Abstract
Zingerone, a component of dried ginger, has known anti-ulcer and bone growth-promoting effects, but its impact on postmenopausal osteoporosis (PO) is unclear. This study investigates the therapeutic potential and underlying mechanisms of zingerone in PO. A concentration-dependent effect identified on osteoclast precursors: at low concentrations, zingerone maintains low ROS levels, enhance proliferation, and facilitates bone remodelling; at high concentrations, it elevates ROS levels, enhances ferroptosis sensitivity, and suppresses osteoclast formation. Zingerone significantly improves bone mass in an ovariectomised mouse model of PO. Metabolomics identifies 869 differential metabolites linked to glutathione and purine metabolism. Transcriptomics highlights pathways including ferroptosis, leukocyte migration, and cell adhesion. In RAW264.7 cells, zingerone modulates p53, enhances ferroptosis sensitivity, increasing ROS and Fe2+, upregulates Sat1, and downregulates Gpx4, suggesting that zingerone may act via p53-mediated ferroptosis, indicating potential clinical utility. Before clinical application, the dose-dependent effects of zingerone on bone remodelling and its underlying mechanisms warrant further investigation.
Similar content being viewed by others
Introduction
Postmenopausal osteoporosis is a prevalent bone metabolic disorder among middle-aged and elderly women, and it is driven primarily by a decrease in oestrogen levels. The reduction in oestrogen levels leads to a progressive decrease in trabecular bone volume, rendering the bone structure fragile and diminishing its resilience to physical stress, thereby increasing the risk of fractures1. Fragility fractures constitute a major cause of disability and mortality in the elderly population. Mitigating the risk of fragility fractures hinges upon the effective management of osteoporosis. Current therapeutic approaches predominantly rely on oestrogen replacement therapy, bisphosphonates, and parathyroid hormone analogues2. However, these modalities present certain limitations and adverse effects3, such as the perceived association of oestrogen replacement therapy with increased risks of cardiovascular events and breast cancer4. Prolonged administration of zoledronic acid increases the incidence of severe atrial fibrillation among patients, whereas some patients may experience severe musculoskeletal pain within days or months of commencing bisphosphonate therapy5. Commonly used parathyroid hormone analogues, such as teriparatide, can induce limb pain, headaches, and nausea6, underscoring the urgent need for novel therapeutic agents.
With the progression of inquiry into traditional Chinese herbal pharmacopeia and its enduring application over past eras, an increasing number of researchers are shifting their focus to the use of Chinese herbal medicine for the treatment of postmenopausal osteoporosis. Studies have revealed that the traditional herb Radix et Rhizoma Coptidis primarily targets key pathways, such as the Hif-1 and Pi3k–Akt pathways, along with oestrogen-related signalling pathways, in bone marrow mesenchymal stem cells, enhancing osteogenesis and angiogenesis by modulating targets such as Jun, Tp53, Akt1, Esr1, and Mark147. Additionally, network pharmacology predictions and experimental validation have demonstrated that the traditional herbal formula Bu Yang Huan Wu Tang (BYD) may treat postmenopausal osteoporosis by activating the β-catenin signalling pathway through oestrogen receptor 1 (ESR1)8. Given the complexity of the components and unclear specific molecules and targets responsible for the therapeutic effects of natural herbs, optimizing and purifying the effective substances of Chinese herbal medicines often enhance their efficacy. The pathogenesis of postmenopausal osteoporosis is significantly influenced by the activities and functions of osteoclast precursor cells, indicating that these cells are pivotal contributors to the progression of this medical condition, with RAW264.7 cells being the most common osteoclast precursor cell line used to assess bone resorption activity during the development of postmenopausal osteoporosis9. We previously conducted a cell phenotype-based high-throughput screen of 2160 bioactive or clinically used compounds from an FDA-approved drug library (Selleck Chem) and found that zingerone significantly inhibits RAW264.7 cells. Zingerone is a bioactive substance derived from Zingiber officinale Roscoe, a plant belonging to the Zingiberaceae family that is native to regions such as China and Southeast Asia. As a commonly used and widely available plant, it can be used to prepare ethnic herbal medicines10, and it has extensive clinical applications in folk medicine and has been confirmed to possess anti-inflammatory properties11 and antioxidant effects12. This study revealed that a traditional Chinese medicine prepared from dried ginger, when combined with processed aconite and roasted liquorice root as the Sini Decoction, can treat symptoms such as limb fatigue, as recorded in the “Chinese Pharmacopoeia” (2020 edition, Volume 1). Zingerone, a key component of dried ginger, has antiulcer, antioxidant, and growth-promoting properties13. However, the mechanism by which zingerone treats postmenopausal osteoporosis by acting on RAW264.7 osteoclast precursor cells remains unclear.
Omics technologies enable the systematic and comprehensive study of the overall and dynamic characteristics of diseases, aiding in the identification of potential biomarkers and the elucidation of disease pathogenesis and drug treatment mechanisms14. Compared with a single analysis, a multiomics integrative analysis provides further validation at the molecular level, effectively advancing the development of novel therapeutic strategies and providing a better understanding of disease pathogenesis and potential therapeutic targets15. In the present study, bone tissue metabolomics combined with transcriptomics revealed the association of zingerone treatment for postmenopausal osteoporosis with the ferroptosis pathway. Further validation indicated that zingerone increases ferroptosis sensitivity through p53, which regulates SAT1 and GPX4, thereby promoting osteoclast precursor cell ferroptosis and achieving the therapeutic purpose of treating postmenopausal osteoporosis.
Results
Zingerone affects RAW264.7 cell proliferation and bone quality in OVX mice
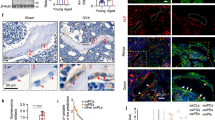
The addition of H2O2 as an exogenous ROS simulates the oxidative stress environment of osteoporotic precursor cells postmenopause and activates the proliferation and differentiation of RAW264.7 cells16. A CCK-8 assay was utilized to assess the effects of various concentrations of zingerone on the viability of H2O2-treated RAW264.7 cells. At a concentration of 400 μM, zingerone significantly reduced the viability of H2O2-treated RAW264.7 cells (Fig. 1A), leading to the selection of 400 μM zingerone for further experiments. Consistent with previous studies, OVX mice were orally administered 100 mg/kg zingerone for two months. An examination of the major organs through a histological analysis with haematoxylin and eosin (H&E) staining did not reveal any considerable organ damage or signs of inflammation (Fig. 1B). The micro-CT evaluation of the distal femurs of the mice (Fig. 1C) revealed a significant reduction in bone mineral density (BMD) in the model group compared with the control group. However, compared with the OVX group, the treatment group presented a substantial increase in trabecular bone density. In addition, key histomorphometric indicators, such as the trabecular thickness (Tb.Th), bone volume fraction (BV/TV), and trabecular separation (Tb.Sp), were significantly improved following zingerone administration (Fig. 1D). The histopathological examination of sagittal sections from the distal femur (Fig. 1E, Figure S2A, B) showed that the femoral tissue from the control group displayed uniform staining, well-aligned trabeculae, an intact osteoblast architecture, consistent growth plate thickness, and no signs of inflammation. Tartrate-resistant acid phosphatase (TRAP) staining revealed a limited number of osteoclasts surrounding the trabeculae. In contrast, the model group presented a reduced trabecular number, localized thinning, enlarged marrow cavities, and disrupted growth plates, accompanied by a marked increase in the number of osteoclasts around the trabecular structures, as observed using TRAP staining. After zingerone treatment, the number and thickness of trabeculae were significantly increased, whereas the number of osteoclasts was notably decreased. As an approach to obtain deeper insights into the mechanism underlying the effect of zingerone on bone metabolism, immunofluorescence staining was performed to assess the expression of osteocalcin (OCN), an osteoblast-specific marker, in bone tissue. The results revealed the significant upregulation of OCN expression in the zingerone-treated group compared with the model group, consistent with the findings from micro-CT and histological analyses (Fig. 1F, Figure S2C). In addition, Western blot analysis revealed that, compared with the control group, the OVX group presented significantly decreased levels of osteogenesis-related proteins, including runt-related transcription factor 2 (RUNX2), alkaline phosphatase (ALP), and bone morphogenetic protein (BMP), along with increased expression of the osteoclast-associated markers matrix metalloproteinase 9 (MMP9) and cathepsin K (CTSK). Zingerone treatment led to a dose-dependent restoration of osteogenic protein expression and the significant suppression of osteoclast-related protein levels (Fig. 1G–J). Collectively, these results suggest that zingerone significantly improves the structural integrity of femoral tissue in OVX mice, particularly by increasing the trabecular number and thickness.
A CCK-8 assay assessing the effect of different concentrations of zingerone on RAW264.7 cell proliferation (n = 3 independent biological replicates). B H&E-stained images of sections of major organs from mice (n = 6 per group). The data are presented as the mean ± standard deviation. Scale bar: 50 μm. C Sections of femoral bone tissue scanned by micro-CT (sagittal and cross-sectional views). Scale bar: 2 mm. D Micro-CT parameters of the femur showing BMD, Tb.Th, BV/TV, and Tb.Sp in the three groups (n = 6 per group). E Histological analysis of femoral tissue sections stained with H&E, Goldner’s trichrome, and TRAP for tissue pathology analysis. Scale bar: 1000 μm. Scale bar: 200 μm. F Immunofluorescence staining of OCN in bone tissue from the NC group, OVX group, and zingerone (Zin) group. Scale bar: 1000 μm. Scale bar: 50 μm. G Western blot analysis verifying the effects of zingerone on the expression of MMP9, RUNX2, ALP, BMP, and CTSK in bone tissue (n = 3). H Quantification of WB in (G). The values are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the model group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to the control group.
Zingerone impacts bone metabolism in OVX mice
Nontargeted metabolomics was employed to observe the effects of zingerone on bone metabolic characteristics in OVX mice. PCA was used to detect outliers and evaluate metabolomic differences (Fig. 2A). A PLS-DA model was subsequently applied to identify differentially abundant metabolites (Fig. 2B). After 200 permutation tests, R2 = 0.92 and Q2 = −0.62 were achieved (Fig. 2C), demonstrating the adaptability and predictive ability of the model. A heatmap was constructed to visualize the alterations in metabolites and capture the metabolic changes more accurately (Fig. 2D). The altered metabolites were input into MetaboAnalyst 5.0 for the pathway analysis, revealing that the major enriched pathways at the metabolomic level between the experimental and model groups were purine metabolism, aspartate metabolism, alanine metabolism, and glutathione metabolism (Fig. 2E). Furthermore, chromatograms showed 869 differentially abundant metabolites after zingerone treatment, and the top 40 significantly upregulated/downregulated metabolites are displayed in a stem plot (Fig. 2F).
A PCA score plot after 8 weeks of zingerone treatment (n = 6 per group). B PLS-DA score plot of zingerone (100 mg/kg) treatment for 8 weeks (n = 6 per group). C The PLS-DA model was validated through 200 permutation tests, demonstrating its effectiveness without overfitting (n = 6 per group). D Heatmap illustrating the effect of zingerone treatment on bone metabolites (n = 6 per group). E Pathway analysis of differentially abundant metabolites. F Stem plot of the top 40 significantly differentially abundant metabolites. Red indicates increased levels after treatment, while blue indicates decreased levels after treatment.
Zingerone alters transcript levels in the bone tissue of OVX mice
An analysis of RNA-seq data was conducted using the Limma package, with a threshold of abs(log2) > 0.2 and an adjusted p-value ≤ 0.05 , to compare differences in gene expression between the model group and the group treated with 100 mg/kg zingerone for 8 weeks. Compared with the model group, the zingerone-treated group presented significant alterations in the expression of 1061 genes, with 572 genes being upregulated and 489 genes being downregulated. The heatmap in Fig. 3A shows the top 100 most significantly differentially expressed genes (DEGs), and their expression trends are depicted in the volcano plot in Fig. 3B. The results of the Gene Ontology (GO) analysis shown in Fig. 3C revealed that these genes play roles in various cellular processes, biological processes, and molecular functions. Additionally, the circular plot of the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Fig. 3D) highlighted 20 enriched pathways associated with differentially expressed genes, including the neutrophil extracellular trap formation pathway (mmu04613), the Rap1 signalling pathway (mmu04015), and the ferroptosis pathway (mmu04216). Further analysis revealed a relatively high proportion of ferroptosis-related genes among the overall correlations. We previously reported that postmenopausal osteoporosis is closely related to ferroptosis and that the inhibition of osteoblast ferroptosis alleviates postmenopausal osteoporosis17, indicating that ferroptosis-related genes may play an important role in the effects of zingerone treatment.
A Heatmap of DEGs (n = 3 independent biological replicates). B Volcano plot of DEGs. Genes were considered significant when the adjusted p-value was ≤ 0.05. C Gene Ontology (GO) enrichment analysis results (red represents biological processes, green represents cellular components, and blue represents molecular functions). D KEGG circlize plot displaying 20 enriched pathways. Integration of metabolomic and transcriptomic analyses elucidates the mechanism of action and targets of zingerone.
The integration of metabolomic and transcriptomic analyses elucidates the mechanism of action and targets of zingerone
Using MetaboAnalyst 5.0, a joint pathway analysis of the metabolomic and transcriptomic data was conducted to establish comprehensive correlations between the DEGs and the differentially abundant metabolites. Pathways such as arachidonic acid metabolism, ferroptosis, and ovarian steroid synthesis were enriched in the joint analysis, further confirming the role of the ferroptosis pathway in the treatment of postmenopausal osteoporosis with zingerone (Fig. 4A).
A Joint pathway analysis based on transcriptomics and metabolomics. B Measurement of ROS levels in the cytoplasm before and after zingerone treatment (n = 3 independent biological replicates). Scale bar: 50 μm. C Average fluorescence intensity of ROS (n = 3 independent biological replicates). D Heatmap of DEGs involved in ferroptosis. E GSEA enrichment plot of the ferroptosis pathway. F Correlation analysis between differentially abundant metabolites and DEGs. G Western blot analysis of p53, SAT1, and GPX4 in RAW264.7 cells, and β-actin was used as a control (n = 3). H Relative level of p53 protein (n = 3 independent biological replicates). I Relative level of SAT1 protein (n = 3 independent biological replicates). J Relative level of GPX4 protein (n = 3 independent biological replicates). The data are presented as the mean ± standard deviation. ***p < 0.001 compared to the model group; ##p < 0.01 and ###p < 0.001 compared to the control group.
Because zingerone is an antioxidant drug and ferroptosis is characterized by a cellular phenotype of oxidative stress, potential discrepancies were observed between the transcriptomic results and zingerone properties. ROS activity was evaluated after treating osteoclast precursor cells with zingerone to validate the transcriptomic results. Zingerone increased the levels of ROS in osteoclast precursor cells (Fig. 4B), and the quantitative analysis showed that the zingerone treatment group presented the highest ROS content (Fig. 4C), suggesting that a zingerone target may be present in osteoclast precursor cells that is independent of its oxidative stress targets. A heatmap of the most important DEGs involved in the ferroptosis pathway and a GSEA plot showing enrichment of the ferroptosis gene set at the top of the sequence (NES = 0.534) were generated to further determine the downstream targets regulated by zingerone in osteoblast precursor cells (Fig. 4D, E). Compared with the model group, the treatment group presented increased expression of the p53 and Sat1 genes but decreased expression of the Gpx4 gene. Because p53 is upstream of Gpx4 and Sat1 in the ferroptosis pathway, p53 may be a downstream target of zingerone in regulating ferroptosis-related processes. The binding affinity of p53 to Gpx4 and Sat1 was predicted.
For Sat1, the sequence of the first site is AGATCTGTCCAGGAAAGCCC, which is located at position 138 on the chromosome and originates from the start of the chromosome as the first base. This site is located on the positive DNA strand and has a high score (191.02), indicating a strong binding affinity for p53. Additionally, the p value for this site was 0, and the E value was also 0, indicating that the prediction of this binding site was statistically significant and not a random event. The sequence of the second site is GATCTGTCCAGGAAAGCCC, located at position 139, which is also on the positive strand. This site had a score of 162.52, which was slightly lower than that of the first site but still indicated significant binding affinity. The p value for this site was 7.5E-5, and the E value was 0.149, supporting the statistical significance of the prediction as a p53 binding site. Although the E value for this site was slightly higher than that for the first site, it was still within an acceptable range (Table 1).
For Gpx4, the DNA sequence of the binding site is CTGCATGTGTATTCATGCCT, located at position 959, which is the 960th base from the starting point of the chromosome. This site is located on the negative strand of DNA and had a high score of 148.59, which indicated that this site may have higher binding affinity for p53. The p value for this site was 0.000375, indicating a statistically significant prediction of p53 binding rather than a random event. However, the E value for this site was 0.74, which was less than 1, suggesting that it may be a functional binding site (Table 2).
Pearson’s correlation analysis was conducted to assess the associations between key target genes and metabolites altered by zingerone treatment and to explore the interplay between the metabolomic and transcriptomic profiles. A correlation heatmap was generated to visualize gene–metabolite interactions, with red representing strong positive correlations and blue denoting strong negative correlations. Notably, glutathione levels were negatively correlated with Gpx4 expression but positively correlated with Sat1 expression (Fig. 4F).
Ferroptosis is a form of programmed cell death involved in various disease processes and is characterized by impaired glutathione metabolism and the excessive accumulation of ROS18. Western blotting was performed to assess the expression levels of ferroptosis-associated proteins, including p53, SAT1, and GPX4. Compared with the model group, zingerone treatment led to increased expression of p53 and SAT1 but decreased expression of GPX4 (Fig. 4G–J). Thus, these findings suggest that zingerone acts on p53 in osteoclast precursor cells, leading to the upregulation of p53 and SAT1 expression and the downregulation of GPX4 expression, thereby participating in the regulation of ferroptosis-related pathways.
Zingerone modulates ferroptosis sensitivity in RAW264.7 cells via p53 regulation
Cells were treated with varying concentrations of zingerone and hydrogen peroxide (H₂O₂), and cell viability was assessed using the CCK-8 assay to investigate the effects of different levels of reactive oxygen species (ROS) on osteoclast precursor cells. Low concentrations of zingerone (0–25 μM) appeared to promote the proliferation of osteoclast precursor cells. However, with increasing concentrations, the inhibitory effects became evident, with significant suppression observed at 400 μM (Fig. 5A). Treatment with H₂O₂ alone at a concentration of 200 μM most effectively promoted cell proliferation. In contrast, higher concentrations resulted in increased cytotoxicity, and severe cellular damage was observed after treatment with 1000 μM H₂O₂ (Fig. 5B). Western blot analysis was performed to assess the expression of osteoclast differentiation markers in the control, 200 μM, and 1000 μM H₂O₂ groups. MMP9 and CTSK were significantly upregulated in response to 200 μM H₂O₂, whereas both markers were markedly downregulated by 1000 μM H₂O₂ (Fig. 5C, D). The subsequent Western blot analysis of ferroptosis-related proteins under the same treatment conditions revealed that p53 and SAT1 were substantially downregulated in the 200 μM group, whereas GPX4 expression was increased. In contrast, the 1000 μM group exhibited the opposite expression pattern (Fig. 5E–F). The cells were divided into normal control, model, treatment, and p53 knockdown groups to further elucidate the modulation of osteoclast precursor cell ferroptosis sensitivity and the mechanism of ferroptosis induced by zingerone. Although a previous study showed that H2O2 promotes the proliferation and differentiation of RAW264.7 cells16, our experiments revealed that H2O2 inhibited ferroptosis in RAW264.7 cells. Based on the joint analysis results and the changes in the expression of the p53-mediated Sat1 and Gpx4 ferroptosis-related genes, p53 may be a target of zingerone in osteoclast precursor cells. RAW264.7 cells were exposed to different concentrations of RSL3, and cell viability was evaluated after 24 hours. The half-maximal inhibitory concentration (IC50), which indicates the RSL3 concentration required to induce the death of 50% of cells, was used to assess ferroptosis sensitivity. IC50 curves were generated for each treatment group, and the corresponding values were calculated. The treatment group required 2.9 μM RSL3 for 50% cell death, whereas the gene knockdown group required 3.5 μM RSL3 for 50% cell death. In addition, p53 knockdown rescued the impaired cell viability caused by RSL3. Thus, zingerone treatment increased the sensitivity of cells to ferroptosis but reduced their sensitivity to ferroptosis after p53 knockdown (Fig. 5G). After zingerone treatment, the highest ROS levels were observed, which led to ferroptosis (Fig. 5H, I). JC-1 staining revealed that the red/green fluorescence ratio of the cells decreased after zingerone treatment, indicating mitochondrial dysfunction, whereas the red/green fluorescence ratio of the cells with p53 knockdown was not affected by zingerone treatment (Fig. 5J, K), indicating that the inhibitory effect of zingerone on RAW264.7 cells was mediated by p53 regulation. The intracellular MDA levels increased after zingerone treatment (Fig. 5L), which indicated excessive oxidation of the cells and increased sensitivity to ferroptosis. Intracellular Fe2+ levels were detected using an iron ion probe and confocal microscopy. Zingerone treatment caused a significant increase in the intracellular Fe2+ level, but p53 knockdown prevented this zingerone-induced increase (Fig. 5M). Further observation by transmission electron microscopy revealed that cells treated with zingerone had smaller mitochondria and no mitochondrial cristae, whereas the mitochondrial morphology of cells after p53 knockdown was not affected by zingerone treatment (Fig. 5N). These results indicate that zingerone increases ferroptosis sensitivity in RAW264.7 cells, indicating that p53 plays a crucial role in the regulatory effect of zingerone on ferroptosis sensitivity in osteoclast precursor cells.
A CCK-8 assay assay of RAW264.7 cells treated with different concentrations of zingerone (n = 3 independent biological replicates). B Cell viability of RAW264.7 cells treated with different concentrations of H₂O₂ was assessed using the CCK-8 assay (n = 3 independent biological replicates). C Western blot analysis verifying the effects of different concentrations of H₂O₂ on the expression of MMP9 and CTSK in RAW264.7 cells (n = 3 independent biological replicates). D Quantification of MMP9 and CTSK protein expression from (C). E Western blot analysis verifying the effects of different concentrations of H₂O₂ on the expression of p53, SAT1, and GPX4 in RAW264.7 cells (n = 3 independent biological replicates). F Quantification of p53, SAT1, and GPX4 protein expression from (E). G Knockdown of p53 with siRNA reduces ferroptosis sensitivity after zingerone treatment (n = 3 independent biological replicates). H Measurement of ROS levels in the cytoplasm before and after zingerone and p53 siRNA treatment (n = 3 independent biological replicates). Scale bar: 50 μm. I Average fluorescence intensity of ROS. J Quantification of JC-1 monomers and aggregates in RAW264.7 cells treated with or without zingerone (n = 3 independent biological replicates). Scale bar: 5 μm. K Fluorescence intensity of JC-1 aggregates/ JC-1 monomers in each group. L MDA levels. M Intracellular FeRhoNox-1 staining in cells transfected for 48 h (n = 3 independent biological replicates). Scale bar: 100 μm. N Observation of cell morphology by transmission electron microscopy after 48 h of treatment (n = 3 independent biological replicates). Scale bar: 1000 μm. Scale bar: 50 μm. The data are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the model group; ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to the control group; ^p < 0.05 and ^^p < 0.01 compared to the treatment group.
Zingerone regulates ferroptosis sensitivity in RAW264.7 cells via the p53-mediated regulation of SAT1 and GPX4 expression
Molecular docking was performed to confirm the binding affinity between zingerone and p53 (Fig. 6A). The results revealed a binding energy of -4.7 kcal/mol for the zingerone–p53 interaction, indicating a strong binding affinity between zingerone and p53. Immunohistochemical staining was performed on femoral tissue samples to determine the changes in the expression of ferroptosis-related proteins (Fig. 6B, Figure S2D). The analysis revealed the GPX4 expression was highest in the OVX group compared with both the control and treatment groups. Conversely, the levels of ACSL4, SAT1, and p53 were significantly lower in the treatment group than in the OVX group (Fig. 6C). Immunofluorescence staining for p53, SAT1, and GPX4 was performed to assess ferroptosis marker expression in bone tissue. Consistent with the transcriptomic data, the zingerone-treated group presented increased expression of p53 and SAT1, while the GPX4 levels were notably reduced compared with those in the OVX group (Fig. 6D, E, Figure S2E, F). RSL3 was used as a ferroptosis inducer to elucidate how zingerone regulates ferroptosis sensitivity in RAW264.7 cells via p53. The cells were divided into normal control, ferroptosis induction, and p53 knockdown with ferroptosis induction groups. The Western blot results showed the upregulation of SAT1 and the downregulation of GPX4 in the ferroptosis induction group. In contrast, upon p53 gene knockdown followed by RSL3 treatment, SAT1 expression decreased, whereas GPX4 expression increased (Fig. 6G, H), suggesting that p53 regulates ferroptosis sensitivity in RAW264.7 cells by modulating SAT1 and GPX4 expression. The cells were subsequently divided into control, model, treatment, and p53 knockdown with drug treatment groups. Western blot analysis revealed that in zingerone-treated cells, the expression of the ferroptosis-related protein SAT1 increased, whereas GPX4 expression decreased. However, in p53-knockdown cells, zingerone treatment did not affect SAT1 or GPX4 expression (Fig. 6I, J), indicating that zingerone-induced ferroptosis in RAW264.7 cells is associated with the p53-mediated regulation of SAT1 and GPX4 expression.
A Molecular docking simulation of p53 with zingerone. B Immunohistochemical staining of bone tissue with antibodies against GPX4, ACSL4, SAT1, and p53 (n = 6 per group). Scale bar: 50 μm. C Quantitative analysis of integrated optical density (IOD) for each marker. D Immunofluorescence staining of ferroptosis-related markers p53 and SAT1 in femoral tissue from the NC, OVX, and Zin groups (n = 6 per group). Scale bar: 50 μm. E Immunofluorescence staining of ferroptosis-related markers GPX4 in femoral tissue from the NC, OVX, and Zin groups (n = 6 per group). Scale bar: 50 μm. F Quantitative analysis of p53, SAT1, and GPX4 expression in bone tissue based on immunofluorescence staining. G Western blot analysis verifying the knockdown efficiency of p53, SAT1, and GPX4 in RAW264.7 cells (n = 3 independent biological replicates). H Quantification of WB in (G). I Western blot analysis of p53, SAT1, and GPX4 expression in RAW264.7 cells after p53 gene knockdown followed by drug treatment (n = 3 independent biological replicates). J Quantification of WB in (I). The data are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the model group; #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to the control group; ^^p < 0.01 compared to the treatment group.
Zingerone inhibits RANKL-induced osteoclastic differentiation by regulating p53–GPX4/SAT1
This study further examined the expression of the osteoclast marker CTSK in bone tissue using immunofluorescence staining to validate the in vitro findings described above. Zingerone treatment led to a significant reduction in CTSK expression in bone tissue compared with that in the OVX group (Fig. 7A, B, Figure S2A). BMMs were harvested from mice, and an in vitro osteoclast differentiation model was established to further investigate the effects of zingerone on osteoclastogenesis. BMMs were cultured in α-MEM containing 30 ng/mL M-CSF and 50 ng/mL receptor activator of RANKL to induce their differentiation into mature osteoclasts. The efficiency of differentiation was verified by TRAP staining, which indicated successful osteoclast formation (Fig. 7C–D). Western blot analysis further demonstrated that the differentiation trend was significantly suppressed upon zingerone treatment (Fig. 7E, F). In addition, after 24 h of treatment with 400 μM zingerone in differentiated cells, the expression level of GPX4 was significantly reduced, whereas the p53 and SAT1 expression levels were markedly increased in osteoclasts (Fig. 7G, H). Notably, knockdown of the p53 gene in osteoclasts attenuated the inhibitory effect of zingerone on osteoclast differentiation markers (Fig. 7I, J) and markedly reduced its proferroptotic effect (Fig. 7K, L). These findings suggest that zingerone may inhibit osteoclast differentiation by promoting ferroptosis. Western blot analysis was performed to detect changes in markers related to endoplasmic reticulum stress-induced apoptosis (caspase-12 and cleaved caspase-12), autophagy (Beclin-1 and LC3B), and pyroptosis (GSDMD and GSDMD-N) as a method to further determine whether zingerone affects other forms of cell death. The results showed no significant differences in the expression of these proteins between the zingerone-treated and model groups (Figure S1). Therefore, this study suggests that zingerone primarily induces ferroptosis in osteoclasts through regulation of the p53/Sat1/Gpx4 signalling axis, thereby exerting its protective effect on postmenopausal osteoporosis.
A Immunofluorescence staining of CTSK in femoral tissue from NC, OVX, and Zin groups (n = 6 per group). Scale bar: 50 μm. Scale bar: 1000 μm. B Quantification of CTSK fluorescence intensity. C Representative TRAP staining of differentiated BMM-derived osteoclasts; TRAP-positive multinucleated cells appear red or purple (n = 3 independent biological replicates). Scale bar: 200 μm. D Quantification of TRAP-positive cells per field. E Western blot analysis of MMP9 and CTSK expression after zingerone treatment (n = 3 independent biological replicates). F Quantification of MMP9 and CTSK from (E). G Western blot analysis of p53, SAT1, and GPX4 expression after zingerone treatment (n = 3 independent biological replicates). H Quantification of p53, SAT1, and GPX4 from (G). I Western blot analysis of MMP9 and CTSK following p53 knockdown and drug treatment (n = 3 independent biological replicates). J Quantification from (I). K Western blot analysis of p53, SAT1, and GPX4 following p53 knockdown and drug treatment (n = 3 independent biological replicates). L Quantification from (K). Data are presented as the mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001 vs. model group; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. control group; ^p < 0.05 and ^^p < 0.01 vs. treatment group.
Discussion
With the increasing ageing population and evolving lifestyle patterns, postmenopausal osteoporosis and its related fracture risk have emerged as significant global public health issues. Chemical synthetic drugs for osteoporosis treatment face challenges, such as long development cycles, high costs, potential side effects, and unclear efficacy. In contrast, traditional Chinese herbal medicine is cost-effective and generally associated with lower toxicity. In a screening study of drugs approved by the FDA, our previous findings were the first to demonstrate the therapeutic potential of zingerone in managing postmenopausal osteoporosis. Osteoclast precursors undergo differentiation into mature osteoclasts in response to RANKL and M-CSF, thereby promoting bone resorption. Once osteoclasts mature, they lose their proliferative capacity, indicating that the proliferation and death of osteoclast precursor cells determine the number of osteoclasts, a major factor in the homeostasis of bone formation and resorption. Dennis J Wu et al. confirmed that in the early stages of postmenopausal osteoporosis, M-CSF promotes the proliferation and survival of RAW264.7 cells, disrupting the balance between bone formation and resorption and leading to osteoporosis19. Therefore, inhibiting the proliferation of osteoclast precursor cells and inducing their death can directly reduce the source of osteoclasts and decrease bone resorption, thereby treating postmenopausal osteoporosis. The present study utilized combined transcriptomic and metabolomic analyses to elucidate the mechanism of action and potential targets of zingerone in treating postmenopausal osteoporosis. Cellular experiments confirmed that zingerone upregulates the expression of the p53 protein in osteoclast precursor cells, regulates the SAT1 and GPX4 pathways, and increases sensitivity to ferroptosis, thereby reversing bone loss in postmenopausal osteoporosis patients.
Postmenopausal osteoporotic bone tissue is in a prolonged state of oxidative stress, leading to the excessive activation of osteoclast precursors and osteoclasts and ultimately leading to increased bone loss. Mitochondria, the primary organelles that regulate the balance between oxidation and reduction, are highly susceptible to oxidative damage. The collapse of the mitochondrial antioxidant system due to cellular oxidative damage leads to ferroptosis, which is characterized by changes in mitochondrial morphology and function, as well as the accumulation of Fe2+ and lipid peroxides20. Oxidative damage to cells leads to mitochondrial morphological changes, such as shrinkage, reduced numbers of cristae, and an increased membrane density. The accumulation of lipid peroxides in mitochondria inhibits system Xc of glutathione (composed of the SLC7A11 catalytic subunit and the SLC3A2 accessory subunit), thereby increasing sensitivity to ferroptosis and promoting its occurrence21. We previously reported a close association between ferroptosis and the occurrence and development of postmenopausal osteoporosis17. The present findings revealed the differential expression of genes in 20 pathways through the transcriptomic analysis, including ferroptosis, leukocyte transendothelial migration, and cell adhesion molecules. Among these pathways, ferroptosis-related genes accounted for a relatively high proportion of the overall correlation. The IC50 curve of RSL3 further confirmed that zingerone increases the sensitivity of osteoclast precursor cells to ferroptosis. Taken together, these findings indicate that the increased sensitivity to ferroptosis and subsequent ferroptosis of osteoclast precursor cells is a specific manifestation of the effectiveness of zingerone in treating postmenopausal osteoporosis. Zingerone induces the accumulation of lipid peroxides, increases oxidative stress, and induces ferroptosis in osteoclast precursor cells.
During bone remodelling, oxidative stress is a critical regulatory factor maintaining the dynamic balance between bone formation and resorption22. Under physiological conditions, moderate levels of ROS act as signalling molecules that promote osteoclast differentiation and stimulate osteoblasts and their precursors to secrete osteogenic factors, such as BMP-2 and osteocalcin, thereby accelerating bone remodelling and supporting tissue renewal and repair23. However, under pathological oxidative stress, such as that observed in postmenopausal oestrogen deficiency or type 2 diabetes, excess ROS accumulation disrupts the balance between bone formation and resorption24. In postmenopausal women, a sharp decrease in oestrogen levels leads to an impaired antioxidant capacity, reduced differentiation of bone marrow-derived MSCs into osteoblasts, and increased osteoclast activity, resulting in decreased bone formation, increased bone resorption, and accelerated bone loss25. In patients with type 2 diabetes, increased oxidative stress not only suppresses the expression of osteogenic genes but also promotes osteoclast differentiation, ultimately contributing to a decreased bone mineral content, impaired bone quality, and a significantly increased fracture risk26,27. Moreover, diabetic conditions have been shown to impair bone repair and delay fracture healing, further aggravating the metabolic imbalance in bone28.
In this study, we found that treatment with low concentrations of H₂O₂ promoted the proliferation of RAW264.7 cells, whereas higher concentrations significantly reduced cell viability. A similar biphasic response was observed with zingerone treatment, suggesting that zingerone may exert a dual regulatory effect on RAW264.7 cell proliferation. This hypothesis was supported by further evidence: low concentrations of zingerone (0–25 μM) promoted RAW264.7 cell proliferation and differentiation, whereas high concentrations (200–400 μM) markedly suppressed proliferation. Additionally, in H₂O₂-treated osteoclasts, the subsequent addition of zingerone (50–400 μM) further increased oxidative stress and induced p53-mediated ferroptosis. The dual effects of zingerone on RAW264.7 cell differentiation and ferroptosis appear to be concentration dependent. A detailed investigation into the dose-dependent effects of zingerone on osteoclasts may provide deeper insights into the mechanisms governing bone remodelling under both physiological and pathological conditions.
Oestrogen deficiency leads to abnormal conversion between oxidized and reduced glutathione, resulting in excessive ROS production and oxidative damage to bone tissue, a characteristic feature of postmenopausal osteoporosis. The KEGG enrichment analysis of metabolomic data revealed pathways associated with zingerone treatment for postmenopausal osteoporosis, such as the glutathione metabolism and purine metabolism pathways. Combined with the joint analysis of transcriptomic data, our findings revealed that zingerone treatment for postmenopausal osteoporosis also affects glutathione metabolism and is associated with the expression of the ferroptosis genes SAT1 and GPX4. Glutathione metabolism involves the catalytic reduction reaction of glutathione and NADPH through the action of the glutathione reductase core enzyme, forming oxidized glutathione and thus maintaining the balance of conversion between oxidized and reduced glutathione. This finding explains why zingerone can effectively ameliorate postmenopausal osteoporosis by fundamentally reversing abnormalities in glutathione metabolism. Moreover, the depletion of glutathione and increased expression of SAT1, coupled with decreased expression of GPX4, induce ferroptosis in mouse pancreatic tumour cells29. In addition to its antioxidant function, glutathione is considered an important ligand for intracellular iron ions and participates in iron metabolism30. SAT1 is a ferroptosis-related gene, and its overexpression is a key feature of ferroptosis, as it promotes ferroptosis by mediating the acetylation of spermidine and spermine31. GPX4 is a glutathione peroxidase that maintains the redox balance within cells, protecting them from oxidative damage32. The present study revealed that after zingerone treatment, the levels of lipid peroxides and ROS increased significantly in osteoclast precursor cells. In addition, GPX4 activity decreased and glutathione accumulation increased, which increased sensitivity to ferroptosis, leading to an inability of these cells to utilize glutathione to alleviate ferroptosis. Similarly, other studies have indicated that upregulation of the SLC7A11 gene in cells leads to cysteine accumulation, causing an abnormal cell structure and osteoclast precursor cell death33. The present findings suggest that zingerone induces ferroptosis in osteoclast precursor cells, which is related to glutathione metabolism mediated by SAT1 and GPX4. Increased SAT1 expression leads to lipid peroxidation and increased ROS levels, whereas decreased GPX4 expression weakens the ability of cells to clear ROS, resulting in an imbalance in glutathione metabolic homeostasis rather than a traditional antioxidant effect.
A joint analysis was performed to further elucidate how zingerone regulates the expression of SAT1 and GPX4, and the results revealed that p53 is a common upstream regulator of both SAT1 and GPX4, suggesting that p53 is a target of zingerone in osteoclast precursor cells. p53 is associated primarily with cell cycle regulation and proliferation34. Studies have shown that Herba Patriniae and its component isovitexin regulate p21 and CDK2 expression by activating the p53 pathway, leading to cell cycle arrest in G0/G1 phase and inhibiting the proliferation of colorectal cancer cells35. Additionally, as a transcription factor, p53 directly upregulates the proapoptotic gene NOXA, inducing apoptosis in mouse embryonic fibroblasts36. Recent research has identified p53 as one of the main regulators of ferroptosis. Studies of cholangiocarcinoma have indicated that the upregulation of p53 expression inhibits GPX4 activity, which leads to excessive oxidative stress in cholangiocarcinoma cells, thereby inducing ferroptosis37. Zoledronic acid, a commonly used drug for treating postmenopausal osteoporosis, also promotes ferroptosis in RAW264.7 cells through the p53/GPX4 pathway38. In the present study, molecular docking experiments revealed a strong binding affinity between zingerone and p53, which was further confirmed by affinity predictions showing the significant binding affinity of p53 for SAT1 and GPX4. Furthermore, zingerone increased the sensitivity of osteoclast precursor cells to ferroptosis and induced ferroptosis by inducing oxidative damage through p53. When p53 expression decreased, the increased sensitivity to ferroptosis induced by zingerone was abrogated by the inhibition of p53, further confirming that p53 may be a downstream target of zingerone in osteoclast precursor cells, independent of the traditional antioxidant properties of zingerone. This mechanism is associated with zingerone regulating SAT1 and GPX4 expression through p53, increasing sensitivity to ferroptosis and inducing ferroptosis.
Methods
Cell culture and reagents
The mouse monocytic leukaemia cell line RAW264.7 was obtained from the Type Culture Collection affiliated with the Cell Bank of the Chinese Academy of Sciences. Zingerone (98%, MB6714) was purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China). Thermo Fisher Scientific (Waltham, MA, USA) supplied H2O2 (3 wt.%, 7722-84-1) and PBS (10010023). Antibodies against RSL3 (99.23%, CM01193), p53 (60283-2-Ig), ACSL4 (26658-1-AP), SAT1 (10708-1-AP), GPX4 (67763-1-Ig), MMP9 (30592-1-AP), CTSK (11239-1-AP), RUNX2 (20700-1-AP), ALP (55319-1-AP), BMP2 (18933-1-AP), Beclin-1 (11306-1-AP), LC3B (14600-1-AP) and β-actin (23660-1-AP) were supplied by Proteintech Group, Inc. (Wuhan, China). Cell Signaling Technology located in Boston, Massachusetts, United States, provided goat anti-rabbit IgG (7074S), goat anti-mouse IgG (7076S), and notably, caspase-3 (9662) cleaved caspase-3 (9661) antibodies. Invitrogen (Carlsbad, CA, USA) provided Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A-11008) and Alexa Fluor® 594-conjugated goat anti-mouse IgG (A-11005). Importantly, antibodies directed against GSDMD-N (DF13758) and GSDMD (AF4012), were acquired from Affinity Biosciences in Changzhou, China. The Fe2+ indicator FeRhoNox-1 (MX4588) was obtained from MedChemExpress (Shanghai, China). Beyotime Biotechnology (Shanghai, China) provided RIPA (Strong) lysis buffer (P0013B), an enhanced BCA protein concentration determination kit (P0010), the MDA lipid oxidation assay kit (S0131S), an advanced apparatus for determining the mitochondrial membrane potential via the JC-1 compound (C2003S), tools dedicated to reactive oxygen species examination (ROS Kit S0033S), a cell proliferation determination kit designated CCK-8 (C0037), the chelator ethylenediaminetetraacetic acid (R0225), and a 4% paraformaldehyde solution used for fixation processes (P0099). Furthermore, Beyotime Biotechnology (Shanghai, China) provided bovine serum albumin (BSA) used as a blocking agent (ST2249), the DAB horseradish peroxidase color development kit for chromogenic detection in immunostaining (P0202), and phenylmethylsulfonyl fluoride (PMSF) used as a protease inhibitor (ST505). p53 small interfering RNA (siRNA) was supplied by Hippobio (Huzhou, China). Thermo Fisher Scientific (Waltham, MA, USA) supplied DMEM (11965092), 10% foetal bovine serum (10099158), Opti-MEM reduced-serum medium (31985070), and Lipofectamine™ 2000 transfection reagent (11668030). The TRAP staining kit (387 A) was obtained from Sigma‒Aldrich (USA). M-CSF (HY-P7085A) was obtained from MedChemExpress (Monmouth Junction, NJ, USA). Glutaraldehyde (G1102), uranyl acetate (G1960), and lead citrate (G1961) were purchased from Servicebio (Wuhan, China). Epoxy resin (14120) and Osmium tetroxide (19150) was obtained from Electron Microscopy Sciences (USA). EDTA decalcifying solution (10%, G1105), Sodium citrate antigen retrieval buffer (G1202) and TRAP staining solution (G1050) were purchased from Servicebio (Wuhan, China). RAW264.7 cells were cultured in DMEM supplemented with 10% foetal bovine serum and maintained in a humidified incubator at 37 °C with 5% CO2.
Cell transfection
Three siRNAs targeting p53 were dissolved in RNase-free water and transfected into the cells using Lipofectamine™ 2000 (Thermo Fisher Scientific, No. 11668030) according to the manufacturer’s protocol. A protein immunoblot analysis was employed to measure p53 levels and assess the transfection efficiency. As a negative control, RAW264.7 cells were transfected with a nontargeting siRNA. Among the siRNA sequences tested, p53-1 had the greatest knockdown efficiency and was therefore chosen for subsequent experiments. The cells were plated in 6-well dishes and cultured to approximately 60% confluence. After 24 h, a mixture of 50 nM siRNA and Lipofectamine™ 2000 reagent was prepared in Opti-MEM reduced-serum medium without antibiotics and incubated at room temperature for 20 minutes. The cells were exposed to the siRNA transfection reagent for 6 h, after which the medium was replaced with fresh culture medium. Cells were cultured at 37 °C within a humidified incubator for 48 h. The specific sequences of the siRNAs used are listed in Table 3.
Cell viability assay
The cells were diluted to a concentration of 10,000 cells per millilitre and distributed into a 96-well plate, with each well containing 100 microlitres. Zingerone was initially dissolved in DMSO at a concentration of 10 mM and further diluted in DMEM supplemented with 10% fetal bovine serum to final concentrations of 50, 100, 200, and 400 µM prior to use. Specifically, a 1 mM intermediate H₂O₂ solution was freshly prepared by diluting 1.13 µL of 3 wt.% H₂O₂ (Thermo Fisher, 7722-84-1) with 998.87 µL of PBS (Thermo Fisher, 10010023), followed by further dilution of 200 µL of the 1 mM solution with 800 µL of PBS to obtain a 200 µM working solution immediately prior to use. Different concentrations of zingerone and H2O2 (diluted to 200 µM) were then added to the culture medium to identify the most suitable concentration for subsequent experiments. Subsequently, 10 μL of CCK-8 solution (Beyotime Biotechnology, C0037) was added to each well and incubated at 37 °C for 2 h, after which the absorbance at 450 nm was measured using a microplate reader to assess cell viability.
Ferroptosis sensitivity experiment
RAW264.7 cells were seeded in a 96-well plate and subjected to modelling, treatment, or p53 knockdown plus drug treatment. RSL3 (Proteintech,CM01193), a ferroptosis inducer, was initially dissolved in DMSO at a stock concentration of 10 mM and subsequently diluted in complete DMEM to a final working concentration of 1 µM. The cells were treated with RSL3 and incubated at 37 °C with 5% CO₂ for 24 h. Afterward, cell viability was determined by measuring absorbance at 450 nm using the CCK-8 assay.
Intracellular ROS detection
RAW264.7 cells were plated in a 6-well plate and divided into control, model, treatment, and experimental groups with p53 knockdown and drug treatment. Following exposure to H2O2 and zingerone, the levels of intracellular reactive oxygen species (ROS) (Beyotime Biotechnology, S0033S) were assessed using a ROS detection kit. DCFH-DA, the principal component of the ROS detection kit, was diluted 1:1000 in serum-free culture medium to prepare a working solution. This diluted solution was then applied to cover the cells, and after an incubation at 37 °C for 30 minutes, the intracellular ROS levels were quantified based on DCFH-DA fluorescence.
Detection of mitochondrial membrane potential
The mitochondrial membrane potential of RAW264.7 cells was evaluated using the JC-1 mitochondrial membrane potential detection kit (Beyotime Biotechnology, C2003S). The cells were plated in a 6-well dish, and the control, model, treatment, and p53 knockdown plus drug groups were established. After 24 h, the cells were treated with JC-1 staining solution and incubated in the dark at 37 °C for 20 min before being analysed.
Measurement of iron ion levels
The iron levels in RAW264.7 cells were analyzed following the protocol for FeRhoNox-1 (Fe2+ indicator) (MedChemExpress, MX4588). FeRhoNox-1 forms irreversible bonds with Fe2 + , resulting in the production of orange‒red fluorescent compounds. Initially, a 1 mM stock solution of FeRhoNox-1 was prepared in DMSO and subsequently diluted to a working concentration with HBSS. After the culture medium was removed, the cells were rinsed twice with HBSS and incubated with the staining solution. They were then maintained at 37 °C with 5% CO₂ for 60 min, washed again with HBSS, and subsequently observed using a confocal laser scanning microscope.
Measurement of MDA
Following the instructions provided in the lipid oxidation (MDA) detection kit protocol (Beyotime Biotechnology, S0131S), the experiment was conducted as follows: 150 μL of cell lysis buffer was initially added to the cells. Upon completion of this procedure, the samples were incubated on ice for 10 min. The cells were scraped and buffer was added; this amalgamation of cellular matter and buffer was transferred to 1.5 mL tubes on ice for preservation. The tubes were vortexed every 10 min for three cycles. The samples were subjected to centrifugation at 12,000 × g at 4 °C for ten minutes, and the resulting supernatants, including the cell lysis buffer, were collected by centrifugation. Next, 100 µl of each lysate was combined with 200 µl of the supplied reagent and incubated at 100 °C in the dark for 15 min. Once the samples reached ambient temperature, they were centrifuged at 1000 g at 25 degrees Celsius for ten minutes. Then, 200 µL of the supernatant of each sample was extracted and transferred to a 96-well plate. A spectrophotometer with precision tuned to optical absorbance at 532 nm was used immediately to analyse the samples.
Transmission electron microscopy observation of mitochondrial structure
For transmission electron microscopy (TEM) observation of mitochondrial structure, RAW264.7 cells were harvested by centrifugation and fixed in 2.5% glutaraldehyde (Servicebio, G1102) at 4 °C overnight. After washing with PBS, the samples were post-fixed with 1% osmium tetroxide (Electron Microscopy Sciences, 19150) for 1 hour, dehydrated through a graded ethanol series, and embedded in epoxy resin (Electron Microscopy Sciences, 14120). Ultrathin sections (60–80 nm) were prepared using an ultramicrotome (Leica Microsystems, Germany) and stained sequentially with 2% uranyl acetate (Servicebio, G1960) and lead citrate (Servicebio, G1961). Sections were examined using a transmission electron microscope (Hitachi, Japan), and high-resolution digital images were captured from five randomly selected fields per group.
Animals and drugs
All animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of China Medical University (Approval No. 2019014). We have complied with all relevant ethical regulations for animal use. Eighteen female C57BL/6 J mice (8 weeks old, weighing 20–25 g, MGI:2164831) were procured for the animal experiments and housed at the Department of Experimental Animal Science at China Medical University. The laboratory maintained controlled conditions, with a temperature of 20–26 degrees Celsius, atmospheric moisture levels of 40% to 70%, an ammonia concentration of 14 mg/m3, a noise level of 60 dB, and a 12 h light/dark cycle. The mice were provided unrestricted access to food and water. After a two-week acclimation period, the mice were randomly divided into three groups (comprising six subjects per cohort): the ovariectomy group (OVX), the zingerone treatment group (Zin), and the sham-operated control group (Sham). The mice in the OVX and Zin groups underwent bilateral ovariectomy under 2–3% isoflurane anaesthesia. Small incisions were made on both dorsolateral sides to expose the ovaries and surrounding fat pads. The oviducts were ligated, and the ovaries were carefully excised. The muscle layers were closed using absorbable sutures, and the skin was sutured with standard surgical thread. The mice in the Sham group underwent the same procedure without ovary removal. The Zin group received zingerone treatment postoperatively. All the mice were administered meloxicam subcutaneously (5 mg/kg/day) for 3 days after surgery for analgesia, and their behaviour was monitored daily to assess signs of pain or distress. Zingerone, dissolved in 0.9% saline to a final concentration of 2 mg/mL, was administered to the mice in the Zin group by oral gavage at a dose of 100 mg/kg body weight per day (equivalent to 1.25 mL for a 25 g mouse), starting three days after surgery. Gavage was performed using a sterile, flexible, ball-tipped feeding needle (20 G × 38 mm) attached to a 2 mL syringe. The mice were gently restrained, and the solution was slowly delivered into the oesophagus to minimize the risk of aspiration. All procedures were performed by trained personnel in accordance with institutional animal care guidelines39. The mice in the OVX group and the sham surgery control group received saline orally.
Sample collection
Following an 8-week treatment protocol, the mice were humanely euthanized via isoflurane anaesthesia combined with cervical dislocation. Cardiac, hepatic, splenic, renal, and bilateral femoral/tibial tissues were collected for the imaging evaluation. Samples were preserved for examination via micro-CT imaging, immunohistochemical staining and immunofluorescence staining. Histopathological investigations were subsequently performed. Femoral metabolomic profiling, RNA extraction, transcriptomic data interpretation, and correlation studies were performed to analyse the samples and obtain insights. All experimental procedures strictly complied with institutional ethical guidelines and animal welfare regulations.
Micro-CT analysis of the femur
After fixation in 4% paraformaldehyde (Beyotime, P0099) at 4 °C for 48 hours, the femur samples were rinsed with PBS and dehydrated through a graded ethanol series. Subsequently, all tissue samples underwent micro-CT scanning with the following parameters: an X-ray tube current of 200 µA, voltage of 85 kV, full specimen scanning, scan resolution of 10.141270 μm, exposure time of 384 ms, and a scan angle of 180 degrees with a rotation step of 0.4°, and a frame averaging of 2. Upon completion of the scanning process, raw projection data were acquired automatically by the micro-CT system and reconstructed into three-dimensional images using NRecon software (version V1.7.4.2, Bruker, Germany). The reconstruction settings were adjusted with the following parameters: smoothing set to 2, beam hardening at 8, and ring artifacts at 25%. Further analysis was conducted using CT Analyser software (version 1.18.8.0, Bruker, Germany), where parameters such as bone mineral density (BMD) were calculated for the tissues.
Histopathological analysis
The hearts, livers, spleens, and kidneys obtained from both the sham surgery group and the treatment groups were preserved in 4% paraformaldehyde, embedded in paraffin after processing, and thereafter sectioned to an 8-micrometer thickness; the samples were stained with haematoxylin and eosin (H&E). The potential adverse effects of zingerone on the major organs of the mice was assessed through a microscopic examination. Femoral tissues devoid of muscle were rapidly collected, rinsed carefully in cold phosphate-buffered saline, the specimens were immersed in a 4% paraformaldehyde solution for a duration extending to twenty-four hours, decalcified in 10% EDTA solution (Servicebio, G1105) at pH 7.4 at room temperature for 2 weeks, with the solution refreshed every 2 days, until the samples became easily penetrable by a needle. After decalcification, the tissues were embedded in paraffin and sectioned at a thickness of 4 μm. Histopathological changes were evaluated using hematoxylin and eosin (H&E) staining, Goldner’s trichrome staining, and tartrate-resistant acid phosphatase (TRAP) staining under an optical microscope.
Immunofluorescence staining of bone tissue
Femoral tissues were fixed in 4% paraformaldehyde for 24 h, followed by decalcification in 10% EDTA solution (Servicebio, G1105) at pH 7.4 at room temperature for two weeks. The decalcifying solution was refreshed every two days, and completion was determined when the specimens could be easily penetrated by a needle. After decalcification, samples were dehydrated through a graded ethanol series and embedded in paraffin. Paraffin-embedded tissues were sectioned at a thickness of 4 μm, and antigen retrieval was performed by heating the sections in sodium citrate buffer (Servicebio, G1202) at 95 °C for 10 minutes. Following sectioning, a 5% bovine serum albumin solution was used to block nonspecific binding sites for one hour; subsequently, these sections were incubated overnight at a temperature of 4 °C with primary antibodies against the p53 (Proteintech, 60283-2-Ig, 1:200), Sat1 (Proteintech, 10708-1-AP, 1:200), Gpx4 (Proteintech, 67763-1-Ig, 1:200), OCN (Proteintech, 23418-1-AP, 1:200), and CTSK (Proteintech, 11239-1-AP, 1:200). The following day, the sections were washed and incubated with Alexa Fluor® 488-conjugated goat anti-rabbit IgG (Invitrogen, A-11008, 1:500) and Alexa Fluor® 594-conjugated goat anti-mouse IgG (Invitrogen, A-11005, 1:500), followed by nuclear counterstaining with DAPI (Beyotime, C1002). Fluorescence images were acquired using a Nikon AXR confocal microscope, Nikon corporation, Japan.
Immunohistochemical staining of bone tissue
After being embedded in paraffin and sectioned, the bone tissue was subjected to deparaffinization, rehydration, and antigen retrieval using 10 mM sodium citrate buffer (pH 6.0) at high temperature. A 10 minute incubation with a 3% H2O2 solution was performed to block endogenous peroxidase activity. The sections were blocked with 3% BSA at ambient temperature for half an hour. After blocking, the sections were incubated overnight at 4 °C with primary antibodies against P53 (Proteintech, 60283-2-Ig, 1:200), SAT1 (Proteintech, 10708-1-AP, 1:200), GPX4 (Proteintech, 67763-1-Ig, 1:200), and ACSL4 (Proteintech, 26658-1-AP, 1:200). The sections were incubated for 1 hour at room temperature with horseradish peroxidase (HRP)-conjugated secondary antibodies, including goat anti-rabbit IgG (CST, 7074S, 1:200) and goat anti-mouse IgG (CST, 7076S, 1:200). The sections were stained with a DAB substrate for visualization, and the nuclei were subsequently counterstained with haematoxylin. Afterwards, the sections then underwent dehydration processes and were mounted. The sections were imaged with an ECLIPSE TE300 microscope from Nikon in Japan.
Protein extraction from bone tissue
The femoral tissues used for protein extraction were obtained from the same mice described in the “Animals and drugs” and “Sample collection” sections, following the completion of the 8-week treatment protocol and humane euthanasia. The collected femurs were rapidly frozen in liquid nitrogen and ground into a fine bone powder using a prechilled mortar and pestle. The resulting bone powder was transferred to 1.5 mL Eppendorf tubes and lysed with precooled RIPA lysis buffer supplemented with 1% PMSF. Lysis buffer was added at a ratio of 500 μL per 50 mg of tissue. The samples were lysed on ice for 30 minutes with vigorous shaking for 10 seconds every 5 minutes to enhance disruption. The lysates were centrifuged for 15 minutes at 12,000 × g and 4 °C; the resulting supernatants were designated comprehensive protein extracts. Protein quantification was subsequently conducted using the BCA assay. The extracted proteins were then subjected to SDS‒PAGE followed by Western blot analysis.
Isolation and culture of primary bone marrow-derived macrophages (BMMs)
Femurs and tibiae used for BMM isolation were collected from the same cohort of C57BL/6 mice described earlier, immediately after euthanasia at the end of the treatment period. Using MEM supplemented with 10% foetal bovine serum, bone marrow cells were extracted from the medullary cavities. The resulting cellular suspension was filtered with a strainer designed for particles measuring 70 micrometres before culture with MEM. This medium included 10% foetal bovine serum supplemented with 1% penicillin‒streptomycin and 30 ng/mL M-CSF. The cells were cultured at 37 °C in a humidified incubator with 5% CO₂. After 3 days, nonadherent cells were removed, and adherent cells were maintained as BMMs for subsequent differentiation or staining experiments.
TRAP staining for osteoclast differentiation
Bone marrow-derived macrophages (BMMs) were induced to differentiate into osteoclast-like cells over a 7-day period by culturing them in MEM supplemented with 30 ng/mL M-CSF and 50 ng/mL RANKL. Postinduction protocols included brief fixation with 4% paraformaldehyde for ten minutes, followed by rinses with PBS. TRAP staining solution (Servicebio, G1050) was added, and the samples were incubated at 37 °C in the dark for 1 hour until a reddish-purple precipitate indicative of TRAP activity became visible. TRAP-positive multinucleated cells were visualized and quantified under an ECLIPSE TE300 microscope (Nikon, Japan) to assess osteoclastogenesis.
Processing and analysis of the femoral metabolomic data
Novogene Co. Ltd. in Beijing, China, conducted the extraction, analysis, data processing, and identification of mouse femoral metabolites. The LC‒MS/MS analysis was performed using a Vanquish UHPLC system (Thermo Fisher, Germany) coupled with a Q Exactive HF‒X mass spectrometer (Thermo Fisher, Germany). Differentially abundant metabolites were identified based on the following criteria: VIP > 1.0, FC > 1.5 or FC < 0.667, and P value < 0.05. The data were transformed using metaX, followed by partial least squares discriminant analysis (PLS-DA) and principal component analysis (PCA) to reveal differences in metabolic patterns among the groups. Hierarchical clustering analysis (HCA) and metabolite correlation analysis were conducted to clarify the relationships between samples and among metabolites. Functional analyses, including a metabolic pathway analysis, were performed to interpret the biological significance of the metabolite correlations. Q2, which represents the model’s predictive ability, was calculated through cross-validation (CV). A high Q2 value indicates strong predictive power, and R2 values close to 1 suggest a well-fitted model.
RNA extraction and transcriptomic data analysis
Novogene Co., Ltd., in Beijing, China, conducted the RNA sequencing analysis of three replicates of mouse femoral tissue. The differential expression analysis was performed using the Limma package for two groups, each with three biological replicates. The differential expression analysis was performed using the Limma package in R, with a cut-off threshold of abs(log2) > 0.2. Genes with a adjp-value < 0.05 were deemed significantly differentially expressed. Significantly enriched genes among the DEGs were identified based on corrected p values less than 0.05. The statistical enrichment of annotated genes was performed using the KEGG pathway database and the clusterProfiler R package. Additionally, gene set enrichment analysis (GSEA) was conducted utilizing the clusterProfiler R package. Fold changes in gene expression were calculated for group comparisons, leading to the generation of gene lists based on |log2FC| changes. Pearson’s correlation analysis was performed to assess the correlations between genes and metabolites, with statistical significance set at a p value of less than 0.05.
Identification of transcription factor binding sites
The DNA sequence 1–2 kb upstream of the gene was considered the promoter sequence of the gene. The mouse Gpx4 and Sat1 genes were queried in the NCBI nucleotide database, and the 2 kb upstream DNA sequence from the starting point was selected as the promoter sequence of the gene. This study used the GRCm39 reference genome to predict and analyse potential p53 binding sites in the target promoter region. The Sat1 promoter is located on the X chromosome and ranges from 154,001,346 to 153,999,346 bases, whereas the Gpx4-related promoter is located on chromosome 10 and ranges from 79,880,999 to 79,882,999 bases; these sequences were downloaded and saved as FASTA files.
JASPAR is an open-source database of transcription factor-binding site information that records the DNA-binding preferences of transcription factors in six major classes of species (fungi, insects, nematodes, plants, chordates, and vertebrates) in the form of position frequency matrices (PFMs) and flexible TF models (TFFMs), which are derived mainly from collections of experimentally defined transcription factor-binding sites published in eukaryotes. The JASPAR database was used to analyse the potential binding sites for the p53 transcription factor at specific chromosomal loci. The analysis was conducted using reference genome version GRCm39.
Molecular docking
The crystal structure of the receptor protein was obtained from the UniProt protein database (https://www.uniprot.org/), and the 3D structure of the compound was acquired from PubChem (https://pubchem.ncbi.nlm.nih.gov/). The docking analysis was subsequently performed using AutoDock, and the results were visualized using PyMOL. The docking parameters were adjusted according to the binding energy of the ligand to assess the docking configurations.
Western blot analysis
The cells were lysed on ice in RIPA buffer for 10 minutes, followed by sonication and centrifugation at 14,000 g for 30 minutes at 4 °C. The resulting supernatants were collected, and the volumes were recorded. Protein concentrations were determined using a BCA assay kit. Proteins were separated on 12.5% SDS‒PAGE gels and transferred onto PVDF membranes. After blocking at room temperature for 1 hour, the membranes were incubated overnight with primary antibodies. The samples were then washed five times with TBST for 5 minutes each before being exposed to secondary antibodies for 1.5 hours at 4 °C. Primary antibodies against P53 (Proteintech, 60283-2-Ig, 1:2000), SAT1 (Proteintech, 10708-1-AP, 1:2000), GPX4 (Proteintech, 67763-1-Ig, 1:2000), MMP9 (Proteintech, 30592-1-AP, 1:2000), CTSK (Proteintech, 11239-1-AP, 1:2000), RUNX2 (Proteintech, 20700-1-AP, 1:2000), ALP (Proteintech, 55319-1-AP, 1:2000), BMP2 (Proteintech, 18933-1-AP, 1:2000), GSDMD-N (Affinity, DF13758, 1:2000), GSDMD (Affinity, AF4012, 1:2000), Beclin-1 (Proteintech, 11306-1-AP, 1:2000), LC3B (Proteintech, 14600-1-AP, 1:2000), caspase-12 (Affinity, AF5196, 1:2000), cleaved caspase-12 (Affinity, DF7735, 1:2000), and β-actin (Proteintech, 23660-1-AP, 1:2000) were used. Secondary antibodies, including goat anti-rabbit IgG (CST, 7074S, 1:2000) and goat anti-mouse IgG (CST, 7076S, 1:2000), were used at the same dilution. β-actin (43 kDa) served as the internal loading control. Enhanced chemiluminescence (ECL) was used for signal detection, and protein bands were visualized using a chemiluminescence imaging system (Analytik Jena AG). Band intensities were quantified using ImageJ software (version 1.8.0; NIH, USA).
Statistics and Reproducibility
GraphPad Prism 9.5.1 (San Diego, California, USA) was used for statistical analysis. Comparisons between multiple groups were conducted using one-way analysis of variance (ANOVA). Significance levels are indicated as follows: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the model group; ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to the control group; ^p < 0.05 and ^^p < 0.01 compared to the treatment group. Data are presented as the mean ± standard deviation (SD). All animal experiments were independently repeated at least twice, with six mice per group in each experiment. All in vitro experiments were performed in triplicate using independent cultures. All data shown in figures are biological replications rather than technical replications.
Ethical approval
All animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of China Medical University (Approval No. 2019014). We have complied with all relevant ethical regulations for animal use.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Supporting data can be requested by contacting the corresponding author. The RNA-seq sequencing data are available on the Gene Expression Omnibus (GEO) of the National Center for Biotechnology Information (NCBI) under the accession number GSE304652. The metabolomics sequencing data are available on the MetaboLights database of the European Bioinformatics Institute (EBI) under the accession number MTBLS12829. Source data in this study can be found in supplementary data 1–8. Figure S1 can be found in supplementary material. All whole-slide scans of bone sections are compiled in Figure S2, which is also available in the supplementary material. The original WB images can likewise be found in the supplementary material.
References
Lin, H. et al. 4D label-free quantitative proteomics analysis to screen potential drug targets of Jiangu Granules treatment for postmenopausal osteoporotic rats. Front Pharm. 13, 1052922 (2022).
Delmas, P. D. Treatment of postmenopausal osteoporosis. Lancet 359, 2018–2026 (2002).
Black, D. M. & Rosen, C. J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med 374, 254–262 (2016).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama 288, 321–333 (2002).
Kuehn, B. M. Long-term risks of bisphosphonates probed. Jama 301, 710–711 (2009).
Nishikawa, A., Ishida, T., Taketsuna, M., Yoshiki, F. & Enomoto, H. Safety and effectiveness of daily teriparatide in a prospective observational study in patients with osteoporosis at high risk of fracture in Japan: final report. Clin. Inter. Aging 11, 913–925 (2016).
Zhao, J. et al. Exploration of the Molecular Mechanism of Polygonati Rhizoma in the Treatment of Osteoporosis Based on Network Pharmacology and Molecular Docking. Front Endocrinol. (Lausanne) 12, 815891 (2021).
Xia, H. et al. Integrated Strategy of Network Pharmacological Prediction and Experimental Validation Elucidate Possible Mechanism of Bu-Yang Herbs in Treating Postmenopausal Osteoporosis via ESR1. Front Pharm. 12, 654714 (2021).
Li, H. et al. Extracellular Vesicles from Human Urine-Derived Stem Cells Ameliorate Particulate Polyethylene-Induced Osteolysis. Int J. Nanomed. 16, 7479–7494 (2021).
Han, Y. A. et al. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother. Res 27, 1200–1205 (2013).
Grzanna, R., Lindmark, L. & Frondoza, C. G. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J. Med Food 8, 125–132 (2005).
Arcusa, R. et al. Potential Role of Ginger (Zingiber officinale Roscoe) in the Prevention of Neurodegenerative Diseases. Front Nutr. 9, 809621 (2022).
Ahmad, B. et al. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). ScientificWorldJournal 2015, 816364 (2015).
Muehlschlegel, J. D. et al. Using next-generation RNA sequencing to examine ischemic changes induced by cold blood cardioplegia on the human left ventricular myocardium transcriptome. Anesthesiology 122, 537–550 (2015).
Hasin, Y., Seldin, M. & Lusis, A. Multi-omics approaches to disease. Genome Biol. 18, 83 (2017).
Liu, M. et al. Curculigoside attenuates oxidative stress and osteoclastogenesis via modulating Nrf2/NF-κB signaling pathway in RAW264.7 cells. J. Ethnopharmacol. 275, 114129 (2021).
Tao, L. et al. Exerkine FNDC5/irisin-enriched exosomes promote proliferation and inhibit ferroptosis of osteoblasts through interaction with Caveolin-1. Aging Cell. 23, e14181 (2024).
Stockwell, B. R. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285 (2017).
Wu, D. J. et al. Autophagy-linked FYVE containing protein WDFY3 interacts with TRAF6 and modulates RANKL-induced osteoclastogenesis. J. Autoimmun. 73, 73–84 (2016).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Manolagas, S. C. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 31, 266–300 (2010).
Chandi, C. M. et al. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem. J. 433, 393–402 (2010).
Chi, Z. et al. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 163, 114834 (2023).
Mengyu, L. et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 61, 102649 (2023).
Yu Z., Jianhong Y. Activation of the PI3K/Akt pathway by oxidative stress mediates high glucose-induced increase of adipogenic differentiation in primary rat osteoblasts. J. Cell. Biochem. 114, 2595–2602 (2013).
Tareck, R. & Stéphanie, L. High Glucose Level Impairs Human Mature Bone Marrow Adipocyte Function Through Increased ROS Production. Front. Endocrinol. 10, 607–621 (2019).
Hiroki, E. et al. Role of tissue factor in delayed bone repair induced by diabetic state in mice. Plos One. 16, e0260754 (2021).
Badgley MA et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Purroy, R., Medina-Carbonero, M., Ros, J. & Tamarit, J. Frataxin-deficient cardiomyocytes present an altered thiol-redox state which targets actin and pyruvate dehydrogenase. Redox Biol. 32, 101520 (2020).
Ou, Y., Wang, S. J., Li, D., Chu, B. & Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl Acad. Sci. USA 113, E6806–e6812 (2016).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Zhong, Z. et al. NFATc1-mediated expression of SLC7A11 drives sensitivity to TXNRD1 inhibitors in osteoclast precursors. Redox Biol. 63, 102711 (2023).
Liu, Y. & Gu, W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ. 29, 895–910 (2022).
Li, J. et al. Herba Patriniae and its component Isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed. Pharmacother. 168, 115690 (2023).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Zeng, C. et al. SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling. Cancer Sci. 113, 3766–3775 (2022).
Qu, X., Sun, Z., Wang, Y. & Ong, H. S. Zoledronic acid promotes osteoclasts ferroptosis by inhibiting FBXO9-mediated p53 ubiquitination and degradation. PeerJ 9, e12510 (2021).
Molavinia, S., Nikravesh, M., Pashmforoosh, M., Vardanjani, H. R. & Khodayar, M. J. Zingerone Alleviates Morphine Tolerance and Dependence in Mice by Reducing Oxidative Stress-Mediated NLRP3 Inflammasome Activation. Neurochem Res 49, 415–426 (2024).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 32200943) and the Shenyang Natural Science Foundation (grant no. 22-321-32-08).
Author information
Authors and Affiliations
Contributions
H.L.: Project administration, validation, and writing – original draft. FM.C: Conceptualization, data curation, methodology, software, supervision, writing and editing original draft. D.L.: Investigation, data curation, and formal analysis. L.T.: Conceptualization, methodology, resources, writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Xiaofeng Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Rosie Bunton-Stasyshyn & David Favero. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Cao, F., Liu, D. et al. Zingerone treats postmenopausal osteoporosis via increased ferroptosis sensitivity by p53-mediated regulation of SAT1 and GPX4 expression. Commun Biol 8, 1367 (2025). https://doi.org/10.1038/s42003-025-08751-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-08751-z