Abstract
Chronic inflammation is known to play a critical role in the development of various diseases, such as osteoporosis. The inflammatory potential of a diet can be evaluated using a well-established scale known as the Dietary Inflammatory Index (DII). This study aimed to investigate the relationship between the DII score and the odds of osteoporosis in Iranian women. The study conducted was a case-control study involving 131 postmenopausal healthy women, as well as 131 women with osteoporosis and osteopenia aged 45–65. Osteoporosis was diagnosed through dual-energy X-ray absorptiometry, which measures bone mineral density (BMD) in the femoral neck bone and lumbar spine. To assess the DII score, a validated semi-quantitative food frequency questionnaire was applied. In comparison to the first tertile of DII score, higher and significant odds of osteoporosis/osteopenia were seen in the last tertile (fully adjusted model (body mass index, age, income, education, physical activity, calcium and vitamin D supplements): odds ratio (OR) = 2.43, 95% confidence interval (CI): 1.19–4.95, Ptrend = 0.023). Also, individuals in the highest DII tertile had higher odds of abnormalities in femoral neck and lumbar spine BMD (fully adjusted model: OR = 2.85, 95% CI: 1.37–5.89, Ptrend = 0.007 and OR = 2.59, 95% CI: 1.29–5.19, Ptrend = 0.009, respectively). Based on our findings, it appears that there may be a connection between following pro-inflammatory diets and the odds of osteoporosis in postmenopausal women.
Similar content being viewed by others
Introduction
Osteoporosis is a condition that affects the skeletal system, causing a decrease in bone mineral density (BMD). It results in macro- and micro-architecture changes and deterioration of the structural geometry of bones. Patients with osteoporosis are more prone to bone fractures1. The Osteoporosis is a worldwide concern. It has been reported that 21.7% of people and 35.3% of women between ages 50 and 85 years are osteoporotic2. The prevalence of osteoporosis in Iranian postmenopausal women is 33.7% 3.
Traditional pathophysiological theories of osteoporosis have focused on nutritional and endocrine factors, such as estrogen deficiency, secondary hyperparathyroidism, and vitamin D inadequacy. However, recent research has yielded novel insights into the underlying mechanisms of osteoporosis, including the intricate interplay between the bone and immune system, the role of inflammation, and the influence of the gut microbiota4.
Chronic inflammation is known to play a critical role in the development of various diseases, such as osteoporosis. Pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and IL-6, play a role in bone remodeling and are therefore implicated in the development of osteoporosis5,6,7.
Food components and the inflammatory potential of a diet can modulate inflammatory biomarkers. Diets based on fruits and vegetables are inversely related to inflammatory biomarkers, while red meat-based Western diets are related to inflammation8,9,10. Many nutrients are associated with lower levels of inflammation. Evidence indicates that magnesium (Mg), carotenoids, flavonoids, fiber, monounsaturated fatty acids (MUFAs), and ω-3 polyunsaturated fatty acids (PUFAs) are associated with decreased levels of inflammatory markers. In contrast, a high ω-6/ω-3 PUFA ratio, high-glycemic index carbohydrates, trans-fatty acids (TFAs), and saturated fatty acids (SFAs) are associated with increased levels of inflammation11.
In this regard, the inflammatory potential of a diet can be evaluated using a well-established scale known as the Dietary Inflammatory Index (DII)12,13,14,15. This index evaluates the potential of pro- and anti-inflammatory characteristics attributed to dietary macronutrients, vitamins, minerals, and other dietary components. In other words, the impact of these nutrients on the levels of six anti-inflammatory and pro-inflammatory mediators, namely IL-1, IL-4, IL-6, C-reactive protein (CRP), TNF-α, and IL-10, is determined12,16.
The DII has been used to evaluate the association between the quality of food intake related to inflammation and some diseases, such as metabolic syndrome17, colorectal cancer18, and asthma19. Few documents show the link between bone health and the dietary inflammatory potential. A cross-sectional study conducted on postmenopausal women revealed that a diet higher in pro-inflammatory foods was found to be connected to lower BMD in the lumbar spine20. Another study showed that an increased DII was related to the risk of fractures21. This study aimed to investigate the relationship between the DII score and the odds of osteoporosis in Iranian women. It was conducted in light of the rising prevalence of osteoporosis, the expensive treatment costs associated with it, and the limited information available about the inflammatory potential of diet for postmenopausal women.
Methods
Study design and participants
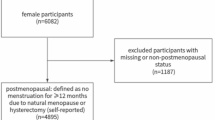
The study conducted was a case-control study involving 131 postmenopausal healthy women, as well as 131 women with osteoporosis and osteopenia aged 45–65. These women were selected from the Bone Densitometry Center in Isfahan city, Iran. The study sample size was computed by Shivappa et al. study (odds ratio (OR) = 2.30, α = 0.05, and power = 80%)20. These participants were admitted to the Bone Densitometry Center in Isfahan, Iran. The ethics committee of Tabriz University of Medical Sciences approved the protocol of the present study (ethical Approval code: IR.TBZMED.REC.1400.114). Some details of the present study were published previously22,23,24.
After being informed about the aim of the study, all participants were given a written consent form. The exclusion criteria for this study included a history of chemotherapy, a history of certain diseases (such as diabetes, renal disease, cancer, and rheumatoid arthritis), the use of alcohol and glucocorticoids, and being premenopausal. Subjects who engaged in intense physical activity (above 3000 metabolic equivalent of task (MET)-minutes/week) and either under-reported or over-reported their calorie intake (less than 800 and more than 4200 kcal/day) were also excluded. Menopause was defined as the cessation of the menstrual cycle for a continuous period of 12 months.
The cases were selected based on standard tools and controlled conditions, with the diagnosis of osteoporosis (including osteoporosis and osteopenia) made by a trained technician. The controls without osteoporosis/osteopenia were chosen according to specific inclusion and exclusion criteria.
BMD measurement
The Horizon Wi (S/N 200451) dual-energy X-ray absorptiometry (DEXA) method was used by a technician to measure the BMD of the lumbar vertebrae and femoral neck, in grams per square centimeter. A physician then determined the bone mass status. The bone mass status was determined using the criteria established by the World Health Organization (WHO)25. According to these criteria, T-score values above − 1 were classified as normal, values between − 1 and − 2.5 were classified as osteopenia, and values below − 2.5 were classified as osteoporosis1,25.
Data collection
A checklist was used to collect information on sociodemographic, contextual, and confounding variables, including socioeconomic status, weight, body mass index (BMI), smoking, use of medications, and dietary supplements that can impact BMD.
For food assessment, a food frequency questionnaire (FFQ) (168 items) was used to assess dietary intake and FFQ was completed by a trained nutritionist. The validity and reliability of the mentioned questionnaire have already been confirmed26. This questionnaire recorded the daily consumption habits of the participants over the past year. Initially, to facilitate participants’ responses, a valid food album and some household measurements (such as teaspoons, tablespoons, cups, plates, etc.) were used to assess the type and portion size of food consumed. Participants were then asked to report the frequency of consumption for each food item (daily, weekly, monthly, or yearly)27. Subsequently, the frequency of consumption was multiplied by the portion size reported by each individual to calculate the intake of each FFQ item in grams. Finally, the Nutritionist IV software was used to calculate nutrient and energy intake28.
DII score calculation
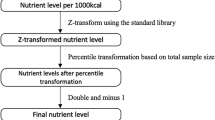
The DII score was determined using the method proposed by Shivappa et al., based on FFQ data13. The DII score was calculated based on the energy-adjusted intake of 29 food components, which include energy, protein, carbohydrates, fiber, omega-6 PUFAs, omega-3 PUFAs, PUFAs, MUFAs, SFAs, cholesterol, total fat, TFAs, iron, Mg, zinc, selenium, vitamin B12, folate, vitamin B6, niacin, riboflavin, vitamin C, beta-carotene, vitamin A, thiamine, vitamin E, caffeine, garlic, and onion. Compared to the original DII, which includes forty-five food items, the FFQ does not assess a range of dietary components. These components include anthocyanidins, flavan-3-ol, flavanones, flavanols, flavones, saffron, eugenol, rosemary, pepper, oregano, turmeric, and ginger. The absence of these items has minimal impact on the DII score because they are typically only found in small amounts in most people’s regular diets13.
At first, the Z-score for each 29 food components were calculated by: (daily intake of each component - mean of the global standard) / the global standard deviation (SD). Then, the Z-score for each component was changed to a percentile. Finally, multiplying by the inflammatory score, the DII score for each component was calculated. Ultimately, the total DII score was determined by adding up all the individual scores. A higher DII score reflects a greater pro-inflammatory potential of the diet29,30.
Calculation of other variables
Height was determined using a stadiometer to the nearest 0.5 cm. Body weight was assess by standard protocols (with the least amount of clothing and excluding shoes), was measured using a digital scale (Seca 700, Hamburg, Germany) with a precision of 100 g. The BMI was calculated by dividing weight (in kilograms) by height (in square meters). A validated International Physical Activity Questionnaire (IPAQ) was applied to assess the participants’ physical activity level31. The subjects were then divided into three groups based on their level of physical activity: intense (> 3000 MET-minutes/week), moderate (600–3000 MET-minutes/week), and low (< 600 MET-minutes/week). This categorization was determined using MET and the standard protocol32.
Statistical analyses
SPSS statistical software version 23 (IBM Corp., Endicott, New York, USA) was used for data analysis. P-value less than 0.05 was considered a significant level. For comparison of qualitative and continuous variables, chi-square and T-test were used for comparisons between cases and controls, respectively. The energy-adjusted method was used to calculate the DII scores. After calculating the DII score, individuals were classified into tertiles according to their DII scores. One-way analysis of variance (ANOVA) was used to assess the intake of nutrients and food groups among the different tertiles of the DII score. A logistic regression test was used to assess the association between DII scores and both BMD and the odds of osteopenia/osteoporosis. Four regression models were considered in this study. The first model, referred to as the crude model, did not have any adjustments. The second model, called model 1, was adjusted for age and BMI. The third model, referred to as model 2, had additional adjustments for physical activity, education, and income, in addition to the adjustments made in model 1. Finally, the fourth model, known as model 3, included adjustments for energy intake, vitamin D and calcium supplements, in addition to the adjustments made in both model 1 and model 2 (potential confounders were selected using previous studies33,34,35.
Results
The study’s flow chart is presented in Fig. 1. A total of 131 cases and 131 controls were included in the analysis.
Table 1 displays the characteristics and dietary intakes of the study participants. The mean (SD) age of the cases was 57.95 ± 5.42 years, while for the controls it was 56.47 ± 5.91 years. Notably, none of the participants had a history of smoking. In the control group, 83.2% had low physical activity level, while the remaining 16.8% reported moderate physical activity level. For the case group, 93.1% had low physical activity level, whereas 6.9% reported moderate physical activity level (p-value = 0.021). There was a significant difference between the two groups in terms of educational level (p-value<0.001), femoral and lumbar BMD (p-value<0.001 for both), intake of fiber (p-value < 0.001), protein (p-value = 0.001), the omega-6/omega-3 ratio (p-value = 0.030), calcium (p-value = 0.008) and Mg (p-value = 0.004). In the current study, the DII score ranged from − 4.30 (indicating the highest anti-inflammatory score) to 5.20 (indicating the highest pro-inflammatory score). The mean DII score demonstrated a statistically significant difference between the control and case groups (p-value = 0.038). The individuals in the case group had a more pro-inflammatory diet.
Table 2 displays the nutrient intake based on the tertiles of the DII score. Individuals in the last tertile (T3), which had higher DII scores, significantly consumed more energy (p-value<0.001), carbohydrates (p-value<0.001), fats (p-value<0.001), SFAs (p-value<0.001), PUFAs (p-value = 0.032), MUFAs (p-value<0.001), cholesterol (p-value = 0.004), vitamin B1 (p-value<0.001), folate (p-value = 0.006), vitamin B12 (p-value<0.001), and sodium (p-value<0.001) compared to those in T1 (the tertile with the lowest DII scores). On the other hand, individuals in T3 had significantly lower intake of potassium (p-value = 0.031), vitamin B6 (p-value = 0.008), vitamin B3 (p-value = 0.034), vitamin C (p-value<0.001), vitamin K (p-value<0.001), beta-carotene (p-value<0.001), vitamin A (p-value<0.001), and fiber (p-value<0.001) compared to T1.
Table 3 indicates the intake of food groups based on tertiles of the DII score. Women in the group with the least pro-inflammatory diet (T1) significantly consumed higher amounts of legumes (p-value = 0.004), vegetables (p-value<0.001), fruits (p-value<0.001), whole grains (p-value = 0.011), and fish & seafood (p-value = 0.001). On the other hand, women following the most pro-inflammatory diet had significantly higher consumption of sugar-sweetened beverages (p-value<0.001), refined grains (p-value<0.001), and dairy (p-value = 0.014) compared to T1 of the DII score.
Table 4 shows the crude and multivariable-adjusted ORs and 95% confidence intervals (CIs) for the osteoporosis/osteopenia odds across the tertiles of DII score. In comparison to the first tertile of DII score, higher and significant odds of osteoporosis/osteopenia were seen in the last tertile, in all models (crude model: OR = 2.20; 95% CI: 1.20–4.04, Ptrend = 0.010; model 1: OR = 2.34; 95% CI: 1.26–4.35, Ptrend = 0.007; model 2: OR = 2.46; 95% CI: 1.22–4.97, Ptrend = 0.018; model 3: OR = 2.43; 95% CI: 1.19–4.95, Ptrend = 0.023). In all models, a lower mean femoral neck and lumbar spine BMD were seen in the last tertile of DII score compared to the first tertile (Femoral neck - crude model: OR = 2.17; 95% CI:1.18-4.00, Ptrend = 0.013); model 1: OR = 2.30; 95% CI:1.23–4.30, Ptrend = 0.009; model 2: OR = 2.91; 95% CI:1.42–5.98, Ptrend = 0.005; model 3: OR = 2.85; 95% CI:1.37–5.89, Ptrend = 0.007 – Lumbar spine - crude model: OR = 2.31; 95% CI: 1.26–4.25, Ptrend = 0.007; model 1: OR = 2.36; 95% CI: 1.28–4.36, Ptrend = 0.006; model 2: OR = 2.61; 95% CI: 1.31, 5.20, Ptrend = 0.008; model 3: OR = 2.59; 95% CI: 1.29–5.19, Ptrend = 0.009).
Discussion
The current research was designed to determine the DII score in 262 Iranian women with osteoporosis and healthy women and assess its association with the odds of osteoporosis. The study’s results showed a positive relationship between pro-inflammatory dietary patterns measured by the DII score, and the odds of osteoporosis. Women diagnosed with osteoporosis had the highest DII score in comparison to women with normal bone health.
The results revealed that women with high DII scores had lower BMD in the lumbar spine and femoral neck. Women with the highest DII score consumed higher amounts of refined grains, sweets, sugar-sweetened beverages, fat, SFAs, and cholesterol compared to women with the lowest DII score. In contrast, the consumption of whole grains, fruits, vegetables, legumes, fish, and seafood, as well as vitamins such as vitamins A, K, and C, in women with the lowest DII score was higher than in women with the highest DII score. Additionally, the findings demonstrated that the intake of protein, calcium, and magnesium in healthy women was higher than in women with osteoporosis.
Several studies have consistently demonstrated that following healthy dietary patterns, such as the Mediterranean diet, can effectively reduce inflammation levels. This particular diet promotes the consumption of foods like fish, nuts, vegetables, and fruits while discouraging the intake of red meats. Conversely, studies have also revealed that adhering to the Western diet, which primarily consists of meat and refined grains, can lead to higher inflammation levels36,37. Specific nutrients such as beta-carotene, vitamin C, fiber, and ω-3 PUFAs are associated with lower levels of inflammation38,39. Also, an excessive level of SFAs in the diet increases IL-1 and IL-6 in the body40. Multiple studies have examined the potential link between DII score and bone health, and these studies consistently found similar results. For instance, a cohort study discovered that female patients who had higher DII scores (indicating a diet with high pro-inflammatory potential) were at a greater risk of developing osteoporosis41. A cross-sectional study conducted in Spain examined the relationship between the inflammatory index of a diet and bone health in young people. The study found that while the inflammatory index of the diet was associated with obesity parameters such as weight and lean mass, there was no association found with osteoporosis42. A study conducted on 160 postmenopausal Iranian women revealed that those with higher scores in DII had significantly lower BMD in the lumbar spine. However, this association may not be applicable to other skeletal sites, such as the femoral neck20. Furthermore, another study reported an association between higher risks of osteoporosis in the femur only and lower bone density with a high DII score43. In our study, women with osteoporosis had low bone density in both the femur and lumbar spine, and a high DII score was associated with decreased bone density in the femur and lumbar spine.
Several biological mechanisms have been introduced to explain the possible impact of diet-induced pro-inflammatory markers on bone diseases. The process of bone resorption may be significantly influenced by these pro-inflammatory mediators5,6,7. Women who are experiencing menopause are at a greater risk of developing osteoporosis. This is likely due to the decrease in estrogen levels, which leads to a disruption in the regulation of inflammatory markers like ILs and the TNF family44,45. Cytokines, such as TNF-α, affect osteoclastogenesis through the receptor activator of nuclear factor kappa B (RANK) ligand (RANKL)-RANK signaling pathway. RANKL serves as the ligand for RANK, and when RANKL binds to RANK receptors on the surface of osteoclasts, it triggers the activation of osteoclastogenesis. RANKL is expressed by osteoblasts and its precursors and is considered a key regulator of osteoclasts’ activation, survival, and differentiation from hematopoietic cells. TNF-α and RANKL synergistically activate nuclear factor kappa-B (NF- κB), which in turn stimulates the production of osteoclasts46,47.
The findings of the study indicated a lower intake of protein, calcium, and magnesium in women with osteoporosis. The bone matrix is made of collagen and other types of proteins, as well as calcium phosphate (hydroxyapatite crystals). Additionally, magnesium plays a role in bone health by activating vitamin D48. Research has shown that adequate protein consumption has a positive relationship with BMD and a reduced risk of hip fracture49,50,51. Intake of protein increases calcium absorption in the intestine. Furthermore, it stimulates insulin-like growth factor-1 (IGF-1) and increases free-fat mass52.
The strengths of this study were face-to-face interviews for data collection and the selection of cases and controls from the same clinics. Also, we controlled for several confounding factors to obtain more accurate results. However, this case-control study had some limitations, including potential selection and recall bias that could affect the associations observed. Additionally, misclassification of participants’ dietary intake due to the use of FFQs cannot be overlooked. However, we utilized validated FFQs. Moreover, we were unable to perform stratified analyses due to the limited sample size. Furthermore, we did not measure biochemical inflammatory indices. The DII is not a direct biochemical inflammatory index; rather, it serves as an estimation of dietary inflammatory potential. Additionally, the unavailability of data regarding the use of hormone replacement therapy was another limitation of the present study.
Conclusions
The findings showed that postmenopausal women who followed diets with higher inflammatory potentials were found to have a significantly higher odds of developing osteoporosis/osteopenia. Avoiding inflammatory foods and consuming more anti-inflammatory foods may reduce the risk and prognosis of osteoporosis. To gain a more thorough understanding of any potential link between the DII score and osteoporosis risk in postmenopausal women, future research should focus on conducting larger studies with longitudinal designs, including intervention studies.
Data availability
Data are available through a reasonable request from the corresponding author.
References
Nuti, R. et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 14, 85–102. https://doi.org/10.1007/s11739-018-1874-2 (2019).
Salari, N. et al. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 16, 609. https://doi.org/10.1186/s13018-021-02772-0 (2021).
Eghbali, T., Abdi, K., Nazari, M., Mohammadnejad, E. & Gheshlagh, R. G. Prevalence of osteoporosis among Iranian Postmenopausal women: a systematic review and Meta-analysis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 15, 11795441211072471. https://doi.org/10.1177/11795441211072471 (2022).
Föger-Samwald, U. et al. Pathophysiology and therapeutic options. Excli j. 19, 1017–1037. https://doi.org/10.17179/excli2020-2591 (2020).
Harmer, D., Falank, C. & Reagan, M. R. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front. Endocrinol. (Lausanne). 9, 788. https://doi.org/10.3389/fendo.2018.00788 (2018).
Barbour, K. E. et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J. Bone Min. Res. 29, 2057–2064. https://doi.org/10.1002/jbmr.2245 (2014).
Mun, H., Liu, B., Pham, T. H. A. & Wu, Q. C-reactive protein and fracture risk: an updated systematic review and meta-analysis of cohort studies through the use of both frequentist and bayesian approaches. Osteoporos. Int. 32, 425–435. https://doi.org/10.1007/s00198-020-05623-6 (2021).
Hart, M. J., Torres, S. J., McNaughton, S. A. & Milte, C. M. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr. J. 20, 24. https://doi.org/10.1186/s12937-021-00674-9 (2021).
Corley, J., Kyle, J. A., Starr, J. M., McNeill, G. & Deary, I. J. Dietary factors and biomarkers of systemic inflammation in older people: the Lothian Birth Cohort 1936. Br. J. Nutr. 114, 1088–1098. https://doi.org/10.1017/s000711451500210x (2015).
Park, K. H. et al. Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metabolism 63, 233–241. https://doi.org/10.1016/j.metabol.2013.10.011 (2014).
Galland, L. Diet and inflammation. Nutr. Clin. Pract. 25, 634–640. https://doi.org/10.1177/0884533610385703 (2010).
Cavicchia, P. P. et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 139, 2365–2372. https://doi.org/10.3945/jn.109.114025 (2009).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hébert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public. Health Nutr. 17, 1689–1696. https://doi.org/10.1017/s1368980013002115 (2014).
Shivappa, N. et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal variation of blood cholesterol study (SEASONS). Public. Health Nutr. 17, 1825–1833. https://doi.org/10.1017/s1368980013002565 (2014).
Kotemori, A. et al. Validating the dietary inflammatory index using inflammatory biomarkers in a Japanese population: a cross-sectional study of the JPHC-FFQ validation study. Nutrition 69, 110569. https://doi.org/10.1016/j.nut.2019.110569 (2020).
Shivappa, N. et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 113, 665–671. https://doi.org/10.1017/s000711451400395x (2015).
Abdurahman, A. A. et al. Association of dietary inflammatory index with metabolic profile in metabolically healthy and unhealthy obese people. Nutr. Diet. 76, 192–198. https://doi.org/10.1111/1747-0080.12482 (2019).
Abulimiti, A. et al. The Dietary Inflammatory Index is positively Associated with Colorectal Cancer Risk in a Chinese case-control study. Nutrients 12 https://doi.org/10.3390/nu12010232 (2020).
Wood, L. G., Shivappa, N., Berthon, B. S., Gibson, P. G. & Hebert, J. R. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin. Exp. Allergy. 45, 177–183. https://doi.org/10.1111/cea.12323 (2015).
Shivappa, N., Hébert, J. R., Karamati, M., Shariati-Bafghi, S. E. & Rashidkhani, B. Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. Eur. J. Nutr. 55, 561–568. https://doi.org/10.1007/s00394-015-0875-4 (2016).
Veronese, N. et al. Pro-inflammatory dietary pattern is associated with fractures in women: an eight-year longitudinal cohort study. Osteoporos. Int. 29, 143–151. https://doi.org/10.1007/s00198-017-4251-5 (2018).
Ghadiri, M. et al. Healthy plant-based diet index as a determinant of bone mineral density in osteoporotic postmenopausal women: a case-control study. Front. Nutr. 9 https://doi.org/10.3389/fnut.2022.1083685 (2023).
Nouri, M. et al. How do carbohydrate quality indices influence on bone mass density in postmenopausal women? A case–control study. BMC Women’s Health. 23, 42. https://doi.org/10.1186/s12905-023-02188-4 (2023).
Ghadiri, M. et al. The relation between dietary quality and healthy eating index with bone mineral density in osteoporosis: a case-control study. BMC Musculoskelet. Disord. 24, 584. https://doi.org/10.1186/s12891-023-06704-3 (2023).
Organization, W. H. Guidelines for prec evaluation and clinical trials in Osteoporosis (World Health Organization, 1998).
Mirmiran, P., Esfahani, F. H., Mehrabi, Y., Hedayati, M. & Azizi, F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public. Health Nutr. 13, 654–662. https://doi.org/10.1017/s1368980009991698 (2010).
Ghafarpour, M., Houshiar-Rad, A. & Kianfar, H. Te manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy. 7, 42–58 (1999).
Nutritionist, I. N-squared computing. Silverton: Nutritionist IV (1998).
Mansouri, F. et al. Dietary inflammatory index could increase the risk of Sarcopenia in patients with chronic kidney disease. Sci. Rep. 14, 15284. https://doi.org/10.1038/s41598-024-65340-6 (2024).
Zare, M. et al. Association between urinary levels of 8-hydroxy-2-deoxyguanosine and F2a-isoprostane in male football players and healthy non-athlete controls with dietary inflammatory and antioxidant indices. Front. Nutr. 9 https://doi.org/10.3389/fnut.2022.1101532 (2023).
Moghaddam, M. H. B. et al. The Iranian version of international physical activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and Stability. World Appl. Sci. J. 18, 1073–1080. https://doi.org/10.5829/idosi.wasj.2012.18.08.754 (2012).
Medina, C., Barquera, S. & Janssen, I. Validity and reliability of the international physical activity guestionnaire among adults in Mexico. Rev. Panam. Salud Publica. 34, 21–28 (2013).
Beck, T. J. et al. Confounders in the association between exercise and femur bone in postmenopausal women. Med. Sci. Sports Exerc. 43, 80–89. https://doi.org/10.1249/MSS.0b013e3181e57bab (2011).
Nevill, A. M. et al. Adjusting bone mass for differences in projected bone area and other confounding variables: an allometric perspective. J. Bone Min. Res. 17, 703–708. https://doi.org/10.1359/jbmr.2002.17.4.703 (2002).
Bailey, R. L. et al. Best practices for conducting Observational Research to assess the relation between nutrition and bone: An International working group summary. Adv. Nutr. 10, 391–409. https://doi.org/10.1093/advances/nmy111 (2019).
Bonaccio, M., Cerletti, C., Iacoviello, L. & de Gaetano, G. Mediterranean diet and low-grade subclinical inflammation: the Moli-Sani study. Endocr. Metab. Immune Disord Drug Targets. 15, 18–24. https://doi.org/10.2174/1871530314666141020112146 (2015).
Barbaresko, J., Koch, M., Schulze, M. B. & Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr. Rev. 71, 511–527. https://doi.org/10.1111/nure.12035 (2013).
Suarez, E. C. & Schramm-Sapyta, N. L. Race differences in the relation of vitamins a, C, E, and β-carotene to metabolic and inflammatory biomarkers. Nutr. Res. 34, 1–10. https://doi.org/10.1016/j.nutres.2013.10.001 (2014).
Micallef, M. A., Munro, I. A. & Garg, M. L. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur. J. Clin. Nutr. 63, 1154–1156. https://doi.org/10.1038/ejcn.2009.20 (2009).
Dumas, J. A. et al. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism 65, 1582–1588. https://doi.org/10.1016/j.metabol.2016.08.003 (2016).
Kim, H. S. et al. Positive association between Dietary Inflammatory Index and the risk of osteoporosis: results from the KoGES_Health Examinee (HEXA) Cohort Study. Nutrients 10 https://doi.org/10.3390/nu10121999 (2018).
Correa-Rodríguez, M. et al. Dietary inflammatory index, bone health and body composition in a population of young adults: a cross-sectional study. Int. J. Food Sci. Nutr. 69, 1013–1019. https://doi.org/10.1080/09637486.2018.1446915 (2018).
Na, W. et al. Association between Inflammatory potential of diet and bone-mineral density in Korean postmenopausal women: Data from Fourth and Fifth Korea National Health and Nutrition Examination Surveys. Nutrients 11, (2019). https://doi.org/10.3390/nu11040885
Malutan, A. M., Dan, M., Nicolae, C. & Carmen, M. Proinflammatory and anti-inflammatory cytokine changes related to menopause. Prz Menopauzalny. 13, 162–168. https://doi.org/10.5114/pm.2014.43818 (2014).
Ji, M. X. & Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl Med. 1, 9–13. https://doi.org/10.1016/j.cdtm.2015.02.006 (2015).
Yao, Z., Getting, S. J. & Locke, I. C. Regulation of TNF-Induced Osteoclast differentiation. Cells 11 https://doi.org/10.3390/cells11010132 (2021).
Zha, L. et al. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 102, 369–374. https://doi.org/10.1016/j.biopha.2018.03.080 (2018).
Erem, S., Atfi, A. & Razzaque, M. S. Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol. 193, 105400. https://doi.org/10.1016/j.jsbmb.2019.105400 (2019).
Rizzoli, R. et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European society for clinical and economical aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 29, 1933–1948. https://doi.org/10.1007/s00198-018-4534-5 (2018).
Groenendijk, I., den Boeft, L., van Loon, L. J. C. & de Groot, L. High Versus low dietary protein intake and bone health in older adults: a systematic review and Meta-analysis. Comput. Struct. Biotechnol. J. 17, 1101–1112. https://doi.org/10.1016/j.csbj.2019.07.005 (2019).
Dolan, E. & Sale, C. Protein and bone health across the lifespan. Proc. Nutr. Soc. 78, 45–55. https://doi.org/10.1017/s0029665118001180 (2019).
Kerstetter, J. E., Kenny, A. M. & Insogna, K. L. Dietary protein and skeletal health: a review of recent human research. Curr. Opin. Lipidol. 22, 16–20. https://doi.org/10.1097/MOL.0b013e3283419441 (2011).
Acknowledgements
The authors of the study want to express their gratitude to Tabriz University of Medical Sciences for their financial support.
Author information
Authors and Affiliations
Contributions
M.G., M.R.A., M.N. and Z.S.; Contributed to writing the first draft. M.N. and B.P.G.; Contributed to all data, statistical analysis, and interpretation of data. B.P.G. and A.A.N.; Contributed to the research concept, supervised the work, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Human ethics and consent to participate
The informed consents were completed by all participants and all experimental protocols were approved by Tabriz university of Medical Science ethical committee (IR.TBZMED.REC.1400.114) and all experiments were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghadiri, M., Gargari, B.P., Ahmadi, M.R. et al. The role of dietary inflammation in the risk of osteoporosis in Iranian postmenopausal women: a case-control study. Sci Rep 15, 5120 (2025). https://doi.org/10.1038/s41598-025-89649-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89649-y
Keywords
This article is cited by
-
Diet-induced inflammation and its association with sarcopenia in an Iranian population: a case-control study
Journal of Health, Population and Nutrition (2025)