Abstract
The aim of this study was to determine the optimal combination of regrowth age and residue height of BRS Capiaçu elephant grass that yields the best balance between the dry mass production (DMP) of the harvested forage and silage quality. Four regrowth ages (75, 90, 105, and 120 days) and two residue heights (10 and 50 cm) were evaluated. The experimental design used a randomized complete block design with a 4 × 2 factorial scheme and 3 replicates. The regrowth age led to a linear increase in DMP. As regrowth age advanced, there were linear increases in the dry matter (DM) concentration, cell wall constituents, and fraction C (acid detergent insoluble nitrogen) of crude protein and reductions in the in vitro digestibility of DM and neutral detergent fiber (IVNDFD) in both the plant and its silage. Lower pH values were observed in silage made from plants harvested at 10 cm. No significant effects of the studied factors were found on the concentrations of organic acids in the silage, except for propionic acid. Management using a 105-day regrowth period and a 10-cm residue height resulted in a better balance between the dry mass production, fermentative profile and nutritional value of silage.
Similar content being viewed by others
Introduction
Elephant grass (Cenchrus purpureus Schum.) is a forage grass commonly cultivated in countries with tropical and subtropical climates. It stands out for its high potential for biomass production, good acceptance by animals, and its versatility in use, whether for grazing, fresh feed in troughs, or preservation as silage1,2,3.
The most recent cultivar launched in Brazil, BRS Capiaçu, can produce approximately 49.8 tons of dry matter (DM)/ha/year. This cultivar is tall (4.2 m) and has erect clumps and thick stems (1.6 cm), which gives it lodging resistance and makes it suitable for mechanized harvesting at the time of ensiling4.
Tropical grasses commonly have a high moisture content (> 700 g/kg fresh matter, FM), which can lead to undesirable fermentations in the ensiled mass, compromising the quality of the resulting silage2,5.
The DM content and nutritional value of elephant grass may vary depending on the adopted management practices. Generally, as regrowth progresses, there is an increase in DM content3,6, which can positively influence fermentation. However, there is a decrease in nutritional value, including a significant reduction in digestibility, crude protein (CP), and total digestible nutrients (TDN). There is also an increase in fibrous constituents and lignin content, along with changes in the medium- and long-chain fatty acid profiles6,7,8.
Associated with regrowth age, increasing the cutting height of the plant may contribute to producing better quality silage. This allows part of the base of the plants’ stems to remain in the field, enabling the ensiling of biomass with a greater share of nutritious plant parts9,10,11. Although manipulating the grass residue height can be an interesting strategy for improving the final silage quality, it can negatively impact the amount of ensiled mass as part of the plant remains in the field.
Therefore, understanding how management strategies affect the quantity and quality of forage produced as well as their effects on silage is essential for establishing efficient management to prioritize both productive characteristics and those associated with the nutritional value of the forage.
To date, there are no reports in the literature demonstrating the effect of different regrowth ages in combination with different residue heights of BRS Capiaçu elephant grass on silage production. Therefore, we hypothesized that regrowth ages and residue heights affect the productive and nutritional characteristics of BRS Capiaçu elephant grass and the fermentative and nutritional characteristics of its silage. Therefore, the objective was to determine the optimal combination of regrowth age and residue height of BRS Capiaçu elephant grass that promotes the best balance between forage mass production and nutritional value alongside the fermentative profile and nutritional quality of its silage.
Methods
Experimental area
The experiment was conducted at the José Henrique Brusqui Experimental Field, which is part of Embrapa Gado de Leite (EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária), situated in the municipality of Coronel Pacheco, MG, Brazil (21°33’22”S, 43°06’15”W), at an average altitude of 410 m. Climatic data were collected at the National Institute of Meteorology (NIMET) automatic meteorological station, which is located 300 m from the experimental area (Supplementary Fig. S1 online). According to the Köppen classification, the climate of the region is classified as monsoon-influenced humid subtropics (Cwa).
The experimental area, which was approximately 500 m² under rainfed conditions, was divided into 24 plots of 20 m², each with a usable area of 9 m² and distributed across 3 blocks. Soil correction, fertilization, and the establishment of BRS Capiaçu elephant grass were carried out according to the recommendations of Pereira et al.12. At of planting, phosphate fertilizer (120 kg/ha of P2O5) was applied in furrows 20–30 cm deep, spaced 1 m apart. When plants reached an average height of 50 cm, 1200 kg/ha of NPK 20-05-20 was broadcast. Twelve months after the establishment of the area, a standardized cut was performed on the experimental plots according to the treatments, and evaluation periods commenced. For maintenance fertilization 300 kg/ha of the formula NPK 20-05-20 was applied throughout the experimental period, divided after each plot cut. Productivity was estimated over one year, while the nutritional value of the forage was assessed only at the time the grass was cut for ensiling.
The treatments were arranged in a 4 × 2 factorial design, with 4 regrowth ages (75, 90, 105, and 120 days) and 2 residue heights above the soil (10 and 50 cm), in a completely randomized block design with 3 replications.
The current study complies with Brazilian ethical regulations. All methods were performed in accordance with relevant guidelines and regulations for plants.
Estimation of forage mass and silage preparation
Ensiling was conducted exclusively on the first elephant grass cut, which occurred between February and April 2019, according to regrowth age. The grass was manually harvested at 08:00 am from the useful area of each experimental plot. The forage was then weighed to determine the green mass and chopped using a stationary forage machine (model EN-9F3B; Nogueira Máquinas Agrícolas, São João da Boa Vista, SP, Brazil), resulting in an average particle size of 1 cm. Approximately 4.5 kg of forage was ensiled in mini PVC silos (150 cm high and 10 cm in diameter) that were previously weighed and equipped with a Bunsen valve to release gases and an electrical conduit ring to collect effluent. The forage was manually compacted with a concrete rod until it reached approximately 100 cm of silo height, simulating an average density of 0.57 g FM/cm2 (570 kg FM/m2). After compaction, a 100 mm PVC pipe filled with concrete (11.6 kg) was placed on top of the forage to apply a constant pressure of 146 g/cm2 replicating the pressure typical in the lower half of a trench silo13. The silos were sealed, weighed again, and stored for 60 days in a protected location at room temperature.
Fermentation profile and dry matter recovery (DMR)
An aqueous extract was prepared by combining 25 g of the plant or silage with 225 mL of saline solution (Ringer Solution, Oxoid®, Hampshire, UK) and homogenizing for 1 min in an industrial blender. A 10-mL aliquot of the extract was filtered through sterile gauze, acidified with 50% H2SO4, and stored at − 20 °C for subsequent analysis of water-soluble carbohydrates (WSC) according to Nelson14.
The pH of the silage was measured using a potentiometer (Tecnal, São Paulo, Brazil) in an extract produced after hydraulic pressing of the silage. A 10-mL aliquot was collected and acidified with 25% metaphosphoric acid to determine the ammonia nitrogen (NH3-N)15, lactic acid (LA), acetic acid (AA), and butyric acid (BA)16 contents. High-performance liquid chromatography (HPLC) was used to analyze the organic acid contents, and the instrument was equipped with a PAD 2998 Detector (Photodiode Array Detector) and a separation system comprising a C18 ODS 80 A reversed-phase column (150 mm × 4.6 mm × 5 μm).
The estimate of DMR was calculated using the following equation proposed by Jobim et al.17. : DMR (%) = DMop/DMclo × 100), where DMR is the DM recovery (%), DMop is the DM of silage at opening (amount of silage in kg × % DM), and DMclo is the DM of forage at closing (amount of forage in kg × % DM).
Microbial population
The populations of lactic acid bacteria (LAB), enterobacteria (ENT), molds, and yeasts were quantified in the forage and silage before acidification using the previously detailed aqueous extract of the sample and sterile saline solution (Ringer Solution, Oxoid, Hampshire). A 10-mL aliquot of the water extract (25 g of sample/225 mL of sterile saline solution) was subjected to serial dilution (10− 1–10− 8). Microorganisms were cultured in sterile Petri plates following the pour-plate plating technique, using De Man, Rogosa, and Sharpe Agar for LAB, Violet Red Bile Agar for ENT, and Potato Dextrose Agar supplemented with 1.5% of 10% tartaric acid (w/v) for molds and yeasts. The plates were incubated aerobically in an oven with the temperature and period determined for each group of microorganisms as follows: ENT, 37 °C/24 h; LAB, 37 °C/48 h; and yeast and molds, 25 °C/72 and 120 h, respectively. Plates with 30–300 colony-forming units (cfu) were counted. For data evaluation and interpretation, the results were converted to a logarithmic scale (log10 cfu).
Nutritive value and fatty acid profile
Forage samples before ensiling and their silage were dried in a forced ventilation oven (55 °C/72 h) and ground in a Willey mill with a 1-mm sieve. The DM (INCT-CA G-003/1 method), crude protein (CP, INCT-CA N-001/1 method), neutral detergent insoluble fiber corrected for ash and protein (NDFap, INCT-CA F-002/1 method), neutral detergent insoluble nitrogen (NDIN, INCT-CA N-004/1 method), acid detergent insoluble nitrogen (ADIN, INCT- CA N-005/1 method), and lignin (INCT-CA F-005/1 method) concentrations were analyzed following Detmann et al.18. The partition of nitrogen and protein fractions, A (nonprotein nitrogen), B1 (true-soluble protein), B2 (insoluble protein), B3 (neutral detergent insoluble protein, but soluble in acid detergent), and C (ADIN, indigestible nitrogen) were calculated according to Licitra et al.19. The composition of fatty acids (FAs) was estimated in a gas chromatograph (Agilent 6890, Agilent Technologies Inc., Santa Clara, CA, USA), from samples that were freeze-dried according to the method proposed by Lopes et al.8.
The in vitro digestibility of DM (IVDMD) and neutral detergent fiber (IVNDFD) were estimated using an ANKOM incubator (Ankom® Technology Corporation, Fairport, NY), following the method proposed by Tilley and Terry20 and adapted by Holden21. The inoculum was collected from two non-castrated Nellore cattle (320 ± 20 kg) with a rumen fistula, fed diets consisting of 60% grass silage and 40% concentrate on a dry matter basis.
The experimental procedures were approved by the rules of the Ethics Committee for the Use of Animals of the EMBRAPA (CEUA, protocol nº 9085021019). The methods were also in accordance with Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines for the reporting of animal experiments.
Statistical analysis
The data obtained for forage before ensiling and silage were analyzed in a factorial scheme using a completely randomized block design. Regrowth ages, residue heights, and the interaction between factors were considered fixed effects, and block and error as random effects. The choice of the covariance matrix was based on the Akaike information criterion22, adopting the following sources of variation: regrowth age, residue height and their interactions according to the following linear model:
Yijkl = µ + Ai + Hj + (AH)ij + Bk + eijkl.
where Yijkl is the response variable; µ is the general constant; Ai is the effect of regrowth age i; Hj is the effect of residue height j; (AH)ij is the interaction of regrowth age i and residue height j; Bk is the effect of block k; and eijkl is the random error assuming an independent normal distribution, NID (0, σ2). The means were compared by Fischer’s minimum significant difference, using the PDIFF option of the LSMEANS command for residue height, and regression analysis for regrowth age, with model selection based on P (P < 0.05) and R2 values. A critical probability level of 0.05 was adopted for type I error using PROC MIXED in SAS version 9.423.
Results
Forage characteristics and dry mass production
The water-soluble carbohydrate concentrations, pH, and microbial populations of elephant grass before ensiling are presented in Supplementary Table S1 online. Forage pH and the ENT population were affected by the interaction between regrowth ages and residue heights (A × H), while yeasts were affected only by regrowth age. The variables WSC, LAB, and molds were not affected (P > 0.05) by the studied factors.
Regrowth age led to a linear increase (P < 0.0001) in forage dry mass production (DMP), reaching an estimated 62.74 tons DM/ha/year at 120 days of age. This represents an approximately 89% increase in productivity compared to the 75-day regrowth age, which yielded 33.14 tons DM/ha/year (Fig. 1). A higher DMP was observed at a residue height of 10 cm compared to 50 cm, with values of 53.6 and 42.3 tons DM/ha/year, respectively.
Fermentation profile, dry matter recovery and microbial population
There was an effect of the interaction (P = 0.0124) on the PA concentration of silage, while the pH and NH3-N concentration were affected by residue height (P = 0.0047) and regrowth age (P = 0.0248), respectively (Table 1). There were no effects (P > 0.05) of the studied factors on residual WSC and other variables of the silage fermentation profile. Higher pH values were recorded in silage produced with grass harvested at 50 cm (Table 1).
A linear reduction (P = 0.0198) in the NH3-N concentration of silage was observed with increasing grass maturity. A similar behavior was observed for the PA concentration of silage. Reductions of approximately 44% (2.83 vs. 1.58 g/kg DM) and 70% (3.59 vs. 1.07 g/kg DM) in PA content were observed at residue heights of 10 (P = 0.0003) and 50 cm (P < 0.0001), respectively, in response to advancing grass maturity (Table 1).
For DMR, the regrowth age had a significant linear effect (P < 0.0001) with an increase of approximately 14% observed as the regrowth age increased from 75 to 120 days. There was no effect of residue height (P = 0.1241) on DMR, with an average value of 892.9 g/kg DM (Table 1).
There was no effect (P > 0.05) of the factors studied on the LAB population of the silage, with an average value observed of 4.03 log10 cfu/g of forage (Table 1). No ENT, molds, or yeasts were detected in the silage (data not shown in Table 1).
Nutritive value and fatty acid profile
Forage and silage DM contents were only affected by regrowth age (P < 0.0001), with increases of 1.427 and 1.413 g/kg for each day of regrowth, respectively (Fig. 2). There were no differences in the DM content of forage (P = 0.1376) and silage (P = 0.1704) at different residue heights, whose average values were 185.8 and 184.3 g/kg FM, respectively.
Contents of dry matter (DM) (a); crude protein (CP) (b); neutral detergent fiber corrected for ash and protein (NDFap) (c), and lignin (d) from the forage and silage of BRS Capiaçu elephant grass, harvested with different regrowth ages (days). Forage: yDM = 1.42680x + 46.71617, R2 = 0.88, (P < 0.0001); yCP = 0.01455x2 − 3.51322x + 253.31083, R2 = 0.76, (P = 0.0044); yNDFap = 0.78009x + 625.96967, R2 = 0.42, (P = 0.0001); yLignin10 = 0.516x + 23.453, R2 = 0.72, (P < 0.0001), yLignin50 = 0.5282x + 13.638, R2 = 0.73, (P < 0.0001). Silage: yDM = 1.41252x + 46.63033, R2 = 0.89, (P < 0.0001); yCP = 0.01545x2 − 3.62108x + 253.30383, R2 = 0.72, (P = 0.0023); yNDFap = 0.65668x + 634.70933, R2 = 0.33, (P = 0.0004); yLignin = 0.50999x + 16.78067, R2 = 0.72, (P < 0.0001). Data that were not significant (P > 0.05) are not shown.
There was an effect of regrowth age on the CP content of forage (P = 0.0044) and silage (P = 0.0023), with data adjustments for a quadratic model. The estimated minimum values were 41.2 and 41.1 g/kg DM at 120 and 117 days of regrowth for forage and silage respectively (Fig. 2). The greater residue height (50 cm) resulted in higher levels (P < 0.05) of CP in forage (55.9 vs. 50.4 g/kg DM) and silage (54.6 vs. 48.2 g/ kg DM).
Linear increases in the NDFap levels were observed in forage (P = 0.0001) and silage (P = 0.0004), with an increase of 0.780 and 0.657 g/kg DM for each day of regrowth, respectively (Fig. 2). Additionally, forage harvested at 10 cm (P = 0.0203; 708.5 vs. 695.6 g/kg DM) and its silage (P = 0.0253; 704.6 vs. 692.8 g/kg DM) presented higher NDFap contents compared to 50 cm.
The lignin content of the forage was affected by the interaction (P = 0.0301). Increases of approximately 39 and 37% were recorded in forage with an increase in regrowth age from 75 to 120 days observed in residues of 10 cm (59.5 vs. 82.9 g/kg DM) and 50 cm (56.9 vs. 77.7 g/kg DM), respectively (Fig. 2). In silage, the lignin content was affected by the regrowth age (P < 0.001), with an approximate increase of 0.51 g/kg DM for each day of regrowth, and by residue height (P = 0.0271) with a higher concentration at 10 cm than at 50 cm (68.4 vs. 64.6 g/kg DM).
There was an effect of the interaction (P = 0.0455) on non-protein nitrogen (fraction A) in the forage. A linear decrease in this fraction was observed with increasing regrowth age of grass harvested at 10 cm. However, at a residue height of 50 cm, no significant linear (P = 0.8245) or quadratic (P = 0.3929) effects were observed across different regrowth ages, with an average value of 248.6 g/kg total nitrogen (total N) (Supplementary Fig. S2 online). In silage, fraction A was affected by regrowth age, showing a reduction of approximately 14% in its concentration, from 523.8 to 452.9 g/kg total N, as the regrowth age increased from 75 to 120 days (Supplementary Fig. S2 online). A higher content of fraction A in the silage was observed at a residue height of 50 cm compared to 10 cm (P = 0.0480, 500.9 vs. 486.2 g/kg total N).
There were no effects of regrowth age on fractions B1 (P = 0.1405), B2 (P = 0.6435), and B3 (P = 0.8076) in the forage, with observed averages of 51.6, 377.0, and 275.1 g/kg total N, respectively (Supplementary Fig. S2 online). For residue height, there was a difference only for fraction B2 (P = 0.0242), with a higher content at a height of 10 cm compared to 50 cm (392.6 vs. 361.4 g/kg total N). In silage, fractions B1 (P < 0.001) and B2 (P = 0.0003) were affected by regrowth age. There was a linear reduction in fraction B1, while fraction B2 increased linearly with advancing grass regrowth age (Supplementary Fig. S2 online). A higher fraction B1 content in the silage was observed at a residue height of 50 cm compared to 10 cm (P = 0.0461; 72.8 vs. 65.0 g/kg total N). In contrast, there were no differences in fraction B2 between residue heights (P = 0.2739), with an average value of 240.8 g/kg total N. The B3 fraction of the silage was not affected by the factors studied, with an average value of 123.3 g/kg total N.
The C fraction of forage (P < 0.001) and silage (P < 0.001) protein increased linearly with advancing regrowth age (Supplementary Fig. S2 online). Forage harvested with a 10-cm residue showed a higher C fraction compared that with a 50-cm residue (P = 0.0003; 70.3 vs. 60.1 g/kg total N). In contrast, in silage, the C fraction was not affected by residue height (P = 0.055), with an average content of 73.3 g/kg total N.
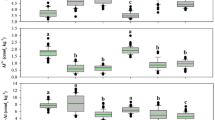
The variables IVDMD and IVNDFD were affected by both regrowth age (P < 0.0001) and residue height (P < 0.05). There was an approximately 27% reduction in IVDMD of forage (from 650.5 to 478.0 g/kg DM) and 23% in silage (from 686.7 to 527.5 g/kg DM) with increasing regrowth age from 75 to 120 days. Similarly, IVNDFD decreased by about 44% in forage (from 489.3 to 272.9 g/kg DM) and 38% in silage (from 541.7 to 335.8 g/kg DM) over the same period (Fig. 3). Higher IVDMD values were observed at a residue height of 50 cm compared to 10 cm for both forage (P < 0.0001; 577.6 vs. 548.0 g/kg DM) and silage (P = 0.0002; 622.6 vs. 594.7 g/kg DM). Similar result was observed for IVNDFD, with values of 394.1 vs. 362.8 g/kg DM (P = 0.0033) for forage and 455.9 vs. 426.1 g/kg DM (P = 0.0016) for silage at heights of 50 and 10 cm, respectively.
In vitro DM digestibility (a) and in vitro NDF digestibility (b) from the forage and silage of BRS Capiaçu elephant grass, harvested with different regrowth ages (days). Forage: yIVDMD = -3.67460x + 921.06850, R² = 0.88, (P < 0.0001); yIVNDFD = -4.54444x + 821.56167, R2 = 0.84, (P < 0.0001). Silage: yIVDMD = -3.3979x + 939.95050, R2 = 0.88, (P < 0.0001); yIVNDFD = -4.33897x + 864.09550, R2 = 0.87, (P < 0.0001). Data that were not significant (P > 0.05) are not shown.
Regrowth age significantly affected (P < 0.05) palmitic (C16:0), stearic (C18:0), oleic (C18:1 cis-9), linoleic, and α-linolenic fatty acids as well as total fatty acids. Palmitic acid concentrations in forage followed a quadratic model (P < 0.0001), whereas a decreasing linear trend was observed (P < 0.0001) in silage with increasing regrowth age (Supplementary Fig. S3 online). Furthermore, a higher palmitic acid content was observed at a residue height of 50 cm compared to 10 cm, with values of 1.733 vs. 1.492 g/kg DM in forage (P < 0.0001) and 1.746 vs. 1.562 g/kg DM in silage (P = 0.0048).
There was a quadratic effect as a function of regrowth ages for stearic acid levels in forage (P < 0.0001) and silage (P = 0.0003), with estimated minimum levels of 0.105 and 0.113 g/kg DM at 117 and 109 days of regrowth, respectively (Supplementary Fig. S3 online). A higher stearic acid content was observed at a residue height of 50 cm compared to 10 cm, with values of 0.137 vs. 0.124 g/kg DM in forage (P = 0.0014) and 0.141 vs. 0.129 g/kg DM in silage (P = 0.0020).
Linear reductions in the oleic and linoleic acid concentrations were observed with increasing regrowth age in both forage and silage (Supplementary Fig. S3 online). The greater residue height (50 cm) provided higher levels of oleic (P < 0.0001; 0.276 vs. 0.234 g/kg DM) and linoleic acids (P < 0.0001; 1.774 vs. 1.562 g/kg DM) in the forage. In silage, linoleic acid was not affected by residue height (P = 0.0602), with an average value of 1.408 g/kg DM. However, silage made from plants harvested at 50 cm residue height had a higher concentration of oleic acid (P = 0.0022; 0.260 vs. 0.227 g/kg DM).
There was a quadratic effect on the α-linolenic acid concentration and total fatty acids in both forage (P = 0.0010 and P = 0.0002, respectively) and silage (P < 0.0001 and P = 0.0076, respectively) (Supplementary Fig. S3 online). Higher levels of α-linolenic acid and total FAs were observed in forage (P < 0.0001; 2.255 vs. 1.770 g/kg DM; and P < 0.0001; 6.438 vs. 5.432 g/kg DM, respectively) and silage (P = 0.0002; 1.896 vs. 1.595 g/kg DM; and P = 0.0006; 5.789 vs. 5.134 g/kg DM, respectively) at a residue height of 50 cm.
Discussion
Understanding forage productivity and quality under different management strategies is crucial due to its impact on animal performance. As the plant advances towards physiological maturity, there is an increase in the proportion of cell wall components, resulting in increased productivity, as reported by Monção et al.6. , Alves et al.3. , and Santana et al.24. and verified in the present study (Fig. 1). However, increasing the cutting height at harvest reduces forage productivity because a portion of the plant’s stem remains in the field and is not included in the harvested forage mass11. In the present study, harvesting the forage plant at a residue height of 50 cm resulted in an approximate 21% reduction in DMP.
Despite the linear increase in DM content with advancing regrowth age (Fig. 2), the content observed at 120 days (215.4 g/kg FM) was lower than that recommended by McDonald et al.25. However, the forage WSC concentrations at different regrowth ages and residue heights remained within the range of 60–80 g/kg DM (Supplementary Table S1 online), as recommended by Woolford26. Similarly, the epiphytic LAB population was higher than the minimum (5 log10 cfu/g) recommended by Pahlow et al.27. (Supplementary Table S1 online), which contributed to fermentation process efficiency, resulting in silage with an adequate fermentation pattern.
The pH values in the silage, ranging from 3.46 to 3.62, were lower than those observed by Kung et al.28. for grass silage (4.3–4.7), reflecting the efficiency of LAB in producing organic acids, particularly LA, which is considered the most effective for acidifying the ensiled mass due to its dissociation constant (pKa = 3.86). In the present study, evidence of the dominance of homofermentative LAB during fermentation was observed, with almost exclusive production of LA. LA concentrations ranged from 40.35 to 59.56 g/kg DM, while AA concentrations were lower (3.80–5.26 g/kg DM) compared to the values reported by Kung et al.28. (10–30 g/kg DM) but similar to those reported by Amaral et al.2. However, the concentrations of these acids were sufficient to ensure adequate preservation of the mass and control of undesirable microorganisms. ENT, molds, and yeasts were not detected in the silage, and low concentrations of BA were recorded (Table 1).
The final PA concentration in well-fermented silage is typically low (< 1 g/kg DM). However, higher concentrations (3–5 g/kg DM) have been observed in silage with a low DM content due to the activity of bacteria of the genus Clostridium28. Although LAB dominated fermentation, in silage made from plants harvested at 75 days, which had a lower DM content, may have occurred secondary fermentation. This could contribute to increased PA and NH3-N concentrations and, consequently, a lower DMR in silage, as previously observed by Santos et al.29. , Silveira et al.30. , and Santos et al.31.
DMR indicates the efficiency of the ensiling process, as it represents the amount of DM preserved in the silo32. Thus, an increase in DMR strongly indicates that losses due to effluents or anaerobic fermentation activity in the silo decrease relative to the increase in DM content. This explains the higher DMR observed in silage produced from plants harvested at 120 days of age (Table 1). The results obtained in the present study corroborate the previous findings of Retore et al.33. , and Santos et al.31. with BRS Capiaçu elephant grass silage.
In the present study, the silage fermentation parameters indicated that LAB were dominant compared to other microorganisms, as previously reported. This dominance is attributed to various mechanisms employed by LAB, including the production of organic acids and bacteriocins34,35.
The non-detection of ENT in silage probably occurred due to the rapid acidification of the ensiled mass (pH < 5.0), making the conditions unsuitable for their growth27. Similarly, the organic acids produced during fermentation, primarily AA and AP, contributed to the reduction in the yeast population in the ensiled mass. At a pH lower than their pKa, these acids are in their undissociated form, which facilitates their entry into microbial cells via passive transport, allowing control of the yeast population28,36,37. Along with the acidic conditions, the non-detection of mold in the silage can be attributed to the adequate compaction of the grass during ensiling, which ensures rapid exhaustion of oxygen inside the silos. This is important because molds are obligate aerobic microorganisms25.
Changes in the plant’s chemical composition, driven by morphophysiological differences with advancing age, led to variations in the nutritional value of the silage produced. The highest CP content and the lowest NDFap and lignin contents were observed in silage produced from plants harvested at 75 days of age (Fig. 2). The main morphophysiological changes occurring in the plant as it matures include stem elongation, leaf senescence, a reduction in the leaf blade-to-stem ratio, and thickening of the cell wall with increased lignification. These changes are driven by the plant’s increased need for supportive tissues6,8,24,38. Therefore, harvesting younger plants at a lower residue height results in a higher proportion of leaf blades in their composition, which is reflected in the higher CP levels and lower concentrations of cell wall constituents39, as verified in the present study (Fig. 2). These changes in chemical composition were crucial in explaining the linear reductions observed in IVDMD and IVNDFD values in both forage and silage in response to advancing plant age and harvest height (Fig. 3), as shown in previous studies3,8,9,30,40.
Understanding how CP fractions change with different management practices is essential, as it can affect ruminal degradation, microbial growth, and the efficiency of feed utilization by the animal41. The reduction in the proportion of fraction A in response to plant maturity can be attributed to changes in morphological characteristics, such as a lower leaf blade-to-stem ratio and a reduction in metabolically active leaves. Therefore, as the plant reaches physiological maturity, nitrogen compounds migrate from the cellular content to the cell wall, resulting in increases in fractions B2, B3, and C, as observed in previous studies41,42. In the present study, there was only an increase in fraction C with advancing plant age (Supplementary Fig. S2 online).
Licitra et al.19. emphasized that fraction C corresponded to nitrogen bound to lignin, tannin-protein complexes, and products of the Maillard reaction, rendering it unavailable to rumen microorganisms. The increase in fraction C with advancing regrowth age and at lower residue heights is most likely associated with an increase in lignin levels under these management conditions (Fig. 2). Despite the increase in fraction C in both forage and silage with advancing regrowth age and lower residue height, the levels remained below the critical threshold proposed by Van Soest and Mason43, of 200 g/kg total N, indicating that the fermentation process occurred properly.
Although the nitrogen compound content was not directly compared in forage and silage, higher values of CP fraction A were observed in silage. This may be attributed to fermentation in the silo, where some of the protein may undergo proteolysis and be converted into non-protein nitrogen28,44.
The FAs evaluated in this study are most common in the lipid fractions of plants, accounting for approximately 90% of the total. These fatty acids are structural components of chloroplast cell membranes, serve as energy and metabolic reserves, and play a role in hormone formation45,46. A decrease in the FAs content was observed in forage with advancing grass maturity, and this trend was similarly reflected in silage. A regrowth age interval of 90–105 days was associated with the most significant reductions in the palmitic, oleic, and linoleic acid contents in the silage. The minimum estimated values for stearic acid, α-linolenic acid, and total fatty acids in the silage were observed at the latest regrowth ages (Supplementary Fig. S3 online). These reductions in FAs levels are associated with leaf senescence and a decrease in the leaf blade-to-stem ratio, which, in turn, leads to a significant reduction in chloroplast membrane lipids, where the FAs in forage are found according to Khan et al.46.
Lipids are characteristic of metabolically active leaves, and the distribution and proportion of FAs in plant tissues vary according to the plant’s stage of development, influenced by its photosynthetic apparatus45. Therefore, cutting intervals that encourage leaf renewal in forage plants lead to beneficial effects on FAs production. This effect was evident in the forage harvested at 75 days of regrowth in the present study. Furthermore, the higher leaf blade-to-stem ratio associated with the greater residue height, reflecting a higher proportion of metabolically active tissues and chloroplasts, may have been a key factor in the higher fatty acid content observed compared to the lower residue height.
In conclusion, the management of forage plants with shorter regrowth ages (75–90 days) and a higher residue height (50 cm) resulted in a forage with improved nutritional value and silage quality, though at the expense of a lower yield in harvested mass. Conversely, extending the regrowth age to 105–120 days, coupled with a lower residue height (10 cm), increased the harvested forage mass and improved dry matter recovery in silages. However, at 120 days, there was a noticeable decline in nutritional value due to elevated lignin content and reduced digestibility of both dry matter and neutral detergent fiber. Therefore, harvesting BRS Capiaçu at a regrowth age of 105 days with a residue height of 10 cm proved to be the optimal management strategy, offering the best balance between harvested forage mass and the fermentation and nutritional parameters of the produced silage. Conserving BRS Capiaçu as silage proved to be an adequate strategy that may reduce labor costs in production, particularly when compared to the demands of daily harvesting and chopping for fresh feeding. In addition, future studies are recommended to assess the productive performance of beef and dairy cattle on diets incorporating BRS Capiaçu silage, to further support its use in animal nutrition.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Bernardes, T. F. & Rêgo, A. C. Study on the practices of silage production and utilization on Brazilian dairy farms. J. Dairy Sci. 97, 1852–1861. https://doi.org/10.3168/jds.2013-7181 (2014).
Amaral, R. C. et al. Novel lactic acid bacteria strains enhance the conservation of elephant grass silage cv. BRS Capiaçu. Anim. Feed Sci. Technol. 264, 114472. https://doi.org/10.1016/j.anifeedsci.2020.114472 (2020).
Alves, J. P. et al. Forage production and quality of BRS Capiaçu as a response of cutting age and nitrogen application. Trop. Anim. Health Prod. 45, 179–186. https://doi.org/10.5398/tasj.2022.45.2.179 (2022).
Pereira, A. V., Ledo, F. J. D. S. & Machado, J. C. BRS Kurumi and BRS Capiaçu- New elephant grass cultivars for grazing and cut-and-carry system. Crop Breed. Appl. Biotechnol. 17, 59–62. https://doi.org/10.1590/1984-70332017v17n1c9 (2017).
Carvalho, M. G. M. et al. Applying the fermentability coefficient concept in tropical grass silages. Anim. Feed Sci. Technol. 314, 115995. https://doi.org/10.1016/j.anifeedsci.2024.115995 (2024).
Monção, F. P. et al. Productivity and nutritional value of BRS Capiaçu grass (Pennisetum purpureum) managed at four regrowth ages in a semiarid region. Trop. Anim. Health Prod. 52, 235–241. https://doi.org/10.1007/s11250-019-02012-y (2020).
Geren, H., Kavut, Y. & Unlu, H. Effect of different cutting intervals on the forage yield and some silage quality characteristics of giant king grass (Pennisetum hybridum) under Mediterranean climatic conditions. Turk. J. Field Crops. 25, 1–8. https://doi.org/10.17557/tjfc.737467 (2020).
Lopes, F. C. F. et al. Chemical composition and fatty acid profile of BRS Capiaçu ensiled at different regrowth ages. Semin. Cienc. Agrar. 42, 1981–2004. https://doi.org/10.5433/1679-0359.2021v42n3Supl1p1981 (2021).
Figueira, D. N., Neumann, M., Ueno, R. K., Galbeiro, S. & Bueno, A. V. I. Forage yield and quality in elephant grass cv. Pioneiro harvested at different cutting height sand times. Semin. Cienc. Agrar. 37, 1017–1027. https://doi.org/10.5433/1679-0359.2016v37n2p1017 (2016).
Kim, D. H. et al. Effects of different cutting height on nutritional quality of whole crop barley silage and feed value on Hanwoo heifers. Asian-Australas J. Anim. Sci. 29, 1265. https://doi.org/10.5713/ajas.16.0099 (2016).
Ferraretto, L. F., Shaver, R. D. & Luck, B. D. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 101, 3937–3951. https://doi.org/10.3168/jds.2017-13728 (2018).
Pereira, A. V. et al. BRS Capiaçu e BRS Kurumi: Cultivo e uso (Embrapa, 2021).
Fransen, S. C. & Strubi, F. J. Relationships among absorbents on the reduction of grass silage effluent and silage quality. J. Dairy Sci. 81, 2633–2644. https://doi.org/10.3168/jds.S0022-0302(98)75821-7 (1988).
Nelson, N. A photometric adaptation of the Somogyi method for determination of glucose. J. Biol. Chem. 153, 375–380 (1944).
Okuda, H., Fujii, S. & Kawashima, Y. A direct colorimetric determination of blood ammonia. Tokushima J. Exp. Med. 12, 11–23 (1965).
Siegfried, V. R., Ruckemann, H. & Stumpf, G. Method for the determination of organic acids in silage by high performance liquid chromatography. Landwirtsch Forsch. 37, 298–304 (1984).
Jobim, C. C., Nussio, L. G., Reis, R. A. & Schmidt, P. Methodological advances in evaluation of preserved forage quality. Rev. Bras. Zootec. 36, 101–119. https://doi.org/10.1590/S1516-35982007001000013 (2007).
Detmann, E. et al. Métodos para Análise de Alimentos (Visconde do Rio Branco, 2012).
Licitra, G., Hernandez, T. M. & Van Soest, P. J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 57, 347–358. https://doi.org/10.1016/0377-8401(95)00837-3 (1996).
Tilley, J. M. A. & Terry, R. A. A two-stage method for the in vitro digestion of forage crops. J. Br. Grassl. Soc. 18, 104–111. https://doi.org/10.1111/j.1365-2494.1963.tb00335.x (1963).
Holden, L. A. Comparison of methods of in vitro dry matter digestibility for ten feeds. J. Dairy. Sci. 82, 1791–1794. https://doi.org/10.3168/jds.S0022-0302(99)75409-3 (1999).
Wolfinger, R. Covariance structure selection in general mixed models. Commun. Stat. Simul. Comput. 22, 1079–1106. https://doi.org/10.1080/03610919308813143 (1993).
SAS. Institute Inc. Versão 9.4. Procedures Guide (SAS Institute Inc, 2013).
Santana, J. C. S. et al. Productive characteristics, chemical composition, in vitro digestibility, and degradation kinetics of two Brachiaria grasses at different regrowth ages. Trop. Anim. Health Prod. 54, 1–12. https://doi.org/10.1007/s11250-022-03341-1 (2022).
McDonald, P., Henderson, A. R. & Heron, S. J. E. Biochemistry of Silage (Marlow Chalcombe, 1991).
Woolford, M. K. The Silage Fermentation (Marcel Dekker, 1984).
Pahlow, G., Muck, R. E., Driehuis, F., Oude Elferink, S. J. W. H. & Spoelstra, S. F. Microbiology of Ensiling. Silage Science and Technology. (American Society of Agronomy, 2003).
Kung Jr, L., Shaver, R. D., Grant, R. J. & Schmidt, R. J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy. Sci. 101, 4020–4033. https://doi.org/10.3168/jds.2017-13909 (2018).
Santos, E. M. et al. Effect of regrowth interval and a microbial inoculant on the fermentation profile and dry matter recovery of guinea grass silages. J. Dairy. Sci. 97, 4423–4432. https://doi.org/10.3168/jds.2013-7634 (2014).
Silveira, T. C. et al. Cutting time and regrowth age affect the quality of elephant grass silage. J. Agric. Stud. 9, 64–83. https://doi.org/10.5296/jas.v9i3.18566 (2021).
Santos, A. S. S. et al. Microsilages elephant grass BRS Capiaçu added with commercial microbial consortium on different days of regrowth. Rev. Mex Cienc. Pecu. 15, 32–48. https://doi.org/10.22319/rmcp.v15i1.6379 (2024).
Santos, R. J. C. D. et al. Elephant grass clones for silage production. Sci. Agric. 70, 6–11. https://doi.org/10.1590/S0103-90162013000100002 (2013).
Retore, M., Alves, J., Orrico Júnior, M. A. P. & Mendes, S. S. Qualidade da silagem do capim-elefante BRS Capiaçu. Embrapa Agropecuária Oeste (2020). https://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1130009
Muck, R. E. et al. Silage review: Recent advances and future uses of silage additives. J. Dairy. Sci. 101, 3980–4000. https://doi.org/10.3168/jds.2017-13839 (2018).
Li, F. et al. Effects of class IIa bacteriocin-producing Lactobacillus species on fermentation quality and aerobic stability of alfalfa silage. Animals 10, 1575. https://doi.org/10.3390/ani10091575 (2020).
Moon, N. J. Inhibition of the growth of acid tolerant yeasts by acetate, lactate and propionate and their synergistic mixtures. J. Appl. Bacteriol. 55, 453–460. https://doi.org/10.1111/j.1365-2672.1983.tb01685.x (1983).
Davidson, P. M. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers (Doyle, M. P., Beuchat, L. R., Montville, T. J. Eds.) (1997).
Rueda, J. A. et al. Morphological composition and fiber partitioning along regrowth in elephant grass CT115 intended for ethanol production. Sci. Rep. 10, 15118. https://doi.org/10.1038/s41598-020-72169-2 (2020).
Lounglawan, P., Lounglawan, W. & Suksombat, W. Effect of cutting interval and cutting height on yield and chemical composition of King Napier grass (Pennisetum purpureum x Pennisetum americanum). APCBEE Proc. 8, 27–31. https://doi.org/10.1016/j.apcbee.2014.01.075 (2014).
Monção, F. P. et al. Yield and nutritional value of BRS Capiaçu grass at different regrowth ages. Semin. Cienc. Agrar. 40, 2045–2056. https://doi.org/10.5433/1679-0359.2019v40n5p2045 (2019).
Leite, R. G. et al. Effects of nitrogen fertilization on protein and carbohydrate fractions of Marandu palisadegrass. Sci. Rep. 11, 1–8. https://doi.org/10.1038/s41598-021-94098-4 (2021).
Braga, A. P. et al. Fractionation of nitrogen compounds and carbohydrates in forages of different ages. Semin. Cienc. Agrar. 39, 819–832. https://doi.org/10.5433/1679-0359.2018v39n2p819 (2018).
Van Soest, P. J. & Mason, V. C. The influence of Maillard reaction upon the nutritive value of fibrous feeds. Anim. Feed Sci. Technol. 32, 45–53. https://doi.org/10.1016/0377-8401(91)90008-G (1991).
Roseira, J. P. S. et al. Effects of exogenous protease addition on fermentation and nutritive value of rehydrated corn and sorghum grains silages. Sci. Rep. 13, 7302. https://doi.org/10.1038/s41598-023-34595-w (2023).
Fernandes, S. A. et al. Perfil de ácidos graxos em alimentos de clima tropical utilizados nas dietas para ruminantes. Bol. Ind. Anim. 64, 19–27 (2007). http://iz.sp.gov.br/bia/index.php/bia/article/view/1250
Khan, N. A. et al. Effect of species and harvest maturity on the fatty acids profile of tropical forages. J. Anim. Plant. Sci. 25, 739–746 (2015).
Acknowledgements
This research was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, APQ-03630-23), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Instituto Nacional de Ciência e Tecnológico – Ciência Animal (INCT-CA), Coordenação de Aperfeiçoamento de Nível Superior (CAPES) and Embrapa Gado de Leite.
Author information
Authors and Affiliations
Contributions
D.L.L. performed the experiment, analyses and prepared the manuscript. D.S.C.P. designed the experiment and contributed to the writing of the manuscript. M.J.F.M. designed the experiment, performed the statistical analyses and contributed to the writing of the manuscript. C.A.M.G. designed the experiment and contributed to the writing of the manuscript. J.P.S.R. performed the chemical composition analyses and contributed to the writing of the manuscript. F.C.F.L. designed the experiment and contributed to the writing of the manuscript. J.S.O. designed the experiment and contributed to the writing of the manuscript. O.G.P. performed the microbiology analyses and contributed to the writing of the manuscript. V.P.S. performed the microbiology analyses and contributed to the writing of the manuscript, and F.H.M.C. designed the experiment, and contributed to the writing of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lelis, D.L., Paciullo, D.S.C., Morenz, M.J.F. et al. Biomass production and silage quality of ensiled BRS Capiaçu elephant grass at different regrowth ages and residue heights. Sci Rep 15, 4016 (2025). https://doi.org/10.1038/s41598-025-88367-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-88367-9