Abstract
Culex quinquefasciatus is a widely spread mosquito species that poses a significant public health threat in many countries. This insect vector is present in the United Arab Emirates (UAE), yet no studies have been conducted on its resistance to any insecticide group. Research shows that controlling mosquitoes is crucial to eliminating mosquito-borne diseases, but when these vectors develop insecticide resistance, the situation can escalate dangerously out of control. This study aimed to identify a knockdown resistance (kdr) mutation L1014F using molecular tools. Additionally, it aimed to assess deltamethrin resistance using the Centers for Disease Control and Prevention (CDC) bottle bioassay. We screened Cx. quinquefasciatus adults (N = 174) for the presence of the mutation using allele-specific PCR (AS-PCR) and DNA sequencing. We detected the mutation and found the kdr allele in all the sampled locations. Furthermore, the CDC bottle bioassay revealed deltamethrin resistance from only one sampling location. To our knowledge, this is the first report of insecticide resistance in Cx. quinquefasciatus in the UAE. Our findings show the need for continued insecticide resistance monitoring for effective mosquito control in the UAE.
Similar content being viewed by others
Introduction
Mosquitoes are important disease vectors found worldwide. One important species is the southern house mosquito, Culex quinquefasciatus. While it may be perceived as a mere nuisance due to its blood-feeding tendencies1,2, this mosquito species holds a more significant role as a carrier of devastating diseases, including the West Nile virus and avian malaria3. These arthropods often facilitate the transmission of infections from one host species to another. The long-standing associations between hosts and pathogens have been disrupted primarily due to extensive human-induced environmental changes, leading to the emergence and resurgence of infectious diseases4,5. The pathogens within a given geographical area, vectors, and density of hosts play a pivotal role in determining the transmission of diseases6,7. Additionally, anthropogenic environmental changes, such as deforestation and climate change, can increase the risk of disease transmission by creating new habitats for mosquitoes and other vectors8. Understanding these organisms’ ecology is essential to developing effective disease prevention and control strategies. As a widespread mosquito, Cx. quinquefasciatus, is found in Asia, Africa, the Middle East, North America, South America, Australia, and New Zealand6. It was reported in UAE9 and neighboring countries such as Oman10, Saudi Arabia11, Qatar12, and Iran13.
Chemical control is a commonly used measure against mosquitoes. For a long time, DDT (dichloro-diphenyl-trichloroethane) was one of the insecticides used for public health initiatives14,15,16,17, while pyrethroids, particularly deltamethrin, are frequently employed in agriculture and control of disease vectors. Yet, there is mounting concern regarding their excessive and, in some cases, careless application, as it may result in heightened resistance18,19,20,21, ultimately jeopardizing the efficacy of vector control measures and rendering them unfeasible in the long run22,23. Two primary mechanisms are responsible for insecticide resistance in Cx. quinquefasciatus. One of these is a change in target site, as seen in the reduced sensitivity of sodium channels, which can be caused by mutations such as kdr (knockdown resistance)24,25,26,27,28. The second mechanism involves an increase in the production of detoxifying enzymes such as esterases, mixed function oxidases (MFOs), and glutathione-S-transferases (GSTs) in resistant insects29,30. DDT and pyrethroids both target the voltage-gated sodium channel (VGSC). By interacting with this channel, they disrupt normal functioning31, causing prolonged channel opening and increasing nerve impulse transmission32. This ultimately leads to paralysis and eventual death of the insect. A specific genetic alteration, a single nucleotide polymorphism (TTA to TTT), within the S6 hydrophobic transmembrane segment of domain-II (IIS6 domain) of VGSC33,34, results in the substitution of leucine with phenylalanine (L1014F)35. This genetic variation reduces the affinity of the target site for insecticides. This resistance mechanism was initially identified in the house fly, Musca domestica, by Milani in 195436 and is known as kdr. The kdr mechanism has been extensively studied for its ability to resist pyrethroids and DDT in various insect species. Many reports of this mutation in Culex mosquitoes exist around the globe, and numerous mutations in insect VGSC have been documented, all of which result in decreased sensitivity to insecticides or neurotoxins37,38,39,40. The kdr mutation in mosquitoes has garnered considerable interest due to pyrethroids’ critical role in worldwide mosquito control initiatives.
In the Middle East, despite Cx. quinquefasciatus being widespread and serving as a vector for various diseases, there is a noticeable lack of research publications addressing its biology, behavior, and disease transmission dynamics. In the UAE, no studies have been conducted on the resistance of Cx. quinquefasciatus to insecticides so far. Only two publications on insecticide resistance exist in the country, both of which focus on the house fly and were produced by our laboratory41,42. The absence of information impedes our understanding of the population dynamics, genetic variations, and potential risks associated with Cx. quinquefasciatus in this part of the world. As a result, it is essential to address this knowledge gap to develop targeted control strategies and implement effective measures to mitigate mosquito-borne diseases within the region. Therefore, it is crucial to encourage increased research efforts and foster collaborations among scientists. This study aimed to identify a resistance kdr mutation (L1014F) in Cx. quinquefasciatus using molecular tools. Additionally, it aimed to assess deltamethrin resistance in Cx. quinquefasciatus adults using a CDC bottle bioassay.
Results
Using primers for the AChE gene, PCR yielded the expected target bands needed for species identification, followed by DNA sequencing that confirmed 100% similarity to Cx. quinquefasciatus strains from India (GenBank accession numbers ON563190.1, ON563188.1, and ON563187.1). The generated AChE gene sequence from this study was deposited in GenBank under accession number PP386733. Similarly, sequencing of the CO1 gene fragment revealed sequences sharing 100% similarity with GenBank entries of Cx. quinquefasciatus from India, Colombia, Turkey and UAE (ON351296.1, MN997468.1, MK713993.1, MK170086.1, respectively). The CO1 gene sequence of this study was submitted to GenBank under accession number PP594439. In addition, the phylogenetic tree confirmed these findings and revealed the DNA sequence of this study in a big cluster of Cx. quinquefasciatus with a node supported by a high bootstrap value (Fig. 1).
Phylogenetic tree of partial nucleotide sequences of the cytochrome oxidase subunit 1 (CO1) mitochondrial gene of Culex quinquefasciatus from Abu Dhabi, UAE, constructed by the Neighbor-Joining method, Kimura 2-parameter (K2P) model, and 1000 replicates of bootstrap. Only bootstrap values > 50% are shown. The phylogenetic analysis includes sequences retrieved from the GenBank using NCBI-BLAST with the highest similarity to the Cx. quinquefasciatus (PP594439) from this study. Aedes albopictus and Culex theileri were used as outgroups. MEGA11 was used to make the tree.
The primers utilized for amplifying the kdr mutation L1014F via two PCR reactions generated target bands measuring 380 bp (Fig. 2). The kdr allele was identified across the three surveyed regions in the genotypes RR or RS (where RR is 1014 F, RS is L1014F, and where SS is L1014). Within the coastal sampling sites, 25.7% of individuals exhibited homozygous resistance (RR) genotype (Fig. 3). Conversely, inland locations depicted a lower prevalence of RR individuals (6.3%). The Oman border locations exhibited 14.3% RR genotype. Overall, the prevalence of RR genotype in all DNA samples was 18.4% (32 out of 174). Furthermore, the percentage of the susceptible genotype (SS) varied across locations, with the highest proportions in the Oman border area (38.1%) and inland region (37.5%), and the lowest along the coast (21.6%). The detailed data of each genotype per location were listed in the supplementary materials (Table S1).
Genotyping of Culex quinquefasciatus from Abu Dhabi, UAE, based on the kdr mutation L1014F using AS-PCR and agarose gel (1.5%) stained with ethidium bromide. Susceptible insects (SS; sus/sus) had a 380-bp band with Cq3. Heterozygous insects (RS; kdr/sus) had a 380-bp with Cq3 and Cq4. Resistant homozygous insect (RR; kdr/kdr) has a 380-bp with Cq4. kdr, Knockdown resistance; M, 100-bp marker (Promega, Madison, WI, USA); AS-PCR, Allele-specific PCR.
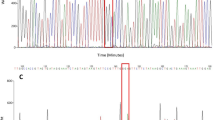
Sequencing the PCR products delineated TTA for SS, TTT for RR insects, and TTW for RS, where W represents T/A (Fig. 4) and the sequences were deposited in the GenBank as PP858882, PP858881, and PP858883, respectively. Accordingly, the chromatograms displayed a singular peak for SS and RR. At the same time, RS depicted two peaks (Fig. 4). Mosquito populations in the three main sampling regions (coastal, inland-desert, and Oman border) adhered to HWE based on χ2 and P-value (0.2303, 0.6312; 0.9741, 0.3236; 0.0077, 0.9297, respectively). Applying the CDC bottle bioassays to susceptible mosquitoes determined the diagnostic time and dose for deltamethrin as 60 min and 0.8 µg/bottle, respectively. Consequently, using these values, deltamethrin resistance was identified in field-collected Cx. quinquefasciatus from only one location (Al Nahyan), constituting 12.5% of the CDC bioassay surveyed locations (Table 1).
Genotyping of the Culex quinquefasciatus from Abu Dhabi, UAE, based on the kdr mutation L1014F using DNA sequencing. Sequences were aligned with the genomic reference sequence (GenBank accession number NC_051862.1) using Unipro UGENE software. The chromatograms were viewed using SnapGene software (www.snapgene.com). The mutation occurs at position number 193,055. Susceptible (SS) insects have the sequence TTA; Resistant homozygous (RR) insects have the sequence TTT; and heterozygous (RS) insects have the sequence TTW, where W is T/A. Chromatograms show a single peak in SS and RR genotypes and two peaks in RS genotypes at the kdr mutation position. kdr, Knockdown resistance; RR, homozygous resistant (kdr/kdr); RS, heterozygous (kdr/sus); SS, homozygous susceptible (sus/sus).
Discussion
The development of insecticide resistance in insect disease vectors, including mosquito populations, presents a significant challenge to global public health efforts24,43. This study focuses on Cx. quinquefasciatus, a key mosquito species in the UAE known to transmit various diseases globally. Hitherto, conventional vector control has relied heavily on insecticides, but the emergence of resistance in mosquito species like Cx. quinquefasciatus threatens their effectiveness. No prior studies have been conducted on insecticide resistance in any mosquito or arthropod species in the UAE, except for two studies on house flies41,42. Therefore, the current study addresses this crucial knowledge gap and provides essential insights to support the global fight against mosquito-borne diseases. The main finding of this study is the identification of deltamethrin-resistant Cx. quinquefasciatus genotypes in Abu Dhabi. In addition, it detected the kdr mutation L1014F in different locations across the study region. Further, this study established diagnostic dose and time for screening Cx. quinquefasciatus for deltamethrin resistance in the UAE using the CDC bottle bioassay.
kdr-mediated target site insensitivity is a prevalent insecticide resistance mechanism in insects44. In mosquitoes, various kdr mutations confer pyrethroid resistance45. For example, Aedes mosquitoes harbor mutations such as V253F, V410L, I1011M, V1016G, V1016I, F1534C, and L982W46. Notably, Cx. quinquefasciatus primarily relies on the L1014F mutation for kdr-based resistance47. In the current study, AS-PCR revealed the presence of the L1014F kdr mutation in some Cx. quinquefasciatus mosquitoes from all sampled locations and this warrants action to avoid reaching higher levels of resistance in the future. A high prevalence of mosquito resistance significantly threatens public health and may cause human deaths worldwide45. Accordingly, the kdr mutation renders Cx. quinquefasciatus mosquitoes less susceptible to pyrethroid insecticides, jeopardizing our ability to control their populations and the spread of mosquito-borne diseases such as Western equine encephalitis, and West Nile fever. The widespread presence of kdr alleles can further fuel resistance development, necessitating the exploration of alternative control methods like genetic approaches48 and biological control49. A shift towards integrated pest management strategies50 that combine these methods with judicious insecticide use is crucial to manage mosquito populations effectively. This calls for establishing resistance management programs for mosquitoes in Abu Dhabi and the rest of the UAE and employing non-chemical control measures as a cornerstone of mosquito control efforts. Our findings generally agree with published studies about insecticide resistance in Cx. quinquefasciatus. Hence, the kdr mutation L1014F was reported in Cx. quinquefasciatus in Mexico51, Colombia52, USA53, India34, Saudi Arabia54, and other countries.
In the current study, the geographic distribution of the resistant genotype RR (kdr/kdr) was not limited to one part of Abu Dhabi; instead, it was spread to the three regions covered in this study (coastal, inland, and Oman border). Insects with the RR genotype survive insecticide exposure and pass on both resistance kdr alleles to offspring, increasing resistance in the mosquito population. This renders insecticides ineffective, necessitating alternative control methods. It should be noted that resistance spread can be a complex process with many factors affecting it. Susceptible migrating insects (SS) can dilute resistance55. Also, the RR insects can mate with susceptible insects and produce heterozygous genotypes (RS), which can contribute to resistance because they carry one copy of the resistance allele56,57. Furthermore, the presence of RR insects may result in the selection of additional resistance mechanisms, leading to the emergence of super-resistant insects58. These insects can be highly challenging to manage and require diverse control strategies.
Allele-specific PCR (AS-PCR) offers a valuable and straightforward approach for detecting resistance mutations in insects59,60. This technique efficiently utilizes PCR and agarose gel electrophoresis to analyze for the presence of mutations. We used AS-PCR successfully in the current study; however, while DNA sequencing, such as Sanger or NGS, can provide definitive confirmation of the specific nucleotide change associated with the kdr mutation, its first-pass success rate may not always be high. Nonetheless, sequencing may offer additional precision when compared to AS-PCR, especially when haplotype-specific positive controls are unavailable. On the other hand, while DNA sequencing offers the highest level of accuracy, its reliance on specialized facilities can limit accessibility, particularly for researchers in resource-limited settings. Therefore, we suggest combining AS-PCR for initial screening followed by DNA sequencing for confirmatory analysis as a powerful strategy for kdr mutation detection, particularly when considering the potential constraints faced by researchers in developing countries. AS-PCR and other surrogate methods offer a cost-effective alternative, allowing researchers to stretch limited resources while enabling broader sampling strategies. When proper positive and negative controls are included, these methods can provide reliable initial results, and they have been instrumental in advancing our understanding of kdr mutations, especially in Aedes aegypti. This approach ensures that resource limitations do not hinder large-scale screening efforts, while DNA sequencing can be reserved for confirmatory purposes to enhance accuracy. Several studies reported the use of DNA sequencing for resistance determination. For example, DNA sequencing was used to identify genomic changes associated with insecticide resistance in Aedes aegypti by deep-targeted sequencing61. Further, whole-genome sequencing was employed to study insecticide resistance loci in malaria mosquitoes62.
Populations of Cx. quinquefasciatus in the three main sampling regions of this study adhered to HWE. Typically deviations can occur due to selection, mutation, genetic drift, gene flow, and non-random mating63. The fact that the populations are currently in equilibrium suggests no strong deviation is caused by these factors at present. However, the presence of the kdr mutation and the RR (kdr/kdr) genotype indicates that insecticide use in Abu Dhabi is exerting selection pressure on mosquito populations. While this has not yet resulted in a significant shift away from HWE, continued insecticide applications may further eliminate susceptible mosquitoes in some treated areas, leaving resistant ones (with the kdr mutation) to reproduce. Over time, this could lead to a shift in allele frequencies and eventual deviation from HWE.
We employed the CDC bottle bioassay, an easy and rapid method for evaluating mosquito resistance to insecticides64. Our study marks the first use of this technique in the UAE to assess insecticide resistance in any mosquito population. The results of the CDC bottle bioassay of the current study suggest its use as a good tool for future resistance research in the UAE because it establishes diagnostic parameters (dose and time) for screening Cx. quinquefasciatus for deltamethrin resistance. Additionally, this method’s findings revealed that only one out of the eight tested mosquito populations exhibited resistance to deltamethrin, with the remaining seven populations demonstrating susceptibility. The DNA sequencing of the deltamethrin-resistant mosquitoes, detected by the CDC bioassay, revealed the presence of kdr mutation L1014F in those insects. In this study, resistance was detected in only one population using the CDC bottle bioassay, despite the presence of RR and RS genotypes in all populations identified by AS-PCR. This difference may be attributed to seasonal variations, as mosquitoes for AS-PCR were collected earlier in the season, while those for the CDC bioassay were collected later. Additionally, population dynamics, such as the influx of susceptible individuals, may have temporarily reduced resistance levels detected by the CDC bioassay. Several studies reported the use of the CDC bottle bioassay to assess resistance in Cx. quinquefasciatus65,66. Beyond its speed, the CDC bottle bioassay is cost-effective, requiring minimal equipment compared to molecular techniques.
Given the vast diversity of mosquito species (> 3500) worldwide67, we employed both morphological and molecular methods for species identification. Our findings confirmed Cx. quinquefasciatus is a prevalent mosquito species in Abu Dhabi, and it was detected in all surveyed locations. This aligns with previous reports documenting Cx. quinquefasciatus as one of the seven Culex species in the UAE9. The research on Cx. quinquefasciatus in the Middle East and North Africa (MENA) region further emphasizes its prevalence and potential disease transmission role. For instance, studies in Sudan identified it as a dominant indoor-resting species along the Nile68. Similarly, studies suggest its potential for West Nile Virus transmission69 and its overall abundance70 in Saudi Arabia. Additionally, studies in Egypt reported its association with Culex pipiens, another medically important mosquito71. Overall, this shows that research on Cx. quinquefasciatus in the UAE can support and integrate with regional and global efforts for mosquito control.
A limitation of this study is that DNA sequencing was not conducted on all AS-PCR samples due to resource constraints, including budgetary and time restrictions. Instead, sequencing was solely employed to validate AS-PCR findings. Nonetheless, we recommend incorporating DNA sequencing for all samples in future studies, contingent upon the availability of resources. This comprehensive approach is anticipated to yield precise identification of kdr mutations and facilitate the discovery of additional mutations within the sequenced gene fragment. Additionally, this study provided insights into insecticide resistance in Cx. quinquefasciatus populations only in Abu Dhabi. Thus, a broader examination across the entire UAE could yield a more comprehensive understanding of the resistance status in Cx. quinquefasciatus populations. Despite these limitations, the findings of this study contributed significant insights into the localized presence of resistant genotypes in Abu Dhabi, laying the groundwork for future research on insecticide resistance in Cx. quinquefasciatus populations across the UAE.
Insecticides such as deltamethrin have been used for a long time to control Cx. quinquefasciatus, and this can lead to resistance development by selecting insects with resistant-related mutations. Our findings revealed the presence of deltamethrin resistance and the kdr mutation L1014F in Cx. quinquefasciatus adults. The resistant genotype RR (kdr/kdr) was found in the three main sampling regions of Abu Dhabi. Hence, it is imperative to study resistance in Cx. quinquefasciatus in all areas of the UAE, to design insecticide resistance monitoring programs and to establish integrated pest management programs for mosquitoes.
Methods
Study sites and sample collection
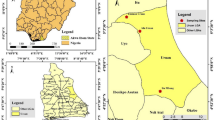
In 2023, mosquito larvae were collected from larval habitats across Abu Dhabi, UAE, to provide a comprehensive representation of the study area. We collected multiple egg rafts from a single location within each area. The samples represented sixteen locations from the three main human-populated regions: coastal, inland-desert, and Oman border (Fig. 5).
Map of United Arab Emirates showing the collection locations of the Culex quinquefasciatus for kdr mutation detection in Abu Dhabi, UAE, 2023. The collection focus was on the more populated areas and less in the desert region. 1, Sadiyat Island; 2, Mina Zayed; 3, Al Bateen; 4, Al Hisn; 5, Khalifa City; 6, Al Mirfa; 7, Madinat Zayed; 8, Liwa; 9, Al Foah; 10, Al Hayer; 11, Al Hili; 12, Al Kahbisi; 13, Al Noud; 14, Um Gafa; 15, Industrial Area; 16, Al Wagan. The UAE map was manually traced and reproduced using Microsoft PowerPoint, based on an image from Worldometers (https://www.worldometers.info/img/maps_c/AE-map.gif).
Mosquito rearing, identification, and use
Mosquito larvae were reared in the laboratory using a standard procedure72 until adults emerged. Mosquitoes were morphologically identified as Cx. quinquefasciatus using keys73,74. In addition, individual morphologically identified adult Cx. quinquefasciatus mosquitoes were selected for molecular identification confirmation using DNA-based molecular tools. In this study, adult Cx. quinquefasciatus mosquitoes were used for two objectives. Firstly, we used live adult mosquitoes (125 insects/test) for the CDC bottle bioassay. Secondly, we stored adult mosquitoes in a -20 °C freezer until used for DNA extraction and PCR for kdr mutation detection.
DNA extraction
For each sampling region, only a subset of the emerging adult mosquitoes was processed due to budget and time constraints. Thus, a total of 174 individuals were used for DNA extraction and AS-PCR. The Oman border region was represented by 84 samples, the coastal region of Abu Dhabi by 74 DNA samples, and the inland-desert region by 16 samples. Genomic DNA (gDNA) was extracted from the mosquito samples individually. For males, the entire body was homogenized, while for females, the abdomen was removed before homogenization to prevent potential DNA contamination from male sperm in mated females. Homogenization was done using a bead homogenizer (Benchmark Scientific, Sayreville, NJ, USA). DNA extraction was conducted using the ReliaPrep™ gDNA Tissue Miniprep System (Promega, Madison, Wisconsin, USA), following manufacturer guidelines. DNA was quantified in each sample using a NanoDrop 2000 UV spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Extracted DNA specimens were stored at − 20 °C.
PCR and Sanger sequencing
After morphological identification, we performed the molecular confirmatory identification of Cx. quinquefasciatus using PCR based on primers targeting two genes, acetylcholinesterase (AChE) and cytochrome c oxidase subunit I (CO1). For the AChE gene, we used specific primers designed to produce a 274 bp band with Cx. quinquefasciatus75. These primers work on the polymorphisms in the second intron of the acetylcholinesterase-2 (ace-2) locus and can unambiguously distinguish among four different species of Culex. We used the forward primer ACEquin and the reverse primer B1246s. The PCR conditions mirrored those detailed by Smith and Fonseca75. For the CO1 gene, we used standard universal DNA barcoding primers LCO1490 and HCO2198 76. The PCR produced a 710 bp band, and thermocycling conditions mirrored those detailed by Folmer et al.76. In addition, we investigated the presence of the kdr mutation L1014F in field-collected mosquitoes using the AS-PCR protocol outlined by Sarkar et al.34. Accordingly, two parallel PCR reactions were run, with one combining primers Cq1, Cq2, and Cq3 (for the detecting susceptible insects, L1014), and the other combining Cq1, Cq2, and Cq4 (for the detecting resistant insects, 1014 F). Reaction mixtures comprised PCR Master-Mix 12.5 µl (GoTaq G2 Green Master Mix, Promega, Madison, Wisconsin, USA), 1 µl of each primer (totaling 3 µl in each reaction), 6.5 µl of nuclease-free water, and 3 µl of DNA sample, with a final reaction volume of 25 µl. PCR conditions mirrored those detailed by Sarkar et al.34. The sequences of all the primers used in this study are presented in Table 2.
For all PCRs, as mentioned earlier, we included a negative control reaction (no DNA template was added) to check for any reagent contamination. Post PCR amplifications, DNA fragments were separated by electrophoresis in 1.5% agarose gels stained with ethidium bromide and visualized under UV light (OmniDOC SAFE Gel Documentation System; Cleaver Scientific, Rugby, UK). Insect genotyping for insecticide resistance was conducted by interpreting gel bands in terms of size and quantity, categorizing genotypes as homozygous susceptible (SS; sus/sus, defined as the L1014 haplotype), homozygous resistant (RR; kdr/kdr, defined as the 1014 F haplotype), and heterozygous (RS; kdr/sus, defined as the L1014F haplotype). The Cq3 and Cq4 primers generated fragments at 380 bp on the gel, where one band with the Cq3 represented SS, one band with the Cq4 represented RR, and two bands with Cq3 and Cq4 represented RS. Moreover, DNA sequencing was employed as a secondary method to confirm mosquito genotypes produced by AS-PCR, involving PCR amplification of mosquito DNA using IIP_F and IIS6_R primers and thermocycling conditions following the protocol detailed in Chamnanya et al.77. Amplified products underwent Sanger sequencing using the above-mentioned forward and reverse primers. The UGENE software78 was used for DNA sequence analysis, and chromatograms were reviewed using the SnapGene software (www.snapgene.com). Sequences underwent multiple alignments using the Clustal Omega algorithm within UGENE, enabling precise comparison to the VGSC gene of the mosquito available on the National Center for Biotechnology Information (NCBI) database (GenBank accession number NC_051862.1). This alignment facilitated the identification of the specific position of the kdr mutation. Additionally, we used a DNA sequence CCACCGTAGTGATAGGAAATTTT as a guide reference sequence for pinpointing genetic variation (kdr mutation) in each mosquito sequence from this study while doing the multiple alignments. In this context, susceptible homozygous (SS) insects had TTA, resistant homozygous (RR) insects had TTT, and heterozygous (RS) insects had TTW (where W is A/T). Chromatograms generated from the sequencing process were uploaded to SnapGene to determine chromatogram shape and quality scores. The chromatograms revealed a single A peak (representing two overlapping A peaks) in SS insects, a single T peak (representing two overlapping T peaks) in RR insects, and two distinct peaks (one A and one T) in RS insects at the kdr mutation position.
CDC bottle bioassay
The active ingredient of the insecticide deltamethrin PESTANAL® (Sigma-Aldrich, St. Louis, MO, USA) was utilized following the CDC bottle bioassay protocol64. Deltamethrin powder was dissolved in acetone to produce a stock solution, from which several serial dilutions (working solutions) were prepared to determine the diagnostic dose and time using susceptible Cx. quinquefasciatus mosquitoes. We obtained the susceptible mosquitoes from Dubai Municipality, UAE; these had been laboratory-bred and not exposed to insecticides for ten years. We reared them under standard conditions in the laboratory. Each CDC bioassay utilized five standard 250-ml DURAN® (DWK Life Sciences GmbH, Mainz, Germany) glass bottles with screw caps. The inside surfaces of four bottles were coated with 1 ml of deltamethrin working solution at appropriate concentrations, while the fifth bottle was treated with acetone only as a control. Each bottle contained 25 mosquitoes (5 days old, both males and females), and mortality was monitored at 15-minute intervals for up to 2 h (time points: 0, 15, 30, 45, 60, 75, 90, 105, and 120 min). After determining the diagnostic dose and time using susceptible mosquitoes, field-collected mosquitoes were tested to determine resistance levels. Mosquitoes were collected from eight locations (Fig. 6).
Map of United Arab Emirates showing the collection locations of the Culex quinquefasciatus for CDC bottle bioassay in Abu Dhabi, UAE, 2023. The collection focus was on the more populated areas and less in the desert region. 1, Al Nahyan; 2, Al Manhal; 3, Al Bateen; 4, Madinat Zayed; 5, Masakin; 6, Al Nabbagh; 7, Mbazzarah Al Khadra; 8, Central District. The UAE map was manually traced and reproduced using Microsoft PowerPoint, based on an image from Worldometers (https://www.worldometers.info/img/maps_c/AE-map.gif).
Statistical analysis
AS-PCR results were processed in a Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) to calculate averages and percentages for each mosquito genotype. Hardy–Weinberg Equilibrium (HWE) was assessed using an online tool79; http://apps.biocompute.org.uk/hwe-mr-calc.html), with Chi-square (χ2) and P-values calculated. The phylogenetic tree was constructed using MEGA11 software80.
Data availability
All data generated or analyzed during the current study are available in the paper, the supplementary file, and the GenBank under the following accession numbers: PP386733, PP594439, PP858882, PP858881, PP858883.
References
Kalimuthu, K., Panneerselvam, C., Murugan, K. & Hwang, J. S. Green synthesis of silver nanoparticles using Cadaba indica lam leaf extract and its larvicidal and pupicidal activity against Anopheles Stephensi and Culex quinquefasciatus. J. Entomol. Acarological Res. 45, 11 (2013).
Muturi, E. J. et al. Blood-feeding patterns of Culex quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme, Kenya. Parasitol. Res. 102, 1329–1335 (2008).
Farajollahi, A., Fonseca, D. M., Kramer, L. D. & Marm Kilpatrick, A. Bird biting mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11, 1577–1585 (2011).
Weiss, R. A. & McMichael, A. J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 10, S70–S76 (2004).
Greger, M. The Human/Animal interface: Emergence and Resurgence of Zoonotic Infectious diseases. Crit. Rev. Microbiol. 33, 243–299 (2007).
Samy, A. M. et al. Climate Change influences on the global potential distribution of the Mosquito Culex quinquefasciatus, Vector of West Nile Virus and Lymphatic Filariasis. PLoS One. 11, e0163863–e0163863 (2016).
Morin, C. W. & Comrie, A. C. Modeled response of the West Nile virus vector Culex quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int. J. Biometeorol. 54, 517–529 (2010).
McClure, K. M., Lawrence, C. & Kilpatrick, A. M. Land use and larval habitat increase Aedes albopictus (Diptera: Culicidae) and Culex quinquefasciatus (Diptera: Culicidae) abundance in lowland Hawaii. J. Med. Entomol. 55, 1509–1516 (2018).
Camp, J. V. et al. Mosquito biodiversity and mosquito-borne viruses in the United Arab Emirates. Parasit. Vectors. 12, 153–153 (2019).
Roberts, D. & Irving-Bell, R. Salinity and microhabitat preferences in mosquito larvae from southern Oman. J. Arid Environ. 37, 497–504 (1997).
Hafez, A. M. & Abbas, N. Insecticide resistance to insect growth regulators, avermectins, spinosyns and diamides in Culex quinquefasciatus in Saudi Arabia. Parasites Vectors. 14, 1–9 (2021).
Kardousha, M. M. Additional records of vector mosquito diversity collected from Al Khor district of North-eastern Qatar. Asian Pac. J. Trop. Disease. 5, 804–807 (2015).
Dehghan, H., Sadraei, J., Moosa-Kazemi, S., Baniani, N. A. & Nowruzi, F. The molecular and morphological variations of Culex pipiens complex (Diptera: Culicidae) in Iran. J. Vector Borne Dis. 50, 111–120 (2013).
Liu, H. et al. Trends in insecticide resistance in Culex pipiens pallens over 20 years in Shandong, China. Parasit. Vectors. 12, 167–167 (2019).
Ahmed, M. & Naqvi, S. N. H. Pesticide Pollution, Resistance and Health hazards. Pesticides - The Impacts Pesticides Exposure. https://doi.org/10.5772/13758 (2011).
Guimarães, R. M., Asmus, C. I. R. F. & Meyer, A. DDT reintroduction for malaria control: the cost-benefit debate for public health. Cad. Saúde. Públ. 23, 2835–2844 (2007).
Chareonviriyaphap, T., Bangs, M. J. & Ratanatham, S. Status of malaria in Thailand. Southeast Asian J. Trop. Med. Public Health. 31, 225–237 (2000).
Walker, K. Cost-comparison of DDT and alternative insecticides for malaria control. Med. Vet. Entomol. 14, 345–354 (2000).
Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 188, S86–S98 (1996).
Michelbacher, A. & Middlekauff, W. New insecticides: effectiveness and limitations of chlorinated hydrocarbon insecticides not yet fully determined. Calif. Agric. 3, 6–12 (1949).
Deedat, Y. D. Problems Associated with the Use of pesticides: an overview. Int. J. Trop. Insect Sci. 15, 247–251 (1994).
Kumar, S., Sharma, A. K., Rawat, S., Jain, D. & Ghosh, S. Use of pesticides in agriculture and livestock animals and its impact on environment of India. Asian J. Environ. Sci. 8, 51–57 (2013).
Messenger, L. A. et al. Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): a nationwide study for insecticide resistance monitoring. Malar. J. 16, 469–469 (2017).
Karunamoorthi, K. & Sabesan, S. Insecticide Resistance in Insect vectors of Disease with Special Reference to mosquitoes: a potential threat to Global Public Health. Health Scope. 2, 4–18 (2013).
Zlotkin, E. The insect voltage-gated sodium channel as target of insecticides. Ann. Rev. Entomol. 44, 429–455 (1999).
Lee, S. E. & Lee, H. S. Insecticide resistance in increasing interest. J. Appl. Biol. Chem. 44, 105–112 (2001).
Vontas, J. et al. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 104, 126–131 (2012).
Williamson, M. S., Denholm, I., Bell, C. A. & Devonshire, A. L. Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica). Mol. Gen. Genet. MGG. 240, 17–22 (1993).
Mouchès, C. et al. Overproduction of detoxifying esterases in organophosphate-resistant Culex mosquitoes and their presence in other insects. Proc. Natl. Acad. Sci. U S A. 84, 2113–2116 (1987).
Hemingway, J., Hawkes, N., Prapanthadara, L., Jayawardenal, K. G. I. & Ranson, H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Phil Trans. R Soc. Lond. B. 353, 1695–1699 (1998).
Gan, S. J. et al. Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: a review. Parasit. Vectors. 14, 315–315 (2021).
Silver, K. S. et al. Voltage-gated Sodium channels as insecticide targets. Adv. Insect Phys. 46, 389–433 (2014).
Sarkar, M., Baruah, I., Srivastava, R. B., Borkotoki, A. & Bhattacharyya, I. K. High-throughput approach to detection of knockdown resistance (kdr) mutation in mosquitoes, Culex quinquefasciatus, based on real‐time PCR using single‐labelled hybridisation probe/melting curve analysis. Pest Manag. Sci. 67, 156–161 (2010).
Sarkar, M., Borkotoki, A., Baruah, I., Bhattacharyya, I. K. & Srivastava, R. B. Molecular analysis of knock down resistance (kdr) mutation and distribution of kdr genotypes in a wild population of Culex quinquefasciatus from India. Tropical Medicine International Health. 14, 1097–1104 (2009).
Xu, Q., Tian, L., Zhang, L. & Liu, N. Sodium channel genes and their differential genotypes at the L-to-F kdr locus in the mosquito Culex quinquefasciatus. Biochem. Biophys. Res. Commun. 407, 645–649 (2011).
Milani, R. Comportamento mendeliano della resistenza alla azione abbatante del DDT: correlazione tran abbatimento e mortalia in Musca domestica L. Riv Parasitol. 15, 513–542 (1954).
Hamid, P. H., Ninditya, V. I., Ghiffari, A., Taubert, A. & Hermosilla, C. The V1016G mutation of the voltage-gated sodium channel (VGSC) gene contributes to the insecticide resistance of Aedes aegypti from Makassar, Indonesia. Parasitol. Res. 119, 2075–2083 (2020).
Soderlund, D. M. Neurotoxicology of pyrethroid insecticides. Adv. Neurotoxicology. 113–165. https://doi.org/10.1016/bs.ant.2019.11.002 (2020).
Yuan, H. et al. High frequency of voltage-gated sodium channel (VGSC) gene mutations in Aedes albopictus (Diptera: Culicidae) suggest rapid insecticide resistance evolution in Shanghai, China. PLoS Negl. Trop. Dis. 17, e0011399–e0011399 (2023).
Burton, M. J. et al. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem. Mol. Biol. 41, 723–732 (2011).
Al-Deeb, M. A. Pyrethroid insecticide resistance kdr gene in the house fly, Musca domestica (Diptera: Muscidae), in the United Arab Emirates. Agricultural Sci. 05, 1522–1526 (2014).
Hamdan, M. et al. Kdr mutations and deltamethrin resistance in house flies in Abu Dhabi, UAE. Parasit. Vectors. 17, 47–47 (2024).
Hemingway, J. & Ranson, H. Insecticide Resistance in Insect vectors of Human Disease. Ann. Rev. Entomol. 45, 371–391 (2000).
Dong, K. et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 50, 1–17 (2014).
Liu, N. Insecticide Resistance in mosquitoes: Impact, mechanisms, and research directions. Ann. Rev. Entomol. 60, 537–559 (2015).
Uemura, N., Itokawa, K., Komagata, O. & Kasai, S. Recent advances in the study of knockdown resistance mutations in Aedes mosquitoes with a focus on several remarkable mutations. Curr. Opin. Insect Sci. 63, 101178 (2024).
Davies, T. G. E., Field, L. M., Usherwood, P. N. R. & Williamson, M. S. A comparative study of voltage-gated sodium channels in the Insecta: implications for pyrethroid resistance in Anopheline and other Neopteran species. Insect Mol. Biol. 16, 361–375 (2007).
Achee, N. L. et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 13, e0006822–e0006822 (2019).
Benelli, G., Jeffries, C. L. & Walker, T. Biological Control of Mosquito vectors: past, Present, and Future. Insects 7, 52 (2016).
Connelly, C. R. R. et al. Integrated Pest Management for Mosquito Reduction around Homes and Neighborhoods: ENY-753/IN1045, 9/2014. EDIS (2014). (2014).
Ponce, G. et al. First report of L1014F kdr mutation in Culex quinquefasciatus in Mexico. Insect Sci. 23, 829–834 (2015).
Maestre-Serrano, R. et al. Susceptibility to pyrethroids and the First Report of L1014F kdr mutation inCulex quinquefasciatus(Diptera: Culicidae) in Colombia. J. Med. Entomol. 57, 1830–1834 (2020).
Yoshimizu, M. H., Padgett, K. & Kramer, V. Surveillance of a kdr resistance mutation in Culex pipiens (Diptera: Culicidae) and Culex quinquefasciatus in California. J. Med. Entomol. 57, 645–648 (2020).
Fang, Y. et al. Molecular analysis of targeted insecticide resistance gene mutations in Field-Caught mosquitos of Medical Importance from Saudi Arabia. J. Med. Entomol. 58, 1839–1848 (2021).
Georghiou, G. P. & Taylor, C. E. Genetic and biological influences in the evolution of Insecticide Resistance. J. Econ. Entomol. 70, 319–323 (1977).
Helps, J. C., Paveley, N. D., White, S. & van den Bosch, F. Determinants of optimal insecticide resistance management strategies. J. Theor. Biol. 503, 110383 (2020).
Levick, B., South, A. & Hastings, I. M. A two-locus model of the evolution of Insecticide Resistance to inform and optimise Public Health Insecticide Deployment Strategies. PLoS Comput. Biol. 13, e1005327–e1005327 (2017).
Guerrero, F. D., Jamroz, R. C., Kammlah, D. & Kunz, S. E. Toxicological and molecular characterization of pyrethroid-resistant horn flies, Haematobia irritans: identification of kdr and super-kdr point mutations. Insect Biochem. Mol. Biol. 27, 745–755 (1997).
Hayashi, K., Yoshida, H. & Ashikawa, I. Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes. Theor. Appl. Genet. 113, 251–260 (2006).
Stenhouse, S. A. et al. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasit. Vectors. 6, 253–253 (2013).
Faucon, F. et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito aedes aegypti by deep targeted sequencing. Genome Res. 25, 1347–1359 (2015).
Lucas, E. R. et al. Whole-genome sequencing reveals high complexity of copy number variation at insecticide resistance loci in malaria mosquitoes. Genome Res. 29, 1250–1261 (2019).
Lachance, J. Hardy–Weinberg Equilibrium and Random mating. Encyclopedia Evolutionary Biology. 208–211. https://doi.org/10.1016/b978-0-12-800049-6.00022-6 (2016).
Brogdon, W. & Chan, A. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. CDC Atlanta USA: CDC Technical Report 28, (2010).
Richards, S. L. et al. Insecticide Susceptibility Screening against Culex and Aedes (Diptera: Culicidae) mosquitoes from the United States. J. Med. Entomol. 55, 398–407 (2017).
Hung, K. Y., Cavanaugh, C., Perezchica-Harvey, G. & Chuzel, G. Insecticide Resistance Bottle Bioassay evaluation of Culex quinquefasciatus mosquitoes from Coachella Valley, 2019. Arthropod Manage. Tests 46, (2021).
Foster, W. A., Walker, E. D., Mosquitoes & Culicidae ). Med. Vet. Entomol. 261–325 doi:https://doi.org/10.1016/b978-0-12-814043-7.00015-7. (2019).
Simsaa, M. A. A. et al. Culex mosquitoes (Diptera: Culicidae) recorded along the Nile River in central and northern Sudan, with a key for the identification of all species of the genus known to occur in the country. Zootaxa 4963, (2021).
Al-Ali, K. H. et al. A study on culex species and culex transmitted diseases in Al-Madinah Al-Munawarah, Saudi Arabia. Parasitol. United J. 1, 101–108 (2008).
Al-Ashry, H., Kenawy, M. A. & Mohammed Shobrak, M. S. Ecological aspects of the bancroftian filariasis vectors, Culex pipiens and cx. Quinquefasciatus (Diptera: Culicidae) in Hail, Saudi Arabia. Int. J. Mosq. Res. 5, 25–32 (2018).
Kenawy, M. A., Ashry, A., Shobrak, M. Y. & H. A. H. & Analysis of the interspecific association between larvae of Culex pipiens and Culex quinquefasciatus, the common and medically important mosquito species (Diptera: Culicidae) in Hail Region, Kingdom of Saudi Arabia. Asian Pac. J. Trop. Disease. 7, 788–791 (2017).
Kauffman, E. et al. Rearing of Culex spp. and Aedes Spp. Mosquitoes. BIO-PROTOCOL 7, (2017).
Becker, N. et al. Mosquitoes and Their Control (Springer Science & Business Media, 2010).
Mathews, G., Derraik, J. G., Walker, M., Knox, R. & Barraclough, R. K. Morphological variation in invasive mosquito Culex quinquefasciatus Say (Diptera: Culicidae) larvae from an urban site in Auckland, New Zealand. New. Z. J. Zool. 44, 342–353 (2017).
Smith, J. L. & Fonseca, D. M. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). Am. J. Trop. Med. Hyg. 70, 339 (2004).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3: 294–299. Link: (1994). https://bit.ly/2x9R9WL.
Chamnanya, S. et al. Novel real-time PCR assay detects widespread distribution of knock down resistance (kdr) mutations associated with pyrethroid resistance in the mosquito, Culex quinquefasciatus, in Thailand. Pestic. Biochem. Physiol. 186, 105172 (2022).
Okonechnikov, K., Golosova, O. & Fursov, M. Ugene Team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28, 1166–1167 (2012).
Rodriguez, S., Gaunt, T. R. & Day, I. N. Hardy-Weinberg equilibrium testing of biological ascertainment for mendelian randomization studies. Am. J. Epidemiol. 169, 505–514 (2009).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Acknowledgements
We thank the United Arab Emirates University for funding this research project; Dubai Municipality for providing susceptible mosquitoes (Culex quinquefasciatus); and Mohamed Al Naqbi and Fawzia Jumma Al Jneibi for their support during the project. We thank Biduth Kundu from the Biology Department for performing Sanger sequencing, and Ameed Salem and Mohamad Khaled for their efforts.
Funding
The funding for this study was provided by the UAE University through joint collaboration Grant no. G00003719.
Author information
Authors and Affiliations
Contributions
Conceptualization: MAA. Methodology: MAA, MHA, MMA. Investigation: ASA, AI, TK, ARG, SS, MH. Formal analysis: ASA, MAA, AI, TK, ARG, SS. Visualization: ASA, MAA. Supervision: MAA, MHA, MMA. Project administration: MAA. Funding acquisition: MAA. Writing—original draft: ASA, MAA. Writing—review & editing: MAA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ali, A.s., Iqbal, A., Kamalanathan, T. et al. The southern house mosquito Culex quinquefasciatus in Abu Dhabi, UAE, is developing resistance to deltamethrin insecticide. Sci Rep 15, 3411 (2025). https://doi.org/10.1038/s41598-025-87843-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-87843-6