Abstract
During the resolution of inflammation, Ly6C+F4/80- monocytes differentiate to Ly6C-F4/80+ macrophages that exert apoptotic cell engulfment (efferocytosis) properties and consequently convert to interferon (IFN)-β-producing macrophages. Here, we report that exposure to IFN-β, or transforming growth factor (TGF)-β, or a deficiency in the pro-apoptotic protein ARTS, results in the conversion of mature macrophages to an Ly6C+F4/80+CCR2+ phenotype in vivo and ex vivo. Deficiency in ARTS or caspase inhibition results in enhanced conversion of macrophages to the Ly6C+ phenotype. Moreover, IFN-β-triggered Ly6C+ macrophages are hyper-efferocytic and express higher levels of the efferocytic receptor CD36. Inhibition of CD36 ligation results in complete abrogation of efferocytosis ex vivo in both Ly6C+ and Ly6C- macrophages. Notably, IFN-β also promotes the emergence of Ly6C+ macrophages during the resolution of liver fibrosis, while transcriptomic analysis further links this rejuvenated phenotype to macrophage subsets found in human inflammatory liver disease. Altogether, our findings indicate IFN-β promotes macrophage conversion to a distinct hyper-efferocytic phenotype that is limited by ARTS and apoptosis during the resolution of inflammation.
Similar content being viewed by others
Introduction
Macrophages, conventionally regarded as essential contributors to the innate immune system, exhibit notable plasticity and can undergo polarization into distinct biological states. This remarkable attribute is evident in their ability to respond to various signals such as cytokines, growth factors, and structural cues. Whether resident in tissues or infiltrating from the bloodstream, macrophages actively participate in the response to infections and tissue repair, while also playing a role in the pathophysiology of diverse diseases, including cancers, autoimmunity, and fibrosis1,2. Macrophage subtypes have been broadly aligned with the bimodal classification into pro-inflammatory, classically activated, M1, or anti-inflammatory, alternatively activated, M23. Today it is clear that the M1 and M2 phenotypes represent more in vitro states under specific stimulants rather than reflecting the exact in vivo continuum of polarization spectra. In fact, macrophage sub-classes distribute between M1 and M2 extremes expressing huge diversity and plasticity in different body tissues in health and disease4,5. This diversity prompted characterizing macrophages based on specific markers as a more relevant approach. Examples include the monocytic myeloid lineage marker Ly6C alone or in combination with other markers mainly the murine macrophage marker F4/80 and the myeloid lineage marker CD11b, among others6.
The resolution of peritonitis is characterized by a macrophage population that is largely derived of monocytes generated from hematopoietic stem cells (HSCs) in the bone marrow7. Their developmental sequence includes, consecutively, the common myeloid precursors (CMPs), the granulocyte-macrophage progenitor (GMP), the common macrophage and dendritic cell (DC) precursor (MDP), and finally the committed monocyte progenitor (cMoP), that gives rise to monocytes8,9. Classical Ly6ChiCCR2+CD62L+CX3CR1loCD43- (Ly6Chi) monocytes (and their parallels CD14+CD16- in humans) constitute the majority of monocytes. The Non-classical Ly6CloCCR2-CD62L-CX3CR1hiCD43+(Ly6Clo) monocytes (and their parallels CD14loCD16+ in humans) originate from Ly6Chi monocytes as precursors in steady state10,11. They were reported as scavengers of the vasculature, patrolling small vessel endothelial walls, and maintaining their integrity8,12. Macrophages originate from blood recruited monocytes upon inflammatory cues. These Bone marrow-derived inflammatory macrophages are the outcome of Ly6C+ monocyte differentiation, which continues the sequence of the described differentiations from CMP. The tissue-infiltrating monocyte first differentiates to an F4/80lo macrophage, and matures progressively to F4/80hi subset13. Albeit, other origins include fetal bone marrow HSCs, liver, and yolk sac progenitors. The pool of macrophages in different tissue niches either in steady state or inflammation represent ontogenetic heterogeneity as well as diversity in molecular cues and circuits that shape tissue microenvironments7,14.
ARTS is a 28 Kd protein that is the product of the Septin 4 gene and is also referred to as Sept4_i215,16. ARTS is a pro-apoptotic protein situated at the mitochondrial outer membrane17. ARTS is a transcriptional target of p53 and initiates apoptosis by binding to and degrading both major anti-apoptotic proteins XIAP (X-linked Inhibitor of Apoptosis Protein) and Bcl-218,19. This results in release of active caspases from their binding to XIAP, permeabilization of the mitochondrial outer membrane (MOMP) and further activation of caspases leading to cell death execution17,19. In resolving inflammation, ARTS is pivotal in steering immune cells (especially neutrophils) toward apoptosis. Consequently, it enables macrophages to perform efferocytosis and transition to a non-inflammatory, reparative state, highlighting its indispensable role in terminating inflammatory responses and promoting tissue healing. Genetic deletion of ARTS impairs this vital process, suggesting its therapeutic targeting could be a frontier in treating unresolved inflammation20.
During the resolution of inflammation, the inflammatory chemokine and cytokine signaling is abrogated to impede neutrophil infiltration and avoid further tissue destruction21. Moreover, neutrophil apoptosis and necroptosis pathways are activated, which lead to the release of chemotactic signals attracting monocytes and macrophages towards their remnants (apoptotic cells)22,23,24. Effectively, the efferocytosis of apoptotic cells by macrophages, in a non-phlogistic fashion, reprograms macrophages towards anti-inflammatory/pro-resolving phenotypes25,26. Previously we have shown that the anti-viral cytokine IFN-β is a key effector produced by satiated macrophages that promotes neutrophil apoptosis, macrophage efferocytosis and reprogramming to pro-resolving and anti-fibrotic phenotypes25,27,28,29,30.
In the current study, we explored the differentiation of F4/80+ resolution phase macrophages. Transcriptomic analysis of peritoneal resolution phase macrophages revealed an elevated monocytic gene signature highlighted by Ly6C in non-phagocytic macrophages (Table 1). In addition, we found the pro-resolving cytokines IFN-β and TGF-β to promote mature macrophage rejuvenation or “dedifferentiation” by inducing Ly6C and reducing F4/80 surface expression. We also show Ly6C+ macrophages are exclusively generated under inflammation-resolution sequel, are limited by apoptosis and the expression of ARTS, and display a CD36-dependent hyper-efferocytic phenotype.
Results
IFN-β promotes the generation of Ly6C+ macrophages during the resolution of inflammation
IFN-β is a potent, recently uncovered pro-resolving macrophage-derived cytokine which facilitates monocyte recruitment and macrophage efferocytosis during the resolution of murine peritonitis31,32. Monocytes differentiate from a Ly6C+ to a Ly6C- phenotype during their maturation to pro-resolving macrophages or upon conversion to non-classical intravascular monocytes8,33.
Unexpectedly, our transcriptomic comparison of non-phagocytic and phagocytic resolution phase macrophages27 has revealed the former express higher levels of most of the genes expressed by Ly6C+ monocyte that are reduced upon their conversion to the Ly6C- phenotype in the BM and blood8. These genes include Cebpe, Ly6c2, Mmp8, Sell, Ccr2, and Fcrls, but not Foxm1 (that showed minimal reduction in blood monocyte Ly6C+ > Ly6C- conversion8). These findings suggest IFN-β promotes the expression of monocyte markers by mature resolution phase monocyte-derived macrophages, thereby converting them to a rejuvenated phenotype.
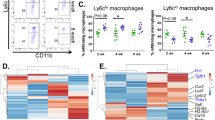
To determine whether resolution phase F4/80+ mature macrophages indeed express surface Ly6C and this is regulated by IFN-β, we collected peritoneal exudates from zymosan A-treated Ifnb+/+ or Ifnb-/- mice and analyzed their marker expression by flow cytometry. All experiments were performed using male mice since females exert delayed and milder pro-resolving responses during peritonitis28. Our results show five prominent monocyte and macrophage subsets recovered at 66 h post peritonitis initiation (PPI), (Fig. 1a-b). These subsets included F4/80+Ly6C+ macrophages that were significantly downregulated by IFN-β deficiency (Fig. 1a-b). In accord, in vivo treatment of Ifnb+/+ mice with IFN-β enhanced the frequency of F4/80+Ly6C+ macrophages and their mean expression of Ly6C, but not F4/80 (Fig. 1c-e). Remarkably, the differentiation of monocytes to mature macrophages and rejuvenated monocytes/macrophages could be recapitulated ex vivo upon culturing of the exudate cells. Five subsets of monocyte-derived cells were detected: monocytes (Ly6C+F4/80-), young (Ly6C+F4/80med), mature (Ly6C-F4/80hi), resolution-promoting (Ly6C-F4/80med), and rejuvenated (Ly6C+F4/80hi) monocytes/macrophages (Supplementary Fig. 1a). Using this setting we could detect a time- and concentration-dependent increase in rejuvenated monocytes/macrophage frequency and Ly6C expression, concomitant with F4/80 down-regulation. Maximal values were obtained at 48 h of culture and 25 ng/ml of IFN-β (Fig. 1f-i). A significant decrease in the frequency of mature macrophages and a minor increase in young macrophages were observed as well with IFN-β treatment.
a Representative density plots of resolution phase myeloid cells from Ifnb+/+ and Ifnb-/- mice 66 h post peritonitis initiation (PPI). b The percentage of Ly6C+ macrophages in resolving exudates of Ifnb+/+ and Ifnb-/- mice 66 h post peritonitis initiation (PPI). N = 13. Significant differences between Ifnb+/+ and Ifnb-/- mice were determined by Student’s t-test. c The percentage of the different myeloid subsets detected in resolving peritoneal exudates following treatment with vehicle or IFN-β in vivo. n = 8. d Ly6C expression by different myeloid subsets in resolving peritoneal exudates following treatment with vehicle or IFN-β in vivo. n = 8. e F4/80 expression by different myeloid subsets in resolving peritoneal exudates following treatment with vehicle or IFN-β in vivo. n = 8. f Representative density plots of resolution phase myeloid cells from peritoneal exudates following incubation with IFN-β, TGF-β, or vehicle for the indicated times. g Percentage of F4/80+Ly6C+ (rejuvenated) macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.04–25 ng/ml of IFN-β. h Ly6C expression of rejuvenated monocytes/macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.04–25 ng/ml of IFN-β. i F4/80 expression of rejuvenated monocytes/macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.04–25 ng/ml of IFN-β. j Percentage of F4/80+ Ly6C+ (rejuvenated) monocytes/macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.4–10 ng/ml of TGF-β. k Ly6C expression of rejuvenated monocytes/macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.4–10 ng/ml of TGF-β. l F4/80 expression of rejuvenated monocytes/macrophages following ex vivo differentiation of resolution phase myeloid cells incubated for the indicated times with 0.4–10 ng/ml of TGF-β. n = 11 for T = 0, T = 48 h control and 25 ng/ml IFN-β, n = 7 for T = 48 h 10 ng/ml TGF-β, n = 5 for T = 24 h control and 25 ng/ml IFN-β and 10 ng/ml TGF-β, n = 3 for T = 72 h control, T = 24, 48, 72 h IFN-β at concentrations of 0.04 and 1 ng/ml, T = 72 h 10 ng/ml TGF-β and T = 24, 48, 72 h TGF-β at concentrations of 0.4 and 5 ng/ml and n = 2 for T = 24, 48, 72 h IFN-β at concentrations of 0.2 and 1 ng/ml. Significant differences between experimental groups in each time point were determined by one-way ANOVA, and Tukey’s HSD. p < 0.05 (∗), 0.01 (∗∗), or 0.001 (∗∗∗). All controls showed significant differences between T = 0 and T = 24 (∗∗). m Representative density plots of resolution phase myeloid cells populations in the resolution phase of CCl4-induced liver fibrosis. Ifnb+/+ and Ifnb-/- mice received CCl4 (CCl4 6 mL/kg, 1:6 dilution in corn oil) by intraperitoneal injection twice weekly for six weeks. 48 h after the last injection some Ifnb-/- mice were treated with rIFN-β (25 ng/mouse). n The ratio of Ly6Cpos/Ly6Cneg macrophages in liver single-cell suspensions from the same treatment, harvested 72 h after the last CCl4 injection. Significant differences between experimental groups was determined by two-tailed Student’s t-test. p < 0.05 (∗). Error bars represent the standard error of the mean (SEM) throughout the figure.
Notably, another pro-resolving cytokine, TGF-β, also induced macrophage rejuvenation (Fig. 1f, j-i; Supplementary Fig. 1e-g) with maximal values at 48 h and 10 ng/ml. Thus, IFN-β promotes resolution phase macrophage Ly6C expression and phenotypic rejuvenation in vivo and ex vivo, while TGF-β promotes this phenomenon ex vivo to a milder level. To determine whether this macrophage rejuvenation phenotype extends to other inflammation resolution setting, we examined monocyte-derived macrophage subsets recovered from the liver of CCl4-treated mice. Our flow cytometric profiling revealed a reduced frequency of F4/80+Ly6C+ macrophages in Ifnb−/− mice, which was restored by treatment with recombinant IFN-β in vivo (Fig. 1m–n)
Resolution phase macrophages dedifferentiate to a unique Ly6C+F4/80hi phenotype
Monocytes differentiate into macrophages upon changes in their metabolic programs and cell surface molecule expression. This includes development into Ly6C+ vs Ly6C- macrophages with entirely different transcriptional programs34. Our results have shown the emergence of Ly6C+ macrophages during the resolution of inflammation, however, it is conceivable that these cells are formed due to a delay in the differentiation of F4/80-Ly6C+ monocytes to F4/80+Ly6C- macrophages. To determine whether resolution phase Ly6C+ macrophages originate from Ly6C+ monocytes or F4/80+Ly6C- macrophages, we sorted Ly6C+ resolution phase monocytes from the rest of the peritoneal cellular content (labelled REST). We labelled the monocytes with CFSE and cultured them and the REST cells separately or in combination (1:4 ratio; Supplementary Fig. 2a-c). Treatments with IFN-β, TGF-β, or anti-IFN-β-neutralizing antibodies were performed to reveal the regulation of phenotype conversion by these cytokines. Flow cytometric analysis after 48 h allowed the delineation of macrophage subsets as previously noted and CFSEhi vs. CFSElo/- populations were analysed separately to distinguish monocytes and REST cells (representative density plots in Supplementary Fig. 2c). Our analysis revealed monocytes were incapable of converting to either rejuvenated monocytes/macrophages or mature ones when cultured alone (Fig. 2a-d). Interestingly, in mixed cultures, monocyte conversion into rejuvenated Ly6C+F4/80+ and mature Ly6C-F4/80+ macrophages, but not to F4/80medLy6C+ young macrophages, was restored (Fig. 2a-d). Rejuvenation was augmented upon treatment with TGF-β or IFN-β in mixed cultures only (Fig. 2a), but with no statistical significance due to the sorting procedure. As expected, the REST cells converted to the unique Ly6C+F4/80hi phenotype as well as to Ly6C-F4/80hi mature macrophages when cultured without monocytes (Fig. 2e). Interestingly, the addition of monocytes to the cell culture in all treatments reduced the frequency of Ly6C+F4/80hi macrophages (Fig. 2e). IFN-β and TGF-β enhanced rejuvenation in both the REST cells and mixed cultures but did not significantly affect other subsets or F4/80 expression (Fig. 2e-h). Anti-IFN-β neutralizing antibodies reduced the frequency of Ly6C+F4/80hi macrophages in vehicle-treated cells (both REST and mixed cultures; Fig. 2e), but due to the sorting procedure these results were not statistically significant. Altogether, our results indicate that mature macrophages, rather than monocytes, are the direct progenitors of the resolution phase Ly6C+F4/80hi macrophage subset, and that TGF-β and IFN-β accelerate dedifferentiation/rejuvenation. Notably, mature macrophages seem to accelerate monocyte differentiation to the Ly6C+F4/80hi phenotype, likely through IFN-β secretion, while monocytes impeded the dedifferentiation of mature macrophages to the Ly6C+F4/80hiphenotype.
a-d Flow cytometry of sorted monocytes (CFSEhi) recovered 66 h PPI and cultured alone or with sorted non-monocytes (REST) and treated with vehicle, anti-IFN-β antibodies, TGF-β, or IFN-β for 48 h. The resulting macrophages were gated as Ly6C+F4/80hi (a), Ly6C+F4/80med (b), or Ly6C-F4/80hi (c) subsets. Ly6C-F4/80hi macrophages were also analyzed for their F4/80 expression (d). e-h Flow cytometry of sorted non-monocytes (CFSE-/low; REST) recovered 66 h PPI and cultured alone or with sorted monocytes and treated with vehicle, anti-IFN-β antibodies, TGF-β, or IFN-β for 48 h. The resulting macrophages were gated as e Ly-6C+F4/80hi, f Ly-6C+F4/80med, or g Ly-6C-F4/80hi subsets. h Ly6C-F4/80hi macrophages analyzed for their F4/80 expression. Results are average±SE from n = 4 for vehicle and TGF-β treatments in CFSEhi cells, n = 3 for vehicle and TGF-β treatment in CFSE-/low cells and n = 2 for anti-IFN-β and IFN-β treatments in CFSE-/lo and CFSEhi cells. p < 0.05 (∗), 0.01 (∗∗), 0.001 (∗∗∗), one-way ANOVA with Tukey’s HSD. Error bars represent the standard error of the mean (SEM) throughout the figure.
IFN-β increases the expression of Ly6C exclusively in peritoneal resolution phase macrophages
Next, we aimed to determine whether increased Ly6C expression following IFN-β exposure is a specific response of peritoneal resolution phase macrophages. To this end we analyzed surface marker expression on splenic resolution phase macrophages (Supplementary Fig. 3a) and compared it to the expression by their peritoneal counterparts. Our results show the Ly6C+F4/80hi macrophages in the spleen express significantly lower levels of F4/80 than their peritoneal counterparts (Supplementary Fig. 3b) supporting them being an intermediate phase in the differentiation of monocytes to macrophages. Moreover, the frequency of this subset did not increase during the resolution phase, while monocyte frequency was reduced (Supplementary Fig. 3c).
To evaluate Ly6C expression by resident macrophages we collected these cells from the peritoneum, spleen, and bone marrow, treated them with IFN-β and determined their surface marker expression. We found that the rejuvenated monocytes/macrophage phenotype was prominent exclusively in peritoneal resolution phase macrophages (Fig. 3a-b). Moreover, IFN-β promoted the frequency of the Ly6C+F4/80+ phenotype and reduced Ly6C-F4/80+ macrophages in peritoneal resolution phase macrophages exclusively, while the frequency of Ly6C-F4/80+ cells from resident peritoneal macrophages was slightly reduced, likely due to cell death in culture (Fig. 3c).
a Representative dot plots of Ly6C and F4/80 expression during ex vivo maturation of peritoneal zymosan A-induced inflammatory macrophages (48 h), or peritoneal, bone marrow, or splenic resident macrophages treated with IFN-β or vehicle for 24 h. Percentages of each quadrant are shown. b Monocyte and macrophage populations at isolation; peritoneal zymosan A-induced inflammatory macrophages (48 h), or peritoneal, bone marrow, or splenic resident macrophages, as indicated. c Monocyte and macrophage populations at 24 h post treatment; peritoneal zymosan A-induced inflammatory macrophages (48 h), or peritoneal, bone marrow, or splenic resident macrophages, as indicated. Results (b, c) are average± SE from n = 3. p < 0.05 (*), 0.01 (**), .001(***), or 0.0001 (****) by one-way ANOVA and Tukey’s HSD. Error bars represent the standard error of the mean (SEM) throughout the figure.
CCR2 and L-selectin expression increases during macrophage maturation and rejuvenation
CCR2 and L-selectin are additional key surface markers of monocytes which we found to be increased in non-phagocytic resolution phase macrophages35. Therefore, we determined the expression of these markers by different resolution phase monocyte and macrophage subsets. Intriguingly, upon monocyte infiltration into the inflamed peritoneum their CCR2 expression drastically diminishes (Fig. 4a, c) to the levels displayed by non-myeloid cells.
a, b Representative histogram plots of CCR2 and CD62L (L-selectin) expression during ex vivo maturation of the different myeloid subsets. c CCR2 expression by different myeloid subsets at 48 h of ex vivo differentiation. d Time course of CCR2 expression by rejuvenated monocytes/macrophages following exposure to different IFN-β concentrations. e Time course of CCR2 expression by rejuvenated monocytes/macrophages following exposure to different TGF-β concentrations. f CD62L expression by different myeloid subsets at 48 h of ex vivo differentiation. g Time course of CD62L expression by rejuvenated monocytes/macrophages following exposure to different IFN-β concentrations. h Time course of CD62L expression by rejuvenated monocytes/macrophages following exposure to different TGF-β concentrations. Results (c-h) are average±SE from n = 6-7 for T = 0 and for T = 48 h and n = 3 for T = 24 h and for 72 h. p < 0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****) by one-way ANOVA and Tukey’s HSD. i Representative density plots of CD93 versus F4/80 on peritoneal cells 72 h after i.p. zymosan A injection. Mice were treated with IFN-β (25 ng/ml) or vehicle during the final 24 h. j Percentage of CD93+ cells within monocyte and macrophage sub-populations. k MFI of CD93 in F4/80+ cells. l Percentages of F4/80+CD93+ sub-populations. Results (j-l) are average±SE from n = 5-6. p < 0.05 (∗), or 0.01 (∗∗) by one-way ANOVA and Tukey’s HSD. Error bars represent the standard error of the mean (SEM) throughout the figure.
Subsequent differentiation into young (Ly6C+F4/80lo and Ly6C-F4/80lo), mature (Ly6C-F4/80hi) and rejuvenated (Ly6C+F4/80hi) monocytes/macrophages coincided with an upregulation of CCR2 expression, with the last two subsets being statistically different (Fig. 4c). Notably, culturing of resolution phase myeloid cells resulted in reduced CCR2 expression in young (Ly6C+F4/80lo) and rejuvenated monocytes/macrophages, but not in the other subsets. L-selectin (CD62L) was similarly expressed on the various resolution phase myeloid subsets albeit with increased expression on mature macrophages compared to young macrophages (both subtypes; Fig. 4b, f). Notably, culturing for 48 h increased L-selectin expression on mature macrophages and did not change significantly on rejuvenated ones (Fig. 4f).
We next explored the impact of resolution phase cytokines (e.g. IFN-β and TGF-β) on CCR2 and L-selectin expression by resolution phase myeloid cells. Notably, CCR2 expression on rejuvenated monocytes/macrophages initially declined with IFN-β treatment (as with vehicle), but later (48–72 h) recovered in a concentration-dependent manner essentially doubling untreated cells (Supplementary Fig. 4a and Fig. 4d). On the other hand, IFN-β reduced L-selectin expression on rejuvenated monocytes/macrophages, but at 72 h reached similar levels to untreated cells (Supplementary Fig. 4b and Fig. 4g). TGF-β did not affect CCR2 expression by rejuvenated monocytes/macrophages and reduced L-selectin expression at 2 ng/ml (Supplementary Fig. 4a-b and Fig. 4e-h). Altogether our results indicate that CCR2 and L-selectin are additional markers of rejuvenated monocytes/macrophages with dynamic regulation by pro-resolving cytokines. Thus, macrophages rejuvenation seems to be a comprehensive differentiation program that differentiated these cells from mature resolution phase macrophages.
To further substantiate the distinct identity of rejuvenated monocytes/macrophages, we examined the expression of CD93, a monocyte-associated surface marker minimally expressed in mature macrophages on resolution phase macrophages and its regulation by IFN-β. Our results show monocyte-to-macrophage differentiation during resolution results in a gradual increase in CD93 expression that was continued upon conversion to the rejuvenated F4/80hiLy6Cpos subset (Fig. 4i). Of interest, IFN-β reduced CD93 expression in all F4/80+ peritoneal macrophages (Fig. 4j-k), but this reached statistical significance only in F4/80medLy6Chi monocytes. A significant reduction in CD93 expression was observed in the total macrophage MFI, due to reduced conversion to the F4/80medCD93med phenotype (Fig. 4k–l). These findings suggest that physiologically IFN-β promotes phenotypic reprogramming toward a CD93+ rejuvenated state, but overdosing with recombinant IFN-β leads to a reduction in CD93 expression, while increasing Ly6C’s. In addition, these results highlight the importance of CD93 in distinguishing monocytes and macrophage subsets beyond the use of F4/80 alone.
ARTS inhibits the differentiation of rejuvenated monocytes/macrophages
Apoptosis-Related protein in the TGF-β Signaling pathway (ARTS) plays a key role in regulating neutrophil apoptosis and consequently macrophage function during the resolution of peritonitis20. However, apoptotic pathways also seem to regulate macrophage phenotypes during inflammation36. Therefore, we sought to determine whether ARTS and apoptotic pathways play a direct role in macrophage rejuvenation. To investigate the impact of ARTS on macrophage differentiation, resolution phase myeloid cells from WT and Sept4/ARTS-deficient mice were treated with vehicle, IFN-β, Q-VD (Pan-caspase inhibitor), or anti-IFN-β antibodies for 48 h (Fig. 5a). Sept4/ARTS-deficient and Q-VD-treated cells showed increased numbers of monocytes and young macrophages, implying the importance of ARTS in inducing apoptosis in these cells (Fig. 5a, d-e). However, Sept4/ARTS-deficient mice displayed reduced numbers of mature macrophages with lower F4/80 expression, as did Q-VD treatment and caspase inhibition in Sept4/ARTS-deficient mice (Fig. 5a, c).
a Representative density plots of myeloid subsets generated during ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ (ARTS+/+) or Sept4/ARTS-/- (ARTS-/-) mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies (as indicated). b Percentage of rejuvenated monocytes/macrophages (F4/80hiLy6C+) following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. c Percentage of mature macrophages (F4/80hiLy6C-) following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. d Percentage of monocytes (F4/80-Ly6Clo) following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. e Percentage of young macrophages (F4/80loLy6Chi) following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. f Ly6C expression on rejuvenated monocytes/macrophages following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. g F4/80 expression on rejuvenated monocytes/macrophages following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. h CCR2 expression on rejuvenated monocytes/macrophages following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. i CD62L expression on rejuvenated monocytes/macrophages following ex vivo maturation of peritoneal exudate cells from Sept4/ARTS+/+ or Sept4/ARTS-/- mice and treated with vehicle, IFN-β, Q-VD, or anti-IFN-β antibodies. Significant differences between experimental groups were determined by one-way ANOVA, and simple main effects analysis was performed using the Tukey HSD. P values are considered statistically significant when p < 0.05 (∗), 0.01 (∗∗), or 0.001 (∗∗∗). Error bars represent the standard error of the mean (SEM) throughout the figure.
We have shown that IFN-β induces rejuvenation of macrophages (Fig. 3). However, our results have indicated that the pro-apoptotic activity of Sept4/ARTS inhibits the generation of the rejuvenated monocyte/macrophage subset (Fig. 5a-b). These results suggest that the rejuvenation of macrophages observed in Sept4/ARTS-deficient mice is mediated by IFN-β. Therefore, we examined whether neutralizing IFN-β using appropriate antibodies will compromise rejuvenation of macrophages in Sept4/ARTS-deficient or Q-VD treated macrophages. Our results show that blocking IFN-β reduces rejuvenation of macrophages in Sept4/ARTS-deficient or Q-VD-treated macrophages (Fig. 5a-b). Moreover, we found that supplementation of Sept4/ARTS-deficient macrophages with IFN-β further increased the number of rejuvenated monocytes/macrophages (Fig. 5a-b). We therefore conclude that inhibition of apoptosis either by loss of ARTS or by treatment with Q-VD results in increased numbers of monocytes and young macrophages, as well as enhanced rejuvenation of macrophages through IFN-β production. Sept4/ARTS-deficient mice exhibited reduction of mature macrophages frequency (Fig. 5c). Similarly, treatment with Q-VD resulted in reduction in the frequency of mature macrophages. Inhibition of caspases in Sept4/ARTS-deficient mice showed further reduction in the frequency of mature macrophages, suggesting ARTS deficiency does not completely abrogate apoptosis signaling. Moreover, increased numbers of monocytes and young macrophages were found in the Sept4/ARTS-deficient cells and in the Q-VD treated cells (Fig. 5d-e). This finding suggests that ARTS is important for induction of apoptosis in these cells.
Next, we evaluated the levels of expression of Ly6C on rejuvenated monocytes/macrophage as well as of F4/80, CCR2, and L-selectin in ARTS-deficient mice. Our results (Fig. 5f-i) indicate that Sept4/ARTS deficiency promoted Ly6C expression on rejuvenated monocytes/macrophages, as did caspase inhibition, in a similar manner to IFN-β treatment. Anti-IFN-β antibodies showed a trend toward decreased expression (Fig. 5f). Thus, the pro-apoptotic function of ARTS seems to inhibit macrophage rejuvenation and Ly6C expression through the inhibition of IFN-β release. Moreover, treatment with Q-VD reduced F4/80 expression on WT rejuvenated monocyte/macrophage as well, thus suggesting that the reduction of this marker on Sept4/ARTS-deficient macrophages is due to ARTS’s pro-apoptotic properties (Fig. 5g). Overall, we found apoptosis inhibition shows significant similarity to the response induced by IFN-β. Notably, IFN-β further decreased F4/80 expression in Sept4/ARTS-deficient rejuvenated monocytes/macrophages. However, anti-IFN-β antibodies had no effect on F4/80 expression on these cells, although it reversed the IFN-β-induced reduction in its expression.
CCR2 expression in rejuvenated Sept4/ARTS-deficient monocytes/macrophages decreased compared to their WT counterparts (Fig. 5h). Surprisingly, CCR2 expression induced by either IFN-β or Q-VD was also decreased in Sept4/ARTS-deficient rejuvenated monocytes/macrophages (Fig. 5h) suggesting ARTS is also regulating IFN-β signaling in addition to its production. L-selectin expression on rejuvenated monocytes/macrophages was not affected by ARTS deficiency, nor did the reduction in L-selectin expression following IFN-β exposure (Fig. 5i), thus suggesting differential regulation of CCR2 and L-selectin by ARTS. Altogether, our results suggest that apoptosis inhibition by ARTS deficiency delays CCR2, but not L-selectin expression, while apoptosis inhibition by Q-VD delays L-selectin expression, but promotes CCR2 expression by resolution phase macrophages. Thus, we indicate important and not entirely overlapping roles for ARTS and the caspase apoptotic pathway in regulating various aspects of macrophage rejuvenation.
Rejuvenated monocytes/macrophages display improved efferocytic capacity through CD36
Efferocytosis is considered imperative for macrophage phenotype conversion from pro-inflammatory, IL-1β/TNF-α-producing toward anti-inflammatory, IL-10-producing cells. Efferocytic activity is increased following exposure to pro-resolving cytokines, like IFN-β32. CD36 is considered pivotal for macrophage and dendritic cell entrapment of apoptotic cells, and its expression is reduced on satiated/non-phagocytic macrophages32,37,38. To determine the efferocytic capacity of rejuvenated monocytes/macrophages we performed phagocytosis assays employing apoptotic cells as targets across various resolution-phase macrophage phenotypes. Our findings in Fig. 6a-e show peritoneal macrophages (48 h PPI) treated either with vehicle or IFN-β show a significant difference in their uptake of labeled apoptotic cells. We observed that Ly6C+ macrophages treated with vehicle exhibited notably lower average efferocytosis levels compared to their Ly6C- counterparts (Fig. 6c). However, following IFN-β treatment, Ly6C+ macrophages revealed higher efferocytosis than the Ly6C- counterparts both in the average engulfment per macrophage and the overall efferocytic index of the culture (Fig. 6c, e). These results suggest the generation of Ly6C+ macrophages is key to the pro-efferocytic activity of IFN-β.
a Representative images of vehicle or IFN-β-treated peritoneal macrophages (48 h PPI) incubated with CypHer5E (red)- labeled apoptotic Jurkat cells (1:5 ratio) and stained with Hoechst (blue) and anti-Ly6C (green). The cells were analyzed by confocal microscopy (X60). b The percentage of Ly6C+ macrophages following incubation ex vivo with apoptotic Jurkat cells. c Violin plots of the average number of apoptotic cells engulfed per macrophage of the Ly6C+ or Ly6C- phenotypes. n = 57 macrophages for vehicle, Ly6C+, n = 627 for vehicle, Ly6C-, n = 355 for IFN-β Ly6C+, and n = 444 cells for IFN-β Ly6C-. d The percentage of non-phagocytic macrophages of the Ly6C+ or Ly6C- phenotypes. n = 15-16. e Efferocytic index of macrophages of the Ly6C+ or Ly6C- phenotypes. f Flow cytometry of the percentage of CD36+ macrophages (upper panel) or CD36 expression (lower panels) by different myeloid cell subsets (48 h PPI). n = 4 mice. g Percentage of F4/80+CD36+ macrophages from peritoneal exudates of Ifnb+/+ and Ifnb-/- mice. n = 19-29 mice. h CD36 expression (MFI) on the surface of F4/80+CD36+ macrophages from peritoneal exudates of Ifnb+/+ and Ifnb-/- mice. n = 19-29 mice. i CD36 expression by F4/80+Ly6C+, F4/80-Ly6C+, or F4/80+Ly6C- myeloid cells from peritoneal exudates of Ifnb+/+ and Ifnb-/- mice. n = 19-29 mice. j Percentage of F4/80+CD36+ macrophages from peritoneal exudates following treatment ex vivo with vehicle or IFN-β (20 ng/ml, 24 h). n = 4 mice. k The percentage of efferocytic macrophages following vehicle or IFN-β treatment (48 h) of peritoneal exudate macrophages, and treatment with the CD36 inhibitor Sulfosuccinimidyl oleate sodium (SSO). N = 5. Statistical significance of differences between indicated samples was determined by two-way ANOVA (for multiple groups) or one-way ANOVA (for comparison between two groups). P values are considered statistically significant when p < 0.05 (∗), 0.01 (∗∗), 0.001 (∗∗∗), 0.0001 (∗∗∗∗). Error bars represent the standard error of the mean (SEM) throughout the figure.
To determine the mechanism by which Ly6C+ macrophages exercise hyper-efferocytosis we analyzed the percentage of CD36+ cells among each of the resolution phase macrophage subtypes. We found that rejuvenated monocytes/macrophages had the highest expression of CD36 (Fig. 6f, upper and lower panels). Moreover, our results (Fig. 6g-h) show a significant reduction (almost 50%) in the percentage of CD36+ macrophages and CD36 mean expression in Ifnb-/- mice (compared to their WT counterparts). Within the macrophages, both mature F4/80+Ly6C- macrophages and rejuvenated F4/80+Ly6C+ macrophages displayed reduced CD36 expression in IFN-β-deficient mice (Fig. 6i). Furthermore, we found that ex vivo treatment with IFN-β of peritoneal exudates induced an increase of CD36 expression in macrophages (Fig. 6j). Our findings provided evidence that efferocytotic activity of macrophages requires CD36 activity. This was revealed upon treatment with the CD36 inhibitor sulfosuccinimidyl oleate sodium (SSO). As SSO abrogated efferocytosis in both vehicle and IFN-β treated macrophages (Fig. 6k). The decline in efferocytosis was less prominent in vehicle than in IFN-β treated macrophages suggesting that IFN-β treated cells became more sensitive to CD36 inhibition.
The expression of CD36 was also augmented by IFN-β treatment in vivo which further suggests IFN-β facilitates CD36 surface expression by rejuvenated macrophages (Fig. 6j). These results suggest IFN-β is upregulating CD36 expression by resolution phase macrophages during the resolution of inflammation. Finally, using escalating concentrations of SSO39, we found a dose-dependent inhibition of efferocytosis in both vehicle and IFN-β-treated macrophages (Fig. 6k). Thus, IFN-β seems to convert macrophages to an Ly6C+ hyper-efferocytic phenotype through the upregulation of CD36.
IFN-β in vivo promotes phagocytosis in rejuvenated monocytes/macrophages, but not CD36 expression
Our results so far have indicated IFN-β is essential for CD36 expression on resolution phase macrophages and provided an ex vivo evidence for enhanced efferocytosis via CD36 that is promoted by IFN-β. Next, we examined whether IFN-β increases coupled macrophage CD36 expression and efferocytotic function during the resolution of inflammation. To this end, we performed flow cytometry of resolution phase myeloid subsets after in vivo exposure to recombinant IFN-β followed by engulfment of PKH2-red as illustrated in Fig. 7a and analysis of the cells based on their PKH2 engulfment and F4/80 expression as detailed in Fig. 7b. The results of this analysis revealed significant differences in phagocytic capacity following IFN-β treatment that culminated in increased frequency of F4/80+PKH2high macrophages (Fig. 7c). As expected this was associated with an increase in Ly6C expression in macrophages (F4/80+) but not in monocytes (F4/80-; Fig. 7d). An analysis based on F4/80 and Ly6C expression (as in Fig. 7e) showed augmented in vivo engulfment capacity for F4/80lowLy6Clow monocytes, F4/80lowLy6Chigh young and F4/80highLy6Clow rejuvenated monocytes/macrophages (Fig. 7f-h) following IFN-β treatment. This was reflected by higher frequency of PKH2hi macrophages. On the other hand, mature (F4/80hiLy6C-) and satiated (F4/80medLy6C-) macrophages did not display increased phagocytic capacity (Fig. 7i-j). These findings indicate that the in vivo pro-efferocytotic impact of IFN-β is confined to young and rejuvenated resolution phase monocytes/macrophages.
a An illustration of the experimental design used to determine myeloid cell phagocytosis following IFN-β treatment in vivo. b Representative density plots of resolution phase myeloid cells following the phagocytosis of PKH2-red. c The percentage of F4/80negPKHpos, or F4/80posPKHhigh/med/low myeloid cells in the peritoneum of IFN-β treated mice. Significant differences between vehicle and IFN-β-treated mice were determined by Student’s t-test. d Surface expression of Ly6C on F4/80neg and F4/80pos cells following IFN-β treatment in vivo. n = 8 mice. e Representative density plots of resolution phase myeloid cell subsets immunostained for F4/80 and Ly-6C. f Flow cytometry of the percentages of the PKHhigh, PKHmed, and PKHlow populations in F4/80medLy6Chigh monocytes following IFN-β treatment in vivo. n = 8 mice. g Flow cytometry of the percentages of the PKHhigh, PKHmed, and PKHlow populations in F4/80lowLy6Chigh young macrophages following IFN-β treatment in vivo. n = 8 mice. h Flow cytometry of the percentages of the PKHhigh, PKHmed, and PKHlow populations in F4/80highLy6Clow rejuvenated monocytes/macrophages following IFN-β treatment in vivo. n = 8 mice. i Flow cytometry of the percentages of the PKHhigh, PKHmed, and PKHlow populations in F4/80lowLy6Cneg macrophages following IFN-β treatment in vivo. n = 8 mice. j Flow cytometry of the percentages of the PKHhigh, PKHmed, and PKHlow populations in F4/80highLy6Cneg mature macrophages following IFN-β treatment in vivo. n = 8 mice. k Flow cytometry of CD36+ cells in the F4/80negPKHpos, or F4/80posPKHhigh/med/low myeloid cells following IFN-β treatment in vivo. n = 8 mice. l Flow cytometry of CD36 MFI in the F4/80negPKHpos, or F4/80posPKHhigh/med/low myeloid cells following IFN-β treatment in vivo. n = 8 mice. Statistical significance of differences between indicated samples was determined by two-way ANOVA (for multiple groups) or one-way ANOVA (for comparison between two groups). P values considered statistically significant when p < 0.05 (∗), 0.01 (∗∗), 0.001 (∗∗∗), or 0.0001 (∗∗∗∗). Error bars represent the standard error of the mean (SEM) throughout the figure. m Functional scheme of the monocyte-derived macrophage polarization states during inflammation and its resolution. Panel a and m were created using BioRender.com under an Academic Individual Plan license.
To determine whether the increased IFN-β-driven phagocytic capacity in vivo was associated with increased expression of CD36 we analyzed the frequency of CD36+ cells in the F4/80negPKHpos, or F4/80posPKHhigh/med/low myeloid subsets following the same experimental regime. Unexpectedly, our findings (Fig. 7k-l) did not show increased CD36 expression in any myeloid subset. In fact, CD36 expression was reduced in two subset, F4/80+PKHhi phagocytic macrophages, and F4/80+PKHlo satiated macrophages when treated with IFN-β. While this result is expected for satiated macrophages, it is not expected for phagocytic macrophages and might indicate IFN-β treatment is inducing the migration of CD36+ macrophages out of the peritoneum, and thereby reduces its expression on the phagocytic subset.
Discussion
The multifaceted roles of monocytes and macrophages in health and disease incites many studies to explore the subtypes of these cells and their dynamics. Our study identifies a novel macrophage subtype, as the product of a unique macrophage differentiation process. These macrophages display a rejuvenated phenotype reflected by high expression of Ly6C and low expression of F4/80. They are generated from mature peritoneal macrophages following exposure to the pro-resolving cytokines IFN-β and TGF-β. Inactivation of apoptotic pathways or the pro-apoptotic ARTS protein results in spontaneous enhancement of this phenotype. Ly6C+ macrophages are hyper-efferocytic ex vivo thorough the expression of CD36, but not in vivo, likely due to the departure of CD36+ macrophages from the peritoneal cavity. Consistently, this process of rejuvenation in response to the pro-resolving IFN-β also takes place in other resolution settings as shown in liver fibrosis.
Previous reports have demonstrated that pro-inflammatory Ly6C+ monocytes, the precursors of inflammatory macrophages, follow an alternative differentiation pathway by transitioning in the circulation to Ly6C- monocytes, which play pivotal roles in scavenging and maintaining the luminal aspect of the endothelium of small blood vessels8,40. The transition from Ly6C+ to Ly6C- monocytes is a mirror image of macrophage rejuvenation during the resolution of inflammation: as Ly6C+ differentiation leads to mature monocytes losing Ly6C expression, rejuvenation fosters a less differentiated macrophage phenotype that regains Ly6C expression. The maturation of Ly6C+ monocytes is distinctive in that the matured monocyte exhibits less specialization compared to its precursor. Typically, cell maturation entails the acquisition of additional capabilities. However, Ly6C- monocytes lose unique functions of their Ly6C+ precursors, such as tissue infiltration and pro-inflammatory activity8. Mildner et al. have reported that the maturation of Ly6C+ to Ly6C- monocytes is dependent on C/EBPβ, as this protein activates the monocytic survival factor Nr4a1. C/EBPβ is indispensable for the proper functioning of macrophages, and its deficiency results in inadequately differentiated macrophages41. Hence, appropriate expression of C/EBPβ ensures the maturation of Ly6C+ monocytes to generate both scavenger Ly6C- cells in the circulation and inflammatory macrophages with the proper capacity for differentiation in inflamed tissue. Notably, C/EBPβ expression was also down-regulated when the Ly6C+ to Ly6C- conversion took place in blood and bone marrow monocytes. However, its role was not evaluated. Our results show that the expression of C/EBPε, but not C/EBPβ, is co-aligning with other monocyte marker upregulation (i.e. Ly6C, CCR2, and L-selectin) in resolution phase macrophages, suggesting it might be a more prominent transcription factor in their rejuvenation and conversion to the Ly6C+ phenotype.
Macrophages display diverse phenotypes coping with different environmental cues and pathophysiological contexts9. In the context of tissue repair macrophages acquire a functional phenotype that is anti-fibrotic to orchestrate proper tissue architecture and limit fibrotic outcomes, as in the case of peritoneal inflammation resolution27,42. In zymosan A-induced peritonitis we previously found macrophages to differentiate in tandem into M2-like and pro-resolving (Mres) phenotypes. Mres macrophages (also termed satiated/CD11blow macrophages), express lower levels of F4/80 (as well as CD11b) compared to their CD11bhigh precursors. They are converted to satiated macrophages from mature phagocytic ones after the latter engulf threshold levels of apoptotic PMNs32,42. This process involves macrophage reprogramming, reducing pro-inflammatory cytokine secretion and boosting anti-inflammatory response. IFN-β, produced by satiated macrophages, in turn enhances macrophage efferocytosis and reprogramming, events that are crucial for inflammation resolution32.
Our current findings reveal, to the best of our knowledge, a novel macrophage subset emerging exclusively in the peritoneum during the resolution of inflammation following IFN-β exposure, expressing an F4/80-to-Ly6C switch in expression. These results imply IFN-β fosters the conversion of mature macrophage to a “younger”, rejuvenated phenotype. We observed enhanced Ly6C expression on mature macrophages and monocytes that mature to macrophages (in the presence of mature macrophages), particularly with IFN-β and TGF-β stimulation, suggesting this expression of Ly6C is likely due to IFN-β and TGF-β secretion from macrophages, which is inhibited by the monocytic subset. Supporting this transition, we further showed that CD93—commonly associated with monocytes—is upregulated in the Ly6C+F4/80+ rejuvenated population, highlighting its potential to distinguish this subset from their precursors, differentiated macrophages. Moreover, we identify high surface levels of the monocyte markers CCR2 and L-selectin on Ly6C+ resolution phase macrophages, underscoring their designation as “rejuvenated macrophages” due to their unique dedifferentiation process. This process seems to be limited by intracellular signaling that involves apoptotic caspases and the pro-apoptotic protein ARTS, as their pharmacological inhibition or genetic deletion exacerbates spontaneous and IFN-β-driven rejuvenation.
Given the pivotal roles of macrophages in immunity and pathophysiology, understanding their differentiation and dedifferentiation processes is essential for complete understanding of their beneficial or detrimental actions. Our findings indicate that during inflammation resolution, mature macrophages can acquire one of three fates: (a) remain in a mature M2-like state with high efferocytosis, (b) transition to CD11blow/Mres after satiation and reprogramming, and migrate to remote lymphoid organs or (c) undergo partial dedifferentiation, leading to the emergence of “rejuvenated” monocytes/macrophages expressing monocyte markers alongside reduced F4/80 and executing superior efferocytic properties to their mature precursors. Our proposed sequence of events suggests that during early resolution, monocyte-derived macrophages differentiate into highly phagocytic CD11bhigh/M2-like cells. Subsequent IFN-β secretion from satiated macrophages prompts CD11blow transition and reduced efferocytosis at the tissue level. A compensatory mechanism consequently generates macrophages with high clearance capacity that adopt the rejuvenated phenotype upon exposure to satiation-generated IFN-β, ensuring continuous efferocytic clearance side-by-side with satiation of a significant portion of the peritoneal macrophage population.
Among the important mediators that balance tissue repair and the resolution of inflammation, IFN-β and TGF-β seem to greatly affect that equilibrium. IFN-β is known to induce opposite effects at different contexts, and thus encourage different cell fates43,44. Regarding macrophage differentiation, we previously reported it promotes macrophage reprogramming away from pro-inflammatory cytokine and towards IL-10 production32. Our findings underscore the pivotal roles of both IFN-β and TGF-β in driving macrophage rejuvenation during inflammation resolution. While IFN-β induces robust rejuvenation and upregulates Ly6C expression in both monocytes and rejuvenated monocytes/macrophages, TGF-β increases the proportion of rejuvenated monocytes/macrophages without intensifying Ly6C expression. Additionally, IFN-β enhances CCR2 expression on rejuvenated monocytes/macrophages, whereas TGF-β restricts both CCR2 and L-selectin expression. Interestingly, IFN-β decreases L-selectin levels, contrary to its ability to promote other monocyte markers, like Ly6C and CCR2. Our data also highlight a reduction in F4/80 expression by rejuvenated monocytes/macrophages, indicative of a comprehensive phenotype conversion, with both IFN-β and TGF-β treatments. These findings suggest that while both cytokines promote rejuvenation, they exert intricate differences in the regulation of specific markers, emphasizing their distinct roles in modulating macrophage phenotypes during inflammation resolution.
While all three monocyte markers we investigated (Ly6C, CCR2, and L-selectin) are highly expressed in rejuvenated monocytes/macrophages, our findings suggest that Ly6C expression marks the final stage of rejuvenation. Initially, peritoneum-infiltrating monocytes express all three markers, but their expression decreases upon differentiation into young (M1-like) macrophages. However, CCR2 and L-selectin are re-expressed on mature (M2-like) macrophages and rejuvenated monocytes/macrophages, while Ly6C re-expression is exclusive to mature macrophages that undergo rejuvenation. Nonetheless, the transcriptome of resolution phase macrophages of either the phagocytic or satiated phenotype share high similarity with monocytes rather than resident peritoneal macrophages, hence reflecting their monocytic origin27. Importantly, Mres that also differentiate from mature macrophages do not express these markers prominently. This strengthens a functional scheme under which rejuvenated monocytes/macrophages originate from mature macrophages rather than inflammatory monocytes, potentially restoring the Ly6C expression of earlier developmental stages. Notably, previous publications indicate a developmental limit in mammalian epithelial cell dedifferentiation, implying that mature macrophages that have become Mres may not change course to rejuvenated monocytes/macrophages (See Fig. 7m for functional scheme).
Apoptosis of polymorphonuclear leukocytes (PMNs) and its regulation by the pro-apoptotic protein ARTS are critical in macrophage differentiation during inflammation resolution20. Our previous study has established the necessity of ARTS in regulating caspase activation, with its deficiency hindering effective macrophage-mediated resolution of inflammation20. This regulatory mechanism was initially perceived as indirect, primarily through promoting PMN apoptosis. Our study reveals that ARTS diminishes the frequency of monocytes and young macrophages during the resolution phase. Worth mentioning, various studies with Sept4/ARTS-deficient mice and suppressed apoptosis showed augmented stem cell populations, which impacted various tissue stem cells45,46,47. Likely, in our setting, resolution phase macrophages from Sept4/ARTS-deficient mice underwent less apoptosis resulting in increased numbers of monocytes and young macrophages. In contrast, our results also revealed decreased mature macrophages compared to wild-type mice. This might be explained by the reported role of caspases in inducing macrophage differentiation alongside their genuine pro-apoptotic roles48,49. This complex role potentially explains ARTS’ seemingly paradoxical function in promoting mature macrophage differentiation. Moreover, apoptotic cells displaying phosphatidylserine and other signals are recognized by phagocytes, impacting macrophage maturation50,51,52. During peritonitis, macrophage apoptosis occurs predominantly via the mitochondrial pathway following the initial wave targeting granulocytes53,54. Macrophage apoptosis is pivotal in regulating their M1/M2 polarization. In cancer, tumor-associated macrophages typically exhibit an M2-like phenotype, while antagonizing inhibitors of apoptosis promotes M1 macrophages55,56. Our findings suggest that apoptosis inhibition promotes rejuvenation and reprogramming, akin to IFN-β function in macrophage differentiation. Furthermore, apoptosis inhibition enhances CCR2 expression on rejuvenated monocytes/macrophages. Caspases, acting as the “executers” of apoptosis, also regulate the immune response. This is done through caspases inducing subsequent release of mtDNA and stimulator of interferon genes (STING)-mediated cytosolic DNA sensing pathway. Caspase inhibition results in substantial release of IFN-β that profoundly activates immune cells, mainly macrophages53,54. This regulatory function maintains the steady state mitochondrial apoptosis “silent”, possibly explaining the effect of IFN-β neutralizing antibodies observed in our study. Additionally, IFN-β and TGF-β promote macrophage viability, with ARTS influencing TGF-β-induced apoptosis57. Our data suggest an extended macrophage lifespan may facilitate prolonged differentiation into rejuvenated monocytes/macrophages, supporting the concept of “rejuvenation” of immune responses. Moreover, rejuvenated monocytes/macrophages exhibit significant CCR2 expression, further augmented by IFN-β. CCR2, in turn, serves as a receptor for several chemoattractants, including CCL2. These findings collectively underscore the tight regulation of macrophage dynamics and rejuvenation by comprehensive regulators of cellular life span and phenotype.
CD36, a commonly-expressed receptor on different macrophage subtypes, orchestrates the engulfment of apoptotic cells during inflammation resolution, a process influenced by the efferocytosis-inducing cytokine IFN-β32. Functioning as a key effector in macrophage-apoptotic cell interactions, CD36 recognizes specific ligands on apoptotic cell surfaces, thereby initiating signaling cascades for inflammatory responses58. Moreover, CD36 acts as an adhesion receptor for thrombospondin (TSP), facilitating macrophage scavenging of apoptotic cells58. Notably, surface expression of CD36 plays a pivotal role in driving monocyte/macrophage differentiation, particularly in response to cytokines and growth factors59,60. The involvement of IFN-β in promoting apoptosis of inflammatory neutrophils and augmenting efferocytosis by resolution phase macrophages32, while CD36 expression is down-regulated in satiated macrophages32 suggest its expression on IFN-β-induced, hyper-efferocytic Ly6C+ macrophages is also controlled. Our results reveal a substantial reduction in CD36 expression in resolution phase macrophages from mice deficient in IFN-β compared to their wild-type counterparts, whereas treatment with IFN-β supplemented this expression. Moreover, mature and rejuvenated monocytes/macrophages display significantly higher expression of CD36 than monocytes, thus indicating the importance of the expression of CD36 in the function of these macrophages as efferocytic cells. These findings also confirm that the expression of CD36 is progressively increased during monocyte/macrophage differentiation.
Ex-vivo efferocytosis assays unveiled heightened apoptotic cell engulfment by resolution phase macrophages following IFN-β treatment, with complete inhibition observed upon CD36 blockade. These findings underscore CD36’s indispensable role in apoptotic cell uptake and its direct involvement in efferocytosis. Furthermore, the decreased CD36 expression in satiated macrophages emphasizes its significance in regulating efferocytosis32. Altogether, our findings not only support the anti-inflammatory nature of macrophage-apoptotic cell interactions but also enhance our understanding of the pivotal role of CD36 in inflammation resolution. Our findings also provided a better understanding of the macrophage population that increases its efferocytic capacity following IFN-β exposure in vivo. These findings underscore that IFN-β increases efferocytosis in the F4/80+ subset of resolution phase macrophages. The only myeloid subsets that presented higher phagocytic capacity in response to in vivo treatment with IFN-β were the rejuvenated and young macrophages, whereas monocytes, mature or satiated macrophages did not show such improvement. Surprisingly, CD36 expression did not increase on the surface of these macrophages suggesting that the CD36hi macrophages might be departing the peritoneal cavity or show strengthened adherence to the peritoneal lining that prevents their recovery.
Noteworthily, to ensure experimental robustness, this study was conducted using male mice. Future research will be important to determine whether the IFN-β–mediated rejuvenation of macrophages is similarly conserved in females. Moreover, while our study provides significant insights into the role of IFN-β and ARTS deficiency in modulating macrophage phenotypes during the resolution of sterile peritonitis in mice and provides evidence of rejuvenation in mice undergoing resolution of CCl4-induced liver fibrosis, further research is needed to determine the extent to which these findings translate to human disease. Interestingly, similar populations of macrophages have been identified in human disease settings, suggesting that our findings may have broader translational relevance. Specifically, in the LiverMap2.0 dataset, based on the foundational single-cell and spatial liver transcriptomics by MacParland and Andrews61,62 (Transcriptomic datasets used in this study are publicly available from GEO under accession numbers GSE240429, GSE243977, GSE115469, GSE185477, GSE136103, GSE245620,GSE247128, and GSE115469), we identified human monocyte and macrophage subclusters that co-express Sell, Ccr2, and Cd36—the same markers enriched in our murine rejuvenated monocytes/macrophage subset during resolution of sterile peritonitis. This shared phenotype anchors their functional similarity. Importantly, these human clusters, particularly the anticipated equivalent “MDM_inflammatory subcluster” (a monocyte-derived macrophage population) are disproportionately represented in donors with liver fibrosis and NASH compared to healthy controls, indicating their expansion in disease states. Furthermore, spatial transcriptomic mapping localizes these cells to periportal regions - vascular and ductular interfaces known to initiate and sustain inflammatory and fibrogenic responses. Given this strategic localization, disease-associated enrichment, and expression of migration- and lipid-handling genes, we infer that these monocyte-derived subsets are likely to play active roles in hepatic inflammation and tissue remodeling. Their phenotypic and contextual parallels with murine resolution-phase macrophages support the translational relevance of our findings. We acknowledge that definitive confirmation of the functional equivalence between murine rejuvenated monocytes/macrophages and human monocyte-derived populations will require future studies, such as cross-species transcriptomic comparisons or ex vivo functional assays in human samples. In summary, our collective results unveil a novel phenomenon in the biology of resolution phase macrophages, namely an IFN-β-governed rejuvenation process. This process likely allows continuation of efficient efferocytosis side-by-side with macrophage satiation and resolution of the inflamed tissue.
Methods
Experimental design
Our objective was to investigate the role of IFN-β in modulating macrophage molecular phenotype, and consequently phagocytosis and CD36 expression during inflammation resolution, with a particular focus on the impact of apoptosis modulation. We employed both in vivo and ex vivo methodologies, treating wild-type and Sept4/ARTS-deficient mice with recombinant IFN-β or TGF-β to assess the effects of pro-resolving cytokines on peritoneal myeloid cell populations. Flow cytometry was utilized to evaluate phagocytic capacity via the engulfment of CypHer-red labeled apoptotic cells, along with the characterization of macrophage subsets based on surface markers, including F4/80, Ly6C, CCR2, and L-selectin. In addition, we aimed to discern the differentiation path of resolution phase Ly6C+ macrophages ex vivo, with initial sorting of Ly6C+ monocytes from the peritoneal cellular content, followed by CFSE labeling and culture experiments to explore their potential for differentiation. This comprehensive design enabled us to elucidate IFN-βs impact on macrophage function and the dedifferentiation process in a robust manner.
Animals
Male C57BL/6 WT mice (7-8 weeks old) were purchased from Harlan Biotech Israel. ARTS/Sept4-/- mice were generously provided by Prof. Hermann Steller, Rockefeller University, NYC, USA. Mice were housed under a 12 h:12 h light-dark cycle and specific pathogen-free conditions, up to 5 mice per cage. Mice were fed standard pellet chow and reverse osmosis water ad libitum. Experiments were approved by the Committee of Ethics, University of Haifa (authorization no. 246/14), and all mice were maintained under specific pathogen-free conditions in the animal facility at the University of Haifa. We have complied with all relevant ethical regulations for animal use.
Murine peritonitis model
Male C57BL/6 WT, Ifnb-/- or ARTS/Sept4-/- mice were injected intra-peritoneally (I.P.) with zymosan A (1 mg/ml, 1 mg/mouse) in sterile PBS. 66 h post zymosan A injection, mice were euthanized using gradual-fill carbon dioxide (CO2) inhalation in accordance with the 2020 AVMA Guidelines for the Euthanasia of Animals. Anesthesia was not used prior to euthanasia, as the procedures did not involve pain or distress, and peritoneal exudates were collected by lavaging their peritoneal cavity with 5 ml of sterile PBS. In some experiments mice were injected with either recombinant IFN-β (25 ng/mouse; BioLegend), or saline 48 h post zymosan injection and were sacrificed 24 h later. Macrophages were recovered from peritoneal exudates stained with PE-conjugated rat anti-mouse F4/80 and isolated with PE selection cocktail, using EasySep kit according to the manufacturer’s instructions (StemCell Technologies).
Murine liver fibrosis
Liver fibrosis was induced in male C57BL/6 WT and Ifnb-/- mice by intraperitoneal injections of 15% CCl4 (v/v in corn oil) at 6.6 ml/kg body weight, twice weekly for 6 weeks. In treatment group, the last CCl4 injection was co-administered with either recombinant IFN-β (25 ng/mouse; BioLegend), or saline. 48 h after the last injection livers were harvested and dissociated with a dissociation net. Cells were recovered and stained for flow cytometric analysis.
Flow cytometry
To determine macrophage subtypes and properties and to evaluate differences between those subtypes under various conditions, exudate cells were immuno-stained (20 min) with the following antibodies: PE-conjugated anti-mouse F4/80 (1:100, BM8; 123143, Biolegend), pacific blue anti-mouse Ly6C (1:200, HK1.4; 128002, Biolegend), Anti-mouse CD62L (1:100, MEL-14;104402, BioLegend), Alexa Fluor 647-conjugated anti-mouse CCR2 (1:100, 150604, BioLegend), and Alexa Fluor 647 anti-mouse CD36 (1:200, HM36; 102610, BioLegend) or APC anti-mouse CD93 (1:100, AA4.1; 136510, Biolegend). Cell populations were evaluated by flow cytometry using FACSCanto II (BD) and analyzed by the FlowJo software (BD Biosciences). Gating strategy: Single cells were gated based on forward and side scatter (FSC/SSC) to exclude debris, eosinophils and doublets. Macrophage and monocyte subsets, such as rejuvenated monocytes/macrophages, mature macrophages, and monocytes, were further identified based on combinations of F4/80 and Ly6C expression, and in some experiments, CCR2, CD62L, CD36, or CD93 markers were used to assess activation or maturation status (Sup Fig. 1).
Detection of apoptosis
Apoptosis of macrophages was determined by staining with Annexin-V-FITC and Propidium Iodide (PI) using MEBCYTO Apoptosis Kit (MBL Laboratories). Apoptosis was evaluated by flow cytometry using FACSCanto II (BD, Biosciences) and analyzed by FlowJo software (BD Biosciences).
Macrophage maturation ex vivo
F4/80+ macrophages were isolated from the peritoneum, spleen or bone marrow of WT mice undergoing peritonitis for 66 h, or unchallenged, using magnetic beads. Then the cells were treated with IFN-β (0.04, 0.2, 1, 5 or 25 ng/ml) or TGF-β (0.4, 2 or 10 ng/ml) for 24, 48 or 72 h in RPMI 1640, supplemented with 10% fetal bovine serum (FBS), 2 µM glutamine, 100 units/ml penicillin and 100 µg/mL streptomycin. In some experiments, WT or ARTS-deficient macrophages were treated with TGF-β (10 ng/ml), IFN-β (25 ng/ml), anti-IFN-β (1 μg/ml), the pan-caspase inhibitor Q-VD (10 μM), or combinations of these agents as indicated, for 48 h. Following each incubation period, the cells were immune-stained for the aforementioned markers and analyzed by flow cytometry as indicated above.
Leukocyte sorting
Peritoneal cells were recovered 66 h post zymosan A injection, and obtained cells were immunostained by PE-conjugated anti-F4/80 and PB-conjugated anti-Ly6C and sorted to Ly6C+ F4/80-and Ly6C-F4/80+/- populations. After sorting isolated monocytes were labeled with CFSE. Peritoneal monocytes or the rest of the exudate myeloid cells were incubated separately or at 1:4 ratio as detailed above. Flow cytometry analysis was performed on monocytes (CFSE+) or REST (CFSElo/-) as indicated. All sorting procedures were performed using BD FACS Aria III according to manufacturer instructions.
Engulfment assay ex vivo
Jurkat T cells were maintained in RPMI-1640 with 10% FBS and antibiotics at 37 °C, 5% CO2 incubator. To generate apoptotic targets, apoptosis was induced using staurosporine (1 µg/ml, 6 h; Sigma). Cells were washed twice with PBS, resuspended in serum free medium and stained with 10 µg/ml CypHer5E Mono NHS Ester. Then, IFN-β-treated macrophages in 8 well chamber slides were incubated in the presence or absence of apoptotic Jurkat cells (1:5 macrophage to apoptotic cell ratio). After 4 h, unbound cells were washed and macrophages were stained with Alexa Fluor 488-conjugated anti-Ly6C (1.5 µg/1 × 106 cells) for 1 hr. Then the cells were fixed with 4% paraformaldehyde +5% sucrose and stained with Hoechst (1:500; Molecular probes) and slides were mounted with SlowFade™ Gold Antifade Mounting (Molecular probes). The slides were imaged using Nikon A1-R confocal laser scanning microscope and engulfment of apoptotic cells was calculated using Nikon NIS-Elements microscope imaging software. In some experiments the CD36 inhibitor SSO was applied at 62.5-125 mM during the engulfment assay.
Phagocytosis assays in vivo
The phagocyte-specific dye PKH26-PCL red was injected I.P. to mice undergoing peritonitis 20 h after IFN-β or vehicle injection and 4 h later (66 h PPI), the peritoneal cells were recovered and immuno-stained as previously described. Flow cytometry was performed for the myeloid populations described above or based on PKH acquisition.
Statistics and reproducibility
Experiments were performed 2-4 times with 4 replicates of each data point as indicated in figure legends. Unpaired two tailed Student’s t test was used for engulfment ex vivo analysis. Results of all other experiments were analyzed by repeated measures ANOVA comparison test, and simple main effects analysis was performed using the Tukey HSD. Values of 0.05 or less were considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated and/or analyzed during the current study are available as supplementary data sheets.
References
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Kazankov, K. et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019).
Mills, C. D., Kincaid, K., Alt, J. M., Heilman, M. J. & Hill, A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164, 6166–6173 (2000).
Locati, M., Curtale, G. & Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev. Pathol. 15, 123–147 (2020).
Zhang, J., Zhou, X. & Hao, H. Macrophage phenotype-switching in cancer. Eur. J. Pharm. 931, 175229 (2022).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Zaid, A. & Ariel, A. Harnessing anti-inflammatory pathways and macrophage nano delivery to treat inflammatory and fibrotic disorders. Adv. Drug Deliv. Rev. 207, 115204 (2024).
Mildner, A. et al. Genomic Characterization of Murine Monocytes Reveals C/EBPβ Transcription Factor Dependence of Ly6C− Cells. Immunity 46, 849–862.e847 (2017).
Italiani, P. & Boraschi, D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front. Immunol. 5, 514 (2014).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Yang, J., Zhang, L., Yu, C., Yang, X.-F. & Wang, H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2, 1–9 (2014).
Michaud, J.-P., Bellavance, M.-A., Préfontaine, P. & Rivest, S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 5, 646–653 (2013).
Hirsch, S., Austyn, J. & Gordon, S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J. Exp. Med. 154, 713–725 (1981).
Bannenberg, G. L. et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 174, 4345–4355 (2005).
National Center for Biotechnology Information. GenBank accession number AF176379, http://www.ncbi.nlm.nih.gov/nuccore/AF176379 (2024).
Larisch, S. et al. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat. Cell Biol. 2, 915–921 (2000).
Edison, N. et al. The IAP-antagonist ARTS initiates caspase activation upstream of cytochrome C and SMAC/Diablo. Cell Death Differ. 19, 356–368 (2012).
Abbas, R. et al. ARTS and small-molecule ARTS mimetics upregulate p53 levels by promoting the degradation of XIAP. Apoptosis 29, 1145–1160 (2024).
Edison, N. et al. Degradation of Bcl-2 by XIAP and ARTS Promotes Apoptosis. Cell Rep. 21, 442–454 (2017).
Maimon, N. et al. The pro-apoptotic ARTS protein induces neutrophil apoptosis, efferocytosis, and macrophage reprogramming to promote resolution of inflammation. Apoptosis 25, 558–573 (2020).
Jones, H. R., Robb, C. T., Perretti, M. & Rossi, A. G. The role of neutrophils in inflammation resolution. Semin Immunol. 28, 137–145 (2016).
Schneider, K. & Arandjelovic, S. Apoptotic cell clearance components in inflammatory arthritis. Immunol. Rev. 319, 142–150 (2023).
Yurdagul, A. Jr., Doran, A. C., Cai, B., Fredman, G. & Tabas, I. A. Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med 4, 86 (2017).
Hosseini, Z. et al. Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. Arterioscler Thromb. Vasc. Biol. 41, 1062–1075 (2021).
Kourtzelis, I. et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat. Immunol. 20, 40–49 (2019).
Silberberg, E., Filep, J. G. & Ariel, A. Weathering the Storm: Harnessing the Resolution of Inflammation to Limit COVID-19 Pathogenesis. Front Immunol. 13, 863449 (2022).
Butenko, S. et al. Transcriptomic Analysis of Monocyte-Derived Non-Phagocytic Macrophages Favors a Role in Limiting Tissue Repair and Fibrosis. Front Immunol. 11, 405 (2020).
Yaseen, H. et al. Galectin-1 Facilitates Macrophage Reprogramming and Resolution of Inflammation Through IFN-β. Front Pharm. 11, 901 (2020).
Butenko, S. et al. ACKR2 limits skin fibrosis and hair loss through IFN-β. Faseb j. 35, e21917 (2021).
Kalkar, P. et al. IFN-β mediates the anti-osteoclastic effect of bisphosphonates and dexamethasone. Front Pharm. 13, 1002550 (2022).
Lee, P. Y. et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 175, 2023–2033 (2009).
Kumaran Satyanarayanan, S. et al. IFN-β is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat. Commun. 10, 3471 (2019).
Zigmond, E. et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 193, 344–353 (2014).
Li, Y.-h. et al. Occurrences and Functions of Ly6Chi and Ly6Clo Macrophages in Health and Disease. Front. Immunol. 13, https://doi.org/10.3389/fimmu.2022.901672 (2022).
Tsou, C.-L. et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Investig. 117, 902–909 (2007).
Zhang, H. et al. Pyroptotic macrophages promote proliferation and chemotherapy resistance of peripheral T-cell lymphoma via TLR4 signaling pathway. Cancer Sci, https://doi.org/10.1111/cas.16180 (2024).
Trahtemberg, U. & Mevorach, D. Apoptotic cells induced signaling for immune homeostasis in macrophages and dendritic cells. Front. Immunol. 8, 1356 (2017).
Fadok, V. A., Warner, M. L., Bratton, D. L. & Henson, P. M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J. Immunol. 161, 6250–6257 (1998).
Drahota, Z. et al. Succinimidyl oleate, established inhibitor of CD36/FAT translocase inhibits complex III of mitochondrial respiratory chain. Biochem. Biophys. Res. Commun. 391, 1348–1351 (2010).
Mildner, A., Marinkovic, G. & Jung, S. Murine monocytes: origins, subsets, fates, and functions. Microbiol. Spectr. 4, mchd-0033–mchd-2016 (2016).
Ruffell, D. et al. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl Acad. Sci. 106, 17475–17480 (2009).
Schif-Zuck, S. et al. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur. J. Immunol. 41, 366–379 (2011).
Czerkies, M. et al. Cell fate in antiviral response arises in the crosstalk of IRF, NF-κB and JAK/STAT pathways. Nat. Commun. 9, 493 (2018).
Molnarfi, N., Gruaz, L., Dayer, J.-M. & Burger, D. Opposite effects of IFNβ on cytokine homeostasis in LPS-and T cell contact-activated human monocytes. J. Neuroimmunol. 146, 76–83 (2004).
García-Fernández, M. et al. Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev. 24, 2282–2293 (2010).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009).
Koren, E. et al. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nat. Commun. 9, 4582 (2018).
Lamkanfi, M., Festjens, N., Declercq, W., Berghe, T. V. & Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 14, 44–55 (2007).
Feinstein-Rotkopf, Y. & Arama, E. Can’t live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis 14, 980–995 (2009).
Ariel, A. & Serhan, C. N. New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Front Immunol. 3, 4 (2012).
Fadok, V. A. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 (2000).
Hoffmann, P. R. et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649–659 (2001).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
Weigert, A. & Brüne, B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide 19, 95–102 (2008).
Morón-Calvente, V. et al. Inhibitor of apoptosis proteins, NAIP, cIAP1 and cIAP2 expression during macrophage differentiation and M1/M2 polarization. PLoS One 13, e0193643 (2018).
Siegel, P. M. & Massagué, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 3, 807–820 (2003).
Parks, B. W. et al. CD36, but not G2A, modulates efferocytosis, inflammation, and fibrosis following bleomycin-induced lung injury [S]. J. Lipid Res. 54, 1114–1123 (2013).
Lopez-Dee, Z., Pidcock, K. & Gutierrez, L. S. Thrombospondin-1: multiple paths to inflammation. Mediat. Inflamm. 2011 (2011).
Han, J., Hajjar, D. P., Febbraio, M. & Nicholson, A. C. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J. Biol. Chem. 272, 21654–21659 (1997).
Andrews, T. S. et al. Single-cell, single-nucleus, and spatial transcriptomics characterization of the immunological landscape in the healthy and PSC human liver. J. Hepatol. 80, 730–743 (2024).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018).
Acknowledgements
We thank Prof. Shohreh Issazadeh-Navikas from the University of Copenhagen, Denmark, for kindly providing the Ifnb-/- mice. We also acknowledge the valuable contributions of the University of Haifa’s administrative staff and our colleagues for their support. This work was supported by the Israel Science Foundation (Grants No. 678/13 and 1207/22 to A.A.), the Rosetrees Trust (A.A.), and the Wolfson Family Charitable Trust (A.A.). S.K.S. was supported by the PBC postdoctoral fellowship from the Israeli Council of Higher Education (MALAG). O.Z.T. and S.S. received the presidential scholarship from the University of Haifa, and O.Z.T. was also awarded the Excellence Scholarship from the Gutwirth Fund. A.Z. was supported by the Dr. Hagdalot-Chicago Foundation scholarship. P.G. received the presidential scholarship from the University of Haifa.
Author information
Authors and Affiliations
Contributions
Conceptualization: A.A., S.L., A.Z. Methodology: O.Z-.T., S.S., H.Y., S.K.S., M.A.Z., E.S., S.S.Z. Investigation: O.Z-.T., S.S., H.Y., S.K.S., M.A.Z., E.S., S.S.Z., P.G. Data Analysis: A.A., S.L., A.Z. Writing—original draft: A.A., S.L., O.Z-.T., A.Z. Writing—review and editing: A.A., S.L., S.S.Z., A.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dario Ummarino. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeituni-Timor, O., Soboh, S., Zaid, A. et al. IFN-β and ARTS deficiency promote the generation of hyper-efferocytic Ly6C+ macrophages in resolving inflammation in male mice. Commun Biol 8, 1269 (2025). https://doi.org/10.1038/s42003-025-08707-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08707-3