti-CD28 group showed significantly lower visual scores compared to the isotype control. Neither higher affinity hGITR-nc-ab-#2 nor lower affinity hGITR-nc-ab-#4 prevented graft rejection and showed no difference in visual score compared to isotype control. (C) and (D) Lower but statistically not significantly different, hCD3 engraftment in blood (C) and spleen (D) were seen with anti-CD28 but not with the non-competitive GITR antibodies. Horizontal bar represents average of the group. Visual scores were compared to isotype control controlled for multiple comparison testing with the original FDR method of Benjamini and Hochberg after a Kruskal-Wallis test. Flow cytometry data were compared to isotype control with Dunnett’s multiple comparisons test after one-way ANOVA. **p < 0.01. h human, mpk milligram per kilogram, i.p. intraperitoneal, nc ligand non-competitive.
GITRL-competitive mechanism May offer benefits in vivo over GITRL-non-competitive mechanism
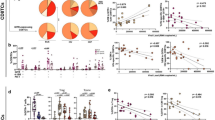
Having failed to observe in vivo effect with hGITR-nc-Ab-#2, we hypothesized that the pharmacology of hGITR-nc-Ab-#2 could exhibit an inverted U-shaped dose-effect curve, and by dosing less antibody, we might observe better efficacy. Hence, instead of the 15 mg/kg dose, we tested hGITR-nc-Ab-#2 at two lower doses, 10 mg/kg and 5 mg/kg in the NSG human skin graft transplant model. Furthermore, we included hGITR-lc-Ab-#3, the GITRL-competitive Ab (Fig. 2E), to investigate if the ligand-competitive mechanism had advantages in vivo over the ligand non-competitive mechanism. We found the visual scores of the grafts were not affected by any of the treatment groups included in this study (Fig. 5A), and neither was the human T cell engraftment in blood or spleen (Fig. 5B, C, respectively). However, the GITRL-competitive Ab, hGITR-lc-Ab-#3, showed some evidence of pharmacology as demonstrated by decreased expression of the activation marker CD69 on CD4 and CD8 T cells, both in circulation and spleen, while the ligand non-competitive hGITR-nc-Ab-#2 had no observable effect on CD69 levels in T cells (Fig. 5D-G). Of interest, following treatment with hGITR-lc-Ab-#3, lower CD69 expression was most detectable on CD8 + cells in circulation (Fig. 5E, p < 0.01 compared to isotype Ab) and on CD4 + T cells in the spleen (Fig. 5F, p < 0.05 compared to isotype Ab). The diminished CD69 expression might reflect lower T cell activation which correlates with a decreased plasma level of IFN-γ, where hGITR-lc-Ab-#3-treated mice have on average a ~ 50% decreased IFN-γ levels compared to isotype Ab treated mice (Fig. 5H, not statistically significant). Taken together, our data show the ligand-competitive mechanism of GITR-inhibition demonstrates pharmacological activity in the context of an in vivo inflammation model, whereas the ligand non-competitive mechanism shows no benefits over an isotype control antibody in the NSG human skin graft transplant model.
GITRL-competitive hGITR blocking antibody ameliorated T cell activation in an NSG human skin graft transplant model, while the non-competitive antibody showed no detectable activity. NSG mice were engrafted with HLA-mismatched human skin grafts and human PBMCs, as described in Fig. 4A. 10 mpk isotype control, 10 and 5 mpk hGITRL non-competitive blocking antibody (hGITR-nc-Ab-#2), 10 mpk GITRL competitive blocking antibody (hGITR-lc-Ab-#3) were given i.p. on day − 3 before PBMC injection followed by twice per week Ab injection for 4 weeks. 4 weeks after the PBMC injection, visual scores were taken for the human skin graft. Blood and spleen samples were harvested for human leukocyte engraftment analysis and CD69 expression was measured by flow cytometry. IFN-γ was measured by MSD. (A) Visual scores assessing the skin graft dryness, scabbing, depression, cracking, and color change showed no significant difference between GITR blocking antibodies and isotype control. Neither ligand non-competitive hGITR-nc-ab-#2 nor ligand competitive hGITR-lc-ab-#3 showed significant hCD3 engraftment reduction in blood (B) and spleen (C) compared to isotype control. (D) No significant difference found between experimental groups in % CD69 + cells in CD4 + T cells in blood. (E) Significant difference in % CD69 + cells in CD8 + T cells in blood between isotype control and hGITR-lc-Ab-#3-10mpk. (f) Significant difference in % CD69 + cells in CD4 + T cells in spleen between isotype control and hGITR-lc-Ab-#3-10mpk. (G) No significant difference found between experimental groups in % CD69 + cells in CD8 + T cells in spleen. (H) No significant difference found between experimental groups in IFN-γ level in the serum. Horizontal bar represents average of the group. (A) Data were compared to isotype control controlled for multiple comparison testing with the original FDR method of Benjamini and Hochberg after a Kruskal-Wallis test. (B–H) Data were compared to isotype control with Šídák’s multiple comparisons test after one-way ANOVA. *p < 0.05, **p < 0.01. h human, mpk milligram per kilogram, i.p. intraperitoneal, nc ligand non-competitive, lc ligand competitive.
GITRL-competitive GITR-antibody is protective in an imiquimod skin inflammation model
Due to the modest but significant pharmacology observed with the GITRL-competitive antibody in the NSG human skin graft rejection model, we decided to probe this mechanism in a mouse model of inflammation. We developed an anti-muGITR antibody, muGITR-lc-Ab-#5, as a monofunctional bi-specific antibody, where one arm of the antibody binds GITR in a ligand-competitive manner, while the other arm is a non-binding arm. The monovalent binding of the antibody was designed to prevent the formation of a linear oligomerization of GITR, and thereby the unintended activation of the GITR pathway. As previously discussed, the skin has high expression of GITR and GITRL genes and proteins, pointing us to the imiquimod skin inflammation model as an ideal in vivo model to study GITR-biology. Mice were challenged with imiquimod daily from day 0 to day 4 topically to the ears. muGITR-lc-Ab-#5 was injected i.p. on day − 2 and day 2, while IL-17 A Ab was used as a positive control Ab in the study27 (Fig. 6A). muGITR-lc-Ab-#5 showed a significant protection from inflammation-induced ear swelling in this model over multiple days compared to isotype Ab, both at 2 mg/kg and 10 mg/kg doses, with the decrease in ear swelling at the end of the study being 20.6% for the 2 mg/kg dose and 35.6% for the 10 mg/kg dose (Fig. 6B, p < 0.0001 and p < 0.001, respectively; data not shown for earlier timepoints). At the conclusion of the study, ears and spleens were immunophenotyped. A decrease in the frequency of CD11b + monocytes (F4/80-Ly6G-CD11c-) was observed in the imiquimod treated ears of mice treated with IL-17 A Ab and 10 mg/kg muGITR-lc-Ab-#5 compared to isotype Ab group (p < 0.05) (Fig. 6C). Most other phenotypic changes in the ear induced by muGITR-lc-Ab-#5 were modest, but interestingly, compared to isotype Ab, in the spleen a decreased CTLA-4 expression on CD4 + T effector cells was observed with muGITR-lc-Ab-#5 treatment in the 2 mg/kg dose group (p < 0.05) but not in 10 mg/kg group (Fig. 6D), suggesting a suppression of T cell activation in the lower dose group since CTLA-4 is up-regulated in activated T cells28. muGITR-lc-Ab-#5 treatment furthermore led to a robust decrease in macrophage frequency (Fig. 6E, p < 0.05 and p < 0.01, for 2 mg/kg and 10 mg/kg doses, respectively) and CD4 + T cell frequency (Fig. 6F, p < 0.01 for both doses) in spleen, showing similar immunomodulatory effects across both dose groups.
GITRL-competitive mouse GITR blocking antibody is protective in an imiquimod skin inflammation model. (A) Schematic of Imiquimod model. 5% imiquimod cream was applied on the left and right ear of 10–12 weeks old female C57Bl/6 mice for 5 days. Mice were dosed i.p. on days − 2 and day 2 with anti-mouse GITR antibody (2 mg/kg, or 10 mg/kg), or isotype control (10 mg/kg), and on day 0 and day 3 with anti-mouse IL17A antibody (20 mg/kg). Ear thickness was measured daily (days 0–5) in triplicate. Mice were sacrificed on day 5. Ear and spleen samples were collected for flow cytometry analysis. (B) Mice treated with muGITR-lc-Ab-#5 at 2mpk and 10mpk and IL-17 A antibody showed significantly less ear swelling compared to isotype control; day 5 measurements. (C) Mice treated with muGITR-lc-Ab-#5 at 10mpk and IL-17 A antibody had less CD11b + monocyte in the ear compared to isotype control. (D) Mice treated with muGITR-lc-Ab-#5 at 2mpk and IL-17 A antibody showed significant lower CTLA4 MFI in Foxp3- CD4 effector T cells in spleen. (E) Mice treated with muGITR-lc-Ab-#5 at 2mpk and 10mpk but not IL-17 A antibody showed significant lower % macrophage in CD11b + cells in spleen. (F) Mice treated with muGITR-lc-Ab-#5 at 2mpk and 10mpk and IL-17 A antibody showed significant lower % CD4 cells in CD3 in the spleen. Horizontal bar represents average of the group. Data were compared to isotype control with Šídák’s multiple comparisons test after one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. m mouse, mpk mg per kilogram, i.p. intraperitoneal, lc ligand competitive, d day.
Discussion
In this study, we demonstrate a previously unknown mechanism of hGITR signaling neutralization using an in-house generated high-affinity monoclonal antibody (mAb) that preserves GITRL binding to the receptor on T cells. Although this ligand non-competitive antibody showed efficacy in vitro, it did not translate into therapeutic benefit in a humanized mouse model of skin graft rejection. Nevertheless, we hypothesize that this mechanism of inhibition could be viable for blocking signaling in other TNFRSF members, as discussed below. Furthermore, data from the imiquimod mouse model of inflammation strongly suggest that targeting GITR with a neutralizing antibody may offer therapeutic benefits in immune-mediated inflammatory diseases.
Therapeutic strategies to inhibit TNFRSF signaling have been in clinical use for over 25 years5, with approved drugs primarily blocking ligand binding (e.g., infliximab and adalimumab) or acting as decoy receptors (etanercept). More recently, alternative approaches have emerged, including antibodies that lock receptors in non-signaling conformations8,9, and small molecules that stabilize non-symmetrical ligand conformations which, despite receptor binding, do not trigger downstream signaling29. Here, we provide evidence for a potentially novel mechanism of TNFRSF neutralization via a ligand non-competitive GITR antibody, which we believe locks the receptor in a non-activated state.
Surprisingly, despite potent in vitro antagonistic activity, our ligand non-competitive mAb did not demonstrate in vivo efficacy. This occurred despite its higher affinity to GITR and greater potency in in vitro assays, where it performed as well as the ligand-competitive mAb comparator. Several factors may contribute to this discrepancy. First, murine GITRL activates its receptor in a dimeric form, whereas human GITRL forms a trimer. This difference in stoichiometry complicates the use of a mechanistically equivalent anti-muGITR surrogate antibody, as bivalent binding could inadvertently activate GITR signaling22. Second, the intense immune response in the human skin graft rejection model may not be suitable for evaluating the activity of our antibody. GITR signaling may play a more critical role in settings of less acute immune dysfunction, as evidenced by the beneficial effects of anti-muGITR mAb in the imiquimod-induced psoriasis model. Notably, while the ligand-competitive GITR antibody showed some efficacy in the skin graft model, this does not preclude a role for ligand non-competitive antibodies in other, less inflammatory contexts.
Considering that our ligand non-competitive antibody preserves GITRL binding to GITR, this raises the question of whether it could lead to unexpected pro-inflammatory signaling rather than the neutral effect we had hypothesized. Interestingly, GITR has been reported to induce reverse signaling through GITRL in GITRL-expressing cells, though the net effect of this signaling remains unclear, with both pro- and anti-inflammatory outcomes described30,31. Thus, it is possible that our mAb indirectly stimulates GITRL-expressing cells, resulting in a pro-inflammatory effect that counteracts the intended anti-inflammatory outcome of GITR blockade.
Alternatively, yet not mutually exclusive, the lack of in vivo efficacy may be explained by increased cell-cell contact between GITR- and GITRL-expressing cells, as demonstrated in vitro, which could amplify pro-inflammatory signaling through other ligand-receptor pairs. If either or both of these mechanisms are valid, they may explain the observed lack of efficacy with this specific ligand non-competitive approach. Nevertheless, we speculate that this neutralization mechanism could be effective for other TNFRSF members that do not signal through their ligands or are activated by soluble rather than membrane-bound ligands.
To elaborate, the soluble recombinant GITRL trimer used in our in vitro studies may underestimate the complexity of inhibiting this pathway in vivo, where GITRL is membrane-associated. It has been shown that receptor clustering for TNFRSF members differs significantly between engagement by soluble versus membrane-bound ligands32. This phenomenon has been recently reviewed and provides a compelling explanation for why membrane-bound ligands generate stronger signaling responses33.
Importantly, this is the first report demonstrating GITR inhibition by an antibody in a mouse model. Previous studies have used GITR-Fc fusion proteins, which may induce reverse signaling. We designed a monovalent bispecific antibody to avoid GITR agonism and observed robust efficacy in the imiquimod-induced psoriasis model, supporting the therapeutic potential of disrupting GITR/GITRL interactions. However, a recent Phase 1 clinical trial of the antagonistic ligand-competitive GITR antibody LY3844583 was terminated due to lack of efficacy in atopic dermatitis34, raising questions about the therapeutic hypothesis, the molecule tested, and/or the disease indication.
In conclusion, we identified a previously undescribed mechanism for inhibiting TNFRSF signaling and provided a high-resolution crystal structure of the antibody–target interaction that demonstrates this mechanism. GITR antibodies employing this ligand-non-competitive mechanism fully blocked GITR signaling in vitro but failed to show efficacy in vivo, potentially due to the membrane-associated nature of GITRL. Taken together, these findings suggest that developing an effective ligand-non-competing GITR antibody for treating autoimmune diseases may not be feasible. Nonetheless, this novel mechanism could be therapeutically relevant for other TNFRSF members that rely more heavily on soluble ligands. Further exploration of alternative strategies to target the GITR/GITRL axis is warranted, as its role in human immune-mediated inflammatory diseases remains to be fully elucidated.
Methods
Antibody generation
Recombinant human GITR protein was used for immunization of mice to generate anti-human GITR antibodies. Hybridomas were generated and screened against human GITR for binding by ELISA and flow cytometry. Epitope binning was performed by GITRL competition ELISA and signaling effect was evaluated by measuring GITRL mediated NFκB signaling in GITR-NFκB-Luciferase-Jurkat cell line (BPS bioscience). Hit hybridoma were sequenced and paired VH and VL sequences were grafted into human IgG1 backbone with mutations in Fc to minimize effector function. The antibodies were then transiently expressed in Expi293 and purified on AKTA purification system (GE) using mabSelect Sure (Cytiva) column.
Recombinant GITRL competition by ELISA
Antibody competition with GITRL were measured by recombinant GITRL competition ELISA. Briefly, 4 µg/ml hGITRL (in PBS-CMF) were coated on clear nunc 384 maxisorp plates overnight. Next day, the plates were washed with a washing buffer (PBS-CMF with 0.01% Tween20) and blocked with blocking buffer (1% BSA in PBS-CMF) and washed. Antibodies were titrated in blocking buffer and incubated with biotinylated recombinant human GITR protein for 1 h then added to blocked and washed plate. After 90 min the plates were washed with wash buffer and streptavidin-HRP was added and incubated for another 30 min. Plates were washed again and TMB was added and neutralized with 2 M HCl. Plates were read on Envision plate reader (Revvity) with detection at Abs450.
Detection and Inhibition of GITRL mediated NFkb signaling in GITR-NFkb-Luciferase-Jurkat cell line
GITRL-GITR mediated NFκB signaling was measured using GITR-NFκB-Luciferase-Jurkat cell line (BPS bioscience). Briefly, GITR-NFκB-Luciferase-Jurkat cell line was cultured in growth medium 2 C (BPS bioscience) in 37 °C with 5% CO2 and seeded into 384-well black walled plate. Recombinant GITRL was titrated, added to the plate, and incubated for 3–5 h at 37 °C with 5% CO2. The protocol for One-Step Luciferase assay system (BPS biosystem) was followed and the plate was read using Envision plate reader (Revvity) to measure luminescence. To measure antibody mediated inhibition of GITRL-GITR signaling, titrated antibodies were incubated with recombinant GITRL at EC50 concentration for 1 h at room temperature prior to addition to the plate containing GITR-NFκB-Luciferase-Jurkat cells.
Characterization of antibody affinity using SPR
The antibody binding kinetics to recombinant human GITR protein were determined using surface plasmon resonance and a Biacore 8 K + instrument (Cytiva). A sensor chip was prepared for anti-Fab antibody capture or reversible streptavidin capture of biotinylated human GITR antigen. Multiple concentrations of antibody, one-armed antibody, or human GITR antigen were prepared in HBS-EP + pH 7.4 buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.05% v/v Surfactant Tween-20) and injected over the sensor chip to measure the association rate. After analyte binding, buffer was flowed to collect dissociation data, and the sensor chip surface was regenerated. Kinetic measurements were done at 37 °C and sensorgrams were double referenced using a control surface and buffer injections. Rate constants and binding affinity were determined using a Langmuir 1:1 model, Biacore Insight Evaluation software, and the equation KD=kd/ka.
Crystal structure
Protein expression and purification DNA encoding of Human GITR extracellular domain (ECD) with a C-terminal tobacco etch virus (TEV) protease cleavage site and IgG1 Fc was cloned into an expression vector. The human GITR ECD sequence used in this study was residues Q26-E161 with a C57S mutation. The vector was transfected into Expi293 cells (Thermo Fisher) for transient expression. Kifunensine (Sigma-Aldrich) at a final concentration of 5 µM was supplemented to the Expi293 cell culture during transfection and culturing to inhibit the α-mannosidase I-mediated glycosylation. The cell culture was harvested 5 days after the transfection and protein was purified using an automated ÄKTA pure (GE Healthcare) chromatographic system. Briefly, conditioned media was first applied over a MabSelect SuRe column (Cytiva) equilibrated in phosphate-buffered saline (PBS), and eluted with 150 mM Glycine, 40 mM NaCl, pH 3.5. The eluate was immediately neutralized with 1 M Tris pH 8. The eluate was then concentrated and subjected to size exclusion chromatography (SEC) using a Superdex 200 column (Cytiva) equilibrated in PBS. The C-terminal Fc tag was then removed from the protein by digestion with AcTEV Protease (Thermo Fisher) for 5 h at room temperature. The Fc was removed by binding the protein to MAb Select Sure resin (Cytiva) and the TEV was removed by binding to Ni NTA Sepharose FF (Cytiva). GITR antibodies were transiently expressed in Expi293 cells and purified using MabSelect SuRe columns as mentioned above. Fab fragments were generated using Pierce Fab preparation kits (Thermo Fisher) and the cleaved Fc fragments were removed using MAb Select Sure resin. Complex formation and crystallization Purified GITR and Ab-#1-Fab proteins were mixed at a 1.2:1 molar ratio and allowed to complex at room temperature for 30 min. N-linked glycosylations were removed by incubating with endoglycosidase H (Endo H; New England Biolabs) for 2 h at 37 °C. The complex was then purified on a Superdex 200 analytical SEC column in Tris-buffered saline (TBS, 50 mM Tris-Cl pH 7.5, 150 mM NaCl). Fractions containing the complex were then concentrated to 12.5 mg/ml. The GITR/Ab-#1-Fab complex was crystallized by sitting drop vapor diffusion method at 20 °C with 1:1 protein solution to reservoir solution of 15% PEG20,000, and 100 mM sodium acetate pH 4.6. The crystals were flash-frozen with liquid nitrogen using 20% glycerol as cryoprotectant. Structure determination X-ray diffraction data were collected at Advanced Light Source (ALS) IMCA-CAT beamline 17-ID. The data were processed with autoPROC35. The structure of the GITR/Ab-#1-Fab complex was solved by molecular replacement with Phaser using the published human GITR structure22 (PDB access code: 7KHD) as the search model. Model building was carried out in COOT36, and refinement was done in BUSTER37. Structural data have been deposited in RCSB with PDB codes 9P8X (GITR/Ab-#1-Fab). All structural figures were prepared using PyMOL (Schrodinger Inc.). Crystal data and refinement statistics are summarized in Table 2.
GITRL-binding assay
Human peripheral blood was collected into heparin-tubes through an internal Pfizer blood donor program. PBMCs were isolated by Ficoll-Paque Plus gradient centrifugation using Sepmate-50 tubes (STEMCELL Technologies) per manufacturer’s recommendation. The PBMCs were incubated at 37 °C with 5% CO2 for 72 h in 96-well round bottom plates in RPMI media with 10% FBS, Pen/Strep, L-glutamine, HEPES, sodium pyruvate, and Pen/Strep (gibco) at 200,000 cells/well, in the presence of Immunocult (25 µl/ml; STEMCELL Technologies) to induce GITR expression. Cells were then incubated with GITR-antibodies in a 9-point 4-fold titration from top concentration of 40 µg/ml for 15 min at 4 °C, followed by washing and then a 15 min incubation of the cells with recombinant GITRL at 4 °C. Following a wash step, the cells were incubated with an anti-GITRL-PE antibody (Miltenyi) for 15 min at 4 °C. The GITRL-PE binding of activated T cells was determined with flow cytometry using FACSymphony A3 (BD Biosciences). The flow cytometry results were analyzed using FlowJo™ v10.7.1 Software (BD Life Sciences). Primary human blood samples were obtained with informed written consent from healthy human adult donors in accordance with Pfizer Inc. Global Colleague Wellness Research Support Program (protocol CW RDP-02) approved by the Advarra IRB. The Pfizer RSP program is protocol number CW RDP-02 and IRB number: 08-33390. All experiments using human samples were approved by and conducted in accordance with the guidelines and regulations of the Pfizer Institutional Biosafety Committee.
Antibody binding to GITR-NFKb-Luciferase-Jurkat cell line by flow-cytometry
Antibody binding to cell surface expressed human GITR was measured by flow-cytometry. Briefly, GITR-NFκB-Luciferase-Jurkat cell line was cultured in growth medium 2 C (BPS bioscience) in 37°C with 5% CO2 and seeded into 384 well plate (Costar). Anti-GITR antibodies were titrated in BD Stain Buffer (PBS CMF with 3% bovine calf serum 0.005% sodium azide), added to the plate and incubated at 4°C for 1 hour. The plate was washed and stained with Rhodamine Red X-conjugated AffiniPure F(ab’)2 Fragment Goat Anti-Human IgG (Jackson ImmunoResearch) at 4 °C for 30 min. The plate was washed, DAPI stain (Thermo Fisher) was added and read on iQUE flow cytometer. Four parameter slope was fitted with GraphPad Prism 10.2.1 (GraphPad Software) with the top two concentrations excluded from curve-fitting due to hook-effect in the staining.
Human PBMC stimulation assay
Human PBMCs (internal Pfizer blood donor program) were incubated at 37 °C with 5% CO2 for 72 h in 96-well round bottom plates in RPMI media (gibco) (with 10% human serum (Sigma-Aldrich) and Pen/Strep (gibco)) at 200,000 cells/well in the presence of Immunocult (25 µl/ml; STEMCELL Technologies), recombinant GITRL-trimer (2 µg/ml; Pfizer), IL-12, IL-15, and IL-18 (0.42 ng/ml, 0.306 ng/ml, and 2.3 ng/ml, respectively; R&D Systems). GITR-antibodies were tested in a 7-point 5-fold titration from top concentration of 300 nM. Supernatant was harvested, and IL-9 and IL-13 levels were quantified using a U-PLEX MSD kit and an MSD Sector reader (Meso Scale Diagnostics). All experiments using human samples were approved by and conducted in accordance with the guidelines and regulations of the Pfizer Institutional Biosafety Committee.
NSG human skin graft transplant model
Mice Female NSG MHC I/II KO mice (The Jackson Laboratory, 025216) were maintained under specific pathogen-free condition in the Pfizer vivarium (Cambridge, MA). Mice were used between 8 and 10 weeks of age at the time of experiment. Human skin graft preparation Freshly excised normal human abdominal skin from cosmetic reduction surgery was obtained from BioIVT (Westbury, NY). The skin was provided at a thickness of 600 µM. 1 cm-diameter skin grafts were generated using skin biopsy punches. HLA-A2 typing was determined by digesting the skin sample to single cell suspension, stained for HLA-A2 and analyzed by flow cytometry. Human Skin graft transplantation GvHD Human skin transplantation was performed similar to previously described38. Meloxicam, gentamicin, and cefazolin were given subcutaneously before the surgery. The animals were anesthetized with 2.5% isoflurane in oxygen. The dorsal fur was removed and the underlying skin cleaned with chlorhexidine swab stick. A 1 cm diameter round shape piece of mouse skin was removed and then a piece of human skin was put on the panniculus carnosus on mouse back skin opening. The graft was then secured with sterile strips, followed by Vaseline gauze and gauze pad, and then wrapped with CoFlex. Mice were put back to a clean cage and recovered on a warm pad. The cages were returned to the holding rack when the mice could stay upright and start walking. Skin grafts healed for 10–14 days and bandages were removed. Eight to ten weeks post-transplant, 10 × 106 human PBMCs from an HLA-A2 mismatched donor (StemCell Technologies) were injected into the mice intravenously. GITR antibodies, an isotype control, or an anti-CD28 antibody were injected i.p. twice per week for 4 weeks, beginning one (Fig. 4) or three (Fig. 5) days prior to the PBMC injection. Body weight and gross skin graft appearance were monitored for four weeks after PBMC injection. At the end of the experiment, visual scores were taken for the human skin graft on each mouse according to the Visual score table (Table 3) and mice were sacrificed with CO2 inhalation. Blood and spleen samples were harvested for human leukocyte engraftment analysis by flow cytometry. IFN-γ was measured by MSD. These experiments are reported in accordance with ARRIVE guidelines. All procedures performed on animals were in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee or through an ethical review process. All experiments using human samples were approved by and conducted in accordance with the guidelines and regulations of the Pfizer Institutional Biosafety Committee.
Imiquimod model
The imiquimod-induced skin inflammation model was performed with 10–12 weeks old female C57Bl/6 mice (Taconic Farms, Germantown, NY). Commercially available 5% imiquimod cream (Sandoz, Princeton, NJ) was applied to the left and right ear for five consecutive days (days 0–4). Mice were dosed by intraperitoneal injection on days − 2 and day 2 with anti-mouse GITR antibody, or isotype control, and on day 0 and day 3 with anti-mouse IL17A antibody (20 mg/kg; R&D Systems). Ear thickness was measured daily (days 0–5) in triplicate on both ears using an engineer’s micrometer (Mitutoyo, IL) to assess swelling and epidermal hyperplasia. On day 5, mice were sacrificed with CO2 inhalation. Tissue processing Ears were cut into small pieces and digested in 3 ml RPMI containing 1 mg/ml Collagenase type IV and 100 mg/ml DNase I (both from Sigma-Aldrich) while shaking at 37 °C for 60 min. The tissue homogenate was then passed through a 70 μm cell strainer using syringe plunger. Cell strainer was rinsed with 10 ml of cold PBS followed by centrifugation (400 x g at 4 °C for 10 min). Single cells were then stained with fluorochrome conjugated antibodies for flow cytometry. Flow cytometric analysis Single cell suspension of homogenized spleen and digested ear tissue was spun down and resuspended in 100 µl of PBS/0.5%BSA/ 2mM EDTA for FACS staining. Cells were incubated with Anti-mCD16/CD32 (Fc block) and then stained with a cocktail of antibodies for 30 min, at 4 °C. Brilliant stain buffer (BD Biosciences) was used in all samples during antibody staining. Near IR Fixable Live/Dead stain (ThermoFisher) was used according to manufacturer’s recommendations to discriminate live cells. Cells were washed twice in FACS buffer and resuspended in Transcription Factor Fixation buffer overnight before staining for intracellular antigens and transcription factors. After staining, cells were resuspended in FACS buffer and analyzed on BD FACSymphony™ A3 Cell Analyzer. This experiment is reported in accordance with ARRIVE guidelines. All procedures performed on animals were in accordance with regulations and established guidelines and were reviewed and approved by an Institutional Animal Care and Use Committee or through an ethical review process.
Statistical analyses
For discrete outcomes, such as visual skin graft scores, comparisons between compound-treated and isotype control-treated mice were performed using the Kruskal–Wallis test, followed by multiple comparison correction using the original false discovery rate (FDR) method of Benjamini and Hochberg. For continuous variables—including flow cytometry data, cytokine levels, and ear thickness measurements—group comparisons were conducted using one-way ANOVA followed by Šídák’s multiple comparisons test. In vitro dose–response data were modeled using a four-parameter logistic (4PL) regression. For in vivo experiments, group sizes were as follows: 8 mice per group in the NSG human skin graft transplant model, and 10 mice per group (20 ears per group for ear thickness measurements) in the imiquimod-induced inflammation model. One mouse in each NSG human skin graft transplant model experiment was not included in the analysis due to a premature death (1 in group ‘hGITR-nc-Ab-#2, 15 mpk’ for Figs. 1 and 4 in group ‘hGITR-nc-Ab-#2–10 mpk’ for Fig. 5). All statistical analyses were performed using GraphPad Prism version 10.4.1 for Windows (GraphPad Software, Boston, Massachusetts, USA; www.graphpad.com).
Data availability
Structural data have been deposited in RCSB with PDB codes 9P8X (GITR/Ab-#1-Fab). All low-dimensional datasets generated during the current study are available from the corresponding author on reasonable request.
References
Conrad, N. et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet 401, 1878–1890. https://doi.org/10.1016/S0140-6736(23)00457-9 (2023).
Miller, F. W. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr. Opin. Immunol. 80, 102266. https://doi.org/10.1016/j.coi.2022.102266 (2023).
Croft, M., Benedict, C. A. & Ware, C. F. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 12, 147–168. https://doi.org/10.1038/nrd3930 (2013).
Hayden, M. S. & Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol. 26, 253–266. https://doi.org/10.1016/j.smim.2014.05.004 (2014).
Veerasubramanian, P. K., Wynn, T. A., Quan, J. & Karlsson, F. J. Targeting TNF/TNFR superfamilies in immune-mediated inflammatory diseases. J. Exp. Med. 221 https://doi.org/10.1084/jem.20240806 (2024).
Bar-Yoseph, H. et al. Infliximab-Tumor Necrosis Factor Complexes Elicit Formation of Anti-Drug Antibodies. Gastroenterology 157, 1338–1351e1338 (2019). https://doi.org/10.1053/j.gastro.2019.08.009
Wajant, H. Principles of antibody-mediated TNF receptor activation. Cell. Death Differ. 22, 1727–1741. https://doi.org/10.1038/cdd.2015.109 (2015).
Torrey, H. et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 10 https://doi.org/10.1126/scisignal.aaf8608 (2017).
Argiriadi, M. A. et al. CD40/anti-CD40 antibody complexes which illustrate agonist and antagonist structural switches. BMC Mol. Cell. Biol. 20, 29. https://doi.org/10.1186/s12860-019-0213-4 (2019).
Cuzzocrea, S. et al. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 19, 1253–1265. https://doi.org/10.1096/fj.04-3556com (2005).
Patel, M. et al. Glucocorticoid-induced TNFR family-related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur. J. Immunol. 35, 3581–3590. https://doi.org/10.1002/eji.200535421 (2005).
Ma, J. et al. Blockade of Glucocorticoid-Induced tumor necrosis Factor-Receptor-Related protein signaling ameliorates murine Collagen-Induced arthritis by modulating follicular helper T cells. Am. J. Pathol. 186, 1559–1567. https://doi.org/10.1016/j.ajpath.2016.02.010 (2016).
Wang, S. et al. Glucocorticoid-induced tumor necrosis factor receptor family-related protein exacerbates collagen-induced arthritis by enhancing the expansion of Th17 cells. Am. J. Pathol. 180, 1059–1067. https://doi.org/10.1016/j.ajpath.2011.11.018 (2012).
Lee, S. K. et al. Glucocorticoid-induced tumour necrosis factor receptor family-related receptor signalling exacerbates hapten-induced colitis by CD4 + T cells. Immunology 119, 479–487. https://doi.org/10.1111/j.1365-2567.2006.02459.x (2006).
Ephrem, A. et al. Modulation of Treg cells/T effector function by GITR signaling is context-dependent. Eur. J. Immunol. 43, 2421–2429. https://doi.org/10.1002/eji.201343451 (2013).
Santucci, L. et al. GITR modulates innate and adaptive mucosal immunity during the development of experimental colitis in mice. Gut 56, 52–60. https://doi.org/10.1136/gut.2006.091181 (2007).
Kim, I. K. et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat. Med. 21, 1010–1017. https://doi.org/10.1038/nm.3922 (2015).
Galle-Treger, L. et al. Costimulation of type-2 innate lymphoid cells by GITR promotes effector function and ameliorates type 2 diabetes. Nat. Commun. 10, 713. https://doi.org/10.1038/s41467-019-08449-x (2019).
Ronchetti, S. et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 34, 613–622. https://doi.org/10.1002/eji.200324804 (2004).
Zappasodi, R. et al. Rational design of anti-GITR-based combination immunotherapy. Nat. Med. 25, 759–766. https://doi.org/10.1038/s41591-019-0420-8 (2019).
Vanamee, E. S. & Faustman, D. L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 11 https://doi.org/10.1126/scisignal.aao4910 (2018).
Wang, F. et al. Structures of mouse and human GITR-GITRL complexes reveal unique TNF superfamily interactions. Nat. Commun. 12, 1378. https://doi.org/10.1038/s41467-021-21563-z (2021).
Vanamee, E. S. & Faustman, D. L. The benefits of clustering in TNF receptor superfamily signaling. Front. Immunol. 14, 1225704. https://doi.org/10.3389/fimmu.2023.1225704 (2023).
Vanamee, E. S. & Faustman, D. L. On the TRAIL of better therapies: Understanding TNFRSF structure-function. Cells 9 https://doi.org/10.3390/cells9030764 (2020).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7 https://doi.org/10.1126/sciadv.abh2169 (2021).
The Human Protein Atlas. (2024). https://www.proteinatlas.org/
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845. https://doi.org/10.4049/jimmunol.0802999 (2009).
Walunas, T. L. et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1, 405–413. https://doi.org/10.1016/1074-7613(94)90071-x (1994).
O’Connell, J. et al. Small molecules that inhibit TNF signalling by stabilising an asymmetric form of the trimer. Nat. Commun. 10, 5795. https://doi.org/10.1038/s41467-019-13616-1 (2019).
Grohmann, U. et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat. Med. 13, 579–586. https://doi.org/10.1038/nm1563 (2007).
Bae, E. M. et al. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol. Immunol. 45, 523–533. https://doi.org/10.1016/j.molimm.2007.05.013 (2008).
Su, Z., Dhusia, K. & Wu, Y. Understanding the functional role of membrane confinements in TNF-mediated signaling by multiscale simulations. Commun. Biol. 5, 228. https://doi.org/10.1038/s42003-022-03179-1 (2022).
Vanamee, E. S., Lippner, G. & Faustman, D. L. Signal amplification in highly ordered networks is driven by geometry. Cells 11 https://doi.org/10.3390/cells11020272 (2022).
C. g. A study of LY3844583 in healthy participants and participants with atopic dermatitis (2022). https://clinicaltrials.gov/study/NCT05486208
Vonrhein, C. et al. Data processing and analysis with the autoproc toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302. https://doi.org/10.1107/S0907444911007773 (2011).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501. https://doi.org/10.1107/S0907444910007493 (2010).
Smart, O. S. et al. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D Biol. Crystallogr. 68, 368–380. https://doi.org/10.1107/S0907444911056058 (2012).
Racki, W. J. et al. NOD-scid IL2rgamma(null) mouse model of human skin transplantation and allograft rejection. Transplantation 89, 527–536. https://doi.org/10.1097/TP.0b013e3181c90242 (2010).
Acknowledgements
The authors thank Donald Bennett for his statistical consultation, Shirin Rahman for her technical contributions to the GITRL-binding experiment, and April Halloran and Kathleen Kelly from the Pfizer Inc. Global Colleague Wellness Research Support Program for obtaining primary human blood samples.
Author information
Authors and Affiliations
Contributions
J.Y., J.M-D., C-S.H., and F.K. wrote first draft of the manuscript. J.Y, J.M-D., M.N.S., M.N-L., A.W., and F.K conceptualized the work. All the authors contributed to design of the work, acquisition and analysis of the work, and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors are current or former employees of Pfizer Inc. and may hold stock and/or stock options with Pfizer Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, J., Min-DeBartolo, J., Huang, CS. et al. Ligand non-competitive GITR antibody prevents formation of the obligatory signal-triggering GITRL: GITR stoichiometry. Sci Rep 16, 2752 (2026). https://doi.org/10.1038/s41598-025-32541-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-32541-6