Abstract
Despite being one of the primary and most effective treatments for advanced stages of lung cancer, chemotherapy drugs like carboplatin have limitations due to their adverse side effects and the development of drug resistance in lung cancer cells. However, recent studies have shown promising results in using small interfering RNAs (siRNAs) as a therapeutic agent for cancer treatment. Hence, this study aimed to investigate the potential of combining siRNA-DLGAP1-AS2 with carboplatin in human lung cancer cell lines. The viability of the cells was assessed using the MTT assay, and apoptosis induction was examined through Annexin V/Pi staining. Additionally, the effect of the combination on cell cycle arrest and colony formation of lung cancer cells was studied. Furthermore, the expression of Bax, Bcl-2, MMP-2, MMP-9, GCLC, and CD44 was evaluated. Our functional analysis revealed that inhibiting the expression of DLGAP1-AS2 increased the sensitivity of lung cancer cells to carboplatin. Moreover, our study demonstrated that the combination of DLGAP1-AS2 inhibition through siRNA-DLGAP1-AS2 transfection and carboplatin treatment had a tumor-suppressive function, inhibiting the progression and proliferation of A549 lung cancer cells. Therefore, it can be concluded that targeting DLGAP1-AS2 using specific siRNA in combination with carboplatin chemotherapy holds promise as a valuable therapeutic approach for lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is a prevalent and highly lethal form of cancer worldwide. The significant fatality rate associated with this illness can be attributed to delayed diagnosis. Patients typically seek medical attention only after experiencing symptoms such as hemoptysis, chest discomfort, or systemic involvement1. Smoking stands as the primary risk factor for lung cancer. Nevertheless, other risk factors unrelated to smoking, including genetic background, lifestyle choices, and environmental and occupational factors, contribute to the likelihood of developing this disease2. Histologically, lung cancer can be divided into two main subtypes: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). The incidence rate of NSCLC is higher than that of SCLC. This disease is considered heterogeneous due to the various molecular mechanisms that lead to the formation of lung tumors3. However, the current treatment options such as surgery, chemotherapy, and radiotherapy have limitations in terms of serious side effects and high recurrence rates. In order to address this issue, the discovery of new biomarkers is crucial to reduce the lethality of lung malignancy and improve the effectiveness of therapy4. By identifying molecular changes and their potential targets, targeted therapies have been developed for lung cancer patients. Some of these changes include DNA methylation, mRNA expression, noncoding RNA expression, and protein expression3,5.
Carboplatin, a platinum-based chemotherapy drug, is commonly used in the treatment of lung cancer. However, the development of acquired and internal drug resistance significantly diminishes its therapeutic effects, posing a serious obstacle in the treatment of this disease6. Therefore, alternative methods such as using interfering RNA (RNAi) have been proposed to enhance the efficiency of lung cancer treatment. By combining RNAi with conventional therapies, it is possible to suppress genes involved in drug resistance7. In the RNAi method, small interfering RNA (siRNA) can specifically regulate the expression of target genes by degrading the corresponding mRNA. This technique holds promise in the field of cancer therapy8. Antisense oligonucleotides (ASOs), which are short, single-stranded, synthetic nucleic acids, can modulate gene expression by binding to specific RNA molecules. ASOs can efficiently target nuclear or cytoplasmic long non-coding RNAs (lncRNAs), while siRNAs are used to target cytoplasmic RNAs or induce transcriptional silencing through chromatin remodeling and histone modification in the nucleus by binding to promoter regions9,10.
lncRNAs that exceed 200 nucleotides in length do not possess the ability to code for proteins. However, they do play a crucial role in modifying chromatin, regulating transcription, and facilitating post-transcriptional processing through their interaction with chromatin-related proteins. The fundamental expression of lncRNAs is vital for various biological processes, including maintaining homeostasis, regulating gene expression, promoting cell differentiation, and facilitating organogenesis in human tissues. Any disruption in the regulation of lncRNAs within the human genome can lead to the development of solid or hematological malignancies11. Studies focusing on lncRNAs in NSCLC have indicated that these molecules may have a central role in the initiation, progression, and response to treatment of lung cancer by influencing different signaling pathways12. For instance, the lncRNA DLGAP1-AS2, which is located on human chromosome 18P11.31, can play a role in various tumors, including colorectal cancer13, gastric cancer14, glioma9, and lung cancer15.This lncRNA is transcribed in the antisense of the DLGAP1 gene and is a long intergenic non-coding RNA9,13. Wang et al. conducted a study investigating the interactions between DLGAP1-AS2, miR-503, and cyclin D1 in NSCLC. They observed that in lung cancer, the expression of DLGAP1-AS2 increases, and this upregulation may lead to an increase in cyclin D1 levels by acting as a sponge for miR-503, thereby promoting lung cancer cell proliferation15. However, to the best of our knowledge, no research has been conducted on the impact of inhibiting DLGAP1-AS2 expression on sensitivity of lung cancer cells to carboplatin and it’s combination with carboplatin on apoptosis, cell cycle regulation, and migration of lung cancer cells.
Hence, we examined the impact of suppressing DLGAP1-AS2 using specific siRNA in conjunction with carboplatin on lung cancer cells (Fig. 1). The findings obtained demonstrated that reducing the expression of DLGAP1-AS2 could augment the sensitivity of lung cancer cells to chemotherapy and enhance carboplatin-induced apoptosis by regulating genes associated with apoptosis. Additionally, the combined administration of siRNA and carboplatin induced cell cycle arrest and inhibited cell migration. These results indicate that the combination of siRNA-DLGAP1-AS2 and carboplatin holds promise as a potential therapeutic approach for the treatment of lung cancer.
Materials and methods
TCGA lung cancer cohort transcriptome data analysis (TCGA-LUAD)
RNAseq gene expression data were collected and analyzed using the UCSC Xena Functional Genomics Explorer (https://xenabrowser.net/) and UALCAN (http://ualcan.path.uab.edu/index.html) online tools. The Cancer Genome Atlas (TCGA) was also used to conduct a receiver operating characteristic (ROC) curve analysis to appraise the DLGAP1-AS2 expression pattern’s potential as a diagnostic biomarker for lung cancer. Patient and control values were derived from TCGA-LUAD data on the expression of these genes in tumor and normal tissue samples. The area under the curve (AUC) was then calculated by a ROC curve analysis performed in GraphPad Prism 9.
Cell culture
The A549 human lung cancer cell line was acquired from the National Cell Bank of Iran, located at the Pasteur Institute in Tehran. These cells were cultivated in RPMI-1640 medium, provided by Gibco in the United States, supplemented with 10% fetal bovine serum (FBS) and antibiotics (including penicillin at a concentration of 100 IU/mL and streptomycin at a concentration of 100 µg/mL). The cultured cells were stored in an incubator set at 37 °C, with a 5% CO2 and 95% humidified atmosphere. Upon reaching approximately 70% confluency, the cells were subcultured. To detach the cells, 0.25% Trypsin/EDTA from Gibco was used, and trypsin activity was subsequently neutralized in complete medium containing 10% FBS. After being subcultured three times to achieve the logarithmic growth phase, characterized by clear cell morphology and stable cell function, the cell lines were ready for further experimentation.
siRNA transfection
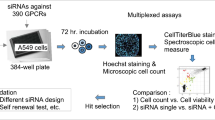
To optimize the dosage of siRNA, scramble siRNA (negative control, 5’ UUCUCCGAACGUGUCACGUUU 3’) and siRNA-DLGAP1-AS2 (Table 1) were transfected separately into A549 cells at varying doses (10, 20, and 40 pmol) using a gene pulser electroporation system (BioRad). Following the provided protocols, the transfection was carried out with a total volume of 5 × 105 A549 cells per 500 µl of electroporation buffer at 160 v for 12.5 ms in a cuvette. Subsequently, 2.5 × 105 transfected A549 cells were cultured in each well of a 6-well plate and incubated at 37 °C for 48 h. After the 48-hour incubation period, q-RT PCR was performed to determine the optimal concentration of siRNA-DLGAP1-AS2. Moreover, the expression of the optimal dose was assessed at 24, 48, and 72 h to determine the ideal transfection time. Additionally, to evaluate the efficiency of cellular uptake, FITC-labeled scramble was transfected into A549 cells and subjected to flow cytometry using the MACSQuant Analyzer 10 (Miltenyi Biotec, Germany). The optimal concentration was selected from the mentioned doses for the remaining tests in the project.
MTT assay
Cytotoxicity associated with siRNA-DLGAP1-AS2 and carboplatin was assessed using the MTT assay. Cells were cultured in 96-well plates at a density of 1 × 104 cells per well for 24 h. Subsequently, the cells were divided into four experimental groups: (1) negative control (transfected with scramble siRNA), (2) transfected with siRNA-DLGAP1-AS2, (3) treated with Carboplatin, and (4) transfected with siRNA-DLGAP1-AS2 and treated with carboplatin simultaneously. Following 24 h of incubation and siRNA-DLGAP1-AS2 transfection, the cells were exposed to varying concentrations of carboplatin ranging from 1 to 800 µg/mL. After another 24 h of incubation, the supernatant was removed and each well was supplemented with 50 µl of MTT solution (2 mg/ml MTT in PBS) and 100 µl of complete culture medium. After 4 hours of incubation, the supernatant was discarded and 100 µl of dimethyl sulfoxide (DMSO) was added to each well to dissolve the MTT formazan crystals. Following an additional 30 min of incubation, the absorbance of each well was measured at a wavelength of 570 nm using an ELISA reader. Cell viability was determined using the following formula, and the resulting IC50 (half maximal inhibitory concentration) was utilized for subsequent experiments:
Apoptosis assay
In order to quantitatively assess the rate of apoptosis induction, we employed a dual staining kit (Exbio, Czech Republic) that consisted of annexin-V-FITC and propidium iodide (PI). A549 cells were transfected with siRNA-DLGAP1-AS2 and plated at a density of 2.5 × 105 cells per well in 6-well plates. After 24 h of incubation, the cells were exposed to carboplatin. Negative controls consisted of cells transfected with scramble siRNA. Following detachment with trypsin/EDTA, the cells were collected and suspended in 100 µL of binding buffer. Subsequently, they were incubated with 5 µL of FITC-conjugated annexin-V and 5 µL of PI for 15 min at room temperature in complete darkness. Stained cells were then examined using flow cytometry (MACSQuant Analyzer 10; Miltenyi Biotec, Germany), and the resulting data was analyzed utilizing the FlowJo software.
Cell cycle assay
Flow cytometry was used to investigate the impact inhibition of DLGAP1-AS2 and carboplatin treatment on cell cycle progression. In the same way as in previous tests, the cells were grouped. Following siRNA-DLGAP1-AS2 transfection, A549 cells were cultured in 6-well plates at a density of 2.5 × 105 cells per well for 24 h and then treated with carboplatin. After 24 h of treatment, the cells were washed, detached and fixed with 80% ethanol. Then, fixed cells were kept overnight at −20 °C. The next day, each group of cells was suspended in 500 µL of PBS containing 5 µL of RNase A and incubated for 30 min at 37 °C. After incubation, DAPI was added to the cells along with Triton. Finally, flow cytometry was used to identify cell cycle arrest, and FlowJo software was used to analyze the data.
Wound-healing assay (scratch)
The migration of cells was assessed using the wound healing method, also known as the scratch test. To perform this assay, 2.5 × 105 A549 cells were transfected and seeded into 24-well plates. After 24 h of incubation, the center of each well was scratched using a yellow pipette tip to simulate a wound. Cell debris was eliminated by washing the wells with PBS. Subsequently, the drug and combination groups were treated with carboplatin. Images were captured from each well at 0, 24, and 72-hour intervals using an inverted microscope (Optica, Italy). The open wound area at 72 h calculated by ImageJ software. During the time intervals, the cells were incubated at 37 °C and 5% CO2 overnight to induce cell migration.
Colony formation
The colony formation ability of A549 cancer cells were evaluated by colony formation test. Cells were cultivated for this purpose in 6-well plates at a density of 103 cells per well, and were divided into the following groups: Negative control, siRNA-DLGAP1-AS2 transfected, carboplatin-treated, combined. After 3–4 days of incubation, the plates were examined for colonies. Wells were then washed with PBS and colonies were fixed using paraformaldehyde (PFA) (4%). 1 ml of crystal violet (Sigma Aldrich, USA) was used to stain the cells, and they were left to incubate at room temperature for 30 min. Wells were washed with PBS and the colonies photographed using a conventional camera after 30 min.
Quantitative RT-PCR (qRT-PCR)
TRIZOL reagent (geneAll biotechnology Seoul, Korea) was used to isolate total cellular RNA according to the manufacturer’s protocol. The nanodrop spectrophotometer (Thermo Fisher Scientific Life Sciences, USA) was used to measure absorbance at wavelengths of 260 nm and 280 nm after extraction to determine the purity and concentration of the RNA. Then, using an mRNA reverse transcription kit (Biofact, Korea), and following the manufacturer’s instructions, cDNA was synthesized in a final volume of 20 µl. Table 1 displays the primer sequences that were utilized. To assess the levels of BAX, BCL-2, MMP2, MMP9, and CD44 expression, qRT-PCR was carried out using BioFACT™ 2X Real-Time PCR Master Mix (Korea) and a set of gene-specific primers in a total reaction volume of 10 µl. The Step One Plus Real-Time PCR System (Applied Biosystems, USA) performed this test in three steps as follows: initial denaturation at 95 °C for 10 min, 45 cycles of denaturation at 95 °C for 10 s. Primer annealing temperature at 62 °C for 30 s. and elongation at 72 °C for 20 s. At the end of each run melting curves were obtained. GAPDH were considered as control genes for lncRNA-DLGAP1-AS2 target and detection genes, respectively. The primer sequences that were utilized in this experiment are listed in Table 2.
Statistical analysis
The experiments were conducted in triplicate. Data analysis was performed using FlowJo, ImageJ, and GraphPad Prism 6 software, along with one-way ANOVA and T-tests. A statistically significant difference was defined as a P-value of 0.05.
Results
DLGAP1-AS2 is upregulated in lung cancer
Ualcan, an online tool utilized for the comprehensive analysis of TCGA data across various cancer types, has unveiled the substantial overexpression of DLGAP1-AS2 in multiple cancers, particularly lung cancer (LUAD and LUSC) (Fig. 2a). To investigate the expression of DLGAP1-AS2 in lung cancer and healthy tissues, the TCGA database was employed. Utilizing the Cancer Genome Atlas (TCGA), analyses have demonstrated a significant elevation of DLGAP1-AS2 in lung cancer samples compared to normal tissues (Fig. 2b). Furthermore, the evaluation of DLGAP1-AS2 expressions through ROC curve analysis has indicated its potential as a diagnostic marker for lung cancer, as depicted in Fig. 2c.
(a) Overexpression of DLGAP1-AS2 was seen in a variety of malignancies, including lung cancer (LUAD, LUSC). (b) DLGAP1-AS2 is upregulated in lung cancer compared to normal samples in terms of its expression. (c) Expressions of DLGAP1-AS2 may be useful as a diagnostic sign for lung cancer (****P<0.0001).
Efficient transfection of siRNA-DLGAP1-AS2 into A549 cells
Following the transfection of A549 cells with varying doses of siRNA-DLGAP1-AS2, qRT-PCR was employed to measure the expression of DLGAP1-AS2 and determine the most effective concentration and duration. Consequently, the inhibition of DLGAP1-AS2 through specific siRNA exhibited a significantly higher downregulation in A549 cells at 20 pmol (p < 0.0001) compared to untransfected control group, siRNA-scramble transfected negative control group, and other concentrations. Thus, the optimal amount of siRNA-DLGAP1-AS2 for subsequent experiments was determined to be 20 pmol. No noticeable difference in viability was observed between untreated cells as control and cells transfected with scramble-siRNA (Fig. 3A). Furthermore, the investigation into the reduction of lncRNA-DLGAP1-AS2 expression in A549 cells at three different time points (24, 48, and 72 h) revealed that the greatest decrease in expression occurred in cells treated with a 20 pmol dose of SiRNA-DLGAP1-AS2 for 48 h, compared to other time points. Consequently, all subsequent tests were conducted within a 48-hour timeframe (Fig. 3B). To evaluate the efficiency of transfection flow cytometry was also utilized. Flow cytometry analysis (Fig. 3C) indicated a transfection rate of 99.6% for FITC-conjugated scramble in A549 cells. The optimal concentration and duration for siRNA-DLGAP1-AS2 transfection in all subsequent experiments were determined to be 20 pmol and 48 h, respectively (Fig. 3).
(A) DLGAP1-AS2 expression levels after transfection with different doses of siRNA. qRT-PCR results showed that dose of 20 pmol significantly decreased expression of the DLGAP1-AS2. (B) The impact of 20 pmol of siRNA-DLGAP1-AS2 on the expression level of lncRNA-DLGAP1-AS2 at 24, 48 and 72 h. At 48 h after transfection of DLGAP1-AS2-siRNA, the greatest decrease in DLGAP1-AS2 expression was observed. (C) The initial efficiency of siRNA-DLGAP1-AS2 transfection after FITC-labeled scramble transfection of A549 cells was evaluated by flow cytometry. The outcomes demonstrated a 99.6% transfection efficiency (ns; not significant, ****P < 0.0001).
Cytotoxicity assay of siRNA-DLGAP1-AS2 transfection
To study the viability and proliferation and of A549 cells following SiRNA-DLGAP1-AS2 transfection, an MTT test was conducted. The results revealed a significant influence on the viability of the transfected cells when the siRNA-DLGAP1-AS2 was transfected after 48 h at the optimal dose of 20 picomoles, in comparison to both control and negative control groups. But there is no significant difference between the viability of control cells and cells transfected with negative control (Fig. 4).
Increasing the sensitivity of A549 cells to carboplatin by reducing the expression of DLGAP1-AS2
The MTT assay was used to examine the toxic effects of carboplatin and siRNA-DLGAP1-AS2 on the viability of A549 cells and inhibitory concentrations of the drug. A549 cells were treated with carboplatin ranging from 1 to 800 µg/ml, which resulted in a persistent decrease in A548 cell viability. The IC50 for carboplatin was 256.6 µg/ml, which reduced cell viability by 50% when compared to the control group. In this study, the combination of carboplatin treatment and SiRNA-DLGAP1-AS2 transfection had a significant effect on the viability of A549 cells. So that, reducing the expression of lncRNA-DLGAP1-AS2 by transfecting siRNA-DLGAP1-AS2 increased the sensitivity of the A549 cells and reduced the IC50 of carboplatin from 256.6 µg/ml in cells that were treated separately to 145 µg/ml in the combined group (Fig. 5).
We used the following formula to investigate the synergistic effect of DLGAP1-AS2-siRNA and carboplatin in combination therapy. Based on the amount of live cells in the treatment group, because the CDI (Coefficient of Drug Interaction) was 0.88 (less than 1), it had synergistic effect.
\({\text{CDI }}=\frac{{AB~\left( {Relative~cell~viability~of~the~combination~group} \right)}}{{A~ \times B~(A~or~B~\left( {Relative~cell~viability~of~the~single~agent~groups} \right)}} \to {\text{CDI}}=\frac{{0.79}}{{0.83~ \times 1.078}}~={\text{ }}0.{\text{88 }}<{\text{ 1}}\)
The combined effects of carboplatin and SiRNA-DLGAP1-AS2 cause A549 cells to undergo apoptosis
To determine whether the combination of siRNA-DLGAP1-AS2 and carboplatin affects the induction of apoptosis, we used the annexin V/PI assay. The findings revealed that both siRNA-DLGAP1-AS2 transfection and carboplatin treatment independently induced apoptosis in A549 cells. However, when used in combination, the treatment resulted in a higher percentage of cell apoptosis compared to individual treatments with siRNA-DLGAP1-AS2 or carboplatin alone. Additionally, quantitative real-time PCR analysis was conducted to evaluate the mRNA levels of Bax and Bcl2, which are apoptosis-related genes. The results demonstrated that both siRNA-DLGAP1-AS2 transfection and carboplatin treatment increased the expression of the Bax gene in both treatment groups compared to the negative control group. Furthermore, the combination treatment exhibited significantly higher expression of the Bax gene compared to the separately treated cells. Moreover, the expression level of Bcl2 was decreased in the treatment group compared to the negative control group. Notably, the lowest levels of Bcl2 were observed in the combined treatment group compared to siRNA-DLGAP1-AS2 transfection or carboplatin treatment alone (Fig. 6).
Results of flow cytometry test using Annexin-V/PI staining method and expression of apoptotic genes. (A) In the combined group of siRNA-DLGAP1-AS2 and carboplatin, a significant increase in apoptosis is observed. (B) A notable increase in the expression level of Bax gene and a notable decrease in the expression level of BCL2 genes were observed in the combined group (**P < 0.01, ***P < 0.001, ****P < 0.0001, ns; not significant).
Regulation of cell cycle in A549 cells by the combined effect of carboplatin and SiRNA-DLGAP1-AS2
We conducted another flow cytometry experiment to examine the effects of SiRNA-DLGAP1-AS2 and carboplatin both individually and in combination on cell cycle arrest. According to our findings, the proportion of G1-phase cells in the transfected group increased, rising from 61.4% in the negative control group to 65.2% in the transfected group. Additionally, the proportion of cells in the sub-G1 phase has increased in the transfected group from 0.41 to 2.37% compared to the negative control group. The proportion of cells in the sub-G1 phase has significantly increased in the carboplatin-treated group, going from 0.41% in the negative control group to 10.5% in the treated group. The percentage of cells arrested in the S phase in the group treated with carboplatin also increased from 22.1% in the negative control group to 30.0% in the treated group. Finally, the combined treatment of SiRNA-DLGAP1-AS2 and carboplatin significantly increased cells in the sub-G1 phase to 25.2% (Fig. 7).
The cell cycle arrest was examined by using a flow cytometry technique. (A) Different groups exhibit cell cycle inhibition, and the combined group induced cell cycle arrest in the sub-G1. (B) The graph represents cell cycle distribution in treatment groups. The results are expressed as the mean of three independent runs (ns = not significant, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Inhibition of migration and invasion of A549 cells by combined effect of carboplatin and SiRNA-DLGAP1-AS2
The wound-healing assay was utilized to investigate the collective impact of siRNA-DLGAP1-AS2 and carboplatin on impeding cell migration. As depicted in Fig. 8, the migration of A549 cells was significantly diminished subsequent to siRNA-DLGAP1-AS2 transfection and carboplatin treatment. The findings further demonstrated that 72 h after the wound was inflicted, the combined treatment exhibited a lower migration rate compared to A549 cells that were individually transfected with siRNA-DLGAP1-AS2 or treated with carboplatin. Additionally, we evaluated the expression levels of MMP2 and MMP9, two genes associated with metastasis, to validate the outcomes of the wound-healing assay. In comparison to the negative control group, the expression levels of these genes were lower in cells treated separately with siRNA-DLGAP1-AS2 or carboplatin. Consequently, the combined treatment demonstrated a more effective reduction in MMP2 and MMP9 expression than individual treatments with siRNA-DLGAP1-AS2 and carboplatin (Fig. 8).
Effect of combination therapy on migration of A549 cells. (A) Downregulation of lncRNA-DLGAP1-AS2 expression and treatment with carboplatin can reduce A549 cell migration. (B) A graph illustrating the open wound area after 72 h. The results are expressed as the mean of three independent runs. (C) qRT-PCR results showed a significant reduction in MMP-2 and MMP-9 mRNA expression in the treatment groups, especially in the combination group (****P < 0.0001, ***P < 0.001, **P < 0.01).
Inhibition of colony formation in A549 cells by combined effect of carboplatin and SiRNA-DLGAP1-AS2
Following siRNA-DLGAP1-AS2 transfection and carboplatin treatment, colony formation ability, and colony size of A549 cells were significantly reduced. According to Fig. 9, it showed a significant decrease in colony formation of cells following siRNA-DLGAP1-AS2 transfection and carboplatin treatment. Also, the results showed that in the combined treatment group, fewer colonies were formed than the cells that were separately transfected with SiRNA-DLGAP1-AS2 or treated with carboplatin. In addition, to confirm the colony formation results, we evaluated the expression level of the CD44 as a colony formation-related gene. According to Fig. 9B, when compared to the negative control group, cells treated with siRNA-DLGAP1-AS2 or carboplatin separately reduced the expression level of CD44. So that, combined treatment reduced CD44 expression more effectively than separate treatment with siRNA-DLGAP1-AS2 and carboplatin (Fig. 9).
Colony assay and the impact of combination therapy on colony formation ability of A549 cells. (A) Reduction of lncRNA-DLGAP1-AS2 expression and treatment with carboplatin can reduce colony formation of A549 cells. (B) Results from qRT-PCR revealed a significant reduction in CD44 mRNA expression in the treatment groups, especially in the combination group (**P < 0.01, ***P < 0.001, ****P < 0.0001).
DLGAP1-AS2 is crucial in various biological processes
The TCGA database was queried using the web resource gepia to identify DLGAP1-AS2-related co-expressed genes. Hence, we identify 200 genes that correlate expression with DLGAP1-AS2. The coexpression network is shown in Fig. 10. Afterwards, these genes were imported into the Enrichr web program to query the KEGG pathway database database (permission obtained)16,17,18, revealing that they play crucial roles in the many pathways. The co-expressed genes and the related pathways are shown in Fig. 10. Also, Fig. 11A, B reveal that ferroptosis and glutathione metabolism emerge as the most significant pathways associated with the lncRNA DLGAP1-AS2, highlighting its potential role in oxidative stress and cell death regulation mechanisms in cancer. The mRNA target and downstream gene, GCLC, is particularly involved in these pathways and shows a notable overexpression in lung adenocarcinoma (LUAD), as demonstrated by TCGA data (Fig. 12A). This overexpression suggests a potential functional interaction between DLGAP1-AS2 and GCLC in promoting cancer cell survival. Further supporting this, qRT-PCR analysis reveals that transfection with DLGAP1-AS2 siRNA effectively downregulates GCLC expression (Fig. 12B), implying that DLGAP1-AS2 may regulate GCLC expression, influencing ferroptosis and glutathione metabolism in lung cancer cells.
(A) TCGA data shows significant overexpression of GCLC in LUAD tissues compared to normal lung tissues, indicating its potential role in tumor progression. (B) qRT-PCR analysis results following DLGAP1-AS2 siRNA transfection in lung cancer cell lines, illustrating a downregulation of GCLC expression. These findings suggest that DLGAP1-AS2 and carboplatin drug regulate GCLC levels, potentially impacting ferroptosis and glutathione metabolism pathways in lung cancer cells.
Discussion
Despite significant advancements in understanding, diagnosing, preventing, and treating cancer in recent years, this disease continues to impact millions of individuals worldwide. As a result, it is crucial to prioritize the fight against cancer through scientific progress on an international scale19. In the realm of cancer therapy, drug resistance poses a significant challenge. The emergence of resistance involves various underlying mechanisms, as each tumor possesses unique characteristics that contribute to its progression20. To better comprehend these processes, it is advisable to explore approaches that hinder the formation of cancer cells resistant to chemotherapeutic drugs and sensitize them to treatment21. Combination therapies, which target key pathways in an additive or synergistic manner, offer improved therapeutic efficacy compared to monotherapy approaches. This not only potentially reduces drug resistance but also provides advantages for cancer treatment. The development of new strategies that target survival pathways and yield affordable, effective, and efficient outcomes is of utmost importance. By employing combination therapy, lower therapeutic doses of individual drugs can be utilized. Additionally, this approach may prevent toxic effects on normal cells while simultaneously enhancing cytotoxic effects on cancer cells. This is particularly relevant if one of the drugs in the combination regimen differs in terms of cytotoxicity in normal cells, thereby safeguarding them from harmful effects22. Therefore, in this study, we have presented a combined chemotherapy-gene therapy protocol for lung cancer. The lung cancer cells were first made sensitive using gene therapy in this treatment method, and then by using the carboplatin drug, we managed to induce the death of A549 cells and significantly reduce their migration ability. When lung cancer cells were first treated with gene therapy by siRNA-DLGAP1-AS2 and then treated with chemotherapy drug carboplatin, the effective dose in IC50 of carboplatin drug decreased dramatically. This finding is critical to minimize damage to healthy cells and tissues adjacent to the tumor. This has been an important step in reducing the side effects of common chemotherapy drugs to treat lung cancer, including carboplatin.
In a study, Soltani et al. demonstrated that when compared to normal specimens, gastric cancer specimens significantly upregulated DLGAP1-AS2. As a result, it has been suggested that DLGAP1-AS2 overexpression could serve as a diagnostic and therapeutic target for gastric cancer and be linked to the progression and metastasis of the disease14. In another study, Chen et al. discovered that upregulation of DLGAP1-AS2 in hepatocellular carcinoma cells predicts poor patient survival. According to the obtained results, the knockdown of this gene can inhibit the invasion and migration of cancer cells by regulating the methylation of the miR-154-5p gene23. Miao et al. showed a new mechanism of how lncRNA DLGAP1-AS2 works in glioma. In this study, reducing the expression of this gene in glioma cells prevented cell proliferation and migration and induced apoptosis. Finally, these researchers confirmed that DLGAP1-AS2 can modulate the proliferation, migration and apoptosis of glioma cells by regulating Yes Associated Protein 1 (YAP1)9. These studies have been consistent with our study in the way that in the present study, gene therapy with siRNA-DLGAP1-AS2 had significant effects on reducing the viability of A549 lung cancer cells.
In this study, we examined that LncRNA-DLGAP1-AS2 affects a number of co-expressed genes and causes disruption in a series of molecular pathways. According to the change in the expression of these genes in cancer, it can help in the diagnosis of the disease and the possibility of treatment in people who have a family history. The upward trend of cancer research indicates the increasing importance of this field, and more studies are needed to determine the exact mechanism of action of these genes. According to a research, GCLC expression is increased in lung adenocarcinoma cell lines, and silencing GCLC accelerates ferroptosis and reduces cancer cell proliferation and invasion24. We also demonstrated in this study that this overexpression indicates a potential functional interaction between DLGAP1-AS2 and GCLC in promoting cancer cell survival. Also, clinical data have shown that high expression of DLGAP1-AS2 is associated with advanced pathological stage and poor prognosis. Functionally, overexpression of DLGAP1-AS2 increases aerobic glycolysis, and knockdown of DLGAP1-AS2 suppresses tumor growth of NSCLC cells25. A study with 5-year follow-up and analysis of DLGAP1-AS2 expression in NSCLC and paired non-tumor tissues from 64 NSCLC patients confirmed the interaction between DLGAP1-AS2 and miR-503. According to the results, DLGAP1-AS2 may regulate miR-503/cyclin D1 in NSCLC and promote cell proliferation26.In our study, the results of qPCR analysis show that transfection with DLGAP1-AS2 siRNA effectively reduces the expression of GCLC, which means that DLGAP1-AS2 may regulate the expression of GCLC, affect ferroptosis and glutathione metabolism in lung cancer cells.
Numerous factors, particularly genetic variations in tumor somatic cells, can contribute to the development of drug-resistant cancer cells. There are several mechanisms through which drug resistance can occur, including multidrug resistance, suppression of apoptosis, enhanced DNA repair, alterations in drug metabolism, epigenetic modifications, changes in drug targets, and gene amplification27. Many chemotherapy treatments function by inducing DNA damage, which leads to apoptosis in dividing cells. However, resistance can arise if cells acquire the ability to repair damage more efficiently or lose their ability to recognize lesions and signal the apoptotic machinery. Additionally, defects in the apoptotic machinery can render tumor cells less susceptible to programmed cell death, making them more resistant to the cytotoxic effects of chemotherapy28. In our study, the combination of gene therapy using SiRNA-DLGAP1-AS2 and chemotherapy resulted in an increased death rate of cancer cells, particularly at low concentrations of the carboplatin drug. This suggests that targeting DLGAP1-AS2 could be a promising strategy for overcoming carboplatin resistance in lung cancer. This finding aligns with the research conducted by Liang et al., who demonstrated that DLGAP1-AS2 can induce resistance to the chemotherapy drug tamoxifen. According to their study, upregulation of this gene can enhance the survival of cancer cells and inhibit apoptosis29. Also, Nan et al. identified DLGAP1-AS2 as an important non-coding RNA involved in chemoresistance in squamous cell carcinoma. In this way, this lncRNA can activate YAP signaling in these cells and cause resistance to cisplatin chemotherapy drug. Therefore, administration of an antisense oligonucleotide targeting DLGAP1-AS2 can sensitize squamous cell carcinoma to cisplatin treatment30.
The abnormal progression of the cell cycle is considered as a significant factor in the growth of cancer cells. In order to address this issue, certain factors can halt the cell cycle by targeting it and subsequently trigger the apoptosis of cancer cells31. The current study examined the synergistic effects of combination therapies on different phases of the cell cycle. Analysis of the cell cycle revealed that inhibiting DLGAP1-AS2 using specific siRNA alone notably increased the number of A549 cells in the G1 and sub-G1 phases. However, when this therapy was combined with carboplatin, the number of cells in the sub-G1 phase significantly increased. Furthermore, the results of the Annexin V/PI assay demonstrated that inhibiting DLGAP1-AS2 and treating with carboplatin alone were both capable of inducing apoptosis in A549 cancer cells. Particularly, when these two treatments were combined, the induction of apoptosis was significantly enhanced at low concentrations of carboplatin. BAX and BCL2 are crucial genes involved in apoptotic cell death and hold clinical importance32. In this study, qRT-PCR results exhibited significant alterations in the expression levels of BAX and Bcl2, indicating the induction of apoptosis in lung cancer cells compared to the control group. These changes towards increased apoptosis were more pronounced in the combined treatment group. In a similar vein, Xiao et al. investigated the role of DLGAP1-AS2 in the radioresistance of rectal cancer stem cells and discovered a link between the inhibition of this gene and apoptosis. According to the findings of their study, inhibiting DLGAP1-AS2 hindered proliferation, migration, and tumorsphere formation, while inducing apoptosis in cancer cells after X-ray irradiation33.
The results achieved from the wound healing test in this study showed that the combined treatment (inhibition of DLGAP1-AS2 by siRNA and chemotherapy with carboplatin) caused a more significant reduction in the ability of A549 cells to migrate than each of these individual treatment methods. In addition, qRT-PCR studies showed that the combined treatment provided considerably reduced the mRNA expression levels of MMP-2 and MMP-9 genes in A549 lung cancer cells. In this regard, Ren et al. showed that knockdown of DLGAP1-AS2 prevented the proliferation, migration and invasion of colorectal cancer cells in vitro and tumor growth in vivo34. In another experiment, Liu et al. presented that knockdown of GALNT10 and DLGAP1-AS2 together can prevent cell proliferation, migration and invasion in cholangiocarcinoma35.
Additionally, it was observed that both treatment approaches exhibited a noteworthy decline in the ability of cancerous cells to form colonies when compared to the control group. However, the combination treatment group displayed a more pronounced reduction in the colony-forming capability of A549 cells. In a similar vein, Lu et al. validated that the suppression of DLGAP1-AS2 led to a significant decrease in the number of colonies formed and the migratory capacity of gastric cancer cells36. Consequently, diminishing the expression of DLGAP1-AS2 not only possesses substantial anti-cancer effects on its own but also holds potential as a promising adjunctive treatment to enhance and augment the efficacy of other anti-cancer therapeutic approaches.
Although the results obtained from this study are very promising due to the silencing of the lncRNA-DLGAP-AS2 gene using related siRNA, transfection of siRNA into cells especially at in vivo conditions has a series of challenges. Against the potential functions of siRNA, there are a series of intracellular and extracellular barriers. For example, the presence of RNases in the intracellular and extracellular spaces causes extra and intravascular degradation of siRNA37,38. Additionally, the nucleotides of an siRNA may bind to unrelated mRNAs and repress gene expression. Processing of a siRNA by the RNA-induced silencing complex (RISC) can result in unwanted silencing of some genes with multiple nucleotide mismatches8. siRNAs can act as miRNAs by using the target recognition mechanisms of endogenous microRNAs (miRNAs). Therefore, partial complementarity is likely to lead to unexpected effects and results. In particular, unwanted gene silencing effects are revealed when long double-stranded RNA is used. Consequently, this off-target effect of siRNA can alter protein level39. Based on the studies, different characteristics of siRNA structure, sequence, and delivery method can cause adverse immune effects by stimulating the immune response40. Some physical characteristics of siRNA make the oral bioavailability of these molecules very weak. Also, intravenous and systemic delivery of siRNA results in rapid renal filtration and urinary clearance. Due to the degradation of siRNA by nucleases, it has a short half-life in vivo, especially if optimized chemical modifications are not introduced on the siRNA molecule41. Therefore, we hope to overcome the existing limitations by conducting more studies in this field. Also, in the near future, we will see the investigation of the combined effect of DLGAP1-AS2 inhibition and chemotherapy drugs under in vivo conditions and animal models, and the study may enter the clinical phases.
Conclusion
The findings of this research are extremely promising, showcasing the remarkable efficacy of DLGAP1-AS2 siRNA gene therapy in enhancing the sensitivity of lung cancer cells to carboplatin. The results obtained indicate that the combination of SiRNA-DLGAP1-AS2 and carboplatin chemotherapy effectively diminishes the migratory ability of cells, triggers apoptosis, and induces cell cycle arrest in A549 cells. These discoveries highlight the significant potential of the suggested combination therapy in providing an effective treatment option for patients with lung cancer.
Data availability
The results will be available upon the reasonable request from the corresponding author.
References
Fan, T., Sun, N. & He, J. Exosome-derived lncRNAs in lung cancer. Front. Oncol. 10, 1728 (2020).
Alberg, A. J., Brock, M. V. & Samet, J. M. Epidemiology of lung cancer: looking to the future. J. Clin. Oncol. 23 (14), 3175–3185 (2005).
Ginn, L. et al. LncRNAs in non-small-cell lung cancer. Non-coding RNA. 6 (3), 25 (2020).
Wang, M. et al. Long non-coding RNAs in non-small cell lung cancer: functions and distinctions from other malignancies. Translational Cancer Res. 8 (7), 2636 (2019).
Inamura, K. Lung cancer: understanding its molecular pathology and the 2015 WHO classification. Front. Oncol. 7, 193 (2017).
Liu, X., Pan, C. G. & Luo, Z. Q. High expression of NFAT2 contributes to carboplatin resistance in lung cancer. Exp. Mol. Pathol. 110, 104290 (2019).
Naghizadeh, S. et al. Overcoming multiple drug resistance in lung cancer using siRNA targeted therapy. Gene 714, 143972 (2019).
Hu, B. et al. Therapeutic siRNA: state of the art. Signal. Transduct. Target. Therapy. 5 (1), 101 (2020).
Miao, W. et al. LncRNA DLGAP1-AS2 modulates glioma development by up-regulating YAP1 expression. J. Biochem. 167 (4), 411–418 (2020).
Grillone, K. et al. A systematic review of non-coding RNA therapeutics in early clinical trials: a new perspective against cancer. J. Transl. Med. 22 (1), 731 (2024).
Jiang, J. et al. The emerging roles of long noncoding RNAs as Hallmarks of Lung Cancer. Front. Oncol. 4156. (2021).
Hu, Q. et al. LncRNA in tumorigenesis of non-small-cell lung cancer: from bench to bedside. Cell. Death Discov.. 8 (1), 1–9 (2022).
Wang, X. et al. Long noncoding RNA DLGAP1-AS2 promotes tumorigenesis and metastasis by regulating the Trim21/ELOA/LHPP axis in colorectal cancer. Mol. Cancer. 21 (1), 1–19 (2022).
Soltani, R. et al. LncRNA DLGAP1-AS2 overexpression associates with gastric tumorigenesis: a promising diagnostic and therapeutic target. Mol. Biol. Rep. 1–10. (2022).
Wang, L. et al. LncRNA DLGAP1-AS2 regulates miR-503/cyclin D1 to promote cell proliferation in non-small cell lung cancer. BMC Pulm. Med. 21 (1), 1–8 (2021).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951 (2019).
Kanehisa, M. et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–d592 (2023).
Biemar, F. & Foti, M. Global progress against cancer—challenges and opportunities. Cancer Biology Med. 10 (4), 183 (2013).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575 (7782), 299–309 (2019).
Leary, M. et al. Sensitization of drug resistant cancer cells: a matter of combination therapy. Cancers 10 (12), 483 (2018).
Mokhtari, R. B. et al. Combination therapy in combating cancer. Oncotarget 8 (23), 38022 (2017).
Chen, K. et al. lncRNA DLGAP1-AS2 knockdown inhibits hepatocellular carcinoma cell migration and invasion by regulating miR-154-5p methylation. BioMed Res. Int. (2020).
Luo, L. et al. Ferroptosis-related gene GCLC is a novel prognostic molecular and correlates with immune infiltrates in lung adenocarcinoma. Cells 11 (21), 3371 (2022).
Zhang, Q. et al. METTL3-induced DLGAP1-AS2 promotes non-small cell lung cancer tumorigenesis through m6A/c-Myc-dependent aerobic glycolysis. Cell. Cycle. 21 (24), 2602–2614 (2022).
Wang, L. et al. LncRNA DLGAP1-AS2 regulates miR-503/cyclin D1 to promote cell proliferation in non-small cell lung cancer. BMC Pulm. Med. 21 (1), 277 (2021).
Mansoori, B. et al. The different mechanisms of cancer drug resistance: a brief review. Adv. Pharm. Bull. 7 (3), 339 (2017).
Samuel, P., Fabbri, M. & Carter, D. R. F. Mechanisms of drug resistance in cancer: the role of extracellular vesicles. Proteomics 17 (23–24), 1600375 (2017).
Liang, X. et al. DLGAP1-AS2 promotes estrogen receptor signalling and confers tamoxifen resistance in breast cancer. Mol. Biol. Rep. 1–9. (2022).
Nan, Y. et al. DLGAP1-AS2-Mediated Phosphatidic Acid Synthesis Confers Chemoresistance via Activation of YAP Signaling. bioRxiv, (2022).
Deep, G. & Agarwal, R. New combination therapies with cell cycle agents. Curr. Opin. Invest. Drugs 9(6), 591 (2008).
Naseri, M. H. et al. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int. 15 (1), 1–9 (2015).
Xiao, S. Y. et al. LncRNA DLGAP1-AS2 promotes the radioresistance of rectal cancer stem cells by upregulating CD151 expression via E2F1. Translational Oncol. 18, 101304 (2022).
Ren, C. et al. DLGAP1-AS2 promotes human colorectal cancer progression through trans-activation of Myc. Mamm. Genome. 33 (4), 672–683 (2022).
Liu, Z. et al. The long noncoding RNA DLGAP1-AS2 facilitates cholangiocarcinoma progression via miR‐505 and GALNT10. FEBS Open. Bio. 11 (2), 413–422 (2021).
Lu, J. et al. Long noncoding RNA DLGAP1-AS2 facilitates Wnt1 transcription through physically interacting with Six3 and drives the malignancy of gastric cancer. Cell. Death Discov.. 7 (1), 1–12 (2021).
Mokhtarzadeh, A. et al. Recent advances on biocompatible and biodegradable nanoparticles as gene carriers. Expert Opin. Biol. Ther. 16 (6), 771–785 (2016).
Mokhtarzadeh, A. et al. Applications of spherical nucleic acid nanoparticles as delivery systems. Trends Mol. Med. 25 (12), 1066–1079 (2019).
Seok, H. et al. Evaluation and control of miRNA-like off-target repression for RNA interference. Cell. Mol. Life Sci. 75, 797–814 (2018).
Meng, Z. & Lu, M. RNA interference-induced innate immunity, off-target effect, or immune adjuvant? Front. Immunol. 8, 331 (2017).
Sarett, S. M., Nelson, C. E. & Duvall, C. L. Technologies for controlled, local delivery of siRNA. J. Controlled Release. 218, 94–113 (2015).
Acknowledgements
We are grateful for financial support from the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Grant number: 71287).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.A. M. , R.M. and , B. B.Data curation: S.G.A. , M.A. and S.N.Formal analysis: S.G.A , M.A. , S.N. , A.A.M.Investigation: S.G.A. , M.A. , S.N. , S.Z.B.M. and A.Y.Methodology: A.A.M.Bioinformatics and systems biology analysis: A.Y.Project administration: A.A.M., B.B.Software: S.G.A. , M.A. , S.N.Supervision: A.A.M. , R.M. , B.B. Validation: A.A.M. , M.A. , S.N.Visualization: S.G.A. , A.A.M. Writing–original draft: S.G.A. , S.Z.B.M , A.Y.Writing–review editing: S.G.A. , B.B. , A.A.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alamdari, S.G., Mohammadzadeh, R., Amini, M. et al. Improvement of carboplatin chemosensitivity in lung cancer cells by siRNA-mediated downregulation of DLGAP1-AS2 expression. Sci Rep 15, 7971 (2025). https://doi.org/10.1038/s41598-025-87649-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87649-6