Abstract
Blue light has been shown to impair spatial memory in mice. However, the underlying neural circuit mechanisms of this impairment remain elusive. Our study revealed that 600-lux blue light disrupted spatial memory retrieval in male mice through the secondary visual cortex (V2M) → the prelimbic cortex (PrL) neural pathway. Specifically, viral tracing and multichannel fiber optic recordings demonstrated that glutamatergic V2M neurons (V2MGlu) projected to GABAergic neurons in the PrL (PrLGABA), thereby inhibiting the excitability of glutamatergic PrL neurons (V2MGlu→PrLGABA→Glu). The selective ablation of V2MGlu neurons projecting to the PrL eliminated the detrimental effects of blue light on spatial memory retrieval. Furthermore, optogenetic or chemogenetic activation of the V2MGlu → PrLGABA→Glu neural circuitry replicated the impairing effects of blue light on spatial memory in mice. Conversely, inhibition of the V2MGlu → PrLGABA→Glu neural circuitry reversed the spatial memory damage induced by blue light. Collectively, our findings elucidate the V2MGlu → PrLGABA→Glu neural circuitry as a potential mediator of blue-light-induced spatial memory impairment in male mice.

Similar content being viewed by others
Introduction
Light can alter cognitive-related brain activity in a wavelength-dependent manner, and the brain is particularly sensitive to blue light1,2,3,4,5. Studies have demonstrated that blue light influences various neurological functions, including circadian rhythms, mood, and cognition6,7,8,9,10,11. For example, brief daytime exposure to blue light can increase cognitive function in humans12 but can impair the spatial memory retrieval of rodents, especially under dim or irregular light conditions13. Long-term dim blue light exposure at night has been shown to induce spatial learning and memory deficits in mice14, mainly due to hippocampal neuron apoptosis, oxidative stress, inflammation, neuron loss, and synaptic dysfunction.
The prelimbic cortex (PrL), a key region of the central nervous system, receives input from several cortical and subcortical areas, including the hippocampus, amygdala, and secondary visual cortex (V2M)15,16,17,18,19,20,21. The PrL plays a significant role in both cognition and emotional processing22,23,24,25,26. It contains glutamatergic (PrLGlu) and GABAergic (PrLGABA) neurons, with the former modulating spatial memory through glutamate release and dopamine interactions27. Functional MRI studies have shown that blue light exposure affects the neural functional connectivity of the DLPFC in humans28,29, suggesting that PrL neuronal functional changes are crucial for facilitating the impact of light on spatial memory. However, it remains unclear whether the PrL mediates blue light-induced spatial memory impairment in mice, and if so, what neural circuit mechanisms are involved.
Based on the preceding findings, we hypothesized that chronic blue light exposure would impair spatial memory in mice, potentially through the PrL. We assessed this hypothesis by applying combined viral tracing, in vivo calcium signal recording, chemical genetics, optogenetics, and patch-clamp techniques to mice. We discovered that chronic blue light exposure reduced the spatial memory of mice. Activating the V2MGlu → PrLGABA→Glu circuitry mimicked the negative effects of blue light on spatial memory, and inhibiting this circuitry reversed these effects. These findings indicate that the V2MGlu → PrLGABA→Glu circuitry is central to blue light exposure-induced impairment of spatial memory in mice.
Results
Exposure to 600 lux blue light impaired spatial memory retrieval
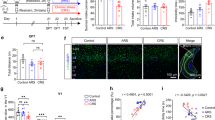
In this work, we explored the effects of different wavelengths of light on spatial memory in mice and their possible neural circuit mechanisms. To do this, we modeled spatial memory impairment in mice exposed to different wavelengths of light (white/blue/green light, 600 lux) for 12 h per day over 7, 14, 21, or 28 consecutive days. Spatial memory was then evaluated in these mice by means of the Y-maze test (Fig. 1A–D). During the Y-maze testing, we observed that 21-day BLED exposure induced the most severe spatial memory impairment in mice, both compared to BLED baseline and to Control, WLED, and GLED groups (Fig. 1E). Figure 1F presents the statistical results of the Y-maze test performed after mice were exposed to different lighting conditions for 21 days. We therefore focused our subsequent investigation on the neural mechanisms underlying this 21-day BLED-induced spatial memory impairment. Furthermore, during the novel object location recall phase, the spatial discrimination index of the BLED group was significantly lower than that of the control group (Fig. 1G–I). While we found that the spatial memory of mice was not significantly impaired after exposure to 600-lux blue light for 4 or 8 h (Supplementary Fig. 1). These findings suggest that exposure to 600-lux blue light, but not light of other wavelengths, impaired the spatial memory of the mice. According to previous research, light exposure can cause anxiety in mice30. Therefore, we also conducted open field test (OFT) and elevated plus maze (EPM) test to evaluate the behavior of the BLED group (Fig. 1J, M). These results revealed that exposure to 600-lux blue light does not cause anxiety-like behavior of the mice (Fig. 1K, L, N, O).
A Schematic diagram of the procedure for exposing mice to different wavelengths of light for 21 days followed by behavioral testing. B Illustration of light exposure in mice. C Schematic of the Y-maze behavioral test in mice. D Heatmap of Y-maze behavioral test in mice. E Statistical graph of Y-maze behavioral test in mice. Control group received 150 lux white light; WLED, GLED, and BLED groups received 600 lux white, green, and blue light, respectively (n = 6 mice per group). F Statistical graph of Y-maze behavioral test in mice exposed to different light for 21 days (n = 6 mice per group). Data analyzed by one-way ANOVA revealed a significant main effect of light exposure on the time spent in the novel arm of the Y-maze (F (3,20) = 7.170, P = 0.0019). Post-hoc Tukey’s test further indicated that the time spent in the novel arm was significantly lower in the BLED group compared to the Control group (P = 0.0037), the GLED group (P = 0.0039), and the WLED group (P = 0.0235). No significant differences were observed in the other pairwise comparisons. G Schematic of the novel object location test (NOL) in mice. Heatmap (H) and Statistical graph (I) of novel object location test results in mice (n = 6 mice per group). During the Encoding phase, an unpaired t-test detected no significant difference between the Control and BLED groups (t (10) = 0.4437, P = 0.6667). During the Recall phase, an unpaired t-test detected a significant difference between the Control and BLED groups (t (10) = 2.278, P = 0.046). J Schematic of the elevated plus-maze behavioral test (EPM) in mice. K, L Heatmap and statistical graph of EPM in mice (n = 6 mice per group). M Schematic of the open field test (OFT) in mice. N, O Heatmap and statistical graph of OFT in mice (n = 6 mice per group). All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; n.s. not significant. The actual time exploring object A or B during encoding and recall session are presented in Supplementary Information (Table 1). Details of the statistical analyses are presented in Supplementary Information (Table 2).
Blue light exposure induced neuronal activation in the V2M and PrL
To explore the mechanism by which long-term blue light exposure impaired spatial memory, we performed c-Fos immunofluorescence staining to detect changes in neuronal activity in the brain regions associated with spatial memory. Compared to the 150-lux white light-exposed control group, the BLED group exhibited an increased number of c-Fos-positive neurons in the primary visual cortex (V1), V2M, and ventral lateral geniculate nucleus (VLGN). In contrast, a lower number of c-Fos-positive neurons was observed in PrL, while no significant changes were detected in hippocampal subregions (CA1, CA2, CA3, and DG) (Fig. 2A, B and Supplementary Fig. 2). Notably, neither continuous 4- nor 8-h blue light exposure for 21 days was sufficient to reduce c-Fos expression in PrL (Supplementary Fig. 3). These findings indicate that these brain regions may play important roles in the ability of blue light exposure to impair the spatial memory of mice.
A Representative images of c-Fos+ immunofluorescence staining in different brain regions for two groups. PrL (prelimbic cortex), V2M (secondary visual cortex), HIP (hippocampus), vLGN (ventrolateral geniculate nucleus), V1 (primary visual cortex) (Scale bar, 200 μm). B Quantitative analysis of c-Fos+ expression in various brain regions. (n = 6 slices per group). C Schematic diagram of anterograde tracing from V2M to PrL. D Representative image of the injection site in V2M (left); representative image of tdTomato+ neurons in PrL (right) (Scale bar, 200 μm). E Representative images of co-labeling of tdTomato+ neurons with GABAergic interneurons or glutamatergic neurons in PrL (Scale bar, 20 μm). F Statistical analysis of co-labeling of tdTomato+ neurons with GABAergic interneurons or glutamatergic neurons in PrL. G Schematic diagram of retrograde tracing from PrL to V2M. H Representative image of the injection site in PrL (left); representative image of tdTomato+ neurons in V2M (right) (Scale bar, 200 μm). I Representative images of co-labeling of tdTomato+ neurons with GABAergic interneurons or glutamatergic neurons in V2M (Scale bar, 20 μm). J Statistical analysis of co-labeling of tdTomato+ neurons with GABAergic interneurons or glutamatergic neurons in V2M. K Schematic diagram of co-labeling of c-Fos+ with GABAergic interneurons and glutamatergic neurons in V2M. L Representative images of c-Fos+ immunofluorescence staining in V2M (left: control group; right: blue light group, Scale bar, 200 μm). M Representative images of co-labeling of c-Fos+ neurons with glutamatergic or GABAergic neurons in the V2M region of the blue light exposure group (Scale bar, 20 μm). N Statistical analysis of co-labeling of c-Fos+ neurons with glutamatergic or GABAergic neurons in the V2M region of the blue light exposure group. All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; n.s. not significant. Details of the statistical analyses are presented in Supplementary information (Table 2).
Previous studies have indicated that V2M neurons can project to the PrL15 and PrL plays an important regulatory role in the spatial memory function of mice13,14,31. Therefore, we focused on the role of the V2M→PrL circuit in regulating the spatial memory of mice. To determine the functional connectivity of the V2M→PrL circuit, we injected the anterograde tracer virus AAV2/1-Cre into the V2M of Ai14 mice (Rosa26-tdTomato; Fig. 2C). After 3 weeks of virus expression, we observed tdTomato-positive signal expression in the PrL (Fig. 2D and Supplementary Fig. 4A–C). Similarly, we injected the retrograde tracer virus retroAAV-Cre into the PrL of Ai14 mice (Fig. 2G). After 3 weeks of virus expression, we observed tdTomato-positive signal expression in the V2M (Fig. 2H and Supplementary Fig. 4D–F). Besides, we employed a retrograde trans-monosynaptic tracing system in which the PrL was infused with AAV-CaMKII-Cre and Cre-dependent helper viruses (AAV-Ef1α-DIO-TVA-mCherry and AAV-Ef1α-DIO-RVG) in C57 mice. After 2 weeks of virus expression, a modified rabies virus (RV), EnvA-pseudotyped RV-∆G-EGFP, was injected into the same site. Following full viral expression, we detected EGFP+ signal in V2M. (Supplementary Fig. 4G–I). Furthermore, immunofluorescence staining revealed that approximately 70% of GABAergic neurons in the PrL colocalized with tdTomato-positive neurons, whereas approximately 30% of glutamatergic neurons colocalized with tdTomato-positive neurons (Fig. 2E, F). In the V2M, most of the tdTomato-positive neurons colocalized with glutamatergic neurons (Fig. 2I, J). Furthermore, we examined the co-labeling of c-Fos-positive signals with glutamatergic neurons and GABAergic neurons in the V2M brain region. Our findings revealed that the increased c-Fos-positive signals in the V2M region predominantly colocalized with glutamatergic neurons (Fig. 2K–N). These results suggest the existence of direct projections V2MGlu → PrLGABA→Glu, providing a structural basis for subsequent functional studies.
Neuronal subtypes involved in light-induced impairment of spatial memory retrieval
Previous research has indicated that glutamate imbalances in the medial prefrontal cortex (mPFC) are linked to cognitive deficits31. To investigate this, we injected the AAV-CaMKII-taCasp3 virus into the PrL of mice to selectively eliminate glutamatergic neurons (Fig. 3A, B). This manipulation resulted in significant spatial memory impairment in these mice compared with the controls treated with artificial cerebrospinal fluid (ACSF) (Fig. 3C, D). We also selectively ablated glutamatergic neurons in the V2M using the same viral approach (Fig. 3, F). Interestingly, when the glutamatergic neurons in the V2M were ablated and the mice were exposed to 600 lux blue light for an extended 21 days, their spatial memory was not significantly affected compared with that of the controls exposed to normal light conditions (Fig. 3G, H). Selective ablation of V2MGlu neurons projecting to PrL prevented the spatial memory impairment caused by BLED exposure (Fig. 3I–L). These findings indicate that blue light may impair spatial memory in male mice by modulating the V2M→PrL neural circuitry, highlighting the crucial role of glutamatergic neurons in the V2M and PrL in spatial memory processing.
A Schematic diagram of PrLGlu neuron ablation. B Expression of PrLGlu neurons in the PrL of C57 mice following injection with ACSF (left) or AAV-CaMkII-taCasp3 virus (right) (Scale bar = 100 µm). C Trajectory maps of mice in the Y-maze. D Quantitative analysis of PrLGlu neurons (left) and behavioral statistics (middle and right) in C57 mice following injection of ACSF or AAV-CaMkII-taCasp3 virus into PrL. (n = 6 mice per group). E Schematic diagram of V2MGlu neuron ablation. F Expression of V2MGlu neurons in the V2M of C57 mice following injection with ACSF (left) or AAV-CaMkII-taCasp3 virus (right) (Scale bar = 100 µm). G Trajectory maps of mice in the Y-maze. H Quantitative analysis of V2MGlu neurons (left, n = 6 slices per group) and behavioral statistics (middle and right) in C57 mice following injection of ACSF or AAV-CaMkII-taCasp3 virus into the V2M region. (n = 6 mice per group). (I) Schematic diagram of V2MGlu-PrL neuron ablation. J Expression of V2MGlu neurons in the V2M region of C57 mice following injection with ACSF (left) or AAV-DIO-taCasp3 and retroAAV-CaMKII-Cre virus (right) (scale bar = 100 µm). K Trajectory maps of mice in the Y-maze. L Quantitative analysis of V2MGlu neurons (left, n = 6 slices per group) and behavioral statistics (middle and right) in C57 mice following injection of ACSF or AAV-DIO-taCasp3 and retroAAV-CaMKII-Cre virus into the V2M region. (n = 6 mice per group). All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; n.s., not significant. The actual time exploring object A or B during encoding and recall session are presented in Supplementary information (Table 1). Details of the statistical analyses are presented in Supplementary information (Table 2).
Effects of chemogenomic modulation of the V2M → PrL neural circuitry on spatial memory
To further establish whether prolonged activation of the V2M→PrL neural circuitry could induce persistent impairments in spatial memory, we utilized chemogenetic methods for validation. In the experimental group, we bilaterally injected the AAV-DIO-hM3Dq-mCherry virus into the V2M of C57 mice, whereas we injected the control group with the AAV-DIO-mCherry virus (Supplementary Fig. 5A–D). We subsequently injected the retroAAV-Cre virus into the ipsilateral PrL of both groups (Fig. 4A). Twenty-one days after the viral injections, V2MGlu neurons projecting to the PrL were activated by daily IP injections of the ligand clozapine-N-oxide (CNO; 2 mg/kg) during BLED sessions (Fig. 4B). Compared with those in the control group, we observed a significant decrease in spatial memory retrieval ability in the experimental mice that expressed hM3Dq following the CNO injections (Fig. 4C).
A Schematic diagram of chemogenetic activation of the V2M-PrL neural circuit. B Representative image of the injection site in the V2M of mice after chemogenetic activation (scale bar, 100 μm). C Bar graphs showing the time, the times and the total distances spent exploring the novel arm of the Y-maze (left) and the spatial discrimination index for novel object location (right) in mice treated with 21 days of CNO, for the mCherry and hM3Dq groups (n = 6 mice per group). D Schematic diagram of chemogenetic inhibition of the V2M-PrL circuit. E Representative image of the injection site in the V2M of mice after chemogenetic inhibition (scale bar, 100 μm). F Bar graphs showing the time, the times and the total distances spent exploring the novel arm of the Y-maze (left) and the spatial discrimination index for novel object location (right) in mice treated with 21 days of CNO and exposed to blue light, for the mCherry and hM4Di groups (n = 6 mice per group). G Representative action potential traces recorded from PrLGlu neurons of mice treated with 21 days of control or blue light exposure. H Bar graph showing the action potential firing frequency of PrLGlu neurons from mice treated with 21 days of control or blue light exposure (n = 18 cells per group). I Bar graph showing the action potential amplitude and the afterhyperpolarization (AHP)、the action potential duration at 50% peak-to-peak excursion (APD50) of PrLGlu neurons from mice treated with 21 days of control or blue light exposure (n = 16 cells per group). All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; n.s. not significant. The actual time exploring object A or B during encoding and recall session are presented in Supplementary information (Table 1). Details of the statistical analyses are presented in Supplementary information (Table 2).
Similarly, we selectively expressed inhibitory chemogenetic receptors in V2M neurons projecting to the PrL. We injected the AAV-DIO-hM4Di-mCherry virus bilaterally into the V2M (Fig. 4D), whereas we injected the control group with the AAV-DIO-mCherry virus (Supplementary Fig. 5E–H). Compare to control group, chemogenetic inhibition of the V2MGlu → PrL pathway reversed spatial memory impairments in mice that received both 600-lux blue light exposure for 21 days and concurrent daily CNO injections to inhibit these neurons (Fig. 4E, F).
To explore changes in the excitability of glutamatergic neurons in the PrL after long-term exposure to blue light, we employed ex vivo brain slice electrophysiology techniques. In current clamp mode, we recorded neuronal firing by injecting current. Compared with that in the control group, the firing frequency of PrLGlu neurons in the BLED-treated group was lower (Fig. 4G, I and Supplementary Fig. 6). These results further confirm that the V2MGlu → PrL circuit mediates blue light-induced spatial memory impairment in mice.
Dissection of the V2MGlu → PrLGABA→Glu neural pathway
To elucidate the functional connectivity of the V2MGlu → PrLGABA pathway, we employed optogenetic activation of glutamatergic neurons in the V2M and simultaneously monitored the calcium signals of GABAergic and glutamatergic neurons in the PrL using fiber photometry. Specifically, we injected the AAV-CaMKII-ChR2-mCherry optogenetic virus into the V2M of C57 mice, while delivering the AAV-CaMKII-GCaMP6m calcium signal virus into the PrL (Fig. 5A, B). After 3 weeks of viral expression, in vivo fiber photometry revealed that upon activation of V2MGlu neurons expressing ChR2 under 473-nm light, the calcium signal fluorescence intensity of PrLGlu neurons expressing GCaMP6m significantly decreased (Fig. 5C, D and Supplementary Fig. 7A, B). We subsequently injected the AAV-CaMKII-ChR2-mCherry and AAV-DIO-GCaMP6m into the V2M and PrL of Vgat-Cre mice, respectively (Fig. 5E, F). After 3 weeks of viral expression, upon the activation of V2MGlu neurons expressing ChR2 under 473-nm light, the calcium signal fluorescence intensity of PrLGABA neurons expressing GCaMP6m rapidly increased (Fig. 5G, H and Supplementary Fig. 7C, D). These results suggest that there is a functional connection between V2MGlu and the PrL and that there may be a microcircuit connection between GABAergic and glutamatergic neurons within the PrL, enabling GABAergic neurons in the PrL to inhibit the excitability of glutamatergic neurons.
A Schematic diagram of in vivo calcium signal fiber photometry recording of PrLGlu. B Representative images of in vivo optogenetic activation of V2MGlu neurons while recording PrLGlu signals (left: V2M fiber implantation site; right: PrL fiber implantation site; scale bar: 200 µm). C Heatmaps showing trials aligned to the onset of photostimulation (473 nm, 100 ms, white dashed line). D Representative traces of GCaMP6m-expressing signals in PrLGlu neurons of C57 mice. The thick green and blue lines indicate the mean, and the light shading represents the standard error of the mean (SEM). E Schematic diagram of in vivo calcium signal fiber photometry recording of PrLGABA. F Representative images of in vivo optogenetic activation of V2MGlu neurons while recording PrLGABA signals (left: V2M fiber implantation site; right: PrL fiber implantation site; scale bar: 200 µm). G Heatmaps showing trials aligned to the onset of photostimulation (473 nm, 100 ms, white dashed line). H Representative traces of GCaMP6m-expressing signals in PrLGABA neurons of Vgat-Cre mice. The thick green and blue lines indicate the mean, and the light shading represents the standard error of the mean (SEM). I Schematic diagram of in vivo calcium signal fiber photometry recording of PrLGlu. J Representative images of in vivo optogenetic activation of V2MGlu neurons while recording GCaMP6m-expressing PrLGlu signals (left: V2M fiber implantation site; right: PrL fiber implantation site; scale bar: 200 µm). K Heatmaps showing trials aligned to the onset of photostimulation (473 nm, 100 ms, white dashed line). L Representative traces of GCaMP6m-expressing signals in PrLGlu neurons of Vgat-Cre mice. The thick red and blue lines indicate the mean, and the light shading represents the standard error of the mean (SEM). M Schematic of viral injection and the recording configuration. N Representative images showing that terminals of ChR2 (red) from the V2M were frequently detected in GABAergic neurons (green) in the PrL. Representative traces (O) and summarized data (P) of light-evoked currents recorded from the PrL before and after CNQX (10 μM). All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; n.s. not significant. Details of the statistical analyses are presented in Supplementary information (Table 2).
To verify this hypothesis, we injected the AAV-CaMKII-hChR2-EGFP virus into the V2M and coinjecting the AAV-CaMKII-GCaMP6m and AAV- flex-taCasp3 viruses into the PrL of C57 mice to eliminate the inhibition of PrLGlu neurons by PrLGABA neurons (Fig. 5I). After 3 weeks of viral expression, most PrLGABA neurons were ablated, and in the taCasp3 group, the activation of V2MGlu neurons resulted in an increase in the calcium signal of PrLGlu neurons (Fig. 5J–L). In contrast, in the group without ablated PrLGABA neurons (the ACSF group), the calcium signal of PrLGlu neurons decreased (Fig. 5K, L and Supplementary Fig. 7E, F). To further characterize the functional connections in this V2M→PrL pathway, we used optogenetics combined with electrophysiology recordings. We found that brief light stimulation of ChR2-containing V2M terminals in the PrL reliably elicited excitatory postsynaptic currents (EPSC), which could be eliminated by administration of the AMPA receptor antagonist 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX) (Fig. 5M–P). These results indicate the existence of a functional V2MGlu → PrLGABA→Glu neural pathway.
Discussion
This study revealed the neural circuitry through which chronic blue light exposure impairs spatial memory in mice. We found that 600-lux blue light affected spatial memory by acting on the V2MGlu → PrLGABA→Glu pathway. Specifically, blue light activated V2MGlu neurons, increasing the excitability of downstream PrLGABA neurons and leading to the inhibition of local PrLGlu neurons and impaired spatial memory.
Light, a fundamental energy source for life, modulates multiple biological processes ranging from emotion and nociception to cognition. Previous study has shown that different wavelengths and intensities of light exposure can affect the spatial memory of mice in various ways. Huang et al.32 found that continuous exposure to 3000-lux white light for 3 weeks improved spatial memory in mice—an effect mediated by a double synaptic circuit between intrinsically photosensitive retinal ganglion cells (ipRGCs) and the thalamic intergeniculate nucleus. Similarly, Shang et al.33 discovered that exposure to 400-lux white light for 4 weeks enhanced spatial memory, which was mediated by neural circuits from ipRGCs to brain regions, such as the amygdala. Nevertheless, few studies have investigated the cognitive impacts of prolonged light exposure. Here, we focused on the blue light, which the retina can specifically perceive1,3,4,5 and may impair human spatial memory13,34,35. Our findings demonstrate that prolonged blue light exposure reduces PrL activity, consequently impairing spatial memory performance. Notably, daily blue light exposure of 4 or 8 h did not significantly affect mice’s spatial memory or PrL brain activity, suggesting that shorter durations are insufficient to induce the sustained neuronal changes required for memory-impairing plasticity. In contrast, 12-h exposure likely triggers distinct synaptic or structural plasticity within the V2M→PrL circuit, resulting in lasting functional alterations.
The PrL is part of the prefrontal cortex and is involved in regulating sensory information and cognitive functions, such as emotional regulation, social behavior, and decision-making9,17,23,24,36,37. It contains glutamatergic and GABAergic neurons, which modulate neuronal activity through AMPA receptors, influencing the excitation/inhibition balance38. PrL activity significantly correlates with spatial memory in mice9,24. Dysfunction of glutamatergic neurons in the PrL can impair spatial memory, as observed with methamphetamine exposure, which inhibits PrLGlu activity27. Additionally, normal aging increases GAT-3 expression in the PrL, affecting GABA levels and memory processes39. Our findings from the selective ablation of PrLGlu neurons support their role in spatial memory regulation.
The PrL does not receive direct input from the retina40,41,42. Instead, the effects of blue light on PrL neurons may be transmitted through visually related brain regions. In this work, retrograde tracing revealed that the PrL received substantial input from the V2M. The V2M processes complex visual features and contributes to spatial navigation and memory through connections with the medial entorhinal cortex43,44. Our electrophysiological results indicate the presence of a direct monosynaptic connection between V2M and PrL, providing a functional basis for the impairment of spatial memory by blue light. Furthermore, chemogenetic inhibition of the V2M→PrL pathway eliminated blue-light-induced spatial memory impairment. Optogenetic activation of V2MGlu neurons increased PrLGABA neuron activity while decreasing PrLGlu neuron activity, confirming the role of the V2MGlu → PrLGABA→Glu circuit in spatial memory impairment. These findings suggest that V2M plays a crucial role in converting visual information into spatial information, providing insights into the effects of blue light on memory and potential nonpharmacological treatments for memory disorders.
Conclusions and implications
In this study, we used behavioral experiments, fiber photometry recordings, and in vivo calcium imaging recordings to investigate the role of the V2MGlu → PrLGABA→Glu circuit in mediating the impact of blue light on spatial memory in mice. While we have made progress in understanding neural mechanisms, limitations exist. First, how the retina perceives blue light and influences V2M neurons remains unknown. Second, whether sleep alters the effect of blue light–induced spatial memory impairment remains to be explored. Additionally, it is unclear whether the V2M→PrL circuit is involved in blue-light-induced spatial memory impairment in humans, which requires further investigation.
Experimental model and subject details
Animals
All experimental animals in this study were 7–10-week-old male SPF-grade male mice with body weights of approximately 23 g. C57BL/6J mice were purchased from Hangzhou Ziyuan Laboratory Animal Co., Ltd (Hangzhou, China), whereas Ai14 (Rosa26-tdTomato) and Vgat-Cre mice were obtained from Jackson Laboratories. All experimental animals were randomly housed in groups of 3–5 per cage under a 12-h light/dark cycle, with an ambient temperature of 23–25 °C and humidity of 40–70%. The mice were provided with free access to water and food. The Animal Ethics Committee of Anhui Medical University approved all the animal experiments.
Spatial memory impairment model
The mice were exposed to white light (WLED group), blue light (BLED group), or green light (GLED group) during the daylight phase (8:00–20:00). This light exposure lasted for 12 h per day throughout the entire experimental period of 21 days. The mice in the control group were exposed to normal lighting conditions in a breeding room with the same light duration and cycle as the experimental group. The light intensity was measured precisely to ensure the consistency and reproducibility of the experimental conditions.
Method details
Y-maze test
The Y-maze test comprises two phases: training and testing. During the training phase, the animals were allowed to freely explore the starting and open arms of the maze for 10 min while the novel arm was blocked. The animals were then returned to their home cages. One hour later, the testing phase began with the novel arm opened. The animals were allowed to explore all three arms of the maze for 5 min. The total time spent in the novel arm and the number of entries into this arm (blocked during training) were measured and compared among groups using VisuTrack software (Shanghai Xinsoft Information Technology Co., Ltd, Shanghai, China). Each mouse was tested once on this test.
Novel object location test
Before the training phase, the mice were allowed to freely explore the open field for 10 min per day. During training, two identical objects were placed in the diagonal corners of the open field, 10 cm from the wall. The mice explored these objects for 10 min in a single training session. The test was conducted 10 min after training. In the test phase, one object was moved to a position parallel to the opposite wall. The percentage of time the mice spent exploring the new location over a 10-min period was calculated to assess their memory of the novel object location using the following formula: [Tm(B) − Tm(A)]/[Tm(B) + Tm(A)], where Tm represents the time spent exploring each object. All the objects were prescreened to ensure that there was no obvious preference from the mice. After each test, the open field and objects were cleaned with 70% ethanol and wiped with paper towels. Each mouse was tested once on this test.
Open field test (OFT)
In the OFT, the mice were placed into the open field arena, and their spontaneous activity within the box (50 × 50 × 40 cm) was recorded for 5 min. After the experiment, the percentage of time spent in the central area of the open field and the number of entries into this area were calculated. Following each animal’s test, the behavioral testing box was thoroughly cleaned with paper, alcohol, and distilled water. Each mouse was tested once on this test.
Elevated plus maze test (EPM)
In the EPM, the mice were placed in the central area of the maze facing the open arm, and their free movement within the maze was recorded for 5 min. After the experiment, the percentage of time each mouse spent in the open arms and the number of entries into the open arms were calculated. Following each animal’s test, the behavioral testing apparatus was thoroughly cleaned with paper, alcohol, and distilled water to ensure that there were no residual traces from previous tests. Each mouse was tested once on this test.
Immunohistochemistry experiment
The mice were deeply anesthetized with an intraperitoneal injection of 8% (w/v) sodium pentobarbital and transcardially perfused with 40 mL of saline followed by 20 mL of 4% paraformaldehyde (PFA). The brains were subsequently fixed in 4% PFA for 12–24 h and then immersed in 20% and 30% (w/v) sucrose solutions at 4 °C until they sank. Coronal sections (40-μm thickness) were collected using a cryostat (Leica, CM3050S, Germany). The sections were washed three times with PBS (5 min each) and incubated in blocking buffer (5% bovine serum albumin, 0.5% Triton X-100, and 5% donkey serum in PBS) for 1 h. Primary antibodies diluted in blocking buffer (3% bovine serum albumin, 0.3% Triton X-100, and 3% donkey serum in PBS) were applied, including anti-c-Fos (1:500, guinea pig, Synaptic Systems), anti-GABA (1:500, rabbit, Sigma, USA), and anti-glutamate (1:500, rabbit, Sigma), and incubated at 4 °C for 24 h. After the samples were washed three times with PBS, secondary antibodies (1:500; goat anti-guinea pig, Invitrogen, Cat# A11076; goat anti-rabbit, Invitrogen, Cat# A21207, USA) conjugated to Alexa 488 and 594 were applied, and the samples were incubated in the dark at 24 °C for 1.5 h. To label the nuclei, the sections were stained with DAPI (1:1000, Sigma, Cat. No. D9542). The sections were mounted with antifade mounting medium (Cat# P0131; Beyotime, Shanghai, China). The fluorescence signals were visualized using a laser confocal fluorescence microscope (Zeiss, LSM980+ Airyscan, Germany).
c-Fos expression mapping
For immunohistochemistry quantification, slice images were captured using a laser confocal microscope (Zeiss, LSM980+ Airyscan) at ×100 magnification. Each c-Fos-labeled region was localized using the reference brain atlas (The Mouse Brain, Paxinos and Franklin, 4th Edition). A laboratory technician blinded to the experimental conditions used ImageJ software (Fiji edition, NIH) to count c-Fos-positive cells. Specifically, each brain section was sampled using a square frame with an area of 0.04 mm2. The same sampled sections were then imported into ImageJ for uniform threshold processing. The number of c-Fos-positive cells was subsequently analyzed. Finally, the data were converted to units of cells per square millimeter for statistical analysis. Adjustments were made to the intensity colocalization with DAPI to ensure accurate counting of cells with moderate to high immunoreactivity. The mean count per mouse was used for statistical analysis.
Stereotaxic surgery and virus injection
Anterograde tracing
Male experimental mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (8%, w/v) and mounted on a stereotactic frame (RWD). The scalp was disinfected with povidone-iodine using cotton swabs, and an incision was made based on the location of the target brain regions. Using the bregma as a reference, the skull was leveled in the anterior–posterior and left–right directions. Holes were drilled above the bilateral target brain regions according to the mouse brain atlas, and the virus was injected into these regions using a microinjection pump and needle.
The adeno-associated virus (AAV) used was purchased from BrainVTA (Wuhan, China). For monosynaptic anterograde tracing, rAAV-hSyn-Cre-WPRE-hGH polyA (PT-0136, AAV2/1, 5.2 × 10¹² vg/mL, 80 nL) was injected into the V2M regions of Ai14 mouse brains (AP: +1.55 mm, ML: −2.58 mm, DV: −0.70 mm). After 3 weeks of viral expression, the mice were perfused transcardially, and brain slices were prepared to track the viral signals. The rates of colocalization of tdTomato⁺ neurons with GABAergic and glutamatergic neurons in the PrL regions were then quantitatively analyzed.
Retrograde tracing
For retrograde tracing, we injected the retrograde tracing virus ScAAV-Cre (ScAAV-hSyn-Cre-WPREs, PT-2334, AAV2/R, 5.2 × 1012 vg/mL, 150 nL) into the PrL (AP: +0.3 mm, ML: +2.34 mm, DV: −2.0 mm) of Ai14 mice. After 21 days of viral expression, the brain slices were imaged using confocal microscopy with the same method used for anterograde tracing. Subsequently, tdTomato⁺ neurons in the V2M were costained with antibodies specific for GABA or glutamate, and the colocalization rates were quantitatively analyzed.
Retrograde monosynaptic tracing
In addition, viruses containing AAV-CaMKII-Cre (rAAV-CaMKII-CRE-WPRE-hGH polyA, PT-0220, AAV2/R, 5.48 × 1012 vg/mL, 100 nL), AAV-DIO-RVG (rAAV-EF1a-DIO-oRVG-WPRE-hGH pA, PT-0023, AAV2/8, 5.47 × 1012 vg/mL, 100 nL) and AAV-DIO-TVA-mCherry (rAAV-EF1a-DIO-mCherry-F2A-TVA-WPRE-hGH pA, PT-0207, AAV2/8, 5.59 × 1012 vg/mL, 100 nL) were injected into PrL of C57 mice. After 2 weeks, RV-EnvA-∆G-EGFP (RV-CVS-EnvA-N2C(∆G)-EGFP, R01002, 2 × 108 IFU/mL, 100 nL) was injected into the PrL of C57 mice. After 1 week, mice were anesthetized and transcranial perfused, and brain slices were prepared for tracing EGFP+ signals.
Ablation virus injection
To investigate how PrL and V2M glutamatergic neurons affect spatial memory in mice, we used a virus-mediated, neuron-specific ablation technique. The AAV-CAMKIIα-taCasp3 virus (rAAV CaMKIIα-taCasp3-TEVpT2A-EGFP-WPRE-hGH pA, AAV 2/9, PT-8142, 5.4 × 1012 vg/mL, 150 nL) was injected into the PrL and V2M of C57BL/6 mice. To specifically ablate V2M glutamatergic neurons projecting to the PrL, we injected retroAAV-CaMKIIα-Cre (rAAV-CaMKIIα-Cre-WPRE-hGH polyA, AAV 2/R, PT-0220, 5.4 × 1012 vg/mL, 150 nL) bilaterally into the PrL and AAV-DIO- taCasp3 (rAAV-EF1a-flex-taCasp3-TEVp-WPRE-hGH polyA, AAV 2/9, PT-0206, 5.53 × 1012 vg/mL, 150 nL) bilaterally into the V2M of C57 mice. The control mice received artificial cerebrospinal fluid (ACSF).
Three weeks after virus injection, the mice were subjected to behavioral testing. Within 30 min of completing the experiment, the mice were perfused, and their brains were harvested for immunofluorescence staining. Target brain sections (PrL and V2M) were costained with a glutamate-specific antibody, and colocalization rates were quantitatively analyzed. The behavioral data were analyzed using GraphPad Prism 10.0 software (GraphPad Software, San Diego, CA, USA) to ensure the reliability and reproducibility of the results.
Chemogenetic virus injection
Specifically, rAAV-Ef1α-DIO-hM4D(Gi)-mCherry-WPRE-pA (AAV 2/9, PT-0043, 5.2 × 1012 vg/mL, 100 nL) and rAAV-Ef1α-DIO-hM3D(Gq)-mCherry-WPRE-pA (AAV 2/9, PT-0042, 5.3 × 1012 vg/mL, 100 nL) were injected into the V2M region, whereas ScAAV-Cre (ScAAV-hSyn-Cre-WPREs, PT-2334, AAV2/R, 5.18 × 1012 vg/mL, 150 nL) was injected into the PrL. The control group received AAV-DIO-mCherry (rAAV-Ef1α-DIO-mCherry-WPRE-pA, AAV2/9, PT-0013, 5.1 × 10¹² vg/mL, 100 nL).
Three weeks postinjection, the mice were anesthetized with isoflurane and given intraperitoneal injections of the chemogenetic ligand clozapine N-oxide (CNO, 2 mg/kg, HY-17366, MCE). Behavioral tests were performed at least 30 min postinjection, with controls following the same protocol. After testing, the mice were euthanized to verify the injection sites, and those with inaccurate targeting were excluded from analysis.
Fiber photometry
To investigate the activity and interactions of neurons in the PrL and V2M, we conducted fiber photometry experiments. For each mouse, AAV-DIO-GCaMP6m or AAV-CaMKIIα-GCaMP6s was injected into the PrL, and optical fibers were implanted to record calcium signals. Similarly, AAV-CaMKIIα-ChR2-mCherry was injected into the V2M, with fibers placed at the injection site and above the PrL to detect calcium signals from V2M glutamatergic neurons and their projections.
To study how optogenetic activation of V2M neurons affects PrL neurons, AAV-CaMKIIα-ChR2-mCherry was injected into the V2M, and AAV-DIO-GCaMP6m was injected into the PrL. Optical fibers were implanted above the PrL in either C57BL/6J or Vgat-Cre mice. To validate intracortical microcircuits in the PrL, we injected AAV-CaMKIIα-ChR2-mCherry into the V2M and a mixture of AAV-CaMKIIα-GCaMP6s and AAV-DIO-taCaspase3 into the PrL of Vgat-Cre mice to eliminate local inhibition by PrL GABAergic neurons.
Three weeks postinjection, the implanted fibers were connected to a laser generator. After 30 min, optogenetic stimulation was performed using blue light (473 nm, 5–8 mW, 15-ms pulse width, 20 Hz) controlled by a Master-8 stimulator. Calcium signals were recorded using a fiber photometry system with 470-nm and 410-nm LED sources for excitation and isosbestic control, respectively. The data were analyzed using MATLAB, and fluorescence changes (ΔF/F) were calculated to generate heatmaps and average calcium signal traces. Mice lacking GCaMP6m signal responses (approximately 15%) were excluded because of potential issues such as failed viral expression, missed target regions, or fiber blockages.
In vitro electrophysiological recordings
Acute brain slice preparation
Male mice aged 6–8 weeks were deeply anesthetized with pentobarbital (80 mg/kg, i.p.) and sacrificed during the dark phase under dim red light. The brains were rapidly removed and placed in ice-cold N-methyl-D-glucamine (NMDG) slicing solution containing: 92 mM NMDG, 30 mM NaHCO3, 1.2 mM KCl, 25 mM D-glucose, 1.2 mM KH2PO4, 5 mM L-ascorbic acid, 20 mM HEPES, 2 mM thiourea, 3 mM Na-pyruvate, 0.5 mM CaCl2, and 1 mM MgSO4 (pH 7.2 ± 0.1; osmolarity 310 ± 5 mOsm/kg). The solution was bubbled with 95% O2/5% CO2 for 10 min and stored in a refrigerator to maintain its ice-cold state. Coronal slices approximately 300 μm thick were obtained using a vibrating microtome (VT1200s, Leica) at a speed of 0.18 mm/s. The slices were then incubated for 30–45 min in ACSF: 1.25 mM KCl, 125 mM NaCl, 1.25 mM KH2PO4, 25 mM NaHCO3, 25 mM D-glucose, 1 mM MgCl2, 2 mM CaCl2, 0.4 mM L-ascorbic acid, and 2 mM Na-pyruvate, bubbled with 95% O2/5% CO2.
Whole-cell patch-clamp recordings
Whole-cell recordings were performed in oxygenated (95% O2 and 5% CO2) ACSF solution with constant perfusion at a rate of 1–2 mL/min. For action potential (AP) recording, the electrodes were filled with a potassium-based internal solution containing the following: 125 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 0.1 mM EGTA, 5 mM ATP-Mg, and 0.5 mM GTP-Na. The pH was adjusted to 7.2 ± 0.1 with potassium hydroxide (KOH), and the osmolarity was adjusted to 310 ± 5 mOsmol/kg with K-gluconate. To verify the effects of blue light exposure on neuronal excitability, we prepared coronal slices containing the PrL from mice after 3 weeks of blue light exposure and performed whole-cell patch-clamp recordings on PrLGlu+ neurons. The neurons were held at −70 mV. The resistance of the recording electrodes was 5–7 MΩ. The cells were visualized using a 40× water-immersion microscope (TH4-200 and U-RFL-T microscope; Olympus, Japan). Data were collected using an Axopatch 700B patch-clamp amplifier, a Digidata 1550B data acquisition system, and pClamp10.7 software (Molecular Devices, USA). The electrophysiological properties of the cells were analyzed using Clampfit 10.7 (Molecular Devices, USA). If the membrane resistance was less than 20 MΩ and the leak current was less than 50 pA during whole-cell recording, the cell was considered healthy and suitable for recording. Otherwise, the data were excluded from the statistical analysis. All the experiments were performed at room temperature (20–25 °C).
Light-evoked response
A laser (Shanghai Fiblaser Technology, China) through an optic fiber (200 mm OD, 0.37 NA, inper) was used to deliver optic stimulation with positioned 0.2 mm above the surface of the target brain region. Light-evoked postsynaptic currents were elicited by 20 ms blue light stimulation of axonal terminals of V2M neurons infected with ChR2-mCherry that projecting to the PrL. The EPSC was recorded with the membrane potential held at −70 mV. To identify the components of the current, 10 mM CNQX (HY-15066, MedChemExpress) was added to the ACSF.
Data acquisition
During the electrophysiological experiments, we amplified the neuronal electrical signals and converted them from analog signals to digital signals via an Axo-patch 700B patch clamp amplifier, a Digidatal 1550B data acquisition system, and Clampfit 10.7 software (Molecular Devices, USA). Throughout the recording process, we applied a low-pass filter of 3 kHz to the data and statistically analyzed the neuronal firing frequencies using Clampfit 10.7 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Supplementary Figures and Tables in the paper can be found in Supplementary information, and the source data behind the graphs in the paper can be found in Supplementary Data. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
Hu, J. et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell 185, 3124–3137.e3115 (2022).
Zaidi, F. H. et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr. Biol. 17, 2122–2128 (2007).
Lee, H. H. & Yeh, S. L. Blue-light effects on saccadic eye movements and attentional disengagement. Atten. Percept. Psychophys. 83, 1713–1728 (2021).
Schilling, T., Soltanlou, M., Nuerk, H. C. & Bahmani, H. Blue-light stimulation of the blind-spot constricts the pupil and enhances contrast sensitivity. PloS ONE 18, e0286503 (2023).
Yang, C. C., Tsujimura, S. I. & Yeh, S. L. Blue-light background impairs visual exogenous attention shift. Sci. Rep. 13, 3794 (2023).
Kumari, J., Das, K., Babaei, M., Rokni, G. R. & Goldust, M. The impact of blue light and digital screens on the skin. J. Cosmet. Dermatol 22, 1185–1190 (2023).
Lin, Z. et al. 40-Hz blue light changes hippocampal activation and functional connectivity underlying recognition memory. Front Hum. Neurosci. 15, 739333 (2021).
Silva, E. H. A. et al. Blue light exposure-dependent improvement in robustness of circadian rest-activity rhythm in aged rats. PloS ONE 18, e0292342 (2023).
An, K. et al. A circadian rhythm-gated subcortical pathway for nighttime-light-induced depressive-like behaviors in mice. Nat. Neurosci. 23, 869–880 (2020).
Silvani, M. I., Werder, R. & Perret, C. The influence of blue light on sleep, performance and wellbeing in young adults: a systematic review. Front. Physiol. 13, 943108 (2022).
Guarana, C. L., Barnes, C. M. & Ong, W. J. The effects of blue-light filtration on sleep and work outcomes. J. Appl. Psychol. 106, 784–796 (2021).
Alkozei, A. et al. Exposure to blue light increases subsequent functional activation of the prefrontal cortex during performance of a working memory task. Sleep 39, 1671–1680 (2016).
Liu, Q., Wang, Z., Cao, J., Dong, Y. & Chen, Y. Insulin ameliorates dim blue light at night-induced apoptosis in hippocampal neurons via the IR/IRS1/AKT/GSK3β/β-catenin signaling pathway. Ecotoxicol. Environ. Saf. 250, 114488 (2023).
Liu, Q., Wang, Z., Cao, J., Dong, Y. & Chen, Y. Dim blue light at night induces spatial memory impairment in mice by hippocampal neuroinflammation and oxidative stress. Antioxidants 11, https://doi.org/10.3390/antiox11071218 (2022).
Cao, P. et al. Green light induces antinociception via visual-somatosensory circuits. Cell Rep. 42, 112290 (2023).
Hallock, H. L. et al. Molecularly defined hippocampal inputs regulate population dynamics in the prelimbic cortex to suppress context fear memory retrieval. Biol. Psychiatry 88, 554–565 (2020).
Gao, F. et al. Elevated prelimbic cortex-to-basolateral amygdala circuit activity mediates comorbid anxiety-like behaviors associated with chronic pain. J. Clin. Investig. 133. https://doi.org/10.1172/jci166356 (2023).
Han, S. et al. Deactivation of dorsal CA1 pyramidal neurons projecting to medial prefrontal cortex contributes to neuropathic pain and short-term memory impairment. Pain 165, 1044–1059 (2024).
Medeiros, A. C. et al. Cannabidiol in the dorsal hippocampus attenuates emotional and cognitive impairments related to neuropathic pain: the role of prelimbic neocortex-hippocampal connections. Prog. Neuropsychopharmacol. Biol. Psychiatry 134, 111039 (2024).
Utashiro, N. et al. Long-range inhibition from prelimbic to cingulate areas of the medial prefrontal cortex enhances network activity and response execution. Nat. Commun. 15, 5772 (2024).
Contreras, C. M. & Gutiérrez-García, A. G. Insulin and fluoxetine produce opposite actions on lateral septal nucleus-infralimbic region connection responsivity. Behav. Brain Res. 437, 114146 (2023).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Tan, S. Z. K., Neoh, J., Lawrence, A. J., Wu, E. X. & Lim, L. W. Prelimbic cortical stimulation improves spatial memory through distinct patterns of hippocampal gene expression in aged rats. Neurotherapeutics 17, 2054–2068 (2020).
Pak, S. et al. Altered synaptic plasticity of the longitudinal dentate gyrus network in noise-induced anxiety. iScience 25, 104364 (2022).
Luo, Y. F. et al. Divergent projections of the prelimbic cortex mediate autism- and anxiety-like behaviors. Mol. Psychiatry 28, 2343–2354 (2023).
Zhang, S. R. et al. A prelimbic cortex-thalamus circuit bidirectionally regulates innate and stress-induced anxiety-like behavior. J. Neurosci. 44, https://doi.org/10.1523/jneurosci.2103-23.2024 (2024).
Ma, J. C. et al. Single-dose methamphetamine administration impairs ORM retrieval in mice via excessive DA-mediated inhibition of PrL(Glu) activity. Acta Pharmacol. Sin. 45, 2253–2266 (2024).
Alkozei, A. et al. Exposure to blue wavelength light is associated with increases in bidirectional amygdala-DLPFC connectivity at rest. Front Neurol. 12, 625443 (2021).
Killgore, W. D. S. et al. Blue light exposure increases functional connectivity between dorsolateral prefrontal cortex and multiple cortical regions. Neuroreport 33, 236–241 (2022).
Wang, G. et al. Short-term acute bright light exposure induces a prolonged anxiogenic effect in mice via a retinal ipRGC-CeA circuit. Sci. Adv. 9, eadf4651 (2023).
Asraf, K., Zaidan, H., Natoor, B. & Gaisler-Salomon, I. Synergistic, long-term effects of glutamate dehydrogenase 1 deficiency and mild stress on cognitive function and mPFC gene and miRNA expression. Transl. Psychiatry 13, 248 (2023).
Huang, X. et al. A visual circuit related to the nucleus reuniens for the spatial-memory-promoting effects of light treatment. Neuron 109, 347–362.e347 (2021).
Shang, M. et al. Moderate white light exposure enhanced spatial memory retrieval by activating a central amygdala-involved circuit in mice. Commun. Biol. 6, 414 (2023).
Meng, Q. et al. Blue light filtered white light induces depression-like responses and temporary spatial learning deficits in rats. Photochem. Photobio. Sci. 17, 386–394 (2018).
Han, R. et al. The effects of blue light on locomotion and cognition in early adult Drosophila melanogaster. J. Exp. Zool. A Ecol. Integr. Physiol. https://doi.org/10.1002/jez.2900 (2025).
Zhao, Y. T. et al. Adaptation of prelimbic cortex mediated by IL-6/STAT3/Acp5 pathway contributes to the comorbidity of neuropathic pain and depression in rats. J. Neuroinflammation 19, 144 (2022).
Lazzerini Ospri, L. et al. Light affects the prefrontal cortex via intrinsically photosensitive retinal ganglion cells. Sci. Adv. 10, eadh9251 (2024).
Fattorini, G. et al. Glutamate/GABA co-release selectively influences postsynaptic glutamate receptors in mouse cortical neurons. Neuropharmacology 161, 107737 (2019).
Gandy, H. M., Hollis, F., Hernandez, C. M. & McQuail, J. A. Aging or chronic stress impairs working memory and modulates GABA and glutamate gene expression in prelimbic cortex. Front Aging Neurosci. 15, 1306496 (2023).
Fernandez, D. C. et al. Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18 (2018).
Zangen, E. et al. Prefrontal cortex neurons encode ambient light intensity differentially across regions and layers. Nat. Commun. 15, 5501 (2024).
Vega-Zuniga, T. et al. A thalamic hub-and-spoke network enables visual perception during action by coordinating visuomotor dynamics. Nat. Neurosci. https://doi.org/10.1038/s41593-025-01874-w (2025).
Liu, Y. et al. Organization of corticocortical and thalamocortical top-down inputs in the primary visual cortex. Nat. Commun. 15, 4495 (2024).
Shao, Q. et al. A non-canonical visual cortical-entorhinal pathway contributes to spatial navigation. Nat. Commun. 15, 4122 (2024).
Acknowledgements
We thank Wenjuan Tao (Anhui Medical University) for critical comments on the manuscript. We thank Hao Huang (Anhui Medical University) for his technical assistance with analyses of calcium imaging data. This study was supported by grants from the National Natural Science Foundation of China (81801317, 32400846), the Natural Science Foundation of Anhui Province (1808085QH277) and the Natural Science Foundation of Universities of Anhui Province (2022AH050752, 2023AH050678).
Author information
Authors and Affiliations
Contributions
Y.Z. and G.A.T.: Conceptualization, investigation, methodology, validation, visualization, writing—original draft and editing. W.P.W.: Methodology, writing—draft and editing. Y.Y.X.: Methodology, funding acquisition, writing—draft and editing. W.B.Z., X.M.C. and X.J.W.: Investigation and methodology. X.Q. and L.C.W.: Conceptualization and visualization, writing—review. C.H.H.: Conceptualization, funding acquisition, visualization, writing—review. S.J.W.: Conceptualization, formal analysis, validation, visualization, writing—original draft and review. P.P.Z.: Conceptualization, formal analysis, investigation, methodology, validation, funding acquisition, visualization, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Robert Stackman Jr and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Tong, G., Wang, W. et al. Neural mechanisms of the V2M–PrL pathway mediate blue light-induced spatial memory impairment. Commun Biol 9, 39 (2026). https://doi.org/10.1038/s42003-025-09304-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-025-09304-0