Abstract
Newborn mammals often encounter painful stimuli (PS)—such as trampling, retrieval by parental incisors, or conspecific aggression—that can disrupt neural and behavioral development. Parental tactile care, including licking and grooming, is thought to buffer these effects, but the underlying mechanisms remain unclear. Using mandarin voles, a highly social species, we showed that postnatal back brushing, a proxy for affiliative tactile stimulation, reversed the negative impact of early tail pinching on emotional and social behaviors. Back brushing notably reversed the reduction in activities of oxytocin (OXT) neurons in the paraventricular nucleus (PVN) and activities of the ventral tegmental area (VTA) induced by early tail pinching. Chemogenetic and optogenetic activation of PVN-VTA OXT neuron terminals reduced anxiety, increased sociality, and enhanced dopamine (DA) release in the nucleus accumbens (NAc) of animals with PS. These effects were reversed by oxytocin receptor (OXTR) antagonism in the VTA. Conversely, inhibition of this circuit produced opposite effects. At the molecular level, brushing reversed PS-induced hypermethylation of Drd1, Drd2, and Nr3c1, as well as broader transcriptomic shifts in the NAc. Our findings uncover a tactile–oxytocin–dopamine pathway underlying resilience to early-life pain and highlight conserved mechanisms through which social touch supports emotional brain development.

Similar content being viewed by others
Introduction
In mammalian neonates, early exposure to painful or noxious stimuli such as rough handling, trampling, or aggressive maternal retrieval may profoundly alter brain development and behavioral trajectories1,2. In humans, childhood maltreatment is a pervasive global issue, with profound repercussions that warrant urgent attention. It manifests in multiple forms, including emotional neglect, physical abuse, sexual violation, and the failure to meet basic needs3. Among these, physical abuse is the most prevalent, capable of inflicting immediate physical harm on children and, in severe instances, posing life-threatening risks4. Furthermore, experiences of physical abuse during childhood are correlated with an elevated risk of developing various mental health disorders and chronic diseases in adulthood5,6. Given these enduring psychological and physiological consequences, addressing and mitigating the adverse effects of neonatal painful stimulation have emerged as a primary focus within the disciplines of neuroscience and clinical therapy.
In mammals, these adverse effects may be buffered by positive tactile stimulation—such as licking and grooming—which enhances neurotrophic signaling and reduces hypothalamic-pituitary-adrenal (HPA) axis activation7,8. The buffering effects of these positive stimulations from parents may be a mechanism via which the brain and behavior develop normally. Research has shown that positive social touch, such as social grooming, provides a stress-buffering effect that transcends mere physical proximity. Significantly, it can alleviate anxiety and stress, thereby enhancing emotional well-being9,10. Similarly, in rats, stroking behavior can alleviate anxiety and reduce corticosterone levels after chronic stress11. Gently stroking activates unmyelinated C-low-threshold mechanoreceptors (C-LTMRs), producing calming and stress-buffering effects. This activation can lower heart rate, reduce anxiety behaviors, and decrease plasma corticosterone levels11,12. Notably, following tissue injury or in chronic pain states, these same afferents or their spinal circuits can undergo plastic changes and contribute to touch-evoked pain (mechanical allodynia)13.In humans, social touch, as a fundamental form of contact, is commonly used to relieve stress, establish a sense of belonging, and convey emotions14. Such social interactions, including patting, stroking, and hugging, can strengthen social bonds, diminish prejudice, and enhance trust, and increase intimacy14,15,16. In mice, positive touch can activate the periaqueductal gray (PAG) tachykinin 1 to PVN OXT pathway, to promote social interaction17. Our recent study found that positive touch can reverse the increased stress susceptibility caused by early pain stimuli, improving emotion and sociality18. However, the neuroendocrine mechanisms underlying these effects remain unclear.
Oxytocin, a pivotal neuropeptide, plays a vital role in mitigating stress through social touch and enhancing prosocial behaviors. Positive touch activates oxytocin - producing neurons in the PVN and suprachiasmatic nucleus (SON), triggering oxytocin release9,19. Skin-to-skin contact during parent-offspring interactions can elevate plasma oxytocin (OXT) levels in offspring, fostering secure parent-offspring bonding20. Furthermore, studies have indicated that massage and gentle tactile stimulation enhance plasma OXT levels and c-Fos expression in OXT neurons19. OXT reduces stress responses via the vagus nerve mechanism21,22. Moreover, oxytocin regulates autonomic responses by inhibiting sympathetic activity and promoting parasympathetic activity23. Recent studies further reveal that oxytocin can enhance prosocial behaviors17,24 possibly via activation of lateral and ventrolateral periaqueductal gray (l/vlPAG) tachykinin 1+ neuron inputs to oxytocin neurons in the PVN17. Therefore, we hypothesize that early social touch may reverse emotional and social behavioral abnormalities caused by early abuse by influencing the oxytocin system.
Several lines of evidence have shown that PVN OXT neurons project to the VTA25,26,27. The midbrain limbic cortical pathway facilitates reward learning and reinforcement by mediating dopamine release from the VTA to the nucleus accumbens (NAc). Dopaminergic signaling from the VTA has been shown to drive social behaviors, such as exploration of unfamiliar conspecifics, same-sex social interactions, and playful behaviors in female rats28. Leah J. Elias and colleagues demonstrated for the first time that somatosensory neurons can activate the brain’s reward centers. In particular, the optogenetic activation of the Mrgprb4 cell line in the genital or dorsal skin of female mice has been shown to be sufficient to induce dopamine release in the NAc, as demonstrated in recent research. Furthermore, their study revealed that mrgprb4-expressing neurons are necessary for dopamine release in males during straddling behavior29. Thus, we predict that early positive touch influences dopamine release by affecting the activity of PVN OXT neurons projecting to the VTA, thereby reversing the decline in sociability caused by early abuse, and pharmacological blocking of OXTR in the VTA may influence the reversal effects of positive touch.
Early life experiences, particularly maltreatment, can also induce long-lasting changes in DNA methylation, which can persist into adulthood and affect various physiological and psychological processes30. The timing and type of maltreatment are crucial factors, with early exposure (before age 11 days) often leading to more severe consequences30. Fujisawa et al. demonstrated that OXTR DNA methylation is linked to alterations in brain volumes in maltreated children, particularly affecting regions associated with stress response, emotional regulation, and social cognition31. Another study by Maud et al. found that OXTR DNA methylation is associated with autism and related social traits32. Oxytocin enhances dopamine release in the nucleus accumbens and promotes social interaction in rats, hinting at a possible mechanism by which early maltreatment could modify DNA methylation at dopamine receptor D2 Gene (DRD2) sites30. Previous study also suggests that Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1) methylation might be a potential biological mediator between childhood maltreatment and depressive symptoms33. The expression product of this gene, the Glucocorticoid receptor, also distributes in the NAc34. In addition, early-life stress can affect microglial transcriptome35, transcriptome in the NAc36, and cell type-specific transcriptomic patterning in the ventral hippocampus37. The NAc receives DA projections from VTA38 and has a high density of OXTR39. This brain region is involved in regulation of emotion and sociality37,39,40. These studies suggest that early-life adversity, such as tail pinching, may alter DNA methylation of dopamine receptors, OXTR, and NR3C1, as well as transcriptomic profiles in the downstream NAc. Positive tactile stimulation, such as back brushing, could reverse these alterations, thereby influencing social behavior and emotional states.
Using the highly social animal model, mandarin voles41,42, the present study tried to reveal neuroendocrine mechanisms underlying the reversal effects of positive tactile stimulation on emotional and social abnormalities induced by early pain stimulation. Two-week early life stress model was implemented, involving tail pinching followed by the application of a soft-bristled brush to mimic early maternal grooming of pups18. The findings indicated that back brushing reversed increases of anxiety and depression-like behavior, reduction of the activity of OXT neurons in the PVN of the brain, and tyrosine hydroxylase (TH) neurons in the VTA induced by tail pinching. It also reversed the alteration of DNA methylation of dopamine receptors, OXTR, and NR3C1, and transcriptomic profiles in the downstream NAc induced by tail-pinching. Utilizing a combination of chemogenetics, optogenetics, and pharmacology, we demonstrated that back brushing induced dopamine release in the NAc via PVNOXT-VTADA pathway, and consequently reduced anxiety and depression-like behavior. The present study elucidates the neural mechanisms by which positive tactile stimulation reverses the adverse effects of early-pain stimulation on emotional and social behaviors. This research identifies specific neurochemical and epigenetic changes in the oxytocin and dopamine pathways, and demonstrates the therapeutic potential of targeting the PVN-VTA circuit for mitigating psychiatric risks associated with early-life stress.
Results
Early positive tactile stimulation reduces stress susceptibility following tail pinching
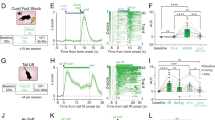
To assess the impact of early tactile stimulation on the stress susceptibility of mandarin voles, we exposed mandarin voles that had received early tactile stimulation to sub-threshold variable stress stimulation and then conducted tests on emotional and social behavior (Fig. 1a). The percentage of time in central area exhibited an interaction effect between back-brushing and tail-pinching treatments (F (1,36) = 6.020, P = 0.019. Figure 1b, c). Compared with the control (Con) group, the tail-pinching (TP) group showed a reduced percentage of time spent in the center area (P = 0.001), while the tail-pinching with back-brushing (TP + BB) showed an increased percentage of time spent in the center area (P = 0.002). The number of entries into the center area exhibited an interaction effect between back-brushing and tail-pinching treatments (F (1,36) = 4.468, P = 0.042. Figure 1b, d). The TP group had fewer entries into the center area compared to the Con group (P = 0.01), whereas the TP + BB group had more entries into the center area (P = 0.05). The total distance traveled showed no interaction between back-brushing and tail-pinching treatments (F (1,36) = 0.068, P = 0.796, Fig. 1b, e). No significant main effects were found in back-brushing (F (1,36) = 1.131, P = 0.295) and tail-pinching (F (1,36) = 0.689, P = 0.412). In the three-chamber sociability tests (TCS), there was a significant interaction between treatment and target in the time spent exploring the unfamiliar1 (U1) and empty (E) chambers (F (3,72) = 3.887, P = 0.012, Fig. 1f, g). Post-hoc analysis indicated that the Con (P = 0.001), Back-brushing (Con+BB) (P < 0.001), and TP + BB (P < 0.001) groups spent significantly more time exploring U1, while the TP group showed no significant difference in time spent exploring U1 and E (P = 0.841). The sociability index exhibited an interaction effect between back-brushing and tail-pinching treatments (F (1,36) = 4.956, P = 0.032. Fig. 1f, h). The TP group had a reduced sociability index for U1 (P = 0.0062), while the TP + BB group had an increased sociability index for U1 (P = 0.0394). In the subsequent social novelty recognition test, a significant interaction between treatment and target in the time spent exploring U1 and unfamiliar 2 (U2) was observed (F (3,72) = 10.391, P < 0.001, Figs. 1f and 1i). Post-hoc analysis showed that the Con (P < 0.001), Con+BB (P < 0.001), and TP + BB (P < 0.001) groups spent significantly more time exploring U2, while the TP group showed no significant difference in time spent exploring U1 and U2 (P = 0.271). The social novelty preference index exhibited an interaction effect between back-brushing and tail-pinching treatments (F (1,36) = 18.011, P < 0.001. Fig. 1f, j). The TP group had a reduced social novelty preference index (P < 0.001), while the TP + BB group had an increased social novelty preference index for U1 (P < 0.001).
a Flowchart of the experimental project. b Representative trajectory heatmap for each group in the open field test (OFT). c Percentage of time spent in the center area of the OFT. d Number of visits to the center area in the OFT. e Total distance traveled in the OFT. f Representative trajectory heatmap for each group in the three-chamber sociability tests (TCS). g Time spent investigating U1 and E in the TCS. h Sociability index in the TCS. i Time spent investigating U2 and U1 in the TCS. j Social novelty preference index in the TCS. The data presented in c, d, e, g, h, i and j were analyzed using two-way ANOVA. ns, P > 0.05 (not significant), *P < 0.05, **P < 0.01, ***P < 0.001. Results were shown as mean ± standard error of the mean (n = 10 for each group).
Early positive tactile stimulation reversed the reduction of the activity of OXT neurons in the PVN of mandarin voles induced by tail pinching
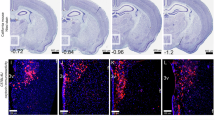
To observe the activation of OXT neurons in the PVN of voles during negative stimulation, a sub-threshold variable stress was applied via a 1-hour tail suspension after all behavioral tests were completed. Ninety minutes later, co-immunostaining for OXT and the immediate early gene c-Fos was performed on brain sections from voles in different groups using immunofluorescence (Fig. 2a). Histological analysis revealed no significant differences in the number of OXT-positive cells among the groups (F (2,15) = 0.196, P = 0.824, Fig. 2b). Approximately 15% of OXT neurons expressed c-Fos in the Con group, 7% in the TP group, and 16% in the TP + BB group (F (2,15) = 6.669, P = 0.008, Fig. 2c). More OXT neurons were activated post-stress in the Con (P = 0.029) and TP + BB (P = 0.011) groups compared to the TP group (Fig. 2c).
a Representative histological images of OXT (green) and c-Fos (red) positive cells in the PVN; blue, DAPI; yellow, co-localized cells. Enlarged views of the boxed areas are shown on the right side of each image, with white arrows indicating the overlap of OXT and c-Fos positive cells (n = 6 for each group). Objective: ×20. Scale bars, 100 μm. b Number of OXT-positive cells per mm² after negative stimulation. c Percentage of OXT neurons in the PVN expressing c-Fos after negative stimulation. d Flowchart of the OXT fiber photometry experiment (Con, n = 6; Con+BB, n = 6; TP, n = 6; TP + BB, n = 5). e Programs for viral injections. f Diagram of the fiber optic cannula location. Objective: ×4. g Post-event histograms (PETHs) of OXT calcium signal z-scores when experimental animals explored an unfamiliar young vole. h Heatmaps illustrating the OXT calcium signal (z-score) in the PVN. i Area under the curve (AUC) of z-scores per second. j Flowchart of the DA fiber photometry experiment (n = 6 for each group). k Programs for viral injections. l Diagram of the fiber optic cannula location. Objective: ×4. m PETHs of DA calcium signal z-scores. n Heatmaps illustrating the DA calcium signal (z-score) in the VTA. o AUC of z-scores per second. The data presented in b and c were analyzed using one-way ANOVA. The data presented in i and o were analyzed using two-way ANOVA. ns, P > 0.05 (not significant), *P < 0.05, **P < 0.01, ***P < 0.001. Results were shown as the mean ± standard error of the mean.
To monitor the real-time activity of PVNOXT neurons, we employed a GCaMP6m virus. The experimental protocol is shown in Fig. 2d, e. In the open field, experimental voles were allowed to explore a wire-mesh enclosure housing a young mandarin vole, and calcium signals were recorded (S1a). The viral expression sites of GCaMP6m are shown in Fig. 2f. PVNOXT GCaMP6m neurons (green) were largely confined to anti-OXT-labeled cells (red). Approximately 81% of OXT neurons were infected by the GCaMP6m virus (Fig. S1c), and about 90% of the neurons infected by the GCaMP6m virus were OXT neurons (Fig. S1d), indicating a high infection rate and specificity of the GCaMP6m virus. The control viral expression sites, infection rates, and specificity are shown in Fig. S1e– S1g. All four groups showed increased fluorescence signals in PVNOXT neurons when exploring an unfamiliar young vole (Fig. 2g, h). The areas under the curves showed no interaction between back-brushing and tail-pinching treatments (F (1,19) = 2.067, P = 0.167, Fig. 2i). The significant main effects were produced by back-brushing (F (1,19) = 76.29, P < 0.001) and tail-pinching (F (1,19) = 16.78, P < 0.001). There was no difference between the Con and Con+BB groups (P = 0.2713). The increase in the TP group was significantly lower than that in the Con group (P < 0.001), while the increase in the TP + BB group was significantly higher than that in the TP group(P = 0.0157). The control virus EGFP signal showed no significant changes upon the occurrence of different behaviors (Interaction: F (1,20) = 0.185, P = 0.672, back-brushing: F (1,20) = 0.002, P = 0.968, tail-pinching: F (1,20) = 0.923, P = 0.348, Fig. S1h - S1j).
Early positive tactile stimulation reversed the reduction of the activity of DA neurons in the VTA of mandarin voles induced by tail pinching
To observe the activation of the VTA in voles during negative stimulation, a 1-hour tail suspension was performed, and 90 minutes later, c-Fos staining was conducted on brain sections from voles in different groups (Fig. S2a). The results showed significant differences in the number of c-Fos positive cells among the groups (Welch F = 20.161, P < 0.001, Fig. S2b). In the Con (P = 0.001) and TP + BB (P = 0.02) groups, more c-Fos neurons were activated post-stress compared to the TP group (Fig. S2b). TH-positive neurons in the VTA were labeled (Fig. S2c). There were significant differences in the number of TH-positive cells among the groups (F (2,15) = 6.138, P = 0.011, Fig. S2d). In the Con (P = 0.028) and TP + BB (P = 0.017) groups, more c-Fos neurons were activated post-stress compared to the TP group (Fig. S2d).
To monitor the real-time activity of VTADA neurons, we employed a GCaMP6s virus. The experimental protocol is shown in Fig. 2j, k. The viral expression sites of GCaMP6s are shown in Fig. 2l. Approximately 78% of DA neurons were infected by the GCaMP6s virus (Fig. S2g), and about 81% of the neurons infected by the GCaMP6s virus were DA neurons (Fig. S2h). The EGFP viral expression sites, infection rates, and specificity are shown in Fig. S2i– S2k. All four groups showed increased fluorescence signals in VTADA neurons when exploring an unfamiliar young vole (Fig. 2m, n). The areas under the curves showed interaction between back-brushing and tail-pinching treatments (F (1,20) = 9.767, P = 0.005, Fig. 2o). There was no difference between the Con and Con+BB groups (P = 0.765). The increase in calcium signals of the TP group was significantly lower than that in the Con group (P < 0.001), while the increase in calcium signals of the TP + BB group was significantly higher than that in the TP group (P = 0.001). The control virus EGFP signal showed no significant changes upon occurring of different behaviors (Interaction: F (1,12) = 0.015, P = 0.905, back-brushing: F (1,12) = 0.002, P = 0.009, tail-pinching: F (1,12) <0.001, P = 0.984, Fig. S2l–S2n).
The impact of chemogenetic activation of PVNOXT neuronal fibers in the VTA combined with pharmacological regulation on emotional and social behavior in voles with tail-pinching
To manipulate neural circuits, we first verified the oxytocin projection from the PVN to the VTA. We injected a retrograde labeling virus, rAAV11-EF1a-EGFP, into the VTA (Fig. 3a, b) and observed the overlap of the EGFP with the somas of OXT neurons in the PVN (Fig. 3c).
a Schematic diagram of the AAV11 viral injection site in the VTA. b Representative histological image showing AAV11 (green) expression in the VTA. Objective: ×4. c Histological image of co-staining for OXT and AAV11. White arrows indicate the overlap of OXT and AAV11 cell bodies. Objective: ×20. Enlarged view of the boxed area on the right side, showing OXT, AAV11, DAPI, and merge. Scale bar, 20 μm. d Flowchart of the pharmacogenetic and pharmacological experiments. e Schematic diagram of viral injection. f Cannula implantation. g Diagram of bilateral cannula placement. Objective: ×4. Representative histological image of anti-TH (green). h Representative trajectory heatmap of each group in the OFT (Tail-pinching-mCherry, n = 7; Tail-pinching-hM3Dq, n = 8; repeat 3 times, with one week between each session). i Percentage of time spent in the center area of the OFT. j Number of visits to the center area of the OFT. k Total distance traveled in the OFT. l Representative trajectory heatmap of each group in the sociality phase of the TCS (Tail-pinching-hM3Dq, n = 8; repeat 3 times, with one week between each session). m Time spent investigating U1 and E in the TCS. n Sociability index of the TCS. o Representative trajectory heatmap of each group in the social recognition phase of the TCS. p Time spent investigating U2 and U1 in the TCS. q Social novelty preference index of the TCS. The data presented in n and q were analyzed using one-way repeated-measures ANOVA. The data presented in i, j, k, m and p were analyzed using two-way repeated-measures ANOVA. ns, P > 0.05 (not significant), *P < 0.05, **P < 0.01, ***P < 0.001. Results shown are mean ± standard error of the mean.
To determine whether chemogenetic activation of PVNOXT neuronal fibers in the VTA, combined with pharmacological regulation, affects the emotional and social behavior of mandarin voles, adult voles that underwent the early tail-pinching model were injected with rAAV-mOXT-hM3D(Gq)-mCherry/ rAAV-mOXT-mCherry into the PVN and had cannulas implanted bilaterally in the VTA. The experimental procedure is shown in Fig. 3d–f. The location of viral expression is shown in Fig. S3a. PVNOXT hM3D(Gq) (red) was relatively confined to anti-OXT labeled cells (green). Approximately 96% of OXT neurons were infected by the hM3D(Gq) virus (Fig. S2b), and almost all neurons infected by the hM3D(Gq) virus were OXT neurons (Fig. S3c), indicating a high infection rate and specificity of the hM3D(Gq) virus. To accurately locate the VTA region, anti-TH and DAPI staining were performed, and Fig. 3g shows a representative image of the cannula position in the VTA. Figure S3d shows an image of the PVN-VTAOXT neuronal fiber projection. CNO administration activated hM3D(Gq) viral fibers, which increased c-Fos expression in the VTA (t = -3.516, P = 0.004, Fig. S3e and S3f), confirming the effectiveness of activation through the hM3D(Gq) virus. Additionally, we found that TH-positive neurons in the VTA region were significantly activated (t = −2.988, P = 0.01, S3e and S3g).
Regarding the impact on emotion, the injection of saline into the VTA region did not affect the percentage of time voles spent in the center area of the open field (P = 0.749, Fig. 3h, i). Injection of CNO to activate PVNOXT fibers in the VTA increased the percentage of time spent in the center area (P = 0.003, Fig. 3h, i). The combined administration of CNO and OXTA had no effect on the percentage of time spent in the center area (P = 0.969, Fig. 3h, i). Neither saline (P = 0.094) nor CNO + OXTR-A (OXTR antagonist) (P = 1) affected the number of entries into the center area, whereas CNO activation of PVNOXT fibers in the VTA increased the number of entries into the center area (P = 0.002, Fig. 3h, j). None of the three treatment methods significantly affected the total distance traveled in the open field test (OFT) (F (2,12) = 1.341, P = 0.298, Fig. 3k).
Regarding the impact on social behavior, during the sociality phase of the TCS, neither saline (P = 0.6261) nor CNO + OXTR-A (P = 0.6288) increased the time voles spent exploring U1. In contrast, CNO activation of PVNOXT fibers in the VTA increased the time voles spent exploring U1 (P = 0.003, Fig. 3l, m). There were no differences in the sociability index among the groups (F(2, 14) = 0.963, P = 0.405, Fig. 3l, n). During the social recognition phase, none of the three treatment methods increased the time voles spent exploring U2 (F (2,14) = 1.708, P = 0.217, Fig. 3o, p). There were no differences in the social novelty preference index among the groups (F (2,14) = 1.587, P = 0.239, Fig. 3o, q). The three treatment methods using the control virus had no significant effect on voles’ sociability (time, F (2,12) = 0.427, P = 0.662; sociability index, F (2,12) = 0.097, P = 0.908; Fig. S3h–S3j) and social recognition (time, F (2,12) = 0.05, P = 0.952; sociability index, F (2,12) = 0.126, P = 0.888; Fig. S3k–m).
The impact of optogenetic activation of PVNOXT-VTA neuronal projection fibers on DA release in the NAc, and on emotional and social behaviors in voles with tail-pinching
To determine whether optogenetic activation of PVNOXT neuronal fibers in the VTA affects DA release in the NAc, as well as emotional and social behavior in mandarin voles, adult voles that underwent early tail-pinch modeling were injected with rAAV-mOXT-ChrimsonR-tdTomato / rAAV-mOXT-mCherry in the PVN and rAAV-CAG-dLight1.1-WPRE-hGH polyA in the NAc. An optical fiber was implanted in the left VTA and left NAc. The experimental procedure is shown in Fig. 4a–c, and the location of viral expression is shown in Fig. S4a. PVN OXT ChrimsonR (red) was relatively confined to anti-OXT-labeled cells (green). Approximately 93% of OXT neurons were infected by the ChrimsonR virus (Fig. S4b), and about 96% of neurons infected by the ChrimsonR virus were OXT neurons (Fig. S4c), indicating a high infection rate and specificity of the ChrimsonR virus. To accurately locate the VTA region, anti-TH and DAPI staining were performed, and Fig. 4d shows a representative image of the optical fiber position in the VTA region. Figure S4d shows an image of the PVNOXT-VTA neuronal fiber projection. Activation of ChrimsonR virus fibers with 598 nm light increased c-Fos expression in the VTA region (t = -3.459, P = 0.004, Fig. S4e and S4f), confirming the effectiveness of activation via the ChrimsonR virus. Additionally, we found that TH-positive neurons in the VTA region were significantly activated (t = −6.916, P < 0.001, Fig. S4e and S4g).
a Flowchart of the optogenetic activation experiment. b Schematic diagram of viral injection (n = 8 for each group). c Schematic diagram of the optical fiber. d Diagram of the optical fiber location. Objective: ×4. e Diagram of the optical fiber and dLight1.1 expression location in the NAc. f PETHs of DA release z-scores under 589 nm light. g Heatmap of DA release. h AUC of z-scores per second for DA release. i Flowchart of the optogenetic activation and pharmacological experiments (n = 8 for each group; repeat 3 times, with one week between each session). j Schematic diagram of viral injection. k Schematic diagram of the optical fiber and canula. l Representative trajectory heatmap of each group in the OFT. m Percentage of time spent in the center area of the OFT. n Number of visits to the center area of the OFT. (o) Representative trajectory heatmap of each group in the sociality phase of the TCS. p Time spent investigating U1 and E in the TCS. q Sociability index of the TCS. r Representative trajectory heatmap of each group in the social recognition phase of the TCS. s Time spent investigating U2 and U1 in the TCS. t Social novelty preference index of the TCS. The data presented in (m, n, p, q, s) and t were analyzed using two-way repeated-measures ANOVA. The data presented in h were analyzed using independent samples t-test. ns, P > 0.05 (not significant), *P < 0.05, ***P < 0.001. Bars without the same letters are significantly different (P < 0.05). Results are shown as mean ± standard error of the mean.
By optogenetically activating the fiber terminals of PVNOXT-VTA, real-time DA release in the NAc region was detected using the dLight1.1 sensor. The location of the optical fiber and viral expression in the NAc are shown in Fig. 4e. When the fiber terminals of PVNOXT-VTA were activated, the signal from the dLight1.1 sensor in the NAc significantly increased (Fig. 4f, g), while there was no change in the mCherry group. The area under the curve (AUC) quantification results were consistent with the above findings (t’ = 10.450, P < 0.001, Fig. 4h).
To determine whether optogenetic activation of PVNOXT neuronal fibers in the VTA affects the emotional and social behavior of mandarin voles and this effect is produced via dopamine system in the NAC, adult voles that underwent the early tail-pinching model were injected with rAAV-mOXT-ChrimsonR-tdTomato/ rAAV-mOXT-mCherry into the PVN, with an optical fiber implanted in the VTA and bilateral cannulas implanted in the NAc (Fig. 4i-k and S4h). The percentage of time in central area exhibited an interaction effect between treatments (F (2,14) = 6.245, P = 0.012. Fig. 4l, m). There were no significant differences among the three mCherry groups (F (2,14) = 0.017, P = 0.983), whereas significant differences were observed among the three ChrimsonR groups (F (2,14) = 21.262, P < 0.001). Optogenetic activation of the PVNOXT-VTA fiber terminals increased the percentage of time that voles spent in the center area of the open field (P = 0.0098), however, injection of Eticlopride (ETIC), a D2 receptor antagonist, into the NAc reversed this increase (P = 0.0363). The number of entries into the center area exhibited an interaction effect (F (2,14) = 6.134, P = 0.012. Fig. 4l, n). There were no significant differences among the three mCherry groups (F (2,14) = 0.664, P = 0.53), whereas significant differences were observed among the three ChrimsonR groups (F (2,14) = 5.952, P = 0.0049). Optogenetic activation of the PVNOXT-VTA fiber terminals increased the percentage of time that voles spent in the center area of the open field (P = 0.0059), however, injection of ETIC into the NAc reversed this increase, showing involvement of dopamine in this effect (P = 0.0049).
Regarding the impact on social behavior, during the sociality phase of the TCS, optogenetic activation of PVNOXT-VTA fiber terminals significantly increased the time voles spent exploring U1 (P = 0.0003, Fig. 4o, p). However, following ETIC injection into the NAC, no significant difference was observed in the time spent exploring E and U1 (P = 0.5171). Optogenetic activation of PVNOXT-VTA fiber terminals has no effect on the sociability index (F (1.738, 12.163) = 3.388, P = 0.073. Fig. 4o, q). During the social recognition phase, optogenetic activation of PVNOXT-VTA fiber terminals significantly increased the time voles spent exploring U2 (P = 0.0171, Fig. 4r, s). However, following ETIC injection into the NAC, no significant difference was observed in the time spent exploring U1 and U2 (P = 0.9892). ETIC significantly attenuates the increase in the social novelty preference index induced by optogenetic activation of PVNOXT-VTA fiber terminals (P = 0.02. Fig. 4r, t).
The impact of optogenetic inhibition of PVNOXT- VTA neuronal projection fibers on DA release in the NAc, and on emotional and social behaviors in voles with tail-pinching
To determine whether optogenetic inhibition of PVNOXT neuronal fibers in the VTA affects DA release in the NAc and the behaviors of mandarin voles, adult voles that underwent TP + BB treatment were injected with PFD-rAAV-mOXT-eNpHR3.0-mCherry-WPRE-hGH polyA/ rAAV-mOXT-mCherry-WPRE-hGH polyA in the PVN and rAAV-CAG-dLight1.1-WPRE-hGH polyA in the NAc. Optical fibers were implanted in the left NAc and bilateral VTA. The experimental procedure is shown in Fig. 5a-c, and the location of viral expression is shown in Fig. S5a. Approximately 93% of OXT neurons were infected with the eNpHR3.0 virus (Fig. S5b), and about 96% of neurons infected with the eNpHR3.0 virus were OXT neurons (Fig. S5c), indicating a high infection rate and specificity of the eNpHR3.0 virus. Figure 5d shows a representative image of the optical fiber position in the VTA region. Figure S5d shows an image of the PVNOXT-VTA neuronal fiber projection. Activation of eNpHR3.0 virus fibers with 598 nm light reduced c-Fos expression in the VTA region (t = -2.838, P = 0.014, Fig. S5e and S5f), confirming the effectiveness of inhibition through the eNpHR3.0 virus. Additionally, we found that TH-positive neurons in the VTA region were significantly inhibited (t = -3.051, P = 0.011, Fig. S5e and S5g).
a Flowchart of the optogenetic inhibition experiment. b Schematic diagram of viral injection (eNpHR3.0, n = 7; mCherry, n = 6). c Schematic diagram of the optical fiber. d Diagram of the optical fiber location. Objective: ×4. e Diagram of the optical fiber and dLight1.1 expression location in the NAc. f (PETHs of DA release z-scores under 589 nm light. g Heatmap of DA release. h AUC of z-scores per second for DA release. i Representative trajectory heatmap of each group in the OFT (eNpHR3.0, n = 7; mCherry, n = 6; repeat 3 times, with one week between each session). j Percentage of time spent in the center area of the OFT. k Number of visits to the center area of the OFT. (l) Representative trajectory heatmap of each group in the sociality phase of the TCS. m Time spent investigating U1 and E in the TCS. n Sociability index of the TCS. o Representative trajectory heatmap of each group in the social recognition phase of the TCS. p Time spent investigating U2 and U1 in the TCS. q Social novelty preference index of the TCS. The data presented in (j, k, m, n, p and q) were analyzed using two-way repeated-measures ANOVA. The data presented in h were analyzed using independent samples t-test. ns, P > 0.05 (not significant), *P < 0.05, **P < 0.01, ***P < 0.001. Bars without the same letters are significantly different (P < 0.05). Results are shown as mean ± standard error of the mean.
By optogenetically inhibiting the fiber terminals of PVNOXT-VTA, real-time DA release in the NAc region was detected using the dLight1.1 sensor. The location of the optical fiber and viral expression in the NAc are shown in Fig. 5e. When the fiber terminals of PVNOXT-VTA were inhibited, the signal from the dLight1.1 sensor in the NAc region significantly decreased (Fig. 5f, g). The signal in the mCherry group remained unchanged. The AUC quantification results were consistent with these findings (t = -7.023, P < 0.001, Fig. 5h).
For emotional detection, optogenetic inhibition of PVNOXT-VTA fiber terminals reduced the percentage of time voles spent in the center area of the open field (F (1,6) = 7.519, P = 0.034, Fig. 5i, j) and the number of entries into the center area (F (1,6) = 35.643, P = 0.001, Fig. 5i, k). The control virus mCherry did not effect on the percentage of time spent in the center area of the open field (F (1,5) = 0.397, P = 0.556, Fig. 5i, j) and the number of entries into the center area (F (1,5) = 0.403, P = 0.554, Fig. 5i, k).
For social behavior detection, during the phase of the TCS to measure levels of sociality, optogenetic inhibition of PVNOXT-VTA fiber terminals was used. The time voles spent exploring U1 and E became indistinguishable (P = 0.524, Fig. 5l, m), and the sociability index showed no significant difference before and after light activation (F (1,6) = 0.651, P = 0.451, Fig. 5l, n). During the phase to test social recognition, optogenetic inhibition of PVNOXT-VTA fiber terminals resulted in no significant difference in the time voles spent exploring U1 and U2 (P = 0.4247, Fig. 5o, p), and the social novelty preference index significantly decreased after light activation (P = 0.002, Fig. 5o, q).
Effects of early tactile stimulation on NAc receptor gene methylation
To assess the impact of early tactile stimulation on the methylation levels of receptor genes in the downstream NAc, we conducted promoter methylation sequencing of several receptor genes associated with emotional and social behaviors. Through sequencing, we identified the gene sequences in the promoter regions of the mandarin vole. The results showed that the methylation level of the dopamine receptor D1 Gene (DRD1) promoter in the TP group was significantly lower than that in the Con group (P = 0.04) and the TP + BB group (P = 0.03, Fig. 6a). For Drd2, the methylation level of the promoter in the TP group was higher than that in the Con group (P < 0.001) and the TP + BB group (P < 0.001, Fig. 6b). The methylation level of the Nr3c1 promoter in the TP group was higher than that in the Con group (P < 0.001) and the TP + BB group (P < 0.001, Fig. 6c). The methylation level of the Oxtr promoter in the TP group was significantly lower compared to the Con group (P < 0.001) and the TP + BB group (P < 0.001, Fig. 6d). The heatmap of methylation levels is shown in Supplementary Fig. S6. The corresponding methylation data are presented in Supplementary File 1.
a Comparison of Drd1 promoter methylation levels. b Comparison of Drd2 promoter methylation levels. c Comparison of Nr3c1 promoter methylation levels. d Comparison of Oxtr promoter methylation levels. e Principal Component Analysis (PCA) plot of the Nac, depicting the clustering of samples from the Con, TP and TP + BB groups. f The number of significantly upregulated or downregulated differentially expressed genes (DEGs) between different groups. g, h Volcano plots of DEGs comparing TP vs. Con and TP + BB vs. TP, respectively. The plots feature horizontal lines at –log10 (false discovery rate, FDR) = 1 and vertical lines at log2 (fold change, FC) = 0, demarcating positive versus negative regulation. Blue dots represent downregulated DEGs, while red dots represent upregulated DEGs. i Hierarchical clustered heatmaps of DEGs. The expression levels were log10 transformed, with high levels of expression indicated in red and low expression in blue. j, k Gene Ontology (GO) classifications of DEGs comparing TP vs. Con and TP + BB vs. TP, respectively. P-value ≤ 0.05. *P < 0.05 (n = 3 for each group, with each sample comprising 3 mice).
Early tactile stimulation-induced changes in gene expression in the NAc
Following behavioral experiments, the NAc brain region was collected for transcriptome sequencing. Principal component analysis (PCA) revealed a significant separation between the Con and TP groups along the second principal component (PC2) (Fig. 6e). Compared to the Con group, the TP group exhibited 255 upregulated genes and 93 downregulated genes (Fig. 6f, g). In contrast, the TP + BB group showed 107 upregulated genes and 234 downregulated genes compared to the TP group (Fig. 6f, h). A heatmap generated from the differentially expressed genes (DEGs) demonstrated distinct clustering between the Con, TP and TP + BB groups.TP group displayed down-regulated (blue) and up-regulated gene expression (red) compared to control group (Fig. 6i) and back brushing (BB) reversed these changes partially indicating reversal effects of back brushing on changes in gene expression profiles induced by TP. Additionally, we found that TP significantly increased the expression of stress-related genes, while TP + BB effectively reversed this phenomenon (Fig. S7a).
To elucidate the functional implications of the DEGs, all DEGs were mapped against the Gene Ontology (GO) database and subjected to enrichment analysis based on a P-value threshold of ≤0.05. We primarily focused on GO terms related to the nervous system. The results indicated that, compared to the Con group, DEGs in the TP group were significantly enriched in processes such as response to stress and stimulus, inflammatory response, glucocorticoid, catecholamine transport, and regulation of nervous system development, neurogenesis and neuron differentiation (Fig. 6j). In comparison to the TP group, DEGs in the TP + BB group were significantly enriched in responses to glucocorticoid, stress, mechanical stimulus, corticotropin releasing hormone (CRH) receptor binding, neurotransmitter receptor regulator activity, D2 dopamine receptor binding, catecholamine transport, and behavioral response to pain (Fig. 6k).
For functional classification and pathway assignment of the DEGs, all DEGs were analyzed against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database using a P-value threshold of ≤ 0.05. The results showed that, compared to the Con group, DEGs in the TP group were significantly enriched in pathways related to Parkinson’s disease, neurodegeneration—multiple diseases, and Alzheimer’s disease (Fig. S7b). In contrast, DEGs in the TP + BB group, compared to the TP group, were significantly enriched in pathways such as neuroactive ligand-receptor interaction and estrogen signaling pathway (Fig. S7c).
Furthermore, we performed Gene Set Enrichment Analysis (GSEA). The results revealed that, compared to the Con group, gene sets in the TP group were enriched in pathways related to mRNA methylation and neurotransmitter receptor regulator activity (Fig. S7d and S7e). In comparison to the TP group, gene sets in the TP + BB group were enriched in the oxytocin signaling pathway and dopaminergic synapse pathway (Fig. S7f and S7g). These findings suggest that TP treatment significantly alters the gene expression profile in the NAc brain region, while BB treatment partially reverses these changes, particularly in pathways associated with stress responses, nervous system function and neurodegenerative diseases.
Discussion
This study reveals the involvement of PVNOXT and VTADA neurons in mediating the reversal effects of back brushing (BB) on maltreatment (tail pinching)-induced emotional and social abnormalities. BB mitigates the adverse effects of pain stimulation on emotional and social behaviors by enhancing the activation of PVNOXT neurons, increasing VTA activity, and reversing alterations in levels of Drd1, Drd2, Nr3c1, and Oxtr gene methylation and transcriptome in the NAc induced by pain stimulation. Manipulation of PVNOXT neurons increased DA release in the NAc. These research findings elucidate the neural mechanisms by which early positive tactile stimulation reverses emotional and social behavioral abnormalities caused by early-life maltreatment, and social behavioral abnormalities caused by early-life maltreatment, and provide potential targets for treatments of neurodevelopmental disorders, such as autism, anxiety and depression, and support positive tactile stimulation as an intervention of these diseases.
We found no significant difference in activity levels of PVN oxytocin neurons between the Con group and the Con+BB group. A plausible explanation is that under the baseline (Con) condition, PVN OXT neurons are already in a relatively high activation state, approaching a “ceiling effect” or saturation. Thus, additional tactile stimulation (BB) may not further increase the population-level Ca²⁺ signal43. It could also be due to the potent “braking system” constituted by GABAergic inputs from the ventromedial hypothalamus onto PVN oxytocin neurons. Under “baseline conditions” devoid of significant physiological or emotional challenges, this braking system remains persistently active, preventing oxytocin neurons from being non-specifically activated by irrelevant or mild stimuli, such as routine grooming behaviors. Only when sufficiently potent stimuli arrive can this inhibition be lifted, thereby triggering neuronal firing44. Research by Natalia Duque-Wilckens and colleagues45 demonstrated that social stress remodels neural circuits, enabling subsequent social interactions to more effectively activate specific pro-social neuronal populations. In our experiments, stress may have shifted the mice’s PVN oxytocin neurons from a “baseline” state to a “heightened state of readiness” or “sensitized” state. In this state, mild social tactile signals, which might otherwise be ignored or filtered out, can now be effectively received and translated into significant action potential firing45. Extensive research has established the foundational role of oxytocin in stress alleviation and the promotion of social bonds9,46. Our findings indicate that within a stress context, the activation of the oxytocin system is triggered as a “buffering mechanism” or “restorative mechanism.” Conversely, in the non-stressed, normal state, the absence of pressure requiring buffering results in a naturally non-significant response of this system to mild tactile stimulation like stroking. Thus, the positive social stimulus did not affect PVN oxytocin neuronal activity in normal mice but increased it in post-stress mice.
In contrast, we observed that voles in the TP + BB group demonstrated reduced anxiety and increased sociability, accompanied by the increased activation of OXT neurons in the PVN during social exploration compared with the TP group, indicating a significant role of OXT in modulating anxiety and social behavior. Previous research has shown that OXT release in the central amygdala can modulate inhibitory circuits, thereby suppressing fear responses and reducing anxiety levels47. OXT has been shown to enhance social behavior in individuals with social anxiety disorder by improving the functional connectivity between the amygdala and the prefrontal cortex48. Furthermore, OXT significantly influences social behavior by modulating neural pathways in the amygdala and hippocampus, potentially through interactions with the serotonin system49. OXT is also believed to influence human social-emotional functions by modulating neural responses related to social cooperation in the brain50. Beyond its well-established roles in modulating anxiety and social interaction, OXT is also recognized as a potent endogenous analgesic. Evidence indicates that OXT can suppress nociceptive processing via PVN-vlPAG-spinal cord pathways and through oxytocin receptors in the spinal dorsal horn, thereby exerting analgesic effects51,52. Given that our experiments involved nociceptive procedures, it is plausible that the anxiolytic and pro-social effects observed here may, at least in part, be mediated through OXT-induced analgesia. In this view, OXT’s dual functions—in reducing aversive nociceptive states and enhancing socio-emotional regulation—are likely to act in concert. These reports support our finding that positive tactile stimulation reverses emotional and social behavioral abnormalities caused by early-life maltreatment via an increase in PVN OXT neuron activity. However, whether an increment of PVN OXT neuron activity was induced by an increase in OXT neurons or the activities of OXT neurons needs further identification. This study relies on fiber photometry to measure bulk population fluorescence, unable to distinguish between cell-number recruitment and per-neuron activation strength. Future work using cellular-resolution techniques such as miniscope could provide more precise insights into the mechanisms of signal enhancement. However, from c-Fos data, we can find that the number of OXT neurons did not change significantly between CON, TP, and TP + BB groups. The TP group has fewer numbers of c-Fos-positive OXT neurons than the CON and TP + BB groups. Thus, we can infer that TP and BB may activate the same OXT neuronal populations to some extent.
Another interesting finding is that voles in the TP + BB group exhibited reduced anxiety and increased sociability, accompanied by increased activation of the VTA and an increase in the number of TH neurons compared with the TP group. Notably, OXT has also been found to facilitate social interaction by enhancing DA release in the nucleus accumbens, suggesting that increased DA release correlates with improved social behavior53. Additionally, studies have shown that chronic social stress can lead to a decrease in mesolimbic dopamine function and a reduction in reward-oriented behaviors, further emphasizing the role of DA in social behavior54. Our results indicate that the therapeutic effects of positive tactile stimulation may be attributed to increased dopaminergic neuron activity, possibly resulting from elevated OXT release.
Our findings confirm that OXT neurons in the PVN can project to the VTA in mandarin voles, a pathway that plays a pivotal role in the regulation of social reward and behavior25. In murine studies, OXT receptors have been identified on dopamine and glutamate neurons within the VTA, which subsequently project to the nucleus accumbens and other regions of the mesolimbic system. This highlights the intricate role of OXT in modulating reward circuits55.
Then, we find that chemogenetic activation of OXT nerve terminals within the VTA reduced levels of anxiety and increased social behavior of TP voles. This effect was abolished by the administration of the OXT receptor antagonist, OXTR-A, in the VTA, suggesting that OXT plays a crucial role in modulating anxiety and social behavior in the VTA. Furthermore, optogenetic inhibition of OXT nerve terminals in the VTA led to diminished social cognition. These findings align with the research conducted by Duque et al.56, which demonstrated that inhibition of OXT neurons results in reduced social behavior and impaired social cognitive abilities. Additionally, dysregulation of the OXT system may be implicated in various psychiatric disorders, such as autism and schizophrenia, which are frequently associated with deficits in social cognition57. However, neither chemogenetic nor optogenetic activation of OXT nerve terminals in the VTA led to significant improvements in social cognition. While some studies have reported that OXT enhances social behavior, its effects on social cognition appear to be relatively limited58. The effects of OXT on social behavior may be attributed to its modulation of emotional and motivational systems, which are influenced by individual characteristics and environmental factors, rather than a direct enhancement of cognitive functions59. While OXT is notably effective in promoting social behavior, its role in social cognition warrants further exploration.
In our optogenetic experiments, we observed that activation of OXT nerve terminals in the VTA led to an increase in real-time DA release in the NAc, whereas inhibition of these terminals resulted in decreased DA release. This suggests a significant interaction between the OXT and DA systems. Activation of oxytocin nerve terminals in the VTA improves anxiety-like and social behaviors, whereas administration of a D2 receptor antagonist in the nucleus accumbens (NAc) reverses this effect. These findings are consistent with what we observed in methylation sequencing and provide solid evidence that the DA system in the NAc is involved in this process. Previous research has demonstrated that OXT can modulate DA release through the activation of specific neural circuits, such as the regulation of DA signaling via GABAergic amacrine cells in the retina60. Consistent with our findings, a study by Lin W. Hung et al. reported that OXT release in the VTA enhanced the activity of specific DA neuronal populations involved in social interaction25. Furthermore, a pivotal study highlights that OXT influences the brain’s reward circuitry, with evidence indicating that OXT receptors in the NAc of prairie voles are essential for the formation of pair bonds61. The involvement of OXT in the VTA, a critical component of the reward circuitry, may be crucial in modulating social interaction cues62,63 and social reward64. In murine models, social interaction is associated with heightened activity of VTA DA neurons that project to the NAc65, with dopamine playing an essential role in motivation-related behaviors66. This interaction is crucial for regulating various behaviors and emotions and may offer novel insights into the treatment of certain neuropsychiatric disorders.
PVNOXT neurons innervate both the VTA and the NAc. Within the VTA, OXT gates DA-neuron excitability via OXTR, rapidly increasing NAc dopamine and shaping social reward-mechanisms directly aligned with our VTA-terminal manipulations and photometry results25,27. In parallel, a direct PVN-NAc OXT pathway has been confirmed, with growing evidence for NAc subregion specificity: PVNOXT-NAc core engagement reduces drug reward and is sensitive to local OXTR antagonism, whereas NAc shell OXTR signaling contributes to pair bonding and maternal behaviors67,68. Taken together, these findings suggest that OXT can influence NAc function via multiple entry points. Given that our manipulations targeted VTA terminals, we interpret our phenotypes primarily through the VTA-dependent OXT-DA mechanism, while acknowledging that PVN-NAc OXT inputs could also be involved in the effects of early tactile stimulation that needs further investigation in the future.
Another interesting finding is that back brushing reversed the decreased methylation of the Drd1 promoter and increased the methylation of the Drd2 promoter within the NAc induced by tail pinching. This finding aligns with a previous study showing that early life stressors, such as prenatal stress, are associated with elevated methylation of the Drd2 promoter, whereas activation of the dopamine 2 receptor (D2R) has been shown to improve long-term depression (LTD) and enhance synaptic plasticity69. In the context of social behavior research, upregulation of D2R expression is posited to foster social interactions. For instance, a study involving prairie voles demonstrated that heightened D2R expression correlated with improved maternal behavior, implying a beneficial role of D2R in modulating social behavior70. Conversely, local microinjection of a D1 receptor antagonist into the central nucleus of the amygdala (CeA) in knockout mice was found to reverse anxiety phenotype71. These findings suggest that D1R and D2R have distinct roles in the regulation of anxiety and social behavior, with reduced expression of D1R and increased expression of D2R potentially linked to diminished anxiety and enhanced sociability, respectively, which is consistent with our behavioral results. In addition, positive tactile stimulation reversed the increase of Nr3c1 (Nuclear Receptor Subfamily 3 Group C Member 1) promoter methylation within the NAc region that affects expression of glucocorticoid receptor (GR), and may subsequently be intricately linked to changes in anxiety and social behavior. Furthermore, a study on rats with allergic airway inflammation demonstrated that increased expression of hippocampal glucocorticoid receptors correlated with a reduction in anxiety-like behaviors, underscoring the potential therapeutic role of GR modulation72. Cause-and-effects of GR in the NAc in the regulation of emotional and social behavior needs further investigation in future studies. Moreover, TP reduced methylation of the Oxtr, and back brushing reversed this reduction. Increasing release of oxytocin in the NAc via optogenetic activation of PVN-NAc pathway reduced anxiety and increased sociality73. Activation of OXTR in the NAc promotes social approach74. Thus, reduced methylation of the OXTR results in increased levels of OXTR induced by TP. This result is not consistent with changes in levels of anxiety, depression, and sociality, back-brushing, and this result needs further explanation. Thus, the changes in Drd1, Drd2, and Nr3c1 gene methylation levels caused by early-life maltreatment and positive tactile stimulation may represent another mechanism by which positive tactile stimulation reverses behavioral abnormalities induced by early-life maltreatment.
Transcriptomic analysis revealed that tail-pinching induces widespread transcriptional changes in the NAc, while back-brushing partially reverses these alterations. Functional enrichment analysis of the DEGs revealed their significant association with nervous response to stress and stimulus, inflammatory response, glucocorticoid, pain, catecholamine transport, and regulation of nervous system development, neurogenesis, and neuron differentiation, CRH and dopamine receptor binding. These findings are consistent with previous studies, indicating that glucocorticoid signaling75,76 and catecholamine dynamics77,78 play crucial roles in stress responses and neural plasticity, which are closely associated with levels of anxiety and depression. Back-brushing may mitigate tail-pinching-induced changes by modulating dopaminergic and stress-related pathways and consequently increase the resilience to stress, and reduce levels of anxiety and depression. These reversal effects maybe due to the activated pathway of PVN OXT neurons to the NAc, increased OXT release to the NAc, and buffering effects of OXT. KEGG pathway analysis further showed that TP significantly enriched DEGs in multiple neurodegeneration-related pathways, which aligns with the observed cognitive impairments in TP-treated mandarin voles79. In contrast, the TP + BB group enriched DEGs in pathways such as neuroactive ligand-receptor interaction and estrogen signaling, suggesting potential neuroprotective and neuromodulator effects. GSEA further indicated that epigenetic modifications and synaptic remodeling are key mechanisms underlying TP-induced effects, while the oxytocin signaling pathway and dopaminergic synapse pathway may contribute to the restorative effects of back-brushing. Furthermore, we found that stress-associated genes such as Interleukin-1 beta (IL1B)80, Arginine Vasopressin (AVP)81, 11β-Hydroxysteroid Dehydrogenase Type 1 (HSD11B1)82, and Complement Component 3 (C3)83 were significantly upregulated in the TP group, whereas back-brushing effectively attenuated the expression of these genes. This is consistent with our speculation that back rushing can improve emotions and social behavior by reducing stress responses. These insights highlight the complex interplay between tactile stimulation, transcriptional regulation, and neural function, providing a deeper understanding of the molecular mechanisms involved in stress response and neuroplasticity.
In conclusion, our findings indicate that positive tactile stimulation can ameliorate anxiety and social behavior disorders in Mandarin voles subjected to early pain stimulation. This effect is mediated through the modulation of PVNOXT and VTADA neurons, which in turn influence dopamine release in the nucleus accumbens, thereby affecting emotional and social behaviors. Tail-pinching induces significant transcriptional changes in the NAc related to stress response, neurodegeneration, and synaptic function, and increased methylation of Drd1, Drd2, Nr3c1, and Oxtr gene, while back-brushing partially reverses these alterations, potentially through the modulation of dopaminergic and oxytocinergic signaling pathways. This study provides novel insights into the neural mechanisms underlying the ameliorative effects of positive tactile stimulation on early-pain stimulation-induced behavioral abnormalities. It also highlights the therapeutic potential of targeting the oxytocin-dopamine pathway for the prevention and intervention of psychiatric disorders associated with early-life stress. It also confirms the positive tactile stimulation as a potential way to prevent adverse effects induced by pain stimulation. Future research should further explore the molecular mechanisms underlying these effects and investigate the therapeutic potential of targeting the oxytocin-dopamine pathway for treating psychiatric disorders associated with early-life stress.
Methods
Animals
The experimental protocols for the animals adhered strictly to the Chinese Animal Welfare Law and were approved by the Experimental Animal Ethics Committee at Shaanxi Normal University (approval number: 2022-041). Mandarin voles (Microtus mandarinus) were housed in the university’s specialized animal facility, maintained under a controlled environment with a 12-hour light-dark cycle and a stable temperature of 24 °C ± 2 °C. Each breeding enclosure housed a pair of mandarin voles, one female and one male. After weaning, each cage accommodated 4 to 5 mandarin voles of the same sex, a configuration maintained until the onset of behavioral assessments. The voles had ad libitum access to a diet of standard feed and carrots. Behavioral assessments were conducted when the voles reached 8 weeks of age, with all experiments conducted exclusively on male mandarin voles.
Early stimulation and stress paradigms at adult
To facilitate valid intra-litter comparisons, pups from the same litter were assigned to distinct experimental groups: Control (Con, n = 10), Control with Back-brushing (Con+BB, n = 10), Tail-pinching (TP, n = 10), and Tail-pinching with Back-brushing (TP + BB, n = 10). Beginning on the eighth postnatal day, the pups underwent tactile stimulation over a two-week period. Daily tail-pinch stress was administered for one minute using a plastic clamp positioned approximately 1 cm from the tail tip, designed to induce discomfort without causing physical harm. Subsequently, the pups’ enclosures were equipped with a heating pad set to 32 °C, and they received gentle brushing from the nape to the base of the tail. This was performed using a soft-bristled, 6 cm wide, fan-shaped brush at a speed of 3 cm per second. The procedure was conducted three times daily, with each session consisting of 100 seconds of brushing followed by a 5-minute interval. At 21 days of age, the pups were weaned and housed separately. Behavioral assessments commenced when the pups reached 56 days of age (Fig. 1a)18.
Mandarin voles that had previously undergone early-life tactile stimulation were later exposed to a series of sub-threshold variable stressors, which typically do not elicit depressive-like behaviors in mandarin voles reared under standard conditions. The stress protocols administered to the mandarin voles over three consecutive days included the following: a 1-hour session of 100 intermittent mild foot shocks at a current of 0.45 mA (with two voles per compartment), a 1-hour period of tail suspension, and a 1-hour episode of restraint within a 50 mL conical centrifuge tube84 (Fig. 1a).
Behavioral testing
Mandarin voles were introduced into the open field test (OFT) apparatus, which measured 50 cm × 50 cm × 50 cm and was illuminated at 200 lux. Behavioral data were recorded using a video camera for a duration of 5 minutes, and subsequent analysis was performed using SuperMaze software (Shanghai Xinruan Information Technology Co., Ltd, China). The analysis focused on two primary parameters: the percentage of time spent in the central area (i.e., the time spent in the central area / the total test time × 100%), and the frequency of entries into the central area. Following each trial, the OFT apparatus was meticulously cleaned with 75% ethanol to eliminate any residual odors or traces that might influence the behavior of subsequent test subjects.
The three-chamber social test, as previously described by Hung et al.23, was conducted using a chamber measuring 60 cm × 40 cm × 20 cm, which was partitioned into three smaller sections of 20 cm × 40 cm × 20 cm each. During the initial habituation phase, the mandarin voles were allowed a 10-minute period to acclimate to the test environment.
During the sociability assessment phase, one compartment was equipped with a wire-mesh enclosure containing an unfamiliar conspecific of the same sex, aged 3-5 weeks, while the other compartment contained an empty wire-mesh enclosure. Each subject vole was placed in the central section and allowed a 10-minute period to explore both side sections freely.
In the subsequent social recognition memory phase, a novel mandarin vole was introduced into the previously unoccupied wire-mesh cage. The test vole was then reintroduced to the apparatus to explore both side sections for an additional 10-minute period.
Behavioral observations were recorded on video, and data analysis was conducted using a video tracking system (Smart3.0, Panlab, USA). The social interaction zones were defined as the 5-cm-wide area immediately in front of a wire-mesh cage and the 8-cm-wide areas on either side of the wire-mesh cage. Sociability was quantified using the sociability index (SI), calculated as follows: [(time spent in the unfamiliar side − time spent in the empty side)/(time spent in the unfamiliar side + time spent in the empty side) × 100%]. The social novelty preference was similarly quantified using the social novelty preference index: [(time spent in the novel side − time spent in the familiar juvenile side) / (time spent in the novel side + time spent in the familiar juvenile side) × 100%]85,86.
Immunofluorescence assays
Upon completion of behavioral testing, Mandarin voles were anesthetized with 0.2 g/ml ethyl carbamate and subsequently perfused intracardially with 40 ml of 0.1 M phosphate-buffered saline (PBS), followed by 20 ml of 4% paraformaldehyde (PFA) to facilitate initial fixation. The brain tissues were then excised and subjected to overnight fixation in 4% PFA. A gradient dehydration process was conducted, involving immersion in 20% sucrose for 24 hours, followed by 30% sucrose for 48 hours. The brain tissues were embedded in Optimal Cutting Temperature compound (OCT, 4583, SAKURA, Japan) and sectioned into 30 μm coronal slices using a freezing microtome (CM1950, Leica, Germany). Continuous brain sections were obtained for histological analysis. The brain slices were treated with 0.3% H2O2 for 25 minutes to inactivate endogenous peroxidases, followed by a 30-minute incubation with 0.5% Triton X-100. After each step, the slices were rinsed with 0.1 M PBS (7 minutes, repeated four times). Subsequently, the slices were blocked with ready-to-use normal goat serum (AR0009, Boster Biological Technology, China) at room temperature for 40 minutes. The slices were then incubated with primary antibodies (anti-OXT, anti-c-Fos, and anti-TH) at 4 °C overnight. After washing with 0.1 M PBS (7 minutes, repeated four times), the tissue slices were incubated with fluorescent secondary antibodies at room temperature for a duration of 2 hours. Following another round of washing with 0.1 M PBS (7 minutes, repeated four times), the slices were stained with DAPI (AR1176-50, Boster Biological Technology, China) for 10 minutes. After a final wash with 0.1 M PBS (7 minutes, repeated four times), the slices were mounted using an aqueous mounting medium (AR1109, Boster Biological Technology, China). Images were acquired using a multispectral tissue quantitative analysis system (OLYMPUS BX43, OLYMPUS, Japan).
The primary antibodies included: rabbit anti-TH (1:2000, ab112, abcam, UK), mouse anti-OXT (1:7500; MAB5296, Millipore, Germany) and rabbit anti-c-Fos (1:1500, ab190289, abcam, UK). The secondary antibodies (1:200): goat anti-mouse IgG 488 (33206ES60) and 647 (33213ES60), and goat anti-rabbit IgG Cy3 (33108ES60), 488 (33106ES60), and 647 (33113ES60) were all purchased from YESEN, China.
Virus preparations
rAAV-mOXT-GCaMP6m-WPRE-hGH polyA (2 × 1012 vg/mL, PT-1898), PFD-rAAV- mOXT-EGFP-WPRE-hGH polyA (2 × 1012 vg/mL, PT-1073), rAAV-TH-GCaMp6s-WPRE-hGH polyA, (2 × 1012 vg/mL, PT-2148), PFD-rAAV-TH-GFP-WPRE-hGH polyA, (2 × 1012 vg/mL, PT-3756), rAAV-mOXT-hM3D(Gq)-mCherry- WPRE-hGHpolyA (2 × 1012 vg/mL, PT-1058), rAAV-mOXT-mCherry-WPRE-hGHpolyA (2 × 1012 vg/mL, PT-7811), rAAV-mOXT-ChrimsonR-tdTomato-WPRE-hGH polyA (5.11 × 1012 vg/mL, PT-10078), rAAV-CAG- dLight1.1-WPRE-hGH polyA (2 × 1012 vg/mL, PT-1138) and PFD-rAAV-mOXT-eNpHR3.0-mCherry-WPRE-hGH polyA (2 × 1012 vg/mL, PT-2812) were purchased from BrainVTA, China. rAAV11-EF1α-EGFP (5 × 1012 vg/mL, BC-0012) was purchased from Brain Case Biotechnology LTD. All AAVs were aliquoted and stored at −80 °C until use.
Stereotaxic surgery
Adult male mandarin voles were anesthetized with 1.5%-3% isoflurane inhalation (R5-22-10, RWD, China) and positioned in a stereotaxic apparatus (68045, RWD, China), with anesthesia maintained throughout the procedure. The hair on the dorsal surface of the head was shaved, and the area was disinfected. A midline incision was made in the skull to expose the anterior and posterior fontanelles. A 10 μL Hamilton microsyringe (7635-01, HAMILTON, Switzerland) was used to level the skull surface based on the positions of the fontanelles. A bone drill (78001, RWD, China) was used to create apertures at the designated injection sites. Then, the viral solution was administered at a controlled rate of 100 nl/min using a microsyringe pump (KDS LEGATO 130, RWD, China). Following the injection, the syringe was elevated by 0.1 mm and maintained in position for 10 minutes to mitigate the risk of viral leakage. The scalp was subsequently sutured. The viral injection sites were as follows: PVN (AP: −0.4, ML: 0, DV: −5.40); VTA (AP: −3.2, ML: ±0.45, DV: −4.7); NAc (AP: +1.4, ML: −1, DV: −4.15).
In experiments involving fiber photometry and optogenetic activation, a fiber optic cannula (diameter: 2.5 mm, depth: 6/5 mm; RWD, China) was implanted 0.1 mm above the target region three weeks after viral injection. For optogenetic inhibition experiments, fiber optical cannulas (diameter: 1.25 mm, depth: 6 mm; RWD, China) were implanted at a 10° angle, positioned 0.1 mm above the bilateral target areas three weeks following viral injection. In pharmacological and chemogenetic experiments, due to the inner cannula extending beyond by 0.5 mm, cannula guide tubes (outer diameter: 0.41 mm; inner diameter: 0.20 mm) were implanted 0.5 mm above the bilateral target areas.
Fiber photometry
To evaluate the calcium signaling of OXT neurons in the PVN across different groups in real-time, 900 nl of rAAV-mOXT-GCaMP6m was injected into the PVN (Con, n = 6; Con+BB, n = 6; TP, n = 6; TP + BB, n = 5). Control mandarin voles received an injection of 900 nl of rAAV-mOXT-EGFP into their PVN (n = 6 for each group). Sub-threshold variable stress was administered for three consecutive days beginning 21 days after injection. Following this, a fiber optic cannula was implanted above the PVN and secured using dental cement (19-7220, HWAYON BIOTECH, China). After a one-week recovery period, the fiber optic cannula was connected to a fiber photometry system (QAXK-FPS-LED, ThinkerTech, China) via a patch cable. A 405 nm emission light (20 μW) served as a control, while a 470 nm emission light (40 μW) was used for the detection of calcium signals. The experimental vole was placed in an open field environment to explore freely for a duration of 10 minutes. Subsequently, a juvenile mandarin vole of the same sex, aged 3-6 weeks, was introduced into a wire-mesh enclosure within the open field. The experimental vole was then allowed an additional 10 minutes of free exploration.
To evaluate the activities of DA neurons in the VTA across different groups in real-time, 600 nl of rAAV-TH-GCaMp6s was injected into the VTA to measure changes in calcium signaling upon different behaviors (n = 6 for each group). Control mandarin voles received an injection of 600 nl of rAAV-TH-GFP into their VTA (n = 6 for each group). The remaining steps are the same as for OXT fiber photometry.
Calcium signals were synchronized with the behavior of the mandarin voles using screen-recording software. The analysis was conducted using MATLAB. Alterations in the calcium signal were quantified using z-scored ΔF/F, calculated as (Vsignal – mean (Vbasal))/std (Vbasal). Vsignal and Vbasal refer to the recorded values at each time point and the recorded values during the baseline period before stimulation, respectively. The area under the curve (AUC) was used to quantify signal changes, with the AUC per second serving as a comparative measure for fluorescence signals associated with behavior and baseline conditions.
Retrograde tracing
To confirm the existence of the PVN-VTA pathway, 300 nl of rAAV11-EF1α-EGFP was bilaterally injected into the VTA. Brain tissues were collected four weeks after the viral injection, and viral expression was verified within the VTA. Overlap of OXT-positive cell staining (red) and virus-infected cells (green) was performed in the PVN region to trace neuronal projections from the PVN to the VTA.
Pharmacogenetics and Pharmacology
To activate PVN-VTA OXT neuronal terminals in the TP animals, 800 nl of rAAV-mOXT-hM3D(Gq)-mCherry was injected into the PVN. Control subjects received an injection of 800 nl of rAAV-mOXT-mCherry into the PVN. Three weeks later, sub-threshold variable stress was applied for three consecutive days. Subsequently, bilateral cannulas were implanted into the VTA. Only voles exhibiting correct viral expression and accurate cannula placement were selected for subsequent analysis. Following a one-week recovery period, the subject voles were anesthetized using isoflurane. Saline (400 nl per side) was administered via a micro-infusion pump at a rate of 0.1 μl/min, with the injection needle remaining in situ for an additional two minutes to facilitate drug diffusion. Twenty minutes later, the subjects underwent OFT and TCS assessments. After a week interval, clozapine N-oxide (CNO, 5 μM, 400 nl per side, CNO-02, BrainVTA, China) was administered for behavioral testing.
To examine the role of oxytocin receptor (OXTR) signaling in the VTA in modulating mood and social behavior through positive tactile stimulation, pharmacological methods were employed to inhibit OXTR function. The subject voles were initially injected with CNO (400 nl per side), followed by an injection of a vehicle containing an OXTR antagonist (OXTR-A, [d(CH2)51, Tyr(Me)2, Thr4, Orn8, des-Gly-NH29]-Vasotocin trifluoroacetate salt) (0.5 ng/200 nl per side) five minutes later. Behavioral testing was conducted 15 minutes post-injection. The dosage and method of administration of OXTR-A were referenced from the research conducted by Yishan Qu et al.41 (TP-mCherry, n = 7; TP-hM3Dq, n = 8; repeat 3 times, with one week between each session).
Optogenetics and Fiber photometry
To determine the real-time effects of PVN-VTA OXT neuronal activation on DA release in the NAc, 800 nl of rAAV-mOXT-ChrimsonR-tdTomato was injected into the PVN of the TP model, and 300 nl of rAAV-CAG-dLight1.1 was injected into the NAc. Control voles received 800 nl of rAAV-mOXT-mCherry in the PVN and 300 nl of rAAV-CAG-dLight1.1 in the NAc. After a three-week period, sub-threshold variable stress was applied over three consecutive days. Subsequently, fiber optic cannulas were implanted into the left VTA and left NAc. Only those voles with accurate viral expression and correct fiber optic cannula placement were selected for further analysis. Tests were conducted one week after recovery. Animals were mildly anesthetized with isoflurane. The fiber optic cannula in the VTA was connected to a 589 nm semiconductor laser from the optogenetic intelligent light source (Aurora 300, NEWDOON INC, China) via an FC/PC adapter and a fiber optic rotary joint. PVN-VTA OXT neuronal terminals were stimulated using laser output parameters of 20 ms pulses at 20 Hz and 10 mW, with a 5-second activation followed by a 15-second rest cycle. The fiber optic cannula in the NAc was connected to the fiber photometry system as previously described. A 405 nm emission light (20 μW) was used as a control, and a 470 nm emission light (40 μW) was used for dLight1.1 detection (n = 8 for each group).
To determine the real-time effects of PVN-VTA OXT neuronal activation on emotional and social behaviors, 800 nl of rAAV-mOXT-ChrimsonR-tdTomato was injected into the PVN of the TP model. Control voles received 800 nl of rAAV-mOXT-mCherry in the PVN. After a three-week period, sub-threshold variable stress was applied over three consecutive days. In order to determine whether the behavioral effect induced by optogenetic activation is through DA system in the NAC, optic fibers were implanted into the left VTA, and the cannulas were implanted bilaterally in the NAc. Tests were conducted one week after recovery. The optic fiber in the VTA was connected to a 589 nm semiconductor laser from the optogenetic intelligent light source. PVN-VTA OXT neuronal terminals were stimulated using laser output parameters of 10 ms pulses at 60 Hz and 60 mW, with a 8-second activation followed by a 2-second rest cycle were used for OFT and TCS. To examine the role of D2 receptor signaling in the NAc in modulating mood and social behavior through positive tactile stimulation, Eticlopride (ETIC, 0.5 μg/side/0.25 μl in saline)87 was employed to inhibit D2 receptor function (n = 8 for each group; repeat 3 times, with one week between each session).
To evaluate the real-time effects of inhibiting PVN-VTA OXT neurons on DA release in the NAc, as well as on emotional and social behaviors, 800 nl of rAAV-mOXT-eNpHR3.0-mCherry was injected into the PVN, and 300 nl of rAAV-CAG-dLight1.1 was injected into the NAc of the TP + BB animals. Control voles received 800 nl of rAAV-mOXT-mCherry in the PVN and 300 nl of rAAV-CAG-dLight1.1 in the left NAc. Subsequently, fiber optic cannulas were bilaterally implanted into the VTA and unilaterally into the left NAc. Inhibition of PVN-VTA OXT neuronal terminals was achieved via bilateral administration of a 589 nm smart light source (10 ms pulses at 60 Hz, 60 mW, with 5 s on and 15 s off cycle), while dLight1.1 signals were recorded in the left NAc. One week later, while inhibiting PVN-VTAOXT neuronal terminals in the VTA (10 ms pulses at 60 Hz, 60 mW, with 8 s on and 2 s off cycle), OFT and TCS assessments were conducted (eNpHR3.0, n = 7; mCherry, n = 6; repeat 3 times, with one week between each session).
Assessment of the virally expressed efficiency
During OXT fiber photometry experiments, sections from the PVN were collected. OXT neurons were identified using anti-OXT antibodies, and images were captured at 20× magnification. The percentage of co-labeled neurons among PVN virus-expressing neurons and OXT-positive neurons was statistically analyzed. For this experiment, the data from each mandarin vole’s brain region sample were presented as the average of three section data points (n = 6 for each group).
After measurement of DA release using fiber photometry experiments, sections from the VTA were collected. DA neurons were identified using anti-TH antibodies, and images were captured at 10× magnification. The percentage of co-labeled neurons among VTA virus-expressing neurons and TH-positive neurons was statistically analyzed. For this experiment, the data from each mandarin vole’s brain region sample were presented as the average of three section data points (n = 6 for each group).
In pharmacogenetic and pharmacological experiments, sections from the PVN and VTA were collected. OXT neurons in the PVN were labeled using anti-OXT antibodies, and 20× images were acquired. The percentage of co-labeled neurons among PVN virus-expressing neurons and OXT-positive neurons was statistically analyzed (n = 8). For the VTA, viral expression fibers were imaged at 10× magnification, and the positions of the cannulas were captured at 4× magnification (using anti-TH to mark the exact location of the VTA). Immunofluorescence double staining was performed in the VTA using anti-TH and anti-c-Fos. The activation levels of TH neurons across different groups were statistically analyzed (mCherry, n = 7; hM3Dq, n = 8). For this experiment, the data from each mandarin vole’s brain region sample were presented as the average of three section data points.
In optogenetic and fiber photometry experiments, sections from the PVN, VTA, and NAc were collected. OXT neurons in the PVN were labeled using anti-OXT antibodies, and 20× images were acquired. The percentage of co-labeled neurons among PVN virus-expressing neurons and OXT-positive neurons was statistically analyzed (n = 8). For the VTA, viral expression fibers were imaged at 10× magnification, and the positions of the cannulas were captured at 4× magnification (using anti-TH to mark the exact location of the VTA). Immunofluorescence double staining was performed in the VTA using anti-TH and anti-c-Fos. The activation levels of TH neurons across different groups were statistically analyzed (n = 8 for each group). In the NAc, viral expression images were taken at 10× magnification to confirm the location of dLight1.1 expression. For this experiment, the data from each mandarin vole’s brain region sample were presented as the average of three section data points.
Target gene Bisulfite sequencing
The NAc tissues were collected from Con, TP, and TP + BB groups (n = 3 for each group, with each sample comprising 3 mice). Mandarin voles were anesthetized by intraperitoneal injection of ethyl carbamate (0.2 g/mL). The NAc was dissected under controlled low-temperature conditions. The isolated tissue was immediately flash-frozen in liquid nitrogen for 30 minutes and subsequently stored at -80°C until further processing. The target gene bisulfite sequencing was conducted by E-Gene Co., Ltd. Briefly, specific validation regions were selected and bisulfite sequencing PCR primers were designed using the online MethPrimer software (www.urogene.org/methprimer/index.html). These primers were designed to recognize regions without CpG sites to avoid amplification bias of methylated versus unmethylated sequences. Primer sequences for methylation sequencing are shown in Table 1. BSP validation experiments were conducted as follows: 500 ng of genomic DNA was converted using the ZYMO EZ DNA Methylation-Gold Kit™ according to the manufacturer’s instructions. After purification of the converted products, PCR amplification was carried out in a final reaction volume of 50 µl consisting of 3 µl purified conversion fractions, 4 µl 2.5 mM dNTP, 5 µl 10×buffer, 1 µl BSP primers, 0.5 µl JumpStart™ Taq DNA Polymerase and 36.5 µl water and the following thermal cycling program was used: 94°C for 1 min, 30 cycles of 94 °C for 10 s, 58°C for 30 s, 72°C for 30 followed by an extension step of 5 min at 72°C and products could be held at 12°C. Following amplification, the PCR products were gel-selected and purified using the QIAquick Gel Extraction Kit (Qiagen) and the purified PCR products were subcloned. The colonies from each region were sequenced on a 3730 Genetic Analyzer (Applied Biosystems) to analyze the methylated cytosine level (n = 3 for each group, one sample per 3 voles).
Adapter sequences were trimmed using Cutadapt. The parameter settings used were “-a AGATCGGAAGAGC -m 50 -u 8 -n 2”.
The cleaned reads were mapped back to the reference amplification region using BSMAP software version 2.90. The parameter settings used were “-v 0.2 -g 0 -n 0”. Methylation ratios were extracted from the BSMAP output (SAM) using a Python script (methratio.py), which is distributed with the BSMAP package. In brief, the methylation level was calculated based on the methylated cytosine (mC) percentage in the whole genome as follows: site methylation level = 100 × (number of sequences with methylated cytosines (mC) / total number of valid sequences).
De novo eukaryotic transcriptome sequencing
The NAc tissues were collected from Con, TP and TP + BB groups as described in methylation analysis (n = 3 for each group, with each sample comprising 3 mice). Total RNA was extracted from the tissue using TRIzol® Reagent. RNA quality was then determined using a 5300 Bioanalyser (Agilent) and quantified using ND-2000 (NanoDrop Technologies). Only highquality RNA samples (OD260/280 = 1.8 ~ 2.2, OD260/230 ≥ 2.0, RIN ≥ 6.5, 28S:18S ≥ 1.0, >1 μg) were used for sequencing library construction.
The purification of RNA, reverse transcription, library construction, and sequencing were carried out at Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China) in accordance with the manufacturer’s instructions (Illumina, San Diego, CA). The preparation of the RNA-seq transcriptome library was conducted in accordance with Illumina® Stranded mRNA Prep, Ligation from Illumina (San Diego, CA) using 1 μg of total RNA. Messenger RNA was then isolated according to the polyA selection method by oligo(dT) beads and subsequently fragmented by fragmentation buffer. Secondly, double-stranded cDNA was synthesized using a SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA) with random hexamer primers (Illumina). Then, the synthesized cDNA was subjected to end-repair, phosphorylation, and ‘A’ base addition according to Illumina’s library construction protocol. Subsequently, the libraries were subjected to size selection, targeting cDNA target fragments of 300 base pairs (bp), employing 2% Low Range Ultra Agarose. Following this, the libraries were amplified by 15 cycles of PCR, utilizing Phusion DNA polymerase (NEB). Quantitation of the paired-end RNA-seq library was conducted using the Qubit 4.0 assay, and the library was subsequently sequenced on the NovaSeq 6000 sequencer, employing 2 × 150 bp read length.
The raw paired-end reads were initially trimmed and quality controlled using the fastp software with the default parameters. Subsequently, the clean data from the samples were used to perform de novo assembly with Trinity. To enhance the quality of the assembly, all the assembled sequences were filtered by CD-Hit and transrate. The assembled transcripts were then searched against the NCBI nonredundant (NR), COG, and KEGG databases using the Diamond program to identify proteins with the highest sequence similarity to the given transcripts and retrieve their function annotations. A typical cut-off E-value of less than 1.0 × 10−5 was set.The BLAST2GO program was then used to obtain GO annotations for the unique assembled transcripts, in order to describe their biological processes, molecular functions, and cellular components. Metabolic pathway analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG).
To identify DEGs (differential expression genes) between two different samples, the expression level of each transcript was calculated according to the transcripts per million reads (TPM) method. RSEM was used to quantify gene abundances. Essentially, differential expression analysis was performed using DESeq2. DEGs with |log2FC | ≥ 1 and FDR ≤ 0.05(DESeq2) were considered to be significantly differentially expressed genes. In addition, functional-enrichment analysis, including GO and KEGG, was performed to identify which DEGs were significantly enriched in GO terms and metabolic pathways at a Bonferroni-corrected P-value ≤ 0.05 compared with the whole-transcriptome background. GO functional enrichment and KEGG pathway analysis were carried out by Goatools and KOBAS, respectively. The RNA-seq data for this study have been submitted to the Sequence Read Archive (SRA). For further details, please refer to the data accessibility.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data analysis
The normality of all data was assessed using the one-sample Kolmogorov-Smirnov test, and the homogeneity of variances was confirmed with the Levene test. Parametric tests were used to analyze data that were normally distributed, while non-parametric tests were applied to the remaining data. Comparisons between two groups were performed using the unpaired t-test. Depending on the specific circumstances, one-way analysis of variance (ANOVA) was employed to compare multiple groups under the same testing conditions. When appropriate, two-way ANOVA or two-way repeated measures ANOVA was employed to compare multiple groups across two different testing conditions. Post-hoc comparisons were performed using Tukey’s test. The levels of significance were set at *p < 0.05, **p < 0.01, and ***p < 0.001. All data were presented as the mean ± standard error of the mean (SEM). Statistical analyses were conducted using SPSS software (V20.0, SPSS Inc., USA). All statistical graphs were generated using GraphPad Prism (V9.0, GraphPad Software, Inc., USA). The raw data is included in Supplementary Data 1.
Data availability
The RNA-seq data for 9 samples have been deposited in the Sequence Read Archive (SRA) under the following accession numbers: Study accession: SUB15734957, Project accession: PRJNA1355321, Run accession: SRP640251, Sample accession numbers: SRR35939851, SRR35939850, SRR35939849, SRR35939848, SRR35939847, SRR35939846, SRR35939845, SRR35939844, SRR35939843. The raw experimental data are provided in the supplementary data 1, which can be accessed upon request from the corresponding author. No restrictions are placed on data access, and all relevant data are available from the corresponding author upon request.
References
Victoria, N. C., Inoue, K., Young, L. J. & Murphy, A. Z. A single neonatal injury induces life-long deficits in response to stress. Dev. Neurosci. 35, 326–337 (2013).
Anand, K. J. & Scalzo, F. M. Can adverse neonatal experiences alter brain development and subsequent behavior?. Biol. Neonate 77, 69–82 (2000).
Bernstein, D. P., Ahluvalia, T., Pogge, D. & Handelsman, L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J. Am. Acad. Child Adolesc. Psychiatry 36, 340–348 (1997).
Wiss, D. A. et al. Association between childhood maltreatment and depressive and anxiety symptoms among men who have sex with men in Los Angeles. J. Urban Health 100, 327–340 (2023).
Nanni, V., Uher, R. & Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169, 141–151 (2012).
Manzari, N., Matvienko-Sikar, K., Baldoni, F., O'Keeffe, G. W. & Khashan, A. S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 54, 1299–1309 (2019).
Meaney, M. J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 (2001).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004).
Tang, Y. et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 23, 1125–1137 (2020).
Wu, Y. E. et al. Neural control of affiliative touch in prosocial interaction. Nature 599, 262–267 (2021).
Walker, S. C. et al. C-low threshold mechanoafferent targeted dynamic touch modulates stress resilience in rats exposed to chronic mild stress. Eur. J. Neurosci. 55, 2925–2938 (2022).
Liu, B. et al. Molecular and neural basis of pleasant touch sensation. Science 376, 483–491 (2022).
Seal, R. P. et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655 (2009).
Saarinen, A., Harjunen, V., Jasinskaja-Lahti, I., Jääskeläinen, I. P. & Ravaja, N. Social touch experience in different contexts: A review. Neurosci. Biobehav. Rev. 131, 360–372 (2021).
Jakubiak, B. K., Fuentes, J. D. & Feeney, B. C. Affectionate touch promotes shared positive activities. Pers. Soc. Psychol. Bull. 49, 939–954 (2023).
Seger, C. R., Smith, E. R., Percy, E. J. & Conrey, F. R. Reach Out and Reduce Prejudice: The Impact of Interpersonal Touch on Intergroup Liking. Basic Appl. Soc. Psychol.
Yu, H. et al. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron 110, 1051–1067.e1057 (2022).
Sun, Y. et al. Early positive tactile stimulation reverses the increase of anxiety and decrease of sociality induced by early chronic mechanical pain in mandarin voles. Integr. Zool. https://doi.org/10.1111/1749-4877.12907 (2024).
Okabe, S., Yoshida, M., Takayanagi, Y. & Onaka, T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci. Lett. 600, 22–27 (2015).
Scatliffe, N., Casavant, S., Vittner, D. & Cong, X. Oxytocin and early parent-infant interactions: A systematic review. Int J. Nurs. Sci. 6, 445–453 (2019).
Marlin, B. J. & Froemke, R. C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 77, 169–189 (2017).
Walker, S. C., Trotter, P. D., Swaney, W. T., Marshall, A. & McGlone, F. P. C-tactile afferents: Cutaneous mediators of oxytocin release during affiliative tactile interactions?. Neuropeptides 64, 27–38 (2017).
Uvnäs-Moberg, K., Handlin, L. & Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 5, 1529 (2014).
Kaye, A. D. et al. Emerging Treatments and Therapies for Autism Spectrum Disorder: A Narrative Review. Cureus 16, e63671 (2024).
Hung, L. W. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017).
Musardo, S., Contestabile, A., Knoop, M., Baud, O. & Bellone, C. Oxytocin neurons mediate the effect of social isolation via the VTA circuits. Elife 11 https://doi.org/10.7554/eLife.73421 (2022).
Xiao, L., Priest, M. F. & Kozorovitskiy, Y. Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. Elife 7 https://doi.org/10.7554/eLife.33892 (2018).
Wang, J. et al. Basal forebrain mediates prosocial behavior via disinhibition of midbrain dopamine neurons. Proc. Natl. Acad. Sci. USA 118 https://doi.org/10.1073/pnas.2019295118 (2021).
Elias, L. J. et al. Touch neurons underlying dopaminergic pleasurable touch and sexual receptivity. Cell 186, 577–590.e516 (2023).
Nance, M. G., Sullivan, K. M. & Puglia, M. H. The impact of the early environment on oxytocin receptor epigenetics and potential therapeutic implications. Pediatr. Res. https://doi.org/10.1038/s41390-024-03563-z (2024).
Fujisawa, T. X. et al. Oxytocin receptor DNA methylation and alterations of brain volumes in maltreated children. Neuropsychopharmacology 44, 2045–2053 (2019).
Maud, C., Ryan, J., McIntosh, J. E. & Olsson, C. A. The role of oxytocin receptor gene (OXTR) DNA methylation (DNAm) in human social and emotional functioning: a systematic narrative review. BMC Psychiatry 18, 154 (2018).
Chen, M. & Cao, C. The mediation effect of glucocorticoid receptor gene (NR3C1) methylation between childhood maltreatment and depressive symptoms in Chinese adolescents: A 2-year longitudinal study. Child Dev. 95, 144–159 (2024).
Wu, B. et al. Glucocorticoid receptor in rat nucleus accumbens: Its roles in propofol addictions. Neurosci. Lett. 662, 115–121 (2018).
Reemst, K. et al. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Transl. Psychiatry 12, 507 (2022).
Parel, S. T. et al. Transcriptional signatures of early-life stress and antidepressant treatment efficacy. Proc. Natl. Acad. Sci. USA 120, e2305776120 (2023).
Kos, A. et al. Early life adversity shapes social subordination and cell type-specific transcriptomic patterning in the ventral hippocampus. Sci. Adv. 9, eadj3793 (2023).
Song, N. et al. NAc-DBS corrects depression-like behaviors in CUMS mouse model via disinhibition of DA neurons in the VTA. Mol. Psychiatry 29, 1550–1566 (2024).
Ross, H. E. et al. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1318 (2009).
Wei, A. et al. Sexually dimorphic dopaminergic circuits determine sex preference. Science 387, eadq7001 (2025).
Qu, Y. et al. Distinct medial amygdala oxytocin receptor neurons projections respectively control consolation or aggression in male mandarin voles. Nat. Commun. 15, 8139 (2024).
Wang, J., Zhang, L., Zhang, P. & Tai, F. Cocaine-induced rewarding properties, behavioural sensitization and alteration in social behaviours in group-housed and postpuberty isolated female mandarin voles. Behav. Pharm. 23, 693–702 (2012).
Carter, J. S., Wood, S. K., Kearns, A. M., Hopkins, J. L. & Reichel, C. M. Paraventricular nucleus of the hypothalamus oxytocin and incubation of Heroin Seeking. Neuroendocrinology 113, 1112–1126 (2023).
Grinevich, V. & Stoop, R. Interplay between Oxytocin and Sensory Systems in the Orchestration of Socio-Emotional Behaviors. Neuron 99, 887–904 (2018).
Duque-Wilckens, N. et al. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol. Psychiatry 83, 203–213 (2018).
Smith, A. S. & Wang, Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol. Psychiatry 76, 281–288 (2014).
Wahis, J. et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 24, 529–541 (2021).
Dodhia, S. et al. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 39, 2061–2069 (2014).
Lan, C. et al. Oxytocinergic Modulation of Stress-Associated Amygdala-Hippocampus Pathways in Humans Is Mediated by Serotonergic Mechanisms. Int. J. Neuropsychopharmacol. 25, 807–817 (2022).
Feng, C. et al. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain Behav. 14, 516–525 (2015).
Iwasaki, M. et al. An analgesic pathway from parvocellular oxytocin neurons to the periaqueductal gray in rats. Nat. Commun. 14, 1066 (2023).
Kawasaki, M., Sakai, A. & Ueta, Y. Pain modulation by oxytocin. Peptides 179, 171263 (2024).
Kohli, S. et al. Oxytocin attenuates phencyclidine hyperactivity and increases social interaction and nucleus accumben dopamine release in rats. Neuropsychopharmacology 44, 295–305 (2019).
Bergamini, G. et al. Chronic social stress induces peripheral and central immune activation, blunted mesolimbic dopamine function, and reduced reward-directed behaviour in mice. Neurobiol. Stress 8, 42–56 (2018).
Peris, J. et al. Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J. Comp. Neurol. 525, 1094–1108 (2017).
Duque-Wilckens, N. et al. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc. Natl. Acad. Sci. USA 117, 26406-26413 (2020).
Sanathara, N. et al. Oxytocin-MCH circuit regulates monosynaptic inputs to MCH neurons and modulates social recognition memory. Neuropharmacology 184, 108423 (2021).
Turcsán, B. et al. Intranasal Oxytocin improves social behavior in Laboratory Beagle Dogs (Canis familiaris) using a custom-made social test battery. Front. Vet. Sci. 9, 785805 (2022).
Froemke, R. C. & Young, L. J. Oxytocin, neural plasticity, and social behavior. Annu Rev. Neurosci. 44, 359–381 (2021).
Hu, S. et al. Activation of oxytocin receptors in mouse GABAergic amacrine cells modulates retinal dopaminergic signaling. BMC Biol. 20, 205 (2022).
Young, L. J. & Wang, Z. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054 (2004).
Buffington, S. A. et al. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 (2016).
Shamay-Tsoory, S. G. & Abu-Akel, A. The social salience hypothesis of Oxytocin. Biol. Psychiatry 79, 194–202 (2016).
Song, Z., Borland, J. M., Larkin, T. E., O'Malley, M. & Albers, H. E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 74, 164–172 (2016).
Gunaydin, L. A. et al. Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551 (2014).
Lammel, S., Lim, B. K. & Malenka, R. C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359 (2014).
Cheng, Y. J. et al. Oxytocinergic input from the paraventricular nucleus to the nucleus accumbens core modulates methamphetamine-conditioned place preference. Nat. Commun. 16, 4808 (2025).
Zhang, L. et al. PVN-NAc Shell-VP Circuit OT and OTR neurons regulate pair bonding via D2R and D1R. J. Neurosci. 45 https://doi.org/10.1523/jneurosci.2061-24.2025 (2025).
Li, Y. et al. Prenatal stress leads to the altered maturation of corticostriatal synaptic plasticity and related behavioral impairments through epigenetic modifications of Dopamine D2 Receptor in Mice. Mol. Neurobiol. 58, 317–328 (2021).
Forero, S. A., Liu, S., Shetty, N. & Ophir, A. G. Re-wiring of the bonded brain: Gene expression among pair bonded female prairie voles changes as they transition to motherhood. Genes Brain Behav. 23, e12906 (2024).
Fernandes, M. F., Lau, D., Sharma, S. & Fulton, S. Anxiety-like behavior in female mice is modulated by STAT3 signaling in midbrain dopamine neurons. Brain Behav. Immun. 95, 391–400 (2021).
Liu, Y. et al. Increased Hippocampal Glucocorticoid Receptor Expression and reduced anxiety-like behavior following Tuina in a rat model with allergic airway inflammation. J. Manipul. Physiol. Ther. 45, 586–594 (2022).
Hou, W. et al. Oxytocin treatments or activation of the paraventricular nucleus-the shell of nucleus accumbens pathway reduce adverse effects of chronic social defeat stress on emotional and social behaviors in Mandarin voles. Neuropharmacology 230, 109482 (2023).
Williams, A. V. et al. Social approach and social vigilance are differentially regulated by oxytocin receptors in the nucleus accumbens. Neuropsychopharmacology 45, 1423–1430 (2020).
Su, C. et al. Glucocorticoid receptor signaling in the brain and its involvement in cognitive function. Neural Regen. Res. 20, 2520–2537 (2025).
Bahtiyar, S., Gulmez Karaca, K., Henckens, M. & Roozendaal, B. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol. Cell Neurosci. 108, 103537 (2020).
Kielbinski, M. et al. Acute stress modulates noradrenergic signaling in the ventral tegmental area-amygdalar circuit. J. Neurochem 164, 598–612 (2023).
Saboory, E., Ghasemi, M. & Mehranfard, N. Norepinephrine, neurodevelopment and behavior. Neurochem. Int. 135, 104706 (2020).
Yang, Q. et al. Extracellular Matrix remodeling alleviates memory deficits in Alzheimer's Disease by Enhancing the Astrocytic Autophagy-Lysosome Pathway.Adv. Sci.11, e2400480 (2024).
Cao, F. et al. Celastrol attenuates Alzheimer's disease-mediated learning and memory impairment by inhibiting endoplasmic reticulum stress-induced inflammation and oxidative stress. Arch. Med. Sci. 21, 538–554 (2025).
Ngala, M. E., Hemmings, S. M. J., Womersley, J. S., Shabangu, T. W. & Qulu-Appiah, L. Social isolation induces sexually aggressive behaviour in male Wistar rats. BMC Neurosci. 26, 15 (2025).
Xu, Y. et al. Chaihu Shugan San exerts antidepressant effects by regulating glucocorticoid metabolism in CUMS Rats and Network pharmacology provides complementary mechanistic insights. ACS Omega 10, 6780–6793 (2025).
Tillmon, H. et al. Complement and microglia activation mediate stress-induced synapse loss in layer 2/3 of the medial prefrontal cortex in male mice. Nat. Commun. 15, 9803 (2024).
Peña, C. J. et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 10, 5098 (2019).
Liu, Y. et al. A circuit from dorsal hippocampal CA3 to parvafox nucleus mediates chronic social defeat stress-induced deficits in preference for social novelty. Sci. Adv. 8, eabe8828 (2022).
Zhang, Y. et al. Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via α7 receptor in hippocampus. Cell Res. 26, 728–742 (2016).
Xu, X. et al. Hypothalamic CRF neurons facilitate brain reward function. Curr. Biol. 34, 389–402.e385 (2024).
Acknowledgements
This research was funded by the STI2030-Majior Projects grant number 2022ZD0205101, the National Natural Science Foundation of China grants numbers 32570560, 32270510 and 31901082, the Natural Science Foundation of Shaanxi Province, China grant number 2020JQ-412, the China Postdoctoral Science Foundation grant number 2019M653534, and the Fundamental Research Funds for Central University grants numbers GK202301012.
Author information
Authors and Affiliations
Contributions
F.T. and Y.S. designed research; Y.S., L.Z., W.Q., H.N., and L.L.M. performed research; L.L., J.L., Y.B., J.X., K.H., X.H., Y.Z., R.J., Q.A., W.C., and L.L.F. analyzed data; Y.S., Z.H., and F.T. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Alexandre Charlet and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Eliana Scemes and Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Y., Qian, W., Zhang, L. et al. Tactile stimulation reverses painful stimuli outcomes via PVN-VTA oxytocin circuitry and dopaminergic regulation. Commun Biol 8, 1799 (2025). https://doi.org/10.1038/s42003-025-09208-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09208-z