Abstract
Vascular calcification is tightly associated with cardiometabolic risk events. PDZK1 (PDZ domain containing 1) has been implicated in protecting from atherosclerosis, however, its relationship with vascular calcification remains unclear. Here we show that the expression levels of PDZK1 are notably boosted in calcified mice aortas, human and mouse vascular smooth muscle cells (VSMCs). PDZK1 deficiency in mice improves aortic calcification mediated by excessive vitamin D3 (VitD3). Consistently, blocking PDZK1 in human and mouse VSMCs effectively alleviates vascular calcification caused by high phosphate (Pi). PDZK1 overexpression in vitro promotes vascular calcification. Mechanistically, PDZK1 positively regulates the expression of β-catenin and its phosphorylated form at serine 552 (p-β-cateninSer552). Rescue experiments confirm that β-catenin is a critical mediator through which PDZK1 exacerbates vascular calcification. PDZK1 extends the half-life of β-catenin and p-β-cateninSer552 by blocking their ubiquitination and degradation. Furthermore, inhibition of PDZK1 attenuates high Pi-induced nuclear translocation of β-catenin whereas PDZK1 overexpression facilitates this process. More deeply, we reveal the direct interaction between PDZK1 and β-catenin is mediated by binding of β-catenin to the PDZ1 domain of PDZK1. These findings elucidate a novel PDZK1/β-catenin axis in vascular calcification progression and provide a potential therapeutic target for treating cardiovascular calcification in high-risk populations.

Similar content being viewed by others
Introduction
Vascular calcification is an independent predictor of cardiovascular events, frequently observed in individuals with chronic renal failure, advanced age, diabetes, and atherosclerosis1. Vascular calcification primarily increases arterial stiffness and dysfunction, which consequently precipitates various cardiovascular complications, ultimately resulting in mortality2. Although using phosphate binders and calcimimetic agents can slow the progression of middle-level aortic calcification in patients with chronic kidney disease3,4, there is currently no causal therapeutic schedule for vascular calcification2. Some experimental drugs in the treatment of vascular calcification are still controversial or lack human experimental evidence5. Hence, a deeper understanding of the mechanistic details underlying vascular calcification’s pathological molecular mechanisms is required to improve therapeutic strategies.
The base mechanisms of current therapies for vascular calcification are the abnormal deposition of calcium and phosphorus6,7,8. PDZK1 (also known as Na+/H+ exchanger regulatory factor 3) has been identified to stabilize renal phosphate transporters, thereby modulating phosphate homeostasis9, the disruption of which constitutes a key mechanism underlying vascular calcification10,11. As a scaffold protein with four PDZ domains12, PDZK1 stabilizes other proteins or promotes their subcellular localization13. To date, the relationship between PDZK1 and cardiovascular diseases has mainly focused on the resistance to atherosclerosis14. PDZK1 is crucial for the trafficking of high-density lipoprotein (HDL) in atherosclerosis by interacting with class B type 1 scavenger receptor (SR-B1) through its PDZ1 and PDZ3 domains15. Notably, although PDZK1 has been identified to inhibit VSMCs proliferation by targeting breakpoint cluster region kinase (Bcr), subsequently preventing arteries from thickening16, the effect of PDZK1 on VSMCs in the vascular calcification process remains unexplored.
VSMCs differentiate into osteoblastic/chondroblast-like cells under calcified stimulation, partially resembling the osteogenesis or chondrogenesis process17. β-catenin, as a pivotal component of pro-osteogenic signaling pathways, has been identified to participate in the regulation of vascular calcification18,19. The activation of β-catenin signaling enhances the promoting effect of periostin20 and warfarin21 on VSMCs calcification. The ubiquitin-proteasome degradation of β-catenin inhibits the calcified marker proteins’ transcription22. The nuclear accumulation of β-catenin, driven by its stabilization, is key to its function23. Phosphorylation of β-catenin at N-terminal Serine 33/37 and Threonine 41 residues (S33/S37/Thr41) (p-β-cateninS33/S37/T41) leads to its ubiquitylation and proteasomal degradation24. Conversely, AMPK phosphorylates β-catenin at Serine 552 (p-β-cateninSer552), stabilizing β-catenin and promoting its nuclear translocation25. Interestingly, there is a PDZ-like target sequence (-D-T-D-L), also known as the type 1 PDZ-binding motif, in the C-terminal region of β-catenin26,27,28. The PDZ protein Erbin directly interacts with the C-terminal PDZ-binding motif of β-catenin28. This study investigates whether PDZK1 interacts with β-catenin and subsequently influences vascular calcification, which constitutes the primary focus of this research.
In the current study, we observe that PDZK1 is upregulated in the calcified mouse aortas, human and mouse VSMCs. The knockdown of PDZK1 attenuates the calcification in both mice and human VSMCs. Mechanically, PDZK1 enhances the stability of β-catenin and p-β-cateninSer552. PDZK1 promotes the nuclear translocation of β-catenin, ultimately aggravating the calcification of mouse VSMCs. Crucially, it is demonstrated that PDZK1 directly interacts with β-catenin via its PDZ1 domain. This study describes a novel excitatory factor, PDZK1, of vascular calcification, which provides a promising target for the therapy of vascular calcification.
Results
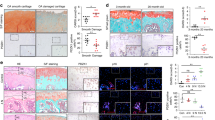
PDZK1 expression is elevated in calcified MOVAS cells and mouse arteries
PDZK1 has been demonstrated to be mainly expressed in endothelium29. Yet, recent evidence showed that PDZK1 is also present in VSMCs16. To explore the correlation between PDZK1 and vascular calcification, the expression of PDZK1 in VSMCs was determined. The observation showed that PDZK1 and the VSMCs marker smooth muscle actin α (α-SMA) were co-expressed in the medial arteries (Supplementary Fig. 1a). Vascular calcification was induced in C57BL/6J mice via subcutaneous injection of VitD3 (500,000 IU/kg/day) for 6 consecutive days, followed by regular feeding for 20 days (Fig. 1a), as previously described30,31. Aortic echocardiographic (ECHO) images revealed that the aortic wall in the VitD3 treatment group was thickened, exhibiting a rough texture and abnormal echo compared to the control group (Fig. 1b). Alizarin red S (ARS) staining of the whole aortas observed a larger purple area representing calcification on the aortas in the VitD3 treatment group compared to the control group (Fig. 1c). Additionally, the Von Kossa staining also showed pronounced brown calcium deposits in response to VitD3 treatment (Fig. 1d). These results indicated that the mice model of vascular calcification was successfully established. Using immunohistochemistry (IHC) analysis, we found that the protein level of PDZK1 was markedly elevated in the calcified aortas (Fig. 1e). Immunofluorescence (IF) staining revealed a more intense PDZK1 fluorescence signal in the aortic rings of VitD3-treated mice compared with those from the control group (Fig. 1f). Likewise, western blotting (WB) analysis demonstrated significantly increased expression of PDZK1 in the calcified aortas, coinciding with the upregulation of osteogenic markers runt-related transcription factor 2 (RUNX2), bone morphogenetic protein 2 (BMP2), Msh homeobox 2 (MSX2) (Fig. 1g). Similarly, mRNA expressions of PDZK1, RUNX2, BMP2, and MSX2 were also elevated in the calcified aortas compared to normal aortas (Fig. 1h).
a The 8–12-week mice were injected with 500,000 IU/kg of VitD3 for 6 consecutive days, followed by regular feeding for 20 days. b Echocardiographic images of aortas from C57BL/6 J mice fed for 20 days after injecting subcutaneously with a controlled solvent or VitD3 for 6 days continuously. Calcified areas are indicated by red arrows. n = 6/group. c–g Dissected the aortas from C57BL/6 J mice injected subcutaneously with a controlled solvent or VitD3. c Alizarin red staining. n = 3/group. d Von Kossa straining. n = 3/group. Scale bar, 200 µm. e IHC of PDZK1 expression. n = 3/group. Scale bar, 100 µm and 200 µm. f Immunofluorescence staining of PDZK1 expression. n = 3/group. g Representative western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). Data are presented as relative fold change to the Control. n = 3/group. h The mRNA levels of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2) in aortas were measured using qRT-PCR. n = 4/group. i Representative western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2) in MOVAS treated with or without Pi (2.6 mM) for 3, 5, or 7 days. Data are presented as a relative fold change to the control. n = 3/group. j The mRNA levels of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2) in MOVAS were measured using qRT-PCR, n = 4/group. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, ns, not significant. The error bar refers to the standard deviation (SD).
To further verify these observations, the mouse aortic smooth muscle cell line MOVAS was cultured in a high-Pi medium for 3, 5, and 7 days to induce VSMCs calcification. PDZK1 was detected to significantly increase in calcified MOVAS cells, accompanied by elevated protein levels of RUNX2, BMP2, and MSX2 (Fig. 1i). Correspondingly, the mRNA levels of PDZK1 and osteogenic markers were substantially elevated in calcified MOVAS cells (Fig. 1j). Stimulation of primary mouse VSMCs with high-Pi medium increased the expression of PDZK1 protein, along with elevated levels of calcification markers (Supplementary Fig. 2a). Similarly, human VSMCs (HVSMCs) were treated with a high-Pi medium to develop an in vitro calcification model. The successful model establishment was confirmed by both alkaline phosphatase (ALP) (Supplementary Fig. 3a) and ARS (Supplementary Fig. 3b) staining. Consistent with the results of mouse aortas and MOVAS cells, the transcriptional mRNA expression levels (Supplementary Fig. 3c) and the protein expression levels (Supplementary Fig. 3d) of PDZK1 and calcification marker factors (including RUNX2, BMP2 and MSX2) in HVSMCs stimulated by high-concentration Pi were significantly increased. These findings suggest an association between increased PDZK1 expression and calcification, both in mice and human VSMCs. However, the precise role of increased PDZK1 expression in vascular calcification remains unclear.
The increased expression of PDZK1 aggravates calcification both in vivo and in vitro
To further elucidate the role of increased PDZK1 in vascular calcification, the mice with deletion of the PDZK1 gene function (Pdzk1−/−)32 were constructed using CRISPR/Cas9 (Supplementary Fig. 4a). Genotypic identification (Supplementary Fig. 4b) and qRT-PCR analysis (Supplementary Fig. 4c) confirmed the successful knockout of PDZK1 in Pdzk1−/− mice. Consistently, WB analysis of multiple tissues from these mice showed significantly suppressed PDZK1 protein expression (Supplementary Fig. 4d–f). Then, 8-12-week-old wild-type (WT) and Pdzk1−/− mice were induced to vascular calcification. ECHO tests revealed a significantly reduced level of aortic wall vagueness and aberrant signals in calcified Pdzk1−/− mice compared to WT mice (Fig. 2a). Additionally, ARS of the whole aortas showed that aortas in Pdzk1−/− mice with calcification exhibited smaller areas of purplish-red calcification versus calcified WT group (Fig. 2b). BMP2 is a critical positive regulator of vascular calcification and has been identified as a representative protein of vascular calcification33,34. IHC analysis revealed the reduced expression of BMP2 in the case of deletion of PDZK1 in the aortas (Fig. 2c). Moreover, WB results showed that PDZK1 knockout also decreased the protein expressions of BMP2, RUNX2, and MSX2 (Fig. 2d), suggesting that PDZK1 deficiency may attenuate vascular calcification.
a Echocardiographic images for WT and Pdzk1−/− mice with vascular calcification. Calcified areas are indicated by red arrows. n = 3/group. b–d Separate aortas from WT mice and Pdzk1−/− mice with or without VitD3. b Alizarin red staining of the whole aorta. n = 3/group. c IHC detected the expression of PDZK1 and BMP2. n = 3/group. Scale bar, 100 µm. d Representative Western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. e–g MOVAS cells transfected with siRNA of PDZK1 combined with or without Pi (2.6 mM) for 7 days. e Representative western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. f ALP staining, n = 3, and g ARS staining. n = 3. Scale bar, 100 µm. h–j MOVAS cells transfected with PDZK1 overexpression plasmid using PcDNA 3.1 as vector, combined with or without Pi (2.6 mM) for 7 days. h Representative western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. i ALP, n = 3, and j ARS staining was used to detect the calcified situation. n = 3. Scale bar, 100 µm. k–m HVSMCs transfected with siRNA of PDZK1 combined with or without Pi (2.6 mM) for 7 days. k Representative WB analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. l ALP staining and m ARS staining. n = 3. Scale bar, 500 µm. n–p HVSMCs transfected with PDZK1 overexpression plasmid using PcDNA 3.1 as vector, combined with or without Pi (2.6 mM) for 7 days. n Representative WB analysis and quantification of PDZK1, RUNX2, BMP2, and MSX2. n = 3. o ALP and p ARS staining were used to detect the calcified situation. n = 3. Scale bar, 500 µm. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, ns, not significant. The error bar refers to SD.
Furthermore, MOVAS cells transfected with siRNA of PDZK1 (si-PDZK1) and PDZK1 overexpression plasmid were induced calcification. We observed that the increased expressions of RUNX2, BMP2, and MSX2 caused by elevated Pi levels were down-regulated by the addition of PDZK1 siRNA (Fig. 2e). ALP and ARS assays indicated that PDZK1 knockdown alleviated high-Pi-induced MOVAS cells calcification (Fig. 2f, g). Overexpression of PDZK1 further promoted the increase of BMP2, RUNX2, and MSX2 proteins induced by high Pi (Fig. 2h). Also, the mRNA levels of BMP2, RUNX2, and MSX2 were elevated by overexpressed PDZK1 (Supplementary Fig. 5a). ALP and ARS assays also demonstrated that PDZK1 overexpression further exacerbated MOVAS cells' calcification induced by high-Pi treatment (Fig. 2i, j). To comprehensively investigate the role of PDZK1 in the calcification of HVSMCs, we also developed species-specific siRNA targeting human PDZK1 and corresponding overexpression plasmids. Our data demonstrated that PDZK1-specific siRNA effectively silenced PDZK1 expression, and this knockdown attenuated the Pi-induced upregulation of RUNX2, BMP2, and MSX2 (Fig. 2k). The anti-calcific effects of PDZK1 deficiency were further validated by reduced ALP activity (Fig. 2l) and diminished ARS-positive calcium deposition (Fig. 2m). Consistent to the results in MOVAS cells, the overexpression of PDZK1 promoted the expression of calcification markers in HVSMCs (Fig. 2n). The addition to HVSMCs of the PDZK1 overexpression plasmid significantly increased Pi-induced ALP activity (Fig. 2o) and the degree of ARS staining (Fig. 2p). Collectively, these data indicate that PDZK1 is involved in calcification both in mice aortas and VSMCs and that increased PDZK1 expression promotes the process of calcification.
PDZK1 aggravates calcification by activating β-catenin
The above results indicate that PDZK1 exacerbated aortic calcification in mice and VSMCs, yet the precise underlying mechanism remains elusive. Previous research demonstrated that the activation of β-catenin can significantly promote vascular calcification35, and β-catenin contains a PDZ-binding motif27. Therefore, to gain deeper insights into the molecular mechanism by which PDZK1 modulated VSMCs calcification, the relationship between PDZK1 and β-catenin was explored. With the increase in the expressions of the protein factors RUNX2, BMP2 and MSX2, which indicate calcification, elevated protein expressions of β-catenin and p-β-cateninSer552 were observed in the Pi-treated MOVAS cells, while p-β-cateninS33/S37/T41 decreased significantly in calcified cells (Fig. 3a). And PDZK1 deletion in Pdzk1−/− mice significantly decreased the protein expressions of total β-catenin and p-β-cateninSer552, instead of p-β-cateninS33/S37/T41, even in the absence of calcification stimuli (Fig. 3b). Additionally, in MOVAS cells subjected to PDZK1 knockdown, the protein expressions of both total β-catenin and p-β-cateninSer552 were also reduced, while the expression of p-β-cateninS33/S37/T41 remained unchanged (Fig. 3c). β-catenin is known to undergo cytoplasm-to-nucleus shuttling, with stabilization and nuclear translocation marking its activation36. Therefore, we determined the total and nuclear expression levels of β-catenin and p-β-catenin of MOVAS cells. Our results revealed a notable decline of nuclear β-catenin and p-β-cateninSer552 in MOVAS cells transfected with PDZK1 shRNA (sh-PDZK1), while nuclear p-β-cateninS33/S37/T41 levels remained largely unaffected (Fig. 3d). PDZK1 overexpression (PDZK1-OE) in MOVAS cells resulted in increased levels of total β-catenin and p-β-cateninSer552 (Fig. 3e), as well as the elevated nuclear levels of β-catenin and p-β-cateninSer552 (Fig. 3f). However, PDZK1 overexpression did not alter the total or nuclear level of p-β-cateninS33/S37/T41. Our findings suggest that PDZK1 contributes to calcification not by directly regulating p-β-cateninS33/S37/T41, but rather through its modulation of β-catenin and p-β-cateninSer552.
a–f Representative western blot analysis and quantification of β-catenin and its phosphorylated protein (p-β-cateninSer552 and p-β-cateninS33/S37/T41), n = 3. Data are presented as relative fold change to Control: a MOVAS cell treated with or without Pi (2.6 mM) for 3, 5, or 7 days. b aortas from Pdzk1−/− mice vs WT mice. c, d The expression of total and nuclear β-catenin and its phosphorylated protein of sh-PDZK1 vs NC. e, f the expression of total and nuclear β-catenin and its phosphorylated protein of PDZK1-OE vs vector. g, h MOVAS transfected with shRNA of PDZK1 and treated with β-catenin recombinant protein combined with or without Pi (2.6 mM) for 7 days. g Representative Western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. h ALP straining. n = 3. i, j MOVAS transfected with PDZK1 overexpression lentivirus and treated with E7386 (an inhibitor of β-catenin) combined with or without Pi (2.6 mM) for 7 days. i Representative western blot analysis and quantification of PDZK1 and osteogenic markers (RUNX2, BMP2, and MSX2). n = 3. j ALP straining. n = 3. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, ns, not significant. The error bar refers to SD.
To assess whether β-catenin acts as a key intermediary in PDZK1-regulated vascular calcification, the recombinant protein of β-catenin (β-catenin) was administered alongside PDZK1 knockdown. Our results showed that the addition of β-catenin recombinant protein could promote the nuclear function of β-catenin to a large extent, specifically manifested in increasing the mRNA levels of downstream RUNX2, BMP2 and MSX2 (Supplementary Fig. 6a) and promoting the transcriptional activity of TCF/LEF (Supplementary Fig. 6b). The reduction of calcification markers (RUNX2, BMP2, and MSX2) protein levels induced by PDZK1 knockdown was further exacerbated in response to the addition of β-catenin recombinant protein (Fig. 3g). The ALP results also demonstrated that the addition of recombinant β-catenin protein rescued the suppression of calcification caused by PDZK1 deficiency (Fig. 3h). E7386, an inhibitor targeting the C-terminal domain of β-catenin, selectively blocks the interaction between β-catenin’s C-terminus and the transcriptional coactivator CREB-binding protein37. MOVAS cells transfected with PDZK1 overexpression plasmid were treated with E7386 (100 nM) for 24 hours, then cultured in Pi-containing medium. The results of WB assays showed that the addition of E7386 mitigated PDZK1 overexpression-worsened MOVAS cells calcification (Fig. 3i). ALP assays indicated that E7386 ameliorated the blue calcified area induced by PDZK1 overexpression (Fig. 3j). These findings suggest that PDZK1 promotes VSMCs calcification through regulating the C-terminal activation of β-catenin.
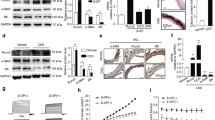
PDZK1 inhibits the ubiquitination of β-catenin and prolongs its half-life
The stabilization of β-catenin facilitates its nuclear transport and induces the transcription of target genes24. β-catenin undergoes ubiquitination-mediated degradation in the absence of Wnt signaling. Therefore, we explored whether PDZK1 stabilizes β-catenin. Cycloheximide (CHX) is a well-known inhibitor of protein synthesis38. Our results revealed that the half-life of β-catenin and p-β-cateninSer552 were significantly shorten by blocking PDZK1 expression (Fig. 4a), while the stability of p-β-cateninS33/S37/T41 remained unchanged (Fig. 4a and Supplementary Fig. 7a). PDZK1 overexpression extended the half-life of β-catenin and p-β-cateninSer552 (Fig. 4b), exerting no significant impact on p-β-cateninS33/S37/T41 (Fig. 4b and Supplementary Fig. 7b). Subsequently, Our data demonstrated that PDZK1 deficiency prevented MG132-induced accumulation of β-catenin and p-β-cateninSer552 (Fig. 4c), indicating that PDZK1 loss promotes ubiquitination-dependent degradation of both β-catenin and p-β-cateninSer552. This effect does not exist on p-β-cateninS33/S37/T41 (Fig. 4c and Supplementary Fig. 7c). Conversely, PDZK1 overexpression markedly enhanced the stabilization of β-catenin and p-β-cateninSer552 (Fig. 4d), while leaving p-β-cateninS33/S37/T41 levels unaltered (Fig. 4d and Supplementary Fig. 7d).
a–d Representative western blot analysis and quantification of β-catenin and its phosphorylated protein (p-β-cateninSer552 and p-β-cateninS33/S37/T41) in MOVAS treated with CHX or MG132. n = 3. CHX (a) and MG132 (c) treated MOVAS transfected with shRNA of PDZK1. CHX (b) and MG132 (d) treated MOVAS transfected with PDZK1 overexpression lentivirus. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, ns, not significant. e Cell lysates from MOVAS cultured with calcified medium after treating with MG132 for 6 hours were pulled down with anti-β-catenin Abs, followed by IB with anti-ubiquitin Abs. n = 3. f Cell lysates from MOVAS transfected with PDZK1 overexpression lentivirus combined with MG132 were pulled down with anti-β-catenin Abs, followed by immunoblotting (IB) with anti-ubiquitin Abs. n = 3. g Cell lysates from MOVAS transfected with PDZK1 ShRNA combined with MG132 were pulled down with anti-β-catenin Abs, followed by immunoblotting (IB) with anti-ubiquitin Abs. n = 3. The error bar refers to SD.
Then, Coimmunoprecipitation (Co-IP) was utilized to determine β-catenin ubiquitination in response to high-Pi stimulation and different PDZK1 levels. Results indicated that Pi stimulation decreased β-catenin ubiquitination (Fig. 4e and Supplementary Fig. 7e). Additionally, PDZK1 overexpression was found to decrease β-catenin ubiquitination levels, suggesting that PDZK1 suppresses ubiquitination-mediated degradation of β-catenin (Fig. 4f and Supplementary Fig. 7f). PDZK1 siRNA treatment significantly enhanced the association between β-catenin and ubiquitin molecules, suggesting that depletion of PDZK1 promotes β-catenin ubiquitination (Fig. 4g and Supplementary Fig. 7g). Collectively, these observations indicated that PDZK1 prolongs the half-life of β-catenin by inhibiting its ubiquitination degradation.
PDZK1 promotes the nuclear translocation of β-catenin
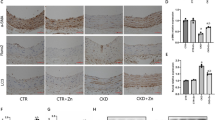
A recent study has demonstrated that suppressing the nuclear translocation of β-catenin inhibits osteogenic differentiation in VSMCs39. In MOVAS cells subjected to PDZK1 knockdown or overexpression, we cultured the cells in either standard growth medium or Pi-containing calcification-inducing medium. Subsequently, cytoplasmic proteins and nuclear fraction proteins were extracted to examine the nuclear translocation status of both PDZK1 and β-catenin. We detected that the high-Pi medium-induced nuclear accumulation of β-catenin was inhibited by silencing PDZK1 in the MOVAS cells (Fig. 5a, b). Conversely, PDZK1 overexpression enhanced the nuclear protein level of β-catenin in high-Pi conditions compared to the empty vector group (Fig. 5c, d). Furthermore, to elucidate whether PDZK1 promotes VSMCs calcification through regulating the nuclear translocation of β-catenin, primary VSMCs were isolated from mice aortas and cultured. Increased nuclear translocation of β-catenin and PDZK1 was observed in VSMCs under calcified conditions, which was reversed by PDZK1 siRNA transfection (Fig. 5e). Overexpressed PDZK1 exhibited heightened nuclear fluorescence of both β-catenin and PDZK1 in response to a high-Pi medium (Fig. 5f). Collectively, these results indicate that PDZK1 facilitates the nuclear translocation of β-catenin.
a–d Representative western blot analysis and quantification of β-catenin and PDZK1 in MOVAS cytoplasmic, nuclear fractions transfected with shRNA of PDZK1 (a, b) and PDZK1 overexpression lentivirus (c, b), and then treated with Pi (2.6 mM) for 7 days. n = 3. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, ns, not significant. e, f Immunofluorescence images of primary mouse VSMCs transfected with PDZK1 siRNA (e) and DZK1-OE plasmids (f) cultured with Pi (2.6 mM) for 7 days. n = 3, Scale bar, 20 µm and 40 µm. The error bar refers to SD.
PDZK1 binds directly to β-catenin via its PDZ1 domain
Although it has been established that PDZK1 regulates the stability of β-catenin, the precise mechanism underlying their interaction remains to be elucidated. Previous studies have indicated that a PDZ-like target sequence (-D-T-D-L) is located in the C-terminal region of β-catenin, implicating that PDZK1 may interact with β-catenin through PDZ domains26,27,28. Co-IP technology was used to analyze the interaction between PDZK1 and β-catenin, and the observation revealed that endogenous PDZK1 binds to β-catenin (Fig. 6a and Supplementary Fig. 8a). Consistently, PDZK1 was readily detectable in β-catenin immunoprecipitates (Fig. 6b and Supplementary Fig. 8b), indicating a physical interaction between PDZK1 and β-catenin. Further investigation under Pi stimulation indicated stronger binding of PDZK1 to β-catenin in MOVAS cultured in the calcified medium compared to those in the normal growth medium (Fig. 6c and Supplementary Fig. 8c, d). Meanwhile, the overexpression of PDZK1 also significantly altered this binding affinity (Fig. 6d and Supplementary Fig. 8e, f). These results collectively indicate that the association between PDZK1 and β-catenin is enhanced under pathological calcifying conditions and can be further augmented by elevated PDZK1 expression.
a, b Co-IP assay of PDZK1 and β-catenin in MOVAS. The lysates were immunoprecipitated with control IgG antibody, anti-PDZK1 antibody (a), or anti-β-catenin antibody (b), followed by immunoblotting (IB) with the indicated antibodies. n = 3. c, d Co-IP assay of PDZK1 and β-catenin in MOVAS cultured with Pi (2.6 mM) for 7 days (c) and transfected with PDZK1 overexpression lentivirus (d). The lysates were immunoprecipitated with control IgG antibody, anti-PDZK1 antibody, followed by IB with anti-β-catenin Abs. n = 3. e Schematic illustration of PDZK1 full length and PDZ1, PDZ2, PDZ3, PDZ4 domain deficiency. f Co-IP assay of PDZK1 domains binding to β-catenin in MOVAS. n = 3. The error bar refers to SD.
To further examine the specific domain responsible for their binding, plasmids lacking specific PDZ domains were constructed, as illustrated in (Fig. 6e). Then, full-length PDZK1 and plasmids lacking PDZ1, PDZ2, PDZ3, and PDZ4 domains were transfected into MOVAS cells. Co-IP analysis indicated that the deletion of PDZ1 blocked the binding of β-catenin, while the deletion of PDZ2, PDZ3, and PDZ4 did not influence the binding (Fig. 6f and Supplementary Fig. 8g). These findings provide compelling evidence that PDZK1 primarily interacts with β-catenin via the PDZ1 domain, highlighting the critical role of this domain in the interaction. Thus, these results suggest that PDZK1 interacts directly with β-catenin.
Discussion
This study explored the essential role of PDZK1 in promoting vascular calcification. Our results detected elevated expression of PDZK1 in calcifying mice aortas and VSMCs. The increase of PDZK1 aggravates VSMCs calcification by enhancing the protein level of β-catenin. PDZK1 is identified in this study as an important regulator of β-catenin and is involved in the ubiquitination and nuclear translocation of β-catenin. Mechanically, PDZK1 binds to β-catenin directly, and the combination of PDZK1 and β-catenin relies on the PDZ1 domain on PDZK1. These findings indicate that PDZK1 is a novel target for vascular calcification, and inhibiting its PDZ1 domain might be a promising therapy for vascular calcification by blocking the accumulation of β-catenin.
Vascular calcification, closely associated with increased cardiovascular events mortality40, is an active process driven by the imbalance between calcium agonists and inhibitors41, particularly elevated serum phosphorus11. Consistently, our results also confirm that high-Pi stimulation in vitro leads to the calcification of VSMCs. The plasma Pi concentration is mainly regulated by sodium-dependent phosphate transporters of the kidney, including NaPi-IIa and NaPi-IIc42. NaPi-IIa and NaPi-IIc have been demonstrated to combine with PDZK19. The Pdzk1−/− mice fed a high-Pi diet showed hyperphosphaturia and attenuated expression of NaPiIIa43. Although PDZK1 has been extensively studied as a key component of the kidney phosphorus transporter, its role in vascular calcification has not been reported. In the current study, we found that PDZK1 increased considerably in calcified VSMCs and aortas of mice. Also, PDZK1 deletion prevents calcification both in vitro and in vivo, while the overexpression of PDZK1 enhances the calcification process of VSMCs. However, these results contrast with previous studies demonstrating that PDZK1 deficiency aggravates atherosclerosis in the apolipoprotein E knockout (Apoe−/−) mice44. We propose that these differences may be associated with the type of cells used. Yu et al. found that PDZK1 in leukocytes protects against apoptosis and necrotic core development in atherosclerotic plaques14. PDZK1 deletion in macrophages has also been demonstrated to inhibit atherosclerosis45. Atherosclerosis primarily involves intimal vascular calcification46. Indeed, we observed that the increased PDZK1 in VSMCs promotes the medial VSMCs calcification. Interestingly, despite the significant atherosclerotic manifestations of PDZK1 and Apoe double knockout mice, single PDZK1 knockout mice did not exhibit aggravated atherosclerosis47. However, our results suggest that the calcification of Pdzk1−/− mice aortas was improved compared to WT mice aortas. Notably, under identical VitD3 induction conditions, female mice exhibited attenuated vascular calcification compared to male counterparts (Supplementary Fig. 9a–c). Our results are consistent with prior clinical observations suggesting that men tend to have more diffuse coronary artery disease48 and exhibit a two-fold higher risk of calcified aortic valve disease49 with more severe calcification at equivalent stenosis levels50,51. The observed differences in calcification levels between sexes may underlie disparities in hormonal profiles52. However, the protective effect of PDZK1 deficiency on calcification severity was detected in both sexes (Supplementary Fig. 9a–c). Mechanistically, PDZK1’s estrogen-responsive nature53,54 and elevated expression in female aortic tissues (consistent with ER-positive breast cancer studies53) create a paradox: while estrogen upregulates PDZK1—a potential pro-calcific factor—it concurrently exerts dominant anti-calcific effects through alternative pathways (e.g., enhanced NO bioavailability, reduced oxidative stress). This dual regulatory role may explain why female mice, despite higher baseline PDZK1 expression, exhibit less severe calcification. Therefore, these findings indicate that calcification increases PDZK1, which intensifies calcification’s progress.
The Wnt/β-catenin pathway is established as the pivotal mechanism underlying vascular calcification55,56,57. Without Wnt signaling (Wnt-off), CK-1 and GSK-3β phosphorylate the Ser33/37/Thr41 of β-catenin, leading to its ubiquitination and degradation. Conversely, Wnt activation (Wnt-on) releases β-catenin from the Axin-APC-GSK-3β-CK-1 destruction complex, phosphorylating the Ser552 of β-catenin’s C-terminus and facilitating its nuclear translocation to activate downstream gene transcription58. Our results have confirmed the role of β-catenin in vascular calcification59. In agreement with the earlier investigations, we observed a remarkable increase of β-catenin and p-β-cateninSer552 in the calcified aortas induced by VitD322. However, the relationship between β-catenin and PDZK1 has not been previously investigated. Herein, this study shows the positive regulation of PDZK1 for β-catenin and p-β-cateninSer552, as their expression decreased upon PDZK1 knockdown and increased upon its overexpression, even under basal physiological conditions, suggesting that PDZK1 mediates the regulation of β-catenin and p-β-cateninSer552 under basal physiological conditions. However, the protein level of p-β-cateninS33/S37/T41 did not change significantly upon different PDZK1 levels. Notably, PDZK1 stabilizes β-catenin not only at the protein level but also modulates its transcriptional activity (Supplementary Fig.10a, b). Then, we investigated that the role of PDZK1 in the aggravation of VSMCs calcification depends on the β-catenin signaling. These results also suggested that the correlation between PDZK1 and β-catenin is universal and, thus, might be used for pathologic or physiological progress besides calcification.
Previous studies have shown that β-catenin binds to PDZ-domain-containing protein via its PDZ-binding motif (-D-T-D-L) on the C-terminal28. PDZK1 contains four PDZ domains: PDZ1, PDZ2, PDZ3, and PDZ460. Therefore, it is reasonable to assume that PDZK1 regulates β-catenin directly. The current study certainly confirmed this hypothesis. We found that endogenous PDZK1 directly binds to β-catenin. Additionally, our observation revealed that PDZK1 binds to β-catenin via its PDZ1 domain. The PDZ1 domain of PDZK1 is a small peptide containing 99 aa. Drugs targeting the PDZ1 domain are novel, promising therapeutic strategies for vascular calcification.
The stability and nuclear translocation of β-catenin are critical for Wnt/β-catenin signaling. Studies have demonstrated that FBXW2 inhibits tumor migration, invasion, and metastasis in lung cancer cells by targeting β-catenin for ubiquitination and degradation23. Moreover, β-catenin ubiquitination has been shown to mitigate vascular calcification22. Recent research has highlighted the significance of β-catenin nuclear translocation in cardiovascular diseases61. For example, CTRP3 inhibits β-catenin nuclear translocation to ameliorate vascular calcification39, and the protective function of ginsenoside Rb1 against vascular calcification is mediated through β-catenin nuclear translocation18. β-catenin ubiquitination and nuclear translocation are crucial targets for vascular calcification intervention. Consequently, our results showed that the combination between β-catenin and ubiquitin was decreased induced by high-Pi stimuli. Therefore, further research is needed to clarify whether the effect of PDZK1 on β-catenin depends on the ubiquitination and nuclear translocation of β-catenin.
This study elucidates that deleting PDZK1 shortens the half-life of β-catenin and p-β-cateninSer552, increasing the ubiquitination process of β-catenin and p-β-cateninSer552. Also, the half-life of β-catenin and p-β-cateninSer552 is prolonged in the presence of PDZK1 overexpression, which is found to inhibit β-catenin and p-β-cateninSer552 ubiquitination. Meanwhile, the Co-IP observation suggests that less ubiquitin binds to β-catenin when PDZK1 is increased, indicating that PDZK1 overexpression suppresses ubiquitin-mediated degradation of β-catenin. The accumulation of β-catenin has been identified to translocate into the nucleus to promote vascular calcification22. Consistently, our results suggest that the high-Pi condition promotes the nuclear translocation of β-catenin. Moreover, PDZK1 downregulation decreases the nuclear β-catenin and p-β-cateninSer552, while increased PDZK1 elevates β-catenin and p-β-cateninSer552 in the nucleus. Furthermore, we have observed more β-catenin translocated into the nucleus in the PDZK1-overexpressed group treated with the high-Pi medium. Notably, PDZK1-increased MOVAS cells also exhibit more nuclear translocation of β-catenin even without high-Pi stimulation. We preliminarily explored the function of the PDZK-β-catenin complex after entering the nucleus. The interaction between β-catenin and activating transcription factor 4 (ATF4) was revealed. ATF4 has been found to promote the transcription of osteogenic factors as a downstream binding transcription factor of β-catenin62. The absence of PDZK1 leads to the blocking of the interaction between β-catenin and ATF4 (Supplementary Fig. 11a), while overexpression of PDZK1 promotes the interaction between the two (Supplementary Fig. 11b). However, this relationship requires further verification to determine whether the PDZK1-β-catenin complex promotes the transcription of calcification promoters through ATF4 after entering the nucleus.
In summary, our findings elucidate the role of PDZK1 in mice vascular calcification driven by VSMCs. PDZK1 deficiency ameliorated vascular calcification in male mice and reduced the degree of calcium deposition in both mouse and human VSMCs. We further investigated the underlying mechanism by which PDZK1 regulates VSMCs' calcification. Our results demonstrated that PDZK1 directly inhibits the ubiquitination and enhances the nuclear translocation of β-catenin through its PDZ1 domain, ultimately inducing calcification in VSMCs. Notably, PDZK1 regulates the ubiquitination and nuclear translocation of β-catenin even in the absence of a high-Pi stimulus, indicating that PDZK1’s regulation of β-catenin might extend to other cellular processes mediated by β-catenin signaling. This study identifies PDZK1 as a novel endogenous promoter of vascular calcification. Importantly, PDZK1 is recognized as an endogenous adaptor protein that stabilizes β-catenin through its PDZ1 domain. The short length of PDZ domains simplifies the therapeutic targeting of vascular calcification using PDZ domains. Thus, we offer new insights into potential treatment strategies for diseases induced by β-catenin, including vascular calcification.
This study employed VitD3 overload to induce vascular calcification, an established method validated in prior research30,31. However, it is crucial to note that these validation studies have been conducted predominantly in rodent models, not humans. Consequently, there are certain difficulties in translating the findings of this study into the clinical treatment of vascular calcification. While VitD3 overload is model-specific, the identified PDZK1/β-catenin pathway provides crucial mechanistic insights into a common phenotypic switch in VSMCs, which might offer novel therapeutic targets for human vascular calcification. In addition, although the findings were validated in vitro using murine and human VSMCs, as well as in mouse aortas ex vivo, the absence of direct analysis in human aortic samples remains a limitation of this study. Future research will incorporate human aortic tissue to directly investigate the correlation between the degree of vascular calcification and PDZK1 levels.
Despite the initial insights into sexual dimorphism of vascular calcification, its primary focus remains on vascular calcification in male mice. Investigation of vascular calcification in females remains insufficient, especially since the underlying mechanism still needs to be verified. Consequently, future work should comprehensively examine PDZK1’s regulation of vascular calcification across sexes to clarify the impact of sex-specific factors and hormonal influences (e.g., estrogen).
Although our study provides a novel target for vascular calcification, the translational feasibility remains to be explored. We found that PDZK1 binds to β-catenin by its PDZ1 domain. Designing the anti-PDZ1 peptide can be a promising therapy for vascular calcification or other diseases induced by β-catenin. Additionally, PDZK1 is not a ubiquitin ligase. Thus, it is needed to reveal the ubiquitin ligase of the complex containing PDZK1 and β-catenin. Our findings reveal a Wnt signaling-independent regulatory role of PDZK1 in modulating β-catenin dynamics. This discovery necessitates further exploration of the PDZK1/β-catenin interplay under activated Wnt/β-catenin signaling conditions and its consequential effects on vascular calcification progression.
Methods
Animal experiments
C57BL/6J mice and Pdzk1−/− mice (provided by Professor Xiaochun Bai, Southern Medical University) were generated with a frameshift mutation in exon 3 (aa 180–256) by CRISPR/Cas9 technology32. Genotyping was performed using PCR with primers listed in Supplementary Table 1. All procedures were approved by the Biomedical Ethics Committee of Kunming Medical University and conducted under SPF conditions. Mice for surgeries were anesthetized with isoflurane (1.5–2%) and euthanized with cervical dislocation. We have complied with all relevant ethical regulations for animal use.
Considering that vascular calcification is associated with the male sex63 and PDZK1 is regulated by estrogen64, male C57BL/6 J mice (8–12 weeks, 20–25 g) were randomly assigned to control or VitD3 groups. The VitD3 group received subcutaneous injection of VitD3 (500,000 IU/kg/day) for 6 days. Mice were euthanized 20 days post-injection, and aortas were harvested for analysis. Sample size was determined based on previous studies30 and ethical guidelines. Group allocation was performed by an independent investigator. The allocation sequence was concealed from personnel involved in animal/subject enrollment. Caregivers/technicians administering interventions were aware of group assignments due to visually distinguishable features and minor weight variations between the two mouse groups. To minimize bias, all procedures were standardized. Outcome assessors who were blinded to group assignments evaluated the results. Unblinding occurred only after completion of primary analyses.
Cell culture
MOVAS cells purchased from SUNNCELL company were maintained in high-glucose DMEM with 10% fetal bovine serum (FBS). Primary mouse VSMCs were isolated from the thoracic aortas of C57BL/6 J mice via collagenase digestion and cultured in DMEM containing 15% FBS, 100 μg/ml mixture of penicillin and streptomycin, and 10 mg/ml type 2 collagenase for one night. The next morning, the cells were centrifuged and cultured with DMEM supplemented with 10% FBS All cells were maintained at 37 °C in water-saturated incubators with 5% CO2.
Reagents
Antibodies and other reagents are listed in the supplementary Table 4.
Plasmids, siRNAs, transfection and lentiviral infection
Plasmids, siRNAs, and lentiviruses for PDZK1 manipulation were obtained from GenePharma (Supplementary Table 3). Lipofectamine 2000 (Invitrogen) was used for transfection according to the manufacturer’s protocols. For gene knockdown or overexpress, cells were infected with the lentivirus and then selected with 1–2 μg/ml puromycin for stable cell lines, followed by various assays 96 h post-infection.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol reagent are reverse-transcribed to cDNA using 5× All-In-One Reverse Transcription Master Mix. qRT-PCR was performed using SYBR Green on an ABI QuantStudio 3 system. The data were analyzed by the ΔΔCT method, and all samples were normalized to β-actin. The primer sequences for target genes are listed in Supplementary Table 2.
Western blot analysis
Total proteins were lysed in RIPA buffer supplemented with phenylmethylsulfonyl fluoride and Protein phosphatase inhibitor. The cytoplasmic and nuclear proteins were extracted using the Nuclear Protein Extraction Kit. Equal amounts of protein mixed with were separated in an SDS-PAGE gel. The protein was subsequently transferred onto 0.45μm PVDF membranes, which were then blocked with 5% non-fat milk or BSA in TBST, followed by incubation with primary antibody at 4 °C overnight. The next day, after 1h incubation with HRP-conjugated secondary antibodies, images were obtained using a chemiluminescence instrument. Band intensity was analyzed with the ImageJ software.
Immunohistochemical staining
Aortic segments were cut into 3 μm in thickness using the technology of paraffin sectioning. Xylene and gradient alcohol were used to remove the paraffin. The sections were blocked with 10% normal goat serum and immediately incubated with primary antibodies or rabbit IgG at 4 °C overnight, followed by incubation with a horseradish peroxidase-labeled anti-rabbit IgG secondary antibody before staining with DAB. Nuclei were counterstained with hematoxylin.
IF staining
Paraffin sections of aortic segments were cut into 3 μm in thickness. The cells cultured in vitro were fixed with 4% paraformaldehyde and permeabilized with 0.3% Triton. The sections or cells were blocked with 10% normal goat serum and incubated with primary antibodies overnight, followed by incubation with fluorescent secondary antibody for 2 hours. The nucleus was stained using DAPI.
Von Kossa staining, ARS and ALP staining
For Von Kossa staining, the paraffin section of aortic segments was cut into 5 μm sections. The section was stained in accordance with the manufacturer’s protocol using a Von Kossa stain kit. The whole aorta from the aortic arch to the common iliac artery was isolated and fixed with 4% paraformaldehyde after the mice's euthanasia. The aortas were soaked overnight in 95% alcohol and subsequently soaked in a 2% ARS-KOH solution for 12 hours. The whole aortas were washed with 2% KOH until calcified areas of the aortas were purplish red, while the non-calcified areas remained milky white. Configured the ALP mixture according to the instructions and treated the fixed cells with ALP staining solution or 0.2% ARS staining.
Coimmunoprecipitation
The plasmid-transfected cells were collected and gently cleaved using lysate from the coimmunoprecipitation kit. Antibodies were added to the cell supernatant for incubation overnight, followed by protein A and G for incubation for 12 h. The precipitate was washed by the wash solution in the coimmunoprecipitation kit and added 20–40 μL loading buffer. Detected the combination by WB.
Echocardiography
Mice requiring an ECHO test were deprived of food and drink for 8 hours, and the chest hair was removed after anesthesia. The probe of the mouse ultrasonic ECG detector was coated with a coupling agent, and the mouse’s aortic arch was detected from the right side of the sternum.
TOP/FOP flash assay
To assess β-catenin-mediated transcriptional activity, MOVAS cells were transiently transfected with the TOP Flash (TCF/LEF-responsive luciferase reporter) or FOP Flash (mutant negative control) plasmids. At 24 h post-transfection, the cells were treated with lithium chloride for 24 h. Cells were treated with recombinant human β-catenin protein or vehicle control for an additional 24 h. Firefly luciferase reporter Gene Detection Kit was used to lyse cells and add fluorescein. The detection was carried out using a multi-functional microplate reader.
Statistics and reproducibility
The data are expressed as the mean ± standard deviation (SD). The significance of the data between the two experimental groups was determined by Student’s t test, and multiple group comparisons were analyzed by one-way ANOVA. All statistical analyses were two-sided, and different cutoff values, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.001 (****) were considered significant. Experiments included at least three biological replicates per group (n ≥ 3). All the statistical analyses were performed via GraphPad Prism 9.0 software for graphs.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that all the other data supporting the findings of this study are available within the paper and its supplementary data 1, and from the corresponding author upon reasonable request.
References
Lanzer, P. et al. Medial vascular calcification revisited: review and perspectives. Eur. Heart J. 35, 1515–1525 (2014).
Lanzer, P. et al. Medial arterial calcification: JACC state-of-the-art review. J. Am. Coll. Cardiol. 78, 1145–1165 (2021).
Chen, N.-C., Hsu, C.-Y. & Chen, C.-L. The strategy to prevent and regress the vascular calcification in dialysis patients. BioMed. Res. Int. 2017, 1–11 (2017).
Raggi, P. et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dialysis Transplant. 26, 1327–1339 (2011).
Tölle, M., Reshetnik, A., Schuchardt, M., Höhne, M. & Van Der Giet, M. Arteriosclerosis and vascular calcification: causes, clinical assessment and therapy. Eur. J. Clin. Investig. 45, 976–985 (2015).
Lomashvili, K. A., Monier-Faugere, M.-C., Wang, X., Malluche, H. H. & O’Neill, W. C. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int. 75, 617–625 (2009).
Toussaint, N. D., Lau, K. K., Strauss, B. J., Polkinghorne, K. R. & Kerr, P. G. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am. J. Kidney Dis. 56, 57–68 (2010).
Martin Vouri, S. & Taggart Blaszczyk, A. Bisphosphonate use in patients undergoing dialysis. Consult. Pharm. 28, 738–741 (2013).
Levi, M. et al. Mechanisms of phosphate transport. Nat. Rev. Nephrol. 15, 482–500 (2019).
Rutsch, F., Nitschke, Y. & Terkeltaub, R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ. Res. 109, 578–592 (2011).
Villa-Bellosta, R. Vascular calcification revisited: a new perspective for phosphate transport. CCR 11, 341–351 (2015).
Zhao, C. et al. Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. 453, 107–121 (2019).
Doyle, D. A. et al. Crystal structures of a complexed and peptide-free membrane protein–binding domain: molecular basis of peptide recognition by PDZ. Cell 85, 1067–1076 (1996).
Yu, P., Qian, A. S., Chathely, K. M. & Trigatti, B. L. PDZK1 in leukocytes protects against cellular apoptosis and necrotic core development in atherosclerotic plaques in high fat diet fed ldl receptor deficient mice. Atherosclerosis 276, 171–181 (2018).
Trigatti, B. L. SR-B1 and PDZK1: partners in HDL regulation. Curr. Opin. Lipidol. 28, 201–208 (2017).
Lee, W. R. et al. PDZK1 prevents neointima formation via suppression of breakpoint cluster region kinase in vascular smooth muscle. PLoS One 10, e0124494 (2015).
Chen, Y., Zhao, X. & Wu, H. Arterial stiffness: a focus on vascular calcification and its link to bone mineralization. Arterioscler. Thromb. Vasc. Biol. 40, 1078–1093 (2020).
Zhou, P. et al. Ginsenoside Rb1 ameliorates CKD-associated vascular calcification by inhibiting the Wnt/β-catenin pathway. J. Cell Mol. Med. 23, 7088–7098 (2019).
Zhang, T. et al. Moscatilin inhibits vascular calcification by activating IL13RA2-dependent inhibition of STAT3 and attenuating the WNT3/β-catenin signalling pathway. J. Adv. Res. 68, 445–457 (2024).
Alesutan, I. et al. Periostin augments vascular smooth muscle cell calcification via β-catenin signaling. Biomolecules 12, 1157 (2022).
Beazley, K. E., Deasey, S., Lima, F. & Nurminskaya, M. V. Transglutaminase 2–mediated activation of β-catenin signaling has a critical role in warfarin-induced vascular calcification. ATVB 32, 123–130 (2012).
Yang, L. et al. Unspliced XBP1 counteracts β-catenin to inhibit vascular calcification. Circ. Res 130, 213–229 (2022).
Yang, F. et al. FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation. Nat. Commun. 10, 1382 (2019).
Stamos, J. L. & Weis, W. I. The -catenin destruction complex. Cold Spring Harb. Perspect. Biol. 5, a007898–a007898 (2013).
Zhao, J., Yue, W., Zhu, M. J., Sreejayan, N. & Du, M. AMP-activated protein kinase (AMPK) cross-talks with canonical Wnt signaling via phosphorylation of β-catenin at Ser 552. Biochem. Biophys. Res. Commun. 395, 146–151 (2010).
Perego, C., Vanoni, C., Massari, S., Longhi, R. & Pietrini, G. Mammalian LIN-7 PDZ proteins associate with β-catenin at the cell–cell junctions of epithelia and neurons. EMBO J. 19, 3978–3989 (2000).
Gujral, T. S., Karp, E. S., Chan, M., Chang, B. H. & MacBeath, G. Family-wide investigation of PDZ domain-mediated protein–protein interactions implicates β-catenin in maintaining the integrity of tight junctions. Chem. Biol. 20, 816–827 (2013).
Ress, A. & Moelling, K. The PDZ protein erbin modulates β-catenin-dependent transcription. Eur. Surg. Res. 41, 284–289 (2008).
Kocher, O. Identification and partial characterization of pdzk1 a novel protein containing pdz interaction domains. Lab. Invest. 78, 117–125 (1998).
Chen, Y. et al. Nidogen-2 is a novel endogenous ligand of LGR4 to inhibit vascular calcification. Circ. Res. 131, 1037–1054 (2022).
Kang, Y. H., Jin, J. S., Yi, D. W. & Son, S. M. Bone morphogenetic protein-7 inhibits vascular calcification induced by high vitamin D in mice. Tohoku J. Exp. Med. 221, 299–307 (2010).
Shao, Y. et al. PDZK1 protects against mechanical overload-induced chondrocyte senescence and osteoarthritis by targeting mitochondrial function. Bone Res. 12, 41 (2024).
Kong, Y. et al. Hyaluronan negatively regulates vascular calcification involving BMP2 signaling. Lab. Investig. 98, 1320–1332 (2018).
Lu, Y. et al. Paraspeckle protein NONO attenuates vascular calcification by inhibiting bone morphogenetic protein 2 transcription. Kidney Int. 105, 1221–1238 (2024).
Zhu, W. et al. The Scavenger Receptor Class B Type I Adaptor Protein PDZK1 Maintains Endothelial Monolayer Integrity. Circ. Res. 102, 480–487 (2008).
Li, X. et al. The transcription factor GATA6 accelerates vascular smooth muscle cell senescence-related arterial calcification by counteracting the role of anti-aging factor SIRT6 and impeding DNA damage repair. Kidney Int. 105, 115–131 (2024).
Oliveira-Paula, G. H. et al. The β-catenin C terminus links Wnt and sphingosine-1-phosphate signaling pathways to promote vascular remodeling and atherosclerosis. Sci. Adv. 10, eadg9278 (2024).
Miao, Y. et al. Cycloheximide (CHX) chase assay to examine protein half-life. Bio Protoc. 13, e4690 (2023).
Liu, D., Cui, X., Lu, R., Hu, H. & Gu, G. CTRP3 is a coronary artery calcification biomarker and protects against vascular calcification by inhibiting β-catenin nuclear translocation to prevent vascular smooth muscle cell osteogenic differentiation. J. Cardiol. 79, 551–558 (2022).
Harper, E. et al. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vasc. Pharmacol. 82, 30–40 (2016).
Lee, S. J., Lee, I.-K. & Jeon, J.-H. Vascular calcification—new insights into its mechanism. IJMS 21, 2685 (2020).
Sasaki, S. et al. Tmem174, a regulator of phosphate transporter prevents hyperphosphatemia. Sci. Rep. 12, 6353 (2022).
Villa-Bellosta, R. et al. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int. 73, 456–464 (2008).
Yesilaltay, A., Daniels, K., Pal, R., Krieger, M. & Kocher, O. Loss of PDZK1 causes coronary artery occlusion and myocardial infarction in paigen diet-fed apolipoprotein E deficient mice. PLoS One 4, e8103 (2009).
Karunakaran, D. Beyond cholesterol homeostasis: a novel role for PDZK1 in macrophage apoptosis and atherosclerosis. Atherosclerosis 276, 168–170 (2018).
Smith, E. R., Hewitson, T. D. & Holt, S. G. Diagnostic tests for vascular calcification. Adv. Chronic Kidney Dis. 26, 445–463 (2019).
Kocher, O. et al. Influence of PDZK1 on lipoprotein metabolism and atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 1782, 310–316 (2008).
Kim, B. S. et al. Sex differences in coronary arterial calcification in symptomatic patients. Am. J. Cardiol. 149, 16–20 (2021).
Gomel, M. A., Lee, R. & Grande-Allen, K. J. Comparing the role of mechanical forces in vascular and valvular calcification progression. Front. Cardiovasc. Med. 5, 197 (2019).
Simard, L. et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation?. Circ. Res. 120, 681–691 (2017).
Nitsche, C., Koschutnik, M., Kammerlander, A., Hengstenberg, C. & Mascherbauer, J. Gender-specific differences in valvular heart disease. Wien. Klin. Wochenschr. 132, 61–68 (2020).
Lai, J. et al. Low serum testosterone level was associated with extensive coronary artery calcification in elderly male patients with stable coronary artery disease. Coron. Artery Dis. 26, 437–441 (2015).
Ghosh, M. G., Thompson, D. A. & Weigel, R. J. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 260, 6367–6375 (2000).
Dunbier, A. K. et al. Relationship between plasma estradiol levels and estrogen-responsive gene expression in estrogen receptor–positive breast cancer in postmenopausal women. JCO 28, 1161–1167 (2010).
Cai, T. et al. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 345, 206–217 (2016).
Yao, L. et al. High phosphorus level leads to aortic calcification via β-catenin in chronic kidney disease. Am. J. Nephrol. 41, 28–36 (2015).
Shao, J. et al. Vascular Bmp–Msx2–Wnt signaling and oxidative stress in arterial calcification. Ann. N.Y. Acad. Sci. 1117, 40–50 (2007).
Goretsky, T. et al. Beta-catenin cleavage enhances transcriptional activation. Sci. Rep. 8, 671 (2018).
Xie, C. et al. BRCC36 prevents vascular calcification in chronic kidney disease through the β-catenin signalling pathway. Exp. Cell Res. 413, 113051 (2022).
Kocher, O. & Krieger, M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr. Opin. Lipidol. 20, 236–241 (2009).
Lee, C.-Y. et al. Increased β-catenin accumulation and nuclear translocation are associated with concentric hypertrophy in cardiomyocytes. Cardiovasc. Pathol. 31, 9–16 (2017).
Hu, Y. et al. The critical role of the piezo1/β-catenin/ATF4 axis on the stemness of Gli1+ BMSCs during simulated microgravity-induced bone loss. Adv. Sci. 10, 2303375 (2023).
Woodward, H. J., Zhu, D., Hadoke, P. W. F. & MacRae, V. E. Regulatory role of sex hormones in cardiovascular calcification. IJMS 22, 4620 (2021).
Kim, H. et al. PDZK1 is a novel factor in breast cancer that is indirectly regulated by estrogen through igf-1r and promotes estrogen-mediated growth. Mol. Med. 19, 253–262 (2013).
Acknowledgements
This project was funded by National Natural Science Foundation of China (grant no. 82260088), Yunnan Provincial Innovative Research Team in Basic and Clinical Study of Coronary Heart Disease of Yunnan Revitalization Talent Support Program (202305AS350030), Yunnan Science and Technology Program (2024AY070001-075; 202401AY070001-030), Bai Xiaochun Expert Workstation (YSZJGZZ-2020040), Major Program of Kunming Science and Technology Innovation Center (2019-1-N-25318000003568), Major Social Development Projects of Yunnan Province (202203AC100007), Scientific Research Fund project of Education Department of Yunnan Province (2024J0171).
Author information
Authors and Affiliations
Contributions
L.S. and N.W. conceived and designed the study; C.L., P.Y., X.L., X.C., and S.M. performed the experiments; L.Y., G.Y., and S.L. analyzed and interpreted data; L.S., J.T., and L.L. provided ideas and critical comments; N.W., P.Y., and C.L. wrote the paper with feedback from all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Viktória Jeney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Jesmond Dalli and Dario Ummarino.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, C., Yang, P., Liu, X. et al. PDZ domain containing protein 1 aggravates mouse vascular smooth muscle cells calcification via PDZ1 domain mediated β-catenin stabilization and nuclear translocation. Commun Biol 8, 1642 (2025). https://doi.org/10.1038/s42003-025-09027-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09027-2